Abstract
Background
Percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) are widely used in the treatment of coronary heart disease, but the best revascularization method for multivessel coronary artery disease (MVD) patients is still controversial. Hybrid coronary revascularization (HCR), together with CABG and PCI, have been proved to be feasible methods, but the long-term effect of HCR is not as clear as CABG.
Method
By October 2020, we retrieved articles from PubMed, Web of science, EMBASE and Cochrane library databases. The main results are based on major adverse cardiovascular and cerebral events (MACCE).
Result
A total of 18 articles (3 randomized controlled trials (RCTs) and 15 observational studies) were included in this meta-analysis. The outcomes of MACCE in the HCR group at perioperative, short-term (30 days to 1 year), medium-term (1 year to 5 years) and long-term (5 years and above) follow-up period were similar to those in the CABG group. The mortality rates of patients in perioperative, short-term and medium-term follow-up were similar to those in the CABG group, but lower than that in the CABG group at long-term follow-up (OR = 0.35, 95% CI 0.18–0.69, p = 0.002). The revascularization rate was higher in the HCR group during the perioperative period (OR = 3.50, 95% CI 2.07–5.94, p < 0.001), short-term (OR = 3.28, 95% CI 1.62–6.64, p < 0.001) and mid-term follow-up (OR = 2.84, 95% CI 1.64–4.92, p < 0.001).
Conclusion
Our results reveal that HCR is a safe and therapeutically effective alternative in treatments for MVD patients. It has not only less short-term adverse effect, but also better long-term effect, especially in death.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13019-022-01903-w.
Keywords: HCR, CABG, MACCE, MVD, Meta-analysis
Introduction
According to the World Health Organization report, in the 2016, Cardiovascular diseases (CVDs) are the number one cause of mortality globally, killing approximately 17.9 million each year [1]. Coronary artery disease (CAD), which can present as angina pectoris, myocardial infarction (MI), and ischemic heart failure [2], is one of the major types of CVDs [3]. Coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) are the two main treatments for patients with multivessel coronary artery disease (MVD) including left main coronary artery (LMCA) disease [2]. CABG results in a lower mortality & MI rate [4–6]. These benefits come from grafting of the left internal mammary artery(LIMA) to the left anterior descending (LAD) artery [7]. However, compared with PCI, CABG is a relatively invasive surgical procedure with a greater possibility of causing immediate complications such as bleeding, stroke, atrial fibrillation (AF), and eventually, prolonged hospitalization [2, 8–10]. PCI is a lesser invasive intervention, which allows the minimum procedural risk and a shorter recovery period. But it increases the possibility of repeat revascularization [2, 10, 11]. Therefore, the optimum revascularization strategy for MVD remains controversial [2, 12, 13].
Hybrid coronary revascularization (HCR), grafting LIMA to LAD along with PCI in the non-LAD vessels, is a less invasive alternative for MVD patients while maintaining durability. HCR may decrease repeat revascularization rate and enhance long term outcomes through benefiting from LIMA-LAD revascularization. As a minimum invasive intervention, HCR reduces risk of bleeding and infection, time of mechanical ventilation, and length of stay [7, 14].
In the recent years, HCR has received considerable attention as being the most suitable revascularization strategy for patients with MVD. In the current meta-analysis of HCR and CABG, no significant change in terms of in-hospital death, MI and stroke was observed between the two groups. Moreover, the need for red blood transfusion, length of hospitalization, length of intensive care unit (ICU) stay, and ventilation time were all better in HCR group [15–17]. HYBRID (Hybrid Revascularization for Multivessel Coronary Artery Disease) trial investigated a 5-year clinical follow-up of patient population that was randomly assigned to HCR group and CABG group. All-cause mortality available for entire cohort were similar in the 2 groups. No significant difference in the rates of MI, repeat revascularization, stroke and major adverse cardiovascular and cerebral events (MACCE) was observed in both the groups [18]. Regrettably, due to the lack of data in clinical trials and meta-analysis including numerous patients with long-term follow-up and various HCR operating conditions, HCR is not implemented broadly in clinical practice [19]. It was suggested that HCR is an alternative for CABG and PCI under specific circumstances listed in American College of Cardiology Foundation/American Heart Association guidelines and American College of Cardiology Foundation/American Heart Association guidelines [20, 21].
Several RCTs and observational studies have compared the long-term outcomes of HCR and CABG. Herein, a meta-analysis was conducted to compare the long-term difference between HCR and CABG in patients with MVD.
Method
Literature search strategy
Systematic literatures were retrieved using these databases following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [22]: PubMed, Web of Science, Embase, and the Cochrane Library database. The search was updated until October, 2020. The research subject was people with CAD. The key words included were “coronary artery disease” OR “coronary disease” OR “cardiovascular disease” OR “heart disease” OR “myocardial infarction” OR “coronary syndrome” OR “multivessel coronary artery disease” OR “left main coronary artery disease” AND “hybrid coronary revascularization” OR “hybrid myocardial revascularization” OR “integrated myocardial revascularization” OR “hybrid revascularization” OR “integrted coronary revascularization” OR “hybrid revascularization” OR “hybrid coronary intervention” OR “hybrid percutaneous coronary intervention”OR “hybrid percutaneous intervention” OR “hybrid coronary artery revoscularization” AND “coronary artery bypass” OR “coronary artery bypass graft” OR “coronary artery bypass grafting” OR “CABG” or “coronary artery bypass surgery”. We supplemented the studies, trials, and review articles manually for potential additional studies. The primary outcomes being analyzed were perioperative, short-term (30 days to 1 year), mid-term (1 year to 5 years) and long-term (5 years and above) MACCE (MI, stroke, mortality, and repeat revascularization). Secondary outcomes focused in individual aspects, more specifically, each period MACCE and in-hospital outcomes.
Inclusion and exclusion criteria
Studies that have met the following criteria were included: (1) original research that compared HCR and CABG for MVD patients; (2) RCTs or observational studies (cohort or case–control studies); (3) relevant data reported. Exclusion criteria were as followed: (1) ongoing studies or with unavailable data; (2) duplicate reports. The latest article will be included / selected if there were duplicate publications. (3) patient with previous experience of coronary revascularization. (4) The language was not English.
Data extraction and quality assessment
Two independent authors evaluated the included RCTs and observational studies via the Cochrane Collaboration’s tool [23] and the Newcastle–Ottawa Quality Assessment Scale [24]. Disagreements were resolved by a third researcher. The following information was extracted: author, publication year, country, sample size, technique of HCR and CABG, extent of CAD, type of stent, staging strategy and study design.
Statistical analysis
We used Review Manager 5.3 (The Cochrane Collaboration) to analyze the extracted data. The odds ratio (OR) and 95% confidence interval (CI) were calculated to establish dichotomous variables while the standardized mean difference (SMD) and the weighted mean difference (WMD) was were calculated for continuous variables. Heterogeneity among studies was quantified using I2 statistic. The fixed effects model was applied when p > 0.1 otherwise the random effects model would be used. p < 0.05 was considered statistically significant.
Result
Eligible studies
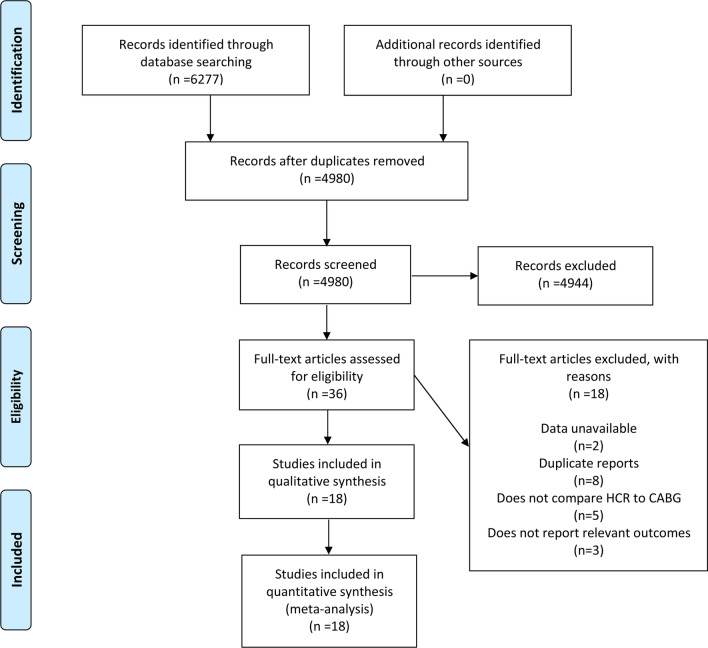
The search strategy originally generated 6,277 relevant clinical records from the four mentioned databases. There were eventually 18 studies (3 RCTs [25–27] and 15 observational studies [28–42]), which fulfilled our inclusion criteria after screening and eligibility assessment, thus were included in the analysis. The detailed searching and selecting process as well as the exclusion criteria can be found in Fig. 1.
Fig. 1.
Flow chart of study selection
Study characteristics and quality
Basic characteristics of all the studies included are shown in Table 1. There were 2041 cases of HCR and 2993 cases of CABG. The majority of patients included had been suffering from MVD. Patients in the HCR group underwent staged or single-staged procedures and the most commonly used stent type was a drug eluting stent (DES). Of all the subjects in the HCR, only LAD bypass received the bypass grafting. Patients in CABG group received on-pump or off-pump procedures. Other detailed characteristics were presented in Additional file 1: Table S1. The RCTs and observational studies were assessed by the Cochrane Collaboration’s tool and Newcastle–Ottawa Quality Assessment Scale, respectively. The results of the risk bias assessment were shown in Additional file 2: Table S2.
Table 1.
Characteristics of all the studies included in the meta-analysis
| Author | Year | Country | Number of patients | HCR technique | CABG technique | Number of CAD | Extent of CAD | Stents | Staging strategy | Study design | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HCR | CABG | ||||||||||
| Hage | 2019 | Canada | 216 | 147 | Robotic-assisted MIDCAB | OPCAB | Double-vessel CAD | LAD with non-LAD lesions | NA | NA | Cohort |
| Patel | 2018 | America | 207 | 207 | MIDCAB | CABG/OPCAB | Double-vessel CAD | LAD with circumflex or RCA | DES | Staged: CABG first | Cohort |
| Qiu | 2019 | China | 52 | 128 | NA | OPCAB | Double-vessel CAD | LAD with non-LAD lesions | DES | NA | Cohort |
| Wu | 2017 | China | 73 | 383 | NA | OPCAB | MVD | LAD with non-LAD lesions | DES | Staged: CABG first | Cohort |
| Di Bacco | 2019 | Italy | 89 | 89 | MIDCAB | NA | NA | NA | NA | Staged: PCI first | Cohort |
| Hannan | 2020 | American | 302 | 302 | MIDCAB | NA | MVD | LAD with non-LAD lesions | NA | Staged | Cohort |
| Shen | 2013 | China | 141 | 141 | MIDCAB | CABG/OPCAB | MVD | LAD with non-LAD lesions | DES | Single-stage | Cohort |
| Modrau | 2020 | Denmark | 103 | 103 | MIDCAB/OPCAB | CABG | MVD | LAD with non-LAD lesions | DES | NA | Cohort |
| Basman | 2020 | America | 100 | 100 | robotic-assisted MIDCAB | CABG/OPCAB | TVD | NA | DES | Staged: CABG first | Cohort |
| Zhao | 2009 | America | 112 | 154 | NA | CABG/OPCAB | NA | NA | NA | Single-stage | Cohort |
| Delhaye | 2010 | France | 18 | 18 | NA | CABG/OPCAB | MVD | LAD with non-LAD lesions | DES | Staged: CABG first | Cohort |
| Harskamp | 2015 | Netherlands | 306 | 918 | MIDCAB | NA | MVD | NA | DES | Staged | Cohort |
| Kon | 2008 | America | 15 | 30 | NA | OPCAB | MVD | LAD with non-LAD lesions | DES | Single-stage | Cohort |
| de Cannière | 2001 | America | 20 | 20 | MIDCAB + PTCA | CABG | Double-vessel CAD | LAD with non-LAD lesions | DES or BMS | Staged: PCI first | Cohort |
| Farid | 2018 | America | 100 | 82 | MIDCAB | MIDCAB | MVD | LAD with non-LAD lesions | DES or BMS | Staged: CABG first | Cohort |
| Gąsior | 2014 | Poland | 98 | 102 | MIDCAB/EACAB | CABG/OPCAB | MVD | LAD with non-LAD lesions | DES | NA | RCT |
| Ganyukov | 2020 | Russia | 49 | 49 | MIDCAB | CABG | MVD | LAD with non-LAD lesions | DES | Staged: CABG first | RCT |
| Esteves | 2020 | Brazil | 40 | 20 | NA | CABG | TVD | NA | NA | Staged: CABG first | RCT |
CABG = coronary artery bypass grafting; HCR = Hybrid coronary revascularization; CAD = coronary artery disease; OPCAB = Off-pump coronary artery bypass grafting; MIDCAB = minimally invasive CABG; DES = drug-eluting stent; MVD = multivessel coronary artery disease; PTCA = percutaneous transluminal coronary angioplasty; BMS = bare metal stent; EACAB = endoscopic atraumatic coronary artery; NA = not available; RCT = Randomized Controlled Trial; TVD = triple-vessel disease; LAD = left anterior descending artery; RCA = right coronary artery
Incidence of MACCE
There was no significant difference in perioperative (≤ 30 days) MACCE between HCR and CABG (OR = 0.90, 95% CI 0.54–1.48, p = 0.67). MACCE were followed up as time went on. 11.3% patients treated with HCR and 8.9% patients with CABG had suffered from MACCE during one-year follow-up (30 days to 1 year) (OR = 1.35, 95% CI 0.73–2.49, p = 0.34). A total of 13.7% patients treated with HCR and 8.7% patients who had undergone CABG have suffered from at least one kind of the MACCE during mid-term follow-up (1 year to 5 years) (OR = 1.25, 95% CI 0.53–2.97, p = 0.61). MACCE occurred in 28.6% patients after HCR and 30.2% patients after CABG during the long-term follow-up (5 years and above) (OR = 0.93, 95% CI 0.61–1.41, p = 0.72). The outcomes of MACCE at short-term, mid-term and long-term follow-up were not statistically dissimilar. Detailed data are shown in Table 2, Table 3.
Table 2.
Summary of In-hospital Outcomes Comparing HCR and CABG
| Subgroup | No. of studies | OR | 95%CI | p | I2 (%) | Effect-model |
|---|---|---|---|---|---|---|
| MACCE | 6 | 0.90 | 0.54–1.48 | 0.67 | 0 | Fixed |
| ICU LOS | 9 | − 13.34 | − 20.27 to (− 6.41) | < 0.001 | 95 | Random |
| Ventilation time | 5 | − 8.69 | − 17.74 to 0.36 | 0.06 | 97 | Random |
| Major bleeding | 2 | 0.36 | 0.11–1.22 | 0.10 | 58 | Fixed |
| Return to work | 2 | − 68.26 | − 77.99 to (− 58.53) | < 0.001 | 0 | Fixed |
| Atrial fibrillation | 8 | 0.58 | 0.36–0.93 | 0.02 | 56 | Random |
| Infection | 6 | 0.24 | 0.09–0.64 | 0.004 | 1 | Fixed |
| cTnI | 3 | − 0.39 | − 1.06 to 0.27 | 0.25 | 83 | Random |
| 25% increase in creatinine | 2 | 0.88 | 0.55–1.39 | 0.57 | 0 | Fixed |
| Cerebrovascular accidents | 2 | 0.87 | 0.21–3.65 | 0.85 | 42 | Fixed |
| Mechanical ventilation > 24 h | 5 | 0.49 | 0.32–0.76 | 0.001 | 31 | Fixed |
| Any transfusion of packed red blood cells | 10 | 0.38 | 0.28–0.51 | < 0.001 | 58 | Random |
| Neurologic event | 2 | 1.24 | 0.15–10.53 | 0.84 | 0 | Fixed |
| Renal failure | 7 | 0.72 | 0.40–1.31 | 0.29 | 51 | Random |
| Operation time | 3 | − 27.86 | − 109.08 to 52.37 | 0.50 | 99 | Random |
|
Extubation in operating room |
2 | 0.13 | 0.04–0.41 | < 0.001 | 0 | Fixed |
| Death | 8 | 1.65 | 0.90–3.02 | 0.11 | 0 | Fixed |
| Myocardial infarction | 12 | 0.77 | 0.45–1.30 | 0.32 | 0 | Fixed |
| Pleural effusion | 2 | 0.44 | 0.20–0.97 | 0.04 | 0 | Fixed |
| Repeat revascularization | 6 | 3.50 | 2.07–5.94 | < 0.001 | 0 | Fixed |
| Hemodialysis | 3 | 0.3 | 0.05–1.75 | 0.18 | 0 | Fixed |
| Platelet transfusion | 2 | 0.41 | 0.18–0.91 | 0.03 | 0 | Fixed |
| Major complications | 3 | 0.40 | 0.16–1.03 | 0.06 | 61 | Random |
| Reopening for bleeding | 8 | 1.11 | 0.80–1.53 | 0.53 | 0 | Fixed |
| Stroke | 7 | 0.92 | 0.44–1.94 | 0.84 | 0 | Fixed |
| Hospital length of stay | 11 | − 1.62 | − 2.38 to (− 0.85) | < 0.001 | 87 | Random |
HCR, hybrid coronary revascularization; CABG, coronary artery bypass grafting; OR, odds ratio; CI, confidence interval; MACCE,major adverse cardiac or cerebrovascular events; ICU LOS, intensive care unit length of stay
The effect measure is standard mean difference (SMD): cTnI. The effect measure is weighted mean difference (WMD): ICU LOS; Ventilation time; Return to work; Operation time; Hospital length of stay
Table 3.
Summary of Follow up Outcomes Comparing HCR and CABG
| Subgroup | No. of studies | OR | 95%CI | p | I2 (%) | Effect-model |
|---|---|---|---|---|---|---|
| One-year | ||||||
| MACCE | 6 | 1.35 | 0.73–2.49 | 0.34 | 0 | Fixed |
| Death | 5 | 1.32 | 0.47–3.70 | 0.59 | 0 | Fixed |
| Myocardial infarction | 6 | 1.31 | 0.64–2.70 | 0.46 | 0 | Fixed |
| Repeat revascularization | 6 | 3.28 | 1.62–6.64 | < 0.001 | 0 | Fixed |
| Stroke | 2 | 3.73 | 0.60–23.02 | 0.16 | 0 | Fixed |
| Within one to five year | ||||||
| MACCE | 4 | 1.25 | 0.53–2.97 | 0.61 | 64 | Random |
| Neurologic event | 2 | 0.29 | 0.07–1.15 | 0.08 | 22 | Fixed |
| Death | 3 | 1.90 | 0.66–5.48 | 0.24 | 0 | Fixed |
| Myocardial infarction | 3 | 1.18 | 0.44–3.14 | 0.74 | 33 | Fixed |
| Repeat revascularization | 4 | 2.84 | 1.64–4.92 | < 0.001 | 0 | Fixed |
| Five-year | ||||||
| MACCE | 3 | 0.93 | 0.61–1.41 | 0.72 | 25 | Fixed |
| Death | 3 | 0.35 | 0.18–0.69 | 0.002 | 15 | Fixed |
| Myocardial infarction | 3 | 0.83 | 0.34–2.04 | 0.68 | 0 | Fixed |
| Repeat revascularization | 4 | 1.05 | 0.71–1.53 | 0.82 | 7 | Fixed |
| Stroke | 2 | 0.57 | 0.16–2.01 | 0.39 | 0 | Fixed |
HCR, hybrid coronary revascularization; CABG, coronary artery bypass grafting; OR, odds ratio; CI, confidence interval; MACCE, major adverse cardiac or cerebrovascular events; ICU LOS, intensive care unit length of stay
Secondary outcomes
Twelve studies referred to the MI. No significant difference was observed to the incidence of MI between HCR group and CABG group during perioperative period (≤ 30 days) (OR = 0.77, 95% CI 0.45–1.30, p = 0.32); short-term (30 days to 1 year) (OR = 1.31, 95% CI 0.64–2.70, p = 0.46), mid-term (1 year to 5 years) (OR = 1.18, 95% CI 0.44–3.14, p = 0.74) and long-term follow-up (5 years and above) (OR = 0.83, 95% CI 0.34–2.04, p = 0.68). Death was reported in eight studies. The mortality in perioperative period (OR = 1.65, 95% CI 0.90–3.02, p = 0.11), short-term (OR = 1.32, 95% CI 0.47–3.70, p = 0.59) and mid-term follow-up (OR = 1.90, 95% CI 0.66–5.48, p = 0.24) between HCR group and CABG group were not significant. Three articles have mentioned the death during long-term follow-up. Patients who had undergone HCR had a lower risk of mortality rate during long-term follow-up compared with those who had undergone CABG (OR = 0.35, 95% CI 0.18–0.69, p = 0.002) (Table 2, Table 3).
Seven articles mentioned repeat revascularization. HCR was associated with a significantly higher risks for repeat revascularization in perioperative period (OR = 3.50, 95% CI 2.07–5.94, p < 0.001), short-term (OR = 3.28, 95% CI 1.62–6.64, p < 0.001)and mid-term follow-up (OR = 2.84, 95% CI 1.64–4.92, p < 0.001). But the prevalence of long-term follow-up repeat revascularization was not significantly different between the two groups (OR = 1.05, 95% CI 0.71–1.53, p = 0.82). Patients treated with HCR did not display a significant reduction in risk of developing a stroke as compared to those who underwent CABG in perioperative period (OR = 0.92, 95% CI 0.44–1.9, p = 0.84), short-term (OR = 3.73, 95% CI 0.60–23.02, p = 0.16) and long-term follow-up (OR = 0.57, 95% CI 0.16–2.01, p = 0.39) (Table 2, Table 3).
Table 2 shows the comparison in in-hospital outcomes. The need for any transfusion of packed red blood cells was significantly lower in the HCR group (OR = 0.38, 95% CI 0.28–0.51, p < 0.001). Moreover, patients in HCR group were associated with a significantly shorter ICU stay (WMD = − 13.34, 95% CI − 20.27 to (− 6.41), p < 0.001) and hospital stay (WMD = − 1.62, 95% CI − 2.38 to (− 0.85), p < 0.001) on average. Results were not significantly different when it comes to development of renal failure (OR = 0.72, 95% CI 0.40–1.31, p = 0.29). The event rate of having AF (OR = 0.58, 95% CI 0.36–0.93, p = 0.02) (Table 2) and infection(OR = 0.24, 95% CI 0.09–0.64, p = 0.004) was higher in CABG group compared with HCR group.
Discussion
An ideal coronary revascularization should offer minimal invasiveness and maximal durability in order to reduce the risk of surgery and increase the survival rate [7, 14]. Under the circumstances, HCR is an alternative for patients with MVD. In this meta-analysis from 15 observational cohort studies and 3 RCTs, we focused on occurrence of MACCE and its components (MI, stroke, mortality, and repeat revascularization) over time. By reviewing the previous meta-analysis, similar conclusions have been raised. To be more detailed, HCR have similar short-term results and lower blood transfusion rates and infection rates than CABG, which our results confirmed again [15]. In addition to the parameters mentioned, which were provided detailly in previous literatures and recent meta-analysis studies, long-term (5 years and above) results in terms of efficacy of HCR vs CABG for treating MVD were assessed by our team for the first time. The outcomes suggested that HCR did not only merit over CABG in short-run, but also had a trend of better long-term results, particularly in survival aspect.
MACCE is the composite endpoint of MI, stroke, mortality, and repeat revascularization. No significant between-group difference of MACCE during the follow-up period has been discovered. However, HCR tended to be hold a lower MACCE incidence in the long-term follow-up. This is possibly because, patients with CABG is superior for repeated revascularization in the short-term follow up while patients with HCR seems to be associated with lower perioperative MI and stroke rates, though not statistically significant. In the long-term follow-up, there is no difference between the two groups in repeated revascularization as well as death, HCR patients have obvious advantages. This outcome is consistent with a prospective randomized pilot study, HYBRID, which is the only randomized trial worldwide with a long-term follow-up [18]. Moreover, previous meta had supported this view. Hu et al. [17] and Sardar et al. [43] respectively revealed that there was no difference in MACCE incidence between HCR group and CABG group in both short-term and mid-term follow-up.
The possible reasons for the advantage in CABG group in early repeated revascularization were as follows. (1) Stents cannot (but bypass grafting can) prevent disease progression near the injured site [44]. (2) Long term Computer Tomography(CT) follow-up indicated that the incidence of primary artery occlusion in MVD patients after transplantation was 3 times higher than that those without transplantation. Thus, due to insufficient target vessels, the progression of coronary heart disease may be an obstacle in of vascular reconstruction in patients after CABG [45]. (3) Due to the recognized low rate of revascularization and increased invasiveness of CABG, patients who presents early atypical chest symptoms after sternotomy may be diagnosed with incision pain, musculoskeletal or pericardial disease and requires medication. Atypical symptoms after PCI are more likely to bring concern about disease progression or stent restenosis. Thus, the possibility of coronary angiography is increased, which has also been demonstrated. Hage et al. [28] pointed out that patients who have underwent CABG did not undergo any routine angiographic evaluation of the graft, while all HCR patients were required with postoperative angiograms, and any patients found with significant anastomotic abnormalities underwent would be required to undergo intervention. Therefore, the rates of graft failure and revascularization in the CABG group may have been underestimated. But there is no significant difference in long-term follow-up. This may due to increase in the risk of saphenous vein graft(SVG) degeneration after the intervention period over time. The graft occlusion rate is approximately 20% in the first year, 30% in 10 years, and almost 70% in 15 years [46]. Non-LAD vascular PCI does not depend on saphenous vein transplantation. Repeat interventions due to stent failure usually occur in the first few years after surgery. With the development of newer generation DES, restenosis rates have been decreased to < 10% overall and < 5% after one year [47]. In summary, non-LAD vessels underwent CABG will present a progressively higher stenosis rate over time while underwent PCI will increase stent stenosis rate slowly in the long term, hence there will be no difference in late repeat revascularization.
Our data seems to have revealed a more pronounced ascent trend in mortality rate in CABG group compared with HCR group as follow-up duration increased. There was no significant difference in perioperative period; short-term, mid-term follow-up between patients underwent HCR and CABG respectively. However, in the long-term follow-up, patients who had undergone HCR had a drastic advantage over those who had undergone CABG. The speculative mortality trends conclusion raised by another study is in accordance with ours [48]. This may be due to the following reasons. (1) A staged CABG-then-PCI strategy, the current most common approach, enable confirm the quality of LIMA–LAD graft at the time of PCI thus defects can be corrected immediately in time [49]. (2) Aortic clamping is avoided during HCR but it is necessary during on-pump CABG, which may increase the risk of cerebral infarction [50]. (3) We recorded the all-cause death, so deaths may not necessarily have occurred by cardiovascular events. Compared with CABG, HCR is less invasive and less harmful to other body systems, which is especially beneficial for elderly patients. (4) we speculate that it is the difference of severity in MACCE that leads to similarity of occurrence probability and differences in death. Previous studies have shown that after stents intervention, angina is reduced, and stroke is mainly in the early stage [28]. Regrettably, no studies have been done to determine whether there is a difference in MACCE degree between these two. (5) Death is a clear indicator for statistical convenience, while MACCE is a mixed event, and leading to errors in assessments. However, Harskamp et al. [51] concluded in a meta-analysis that there was no difference in mortality rate between the two groups. He illustrated that the main concern with HCR is the quality of the anastomosis, especially the long-term adverse clinical outcome is related to LIMA deficiency. Nevertheless, the failure rate of LIMA after HCR was significantly higher than that of CABG in the long-term follow-up. However, LIMA failure is considered to be more related to repeat revascularization and has no direct link with mortality. Harskamp et al. also have only included six observational studies which definitely present a large risk of bias due to the limited amount of literature. This can result from many more patients have appeared with more severe disease that refuses CABG due to the more invasive nature [43]. Therefore, his conclusion is considered to be controversial and awaits further confirmation.
Besides, there was no difference in stroke and MI in each period between two groups. This conclusion is consistent among all existing RCTs [25, 26]. Sardar et al. [43] indicated that, comparing with traditional coronary artery bypass grafting, the risk of stroke after arterial embolization is lower in theory. Our data also showed a lower but not statistically significant perioperative risk for stroke in the HCR group, and this may be because the avoidance of aortic valve surgery and cardiopulmonary bypass in HCR. The atherosclerotic plaque displacement during aortic surgery has been considered as the major cause of stroke [52]. Also, there is a significant difference in the degree of cardiac surgery between HCR and CABG. The heart is in a natural position in HCR, but needs to be rotated frequently in CABG, which may affect the hemodynamic state. In addition, the stent placement time is less than 30 s, thus coronary artery occlusion during HCR is limited to the one that requires to place a single LIMA-LAD graft. In contrast, CABG requires a 8–12 min of coronary artery occlusion in each of the three or four distal anastomoses, resulting in a total ischemic time of estimately 25–40 min [17].
In a large number of randomized trials, the combination of clopidogrel and aspirin has been proved to drastically reduce the incidence of stent thrombosis in PCI patients [53]. Together with antiplatelet therapy, HCR avoids sternotomy and is less invasive, compared with CABG. These advantageous characteristics of HCR are widely believed also by other peer researchers to be capable to result in lower blood transfusion rate, shorter ventilation time and shorter ICU as well as other kinds of, hospital stay in HCR group. Patients are able to have a higher quality of life and greater satisfaction [28, 29].
We found that the rate of AF was relatively low in the perioperative period of HCR. A series of reports investigated that the incidence of AF after minimally invasive CABG (MIDCAB) is between 4 and 23% [29]. These differences may result from subtle though unidentified differences in surgical or anesthetic techniques. As there will be fine precautions designed in advance to control bleeding during MIDCAB, the pericardial window into LAD should be as small as possible, leading to a low incidence of postoperative AF.
In general, HCR has better perioperative results with a good trend of long-term survival rate, but its repeat revascularization rate is higher in the short- and medium-term period. Although repeat revascularization is often evaluated, it is far less important for patients than other endpoints, such as death, MI. It is a complicated to define whether repeating revascularization for patients with stable condition is essential or not. Among all the influential factors, the most vital ones are instructions and preferences, while other endpoints are more qualitative rather than quantitative assessed [53]. Therefore, even if HCR has a higher revascularization rate in the short- and medium- term perspective, its feasibility can still be proposed in treatments for MVD patients.
Though in theory, HCR combines the advantages of both procedures, it is still suggested that HCR actually combines the unfavorable disadvantages. For example, performing two types of invasive surgery in a short period of time requires a high degree of cooperation between the two surgical teams, not mentioning the higher cost. Therefore, HCR needs to be generalized with greater advantages in follow-up.
Our study also has some limitations. Firstly, there are only three RCTs compareing the safety of HCR and CABG while more observational studies have been included. Therefore, chaos between the trial population and selection bias may affect the results. Secondly, the definition of MACCE varies from study to study. Most studies define MACCE as a composite endpoint of MI, stroke, mortality, and repeat revascularization. Others replace repeat revascularization with target vessel revascularization (TVR), or delete stroke. After all, there are few studies with different definitions, hence the general speculation is not significant.
Conclusion
In summary, HCR is relatively less invasive with better perioperative results, and hold a higher trend in long-term survival rate for MVD patients. HCR has the same therapeutic effect but lower long-term mortality incidence compared with CABG. Therefore, we believe that HCR is a promising alternative methods for MVD patients though more long-term RCTs with large-scale are needed in the future.
Supplementary Information
Additional file 1: Table S1. Characteristics of all the studies included in the meta-analysis.
Additional file 2: Table S2. Quality assessment of studies included.
Acknowledgements
Not applicable.
Abbreviations
- PCI
Percutaneous coronary intervention
- CABG
Coronary artery bypass grafting
- MVD
Multivessel coronary artery disease
- HCR
Hybrid coronary revascularization
- MACCE
Major adverse cardiovascular and cerebral events
- RCT
Randomized controlled trial
- CVD
Cardiovascular diseases
- CAD
Coronary artery disease
- LMCA
Left main coronary artery
- LIMA
Left internal mammary artery
- LAD
Left anterior descending
- ICU
Intensive care unit
- AF
Atrial fibrillation
- DES
Drug-eluting stent
- MI
Myocardial infarction
- MIDCAB
Minimally invasive CABG
- OR
Odds ratio
- CI
Confidence interval
- SMD
Standardized mean difference
- WMD
Weighted mean difference
- CT
Computer Tomography
Author contributions
All authors helped to perform the research. BC, LY, and KZ manuscript writing; ND and YS performing procedures and data analysis; JL contribution to writing the manuscript; RS contribution to drafting conception and design. All authors read and approved the final manuscript.
Funding
This study was supported by Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (Grant No. 2022KY343).
Availability of data and materials
Data supporting findings reported in this study are available in the supplementary materials.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare there was no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li Yu and Keying Zhu contributed equally to this work
References
- 1.Anderson JL, Morrow DA. Acute Myocardial Infarction. N Engl J Med. 2017;376(21):2053–2064. doi: 10.1056/NEJMra1606915. [DOI] [PubMed] [Google Scholar]
- 2.Neumann F-J, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):2546. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 3.Malakar AK, et al. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234(10):16812–16823. doi: 10.1002/jcp.28350. [DOI] [PubMed] [Google Scholar]
- 4.Head SJ, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet (London, England) 2018;391(10124):939–948. doi: 10.1016/S0140-6736(18)30423-9. [DOI] [PubMed] [Google Scholar]
- 5.Stone GW, et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med. 2019;381(19):1820–1830. doi: 10.1056/NEJMoa1909406. [DOI] [PubMed] [Google Scholar]
- 6.Umakanthan J, et al. Barriers to the universal adoption of bilateral internal mammary artery grafting. Int J Surg (Lond, Engl) 2015;16(Pt B):179–182. doi: 10.1016/j.ijsu.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 7.McKiernan M, Halkos ME. Hybrid coronary revascularization: are we there yet? Curr Opin Cardiol. 2020;35(6):673–678. doi: 10.1097/HCO.0000000000000784. [DOI] [PubMed] [Google Scholar]
- 8.Palmerini T, et al. Risk of stroke with percutaneous coronary intervention compared with on-pump and off-pump coronary artery bypass graft surgery: evidence from a comprehensive network meta-analysis. Am Heart J. 2013;165(6):215. doi: 10.1016/j.ahj.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Farkouh ME, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367(25):2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 10.Morice M-C, et al. Outcomes in patients with de novo left main disease treated with either percutaneous coronary intervention using paclitaxel-eluting stents or coronary artery bypass graft treatment in the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial. Circulation. 2010;121(24):2645–2653. doi: 10.1161/CIRCULATIONAHA.109.899211. [DOI] [PubMed] [Google Scholar]
- 11.Desai ND, et al. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N Engl J Med. 2004;351(22):2302–2309. doi: 10.1056/NEJMoa040982. [DOI] [PubMed] [Google Scholar]
- 12.Patel MR, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: A report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 2019;157(3):e131–e161. doi: 10.1016/j.jtcvs.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Serruys PW, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 14.Moreno PR, et al. The hybrid coronary approach for optimal revascularization: JACC review topic of the week. J Am Coll Cardiol. 2020;76(3):321–333. doi: 10.1016/j.jacc.2020.04.078. [DOI] [PubMed] [Google Scholar]
- 15.Nolan S, et al. Hybrid coronary revascularization vs complete coronary artery bypass grafting for multivessel coronary artery disease: a systematic review and meta-analysis. J Invasive Cardiol. 2018;30(12):E131–E149. [PubMed] [Google Scholar]
- 16.Reynolds AC, King N. Hybrid coronary revascularization versus conventional coronary artery bypass grafting: Systematic review and meta-analysis. Medicine. 2018;97(33):e11941. doi: 10.1097/MD.0000000000011941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu F-B, Cui L-Q. Short-term clinical outcomes after hybrid coronary revascularization versus off-pump coronary artery bypass for the treatment of multivessel or left main coronary artery disease: a meta-analysis. Coron Artery Dis. 2015;26(6):526–534. doi: 10.1097/MCA.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tajstra M, et al. Hybrid coronary revascularization in selected patients with multivessel disease: 5-year clinical outcomes of the prospective randomized pilot study. JACC Cardiovasc Interv. 2018;11(9):847–852. doi: 10.1016/j.jcin.2018.01.271. [DOI] [PubMed] [Google Scholar]
- 19.Harskamp RE, et al. Practice patterns and clinical outcomes after hybrid coronary revascularization in the United States: an analysis from the society of thoracic surgeons adult cardiac database. Circulation. 2014;130(11):872–879. doi: 10.1161/CIRCULATIONAHA.114.009479. [DOI] [PubMed] [Google Scholar]
- 20.Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur J Cardio-thoracic. 2014;46(4):517–592. doi: 10.1093/ejcts/ezu366. [DOI] [PubMed] [Google Scholar]
- 21.Fihn SD, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):251. doi: 10.1016/j.jacc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Research Ed) 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed) 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 25.Gąsior M, et al. Hybrid revascularization for multivessel coronary artery disease. JACC Cardiovasc Interv. 2014;7(11):1277–1283. doi: 10.1016/j.jcin.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 26.Ganyukov V, et al. Randomized clinical trial of surgical vs. percutaneous vs. hybrid revascularization in multivessel coronary artery disease: residual myocardial ischemia and clinical outcomes at one year-hybrid coronary REvascularization versus stenting or surgery (HREVS) J Interv Cardiol. 2020;2020(250):5458064. doi: 10.1155/2020/5458064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esteves V, et al. Late clinical outcomes of myocardial hybrid revascularization versus coronary artery bypass grafting for complex triple-vessel disease: long-term follow-up of the randomized MERGING clinical trial. Catheterization Cardiovasc Interv. 2021;97(2):259–264. doi: 10.1002/ccd.28710. [DOI] [PubMed] [Google Scholar]
- 28.Hage A, et al. Hybrid coronary revascularization versus off-pump coronary artery bypass grafting: comparative effectiveness analysis with long-term follow-up. J Am Heart Assoc. 2019;8(24):e014204. doi: 10.1161/JAHA.119.014204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel NC, et al. Short- and intermediate-term outcomes of hybrid coronary revascularization for double-vessel disease. J Thorac Cardiovasc Surg. 2018;156(5):251. doi: 10.1016/j.jtcvs.2018.04.078. [DOI] [PubMed] [Google Scholar]
- 30.Qiu J, et al. Hybrid coronary revascularization versus off-pump coronary artery bypass grafting and percutaneous coronary intervention for the treatment of two-vessel coronary artery disease with proximal left anterior descending artery stenosis. J Thorac Dis. 2019;11(6):2402–2409. doi: 10.21037/jtd.2019.05.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu S, et al. Mid-term follow-up outcomes of 2-staged hybrid coronary revascularization compared with off-pump coronary artery bypass for patients with multivessel coronary artery disease. Wideochirurgia i inne techniki maloinwazyjne = Videosurgery and other miniinvasive techniques. 2017;12(2):178–185. doi: 10.5114/wiitm.2017.66803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Bacco L, et al. Long-term follow-up of total arterial versus conventional and hybrid myocardial revascularization: a propensity score matched analysis. Cardiovasc Revascul Med Including Mol Interv. 2019;20(1):22–28. doi: 10.1016/j.carrev.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Hannan EL, et al. Hybrid coronary revascularization versus conventional coronary artery bypass surgery: utilization and comparative outcomes. Circ Cardiovasc Interv. 2020;13(10):e009386. doi: 10.1161/CIRCINTERVENTIONS.120.009386. [DOI] [PubMed] [Google Scholar]
- 34.Shen L, et al. One-stop hybrid coronary revascularization versus coronary artery bypass grafting and percutaneous coronary intervention for the treatment of multivessel coronary artery disease: 3-year follow-up results from a single institution. J Am Coll Cardiol. 2013;61(25):2525–2533. doi: 10.1016/j.jacc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Modrau IS, et al. Outcome of hybrid compared to conventional revascularization in multivessel coronary artery disease. Scand Cardiovasc J SCJ. 2020;54(6):376–382. doi: 10.1080/14017431.2020.1821910. [DOI] [PubMed] [Google Scholar]
- 36.Basman C, et al. Long-term survival in triple-vessel disease: Hybrid coronary revascularization compared to contemporary revascularization methods. J Card Surg. 2020;35(10):2710–2718. doi: 10.1111/jocs.14891. [DOI] [PubMed] [Google Scholar]
- 37.Zhao DX, et al. Routine intraoperative completion angiography after coronary artery bypass grafting and 1-stop hybrid revascularization results from a fully integrated hybrid catheterization laboratory/operating room. J Am Coll Cardiol. 2009;53(3):232–241. doi: 10.1016/j.jacc.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Delhaye C, et al. Hybrid revascularization, comprising coronary artery bypass graft with exclusive arterial conduits followed by early drug-eluting stent implantation, in multivessel coronary artery disease. Arch Cardiovasc Dis. 2010;103(10):502–511. doi: 10.1016/j.acvd.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Harskamp RE, et al. Comparative effectiveness of hybrid coronary revascularization vs coronary artery bypass grafting. J Am Coll Surgeons. 2015;221(2):25. doi: 10.1016/j.jamcollsurg.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Kon ZN, et al. Simultaneous hybrid coronary revascularization reduces postoperative morbidity compared with results from conventional off-pump coronary artery bypass. J Thorac Cardiovasc Surg. 2008;135(2):367–375. doi: 10.1016/j.jtcvs.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Cannière D, et al. Combination of minimally invasive coronary bypass and percutaneous transluminal coronary angioplasty in the treatment of double-vessel coronary disease: two-year follow-up of a new hybrid procedure compared with "on-pump" double bypass grafting. Am Heart J. 2001;142(4):563–570. doi: 10.1067/mhj.2001.118466. [DOI] [PubMed] [Google Scholar]
- 42.Farid S, et al. Long-term outcome of patients undergoing minimally invasive direct coronary artery bypass surgery: a single-center experience. Innovations (Philadelphia, Pa) 2018;13(1):23–28. doi: 10.1097/imi.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 43.Sardar P, et al. Hybrid coronary revascularization versus coronary artery bypass grafting in patients with multivessel coronary artery disease: a meta-analysis. Catheteriz Cardiovasc Interv. 2018;91(2):203–212. doi: 10.1002/ccd.27098. [DOI] [PubMed] [Google Scholar]
- 44.Lamelas P, et al. Limitations of repeat revascularization as an outcome measure: JACC review topic of the week. J Am Coll Cardiol. 2019;74(25):3164–3173. doi: 10.1016/j.jacc.2019.10.041. [DOI] [PubMed] [Google Scholar]
- 45.Yoon S-H, et al. Risk of new native-vessel occlusion after coronary artery bypass grafting. Am J Cardiol. 2017;119(1):154. doi: 10.1016/j.amjcard.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Sabik JF, et al. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann Thorac Surg. 2005;79(2):256. doi: 10.1016/j.athoracsur.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 47.Alexander JH, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294(19):2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 48.Fan M-K, et al. Clinical outcomes of revascularization strategies for patients with MVD/LMCA disease: a systematic review and network meta-analysis. Medicine. 2015;94(42):e1745. doi: 10.1097/MD.0000000000001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanagawa B, et al. What is the state of hybrid coronary revascularization in 2018? Curr Opin Cardiol. 2018;33(5):540–545. doi: 10.1097/HCO.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 50.Kotoh K, et al. Predictors of early postoperative cerebral infarction after isolated off-pump coronary artery bypass grafting. Ann Thorac Surg. 2007;83(5):1679–1683. doi: 10.1016/j.athoracsur.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 51.Harskamp RE, et al. Clinical outcomes after hybrid coronary revascularization versus coronary artery bypass surgery: a meta-analysis of 1,190 patients. Am Heart J. 2014;167(4):585–592. doi: 10.1016/j.ahj.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Lev-Ran O, et al. No-touch aorta off-pump coronary surgery: the effect on stroke. J Thorac Cardiovasc Surg. 2005;129(2):307–313. doi: 10.1016/j.jtcvs.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 53.Steinhubl SR, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Characteristics of all the studies included in the meta-analysis.
Additional file 2: Table S2. Quality assessment of studies included.
Data Availability Statement
Data supporting findings reported in this study are available in the supplementary materials.