Abstract
Background
Chromosome aberrations of 10p monosomy and 10q trisomy resulting from parental pericentric inversion 10 are extremely rare, and to date, very few reports have been published on the matter.
Case Presentation
A 30-year-old pregnant woman with recurrent pregnancy loss is enrolled in this research. In this pregnancy, spontaneous abortion occurred in the first trimester of her pregnancy. Chromosomal microarray analysis of the abortion tissue showed a partial 10p monosomy (arr[GRCh37] 10p15.3p11.21(100,047_34,848,853) × 1) and a duplication of 10q (arr[GRCh37] 10q26.13q26.3(126,093,990_135,426,386) × 3). Further parental karyotype analysis indicated that the chromosomal abnormalities in the fetus was resulted from paternal pericenric inversion inv(10)(p11.21q26.13). This study presents the first case of a large deletion of 10p combined with 10q trisomy, resulting in pregnancy loss. Of these two manifestations, the large deletion of chromosome 10p may be the primary reason for spontaneous abortion in this subject.
Conclusions
This study presents the first case of partial 10p monosomy associated with 10q trisomy in Chinese population. It provides more information on the chromosome aberration of 10p monosomy and 10q trisomy and further strengthens the application value of microarray in the molecular etiological diagnosis of recurrent spontaneous abortion.
Keywords: Recurrent spontaneous abortion, Molecular cytogenetics, Monosomy 10p, Trisomy 10q, Pericentric inversion
Background
Novel genetic abnormalities in the fetus and parental inheritance are a common cause of accidental or recurrent spontaneous abortion (RSA). These irregularities include fetal aneuploidy abnormalities, copy number variants, single-gene diseases, etc. [1] However, the most common parental inheritance factors are the balanced translocation of parental chromosomes, or Robertson translocation and inversion. In RSA cases, 5% of parents have chromosomal rearrangements, including balanced translocations, and Robertson translocations and inversions [2]. In recent years, chromosomal microarray technology has gradually replaced karyotype analysis in the genetic analysis of spontaneous abortion, with advantage of automatic and easily identification of chromosomal abnormalities, as well as increased detection rate of chromosomal abnormalities without cell culture. [3, 4]
Terminal chromosomal duplication and deletion that occurs in the same chromosome is usually the result of parental inversion. Chromosomal pericentric inversion commonly forms a unique inversion loop in the first meiosis, resulting in four different gametes, including a normal chromosome and an inverted chromosome. It also causes two kinds of chromosomal recombinants with deletions and duplications of distal regions [5]. In general, the smaller of the inverted segment, the larger of the duplication and deletion segments in the gametes. This consequently results in a tendency towards infertility, miscarriage, and stillbirth. This chromosomal rearrangement also leads to the production of unbalanced gametes including 10p monosomy associated with 10q trisomy and opposite terminal chromosomal rearrangements. However, few reports are available on the pericentric inversion of chromosome 10.
In this study, we identified a recombinant chromosome l0, which was caused by a paternal pericentric inversion inv(10)(p11.21q26.13). This was the first-ever observation of a large deletion of chromosome 10p associated with 10q trisomy, resulting in spontaneous abortion in the first trimester. Besides, we reviewed the molecular cytogenetics of chromosomal abnormalities resulting from familial pericentric inversion 10 (Table 1).
Table1.
Molecular cytogenetics findings of recombinant chromosome 10 resulting from familial pericenric inversion
| Dutrillaux et al.[6] | Rodriguez et al.[7] | Ohba et al.[8] | Kulharya et al.[9] | Kozma and Meck [10] | Ciuladaite et al.[5] | Our case | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient1 | Patient2 | Patient3 | Patient1 | Patient2 | Patient1 | Patient2 | |||||
| Age/Sex | Newborn/Male | Newborn /Male | Newborn /Female | Newborn /Male | 2.5/Male | Fetus/Female | 4/Female | 36/Male | 13/Female | 28/Female | Fetus/Male |
| Trisomic segment | 10q24 → q26 | 10q24 → qter | 10p11.2 → pter | 10p11.2 → pter | 10p11.2 → pter | 10p11.2 → pter | 10p11.2 → pter | 10p11.2 → pter | 10p15.1 → pter | 10q26.12 → qter | 10q26.13 → qter |
| Monosomic segment | 10p15 → pter | 10p15 → pter | 10q25.2 → qter | 10q26 → qter | 10q26 → qter | 10q26 → qter | 10q26 → qter | 10q26 → qter | 10q26.12 → qter | 10p15.1 → pter | 10p11.21 → pter |
| Breakpoints | 10p15-q24 | 10p15-q24 | 10p11.2-q25.2 | 10p11.2-q26 | 10p11.2-q26 | 10p11.2-q26 | 10p11.2-q26 | 10p11.2-q26 | 10p15.1-q26.12 | 10p15.1-q26.12 | 10p11.21-q26.13 |
| Inheritance | Maternal | Maternal | Maternal | Maternal | Maternal | Maternal | Maternal | Maternal | Maternal | Maternal | Paternal |
| Clinical phenotypes | Facial deformities, finger deformities, psychomotor development delay, severe hypotonia | Facial deformities, long thin fingers | Facial deformities, clinodactyly of the fifth fingers, clenched hands, club foot, ocular abnormalities | Facial deformities, hypotonic and can not rolled over | Facial deformities, right exotropia, bilateral single palmar creases and fifth finger clinodactyly, absent fifth toenails, moderate motor delay | stillborn occurred after delivery | Facial deformities, increased length of the palm, foot deformities, poor physical growth, severe to profound intellectual disability | Facial deformities, unilateral cleft and corresponding absent tooth, partial hearing loss, and a heart murmur, growth and intellectual disability, increased palm length | Facial deformities, psychomotor and language development delay, strabismus, malocclusion of teeth, tapering fingers, transversal crease in the left palm, deformed feet | Psychomotor development delay, obesity, arched eyebrows, low-set ears, short philtrum | Spontaneous abortion occurred in the first trimester |
Methods
Approximately 2 ml of peripheral blood was collected from both parents. Parental chromosome karyotype analysis was conducted using G-banding technology with approximately 300 bands based on the automatic chromosome harvesting system Sinochrome Chromprep II (Shanghai Lechen Biotechnology Co., Ltd, China). The International System for Human Cytogenomic Nomenclature (ISCN 2020) was used as the reference for delineates karyotypes. The QIAamp DNA Blood Kit (QIAGEN, Germany) was utilized to extract genomic DNA from the abortion tissue and parental peripheral blood of the foetus. SNP array analysis was conducted using Affymetrix CytoScan 750 K, according to the Affymetrix CytoScan Assay user guide (http://www.thermofisher.com). The chromosomal copy number variants were described according to the Human NCBI Build GRCh37 (hg19/2009).
Case presentation
A 30-year-old pregnant woman (gravida 4, para 0) from Quanzhou City, Fujian province, China came to Quanzhou Women’s and Children’s Hospital because of spontaneous abortion and adverse pregnancy history. Her husband was 31 years old. There was no family history of hereditary disease and the couple denied any consanguinity. At her first pregnancy, termination was conducted at a gestational age of 13+ weeks due to nuchal translucency (NT) thickening (7 mm) and fetal oedema. A spontaneous abortion then occurred at 8+ weeks during the second pregnancy. The third pregnancy was terminated at gestational age of 11+ weeks due to NT thickening (11.2 mm) and hygroma. Unfortunately, the abortion tissues were not available. In the most recent pregnancy, early threatened abortion occurred at gestational age of 8+2 weeks of pregnancy, and aborted tissue was discharged at gestational age of 8+5 weeks. Molecular investigation was further performed after termination.
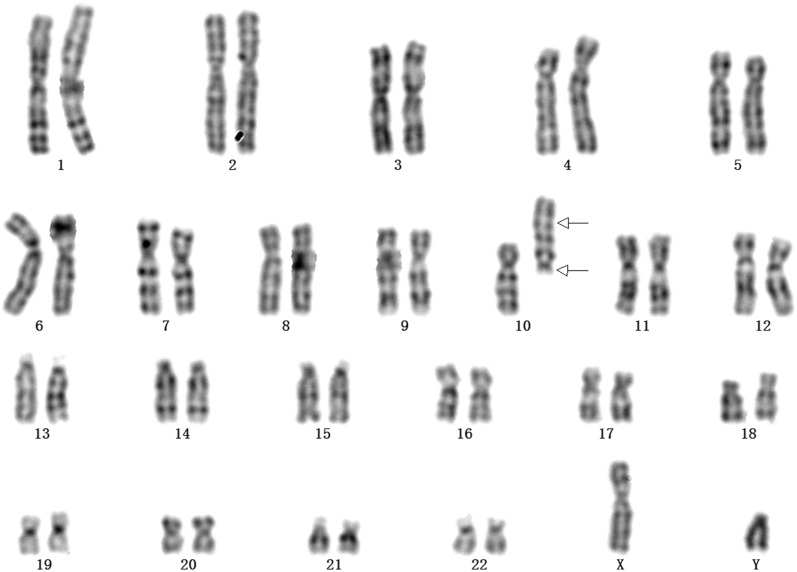
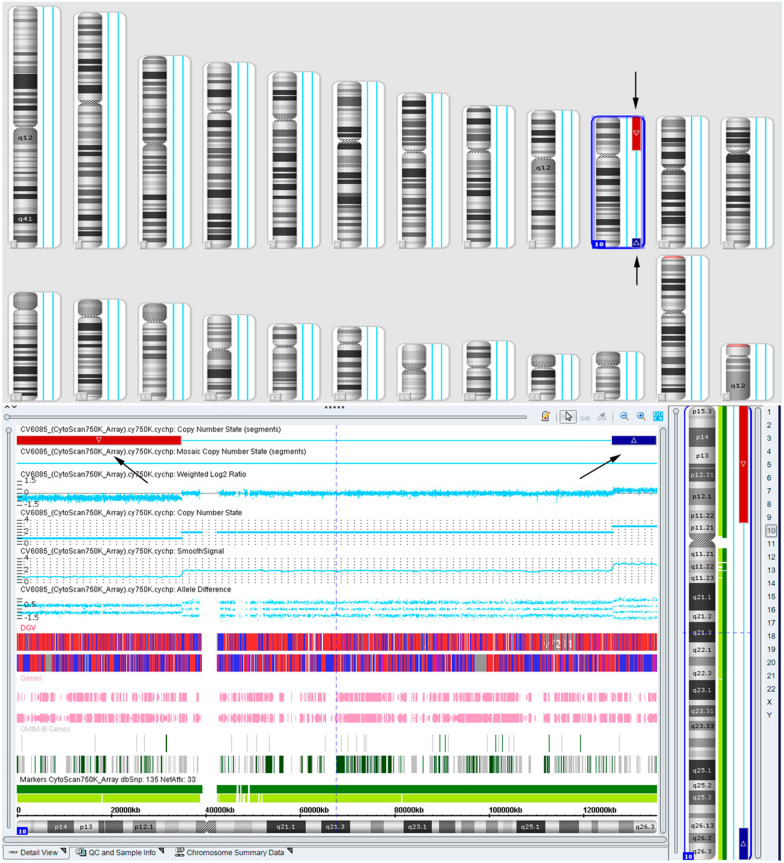
The pregnant woman received prenatal clinical consultation and parental karyotype analysis was further performed. Chromosome karyotype analysis revealed a karyotype of 46,XY,inv(10)(p11.21q26.13) in the prospective father (Fig. 1), and a karyotype of 46,XX in the prospective mother. The SNP array analysis of the abortion tissue showed a 34.7-Mb deletion in 10p11.21p15.3 region (arr[GRCh37] 10p15.3p11.21(100,047_34,848,853) × 1) of chromosome 10 (Fig. 2). Additionally, we observed a 9.3-Mb duplication of chromosome 10q26.13q26.3 region (arr[GRCh37] 10q26.13q26.3(126,093,990_135,426,386) × 3), as illustrated in Fig. 2. The deletion of chromosome 10p11.21p15.3 contained 32 Online Mendelian Inheritance in Man (OMIM) genes and the 10q26.13q26.3 duplication covered 35 OMIM genes. The SNP array was performed in the parents and no copy number variants were observed.
Fig. 1.
The result of chromosome karyotype in the prospective father. The arrows indicated the breakpoints of inversion of chromosome 10. The upper arrow elicited the breakpoint of 10p11.21 and the lower arrow indicated the location of 10q26.13, and the karyotype of the prospective father was described as 46,XY, inv(10)( p11.21q26.13)
Fig. 2.
The SNP array detection results of in the fetus. The arrows indicated the locus of duplication and deletion segments. The red bar represents 10p11.21p15.3 deletion and the blue bar indicates the duplication of 10q26.13q26.3
Discussion and conclusions
Few studies are available on the familial recombination of a pericentric inversion of chromosome 10, a condition which usually results in unbalanced gametes including monosomy 10p associated with trisomy 10q and opposite terminal chromosomal rearrangements. The smaller the inverted segment would lead to more severe phenotype. To our knowledge, this is the first case with large deletion of 10p result from paternal pericentric inversion inv(10)(p11.21q26.13) and leading to spontaneous abortion in the first trimester.
As displayed in Table 1, mostly of the present cases harbored trisomy 10p and monosomy 10q and exhibiting the typical features of trisomy 10p, which was a well-delineated chromosomal abnormality characterization by facial deformities, finger deformities, as well as growth and intellectual disabilities. A previous study conducted by Chen et al. [11] demonstrated a fetus with a 31.65-Mb duplication of 10p15.3p11.22 and a 3.07-Mb deletion of 10q26.3 resulting from paternal pericentric inversion, and the pregnancy was subsequently terminated at gestational age of 23 weeks with the fetus displaying facial dysmorphism of hypertelorism, large low-set ears, a broad and long nasal bridge, a long face, and micrognathia, which were consistent with partial trisomy 10p syndrome.
Recombination in chromosome 10 with partial monosomy 10p and trisomy 10q resulting from parental pericentric inversion is less common. At present, only three cases of partial 10p deletion and 10q duplication resulting from pericentric inversion have been reported in our best knowledge. [5–7] Distal trisomy 10q is a well-recognized syndrome, with typical features including growth retardation, hypotonia, mild to severe intellectual disabilities, and mild to severe psychomotor retardation [12]. Studies revealed that partial 10p deletion and 10q duplication mainly exhibit clinical features of trisomy 10q, which usually present a small deletion in the distal of chromosome 10p [5–7]. However, our case displayed a significant deletion of 10p, which was the first case of a large deletion of chromosome 10p associated with partial trisomy 10q resulting from pericentric inversion. In this study, a deletion fragment range from 10p11.21 to the distal with a 34.7-Mb deletion was identified, which is a rare chromosomal abnormality, and it includes three defined distinct contiguous gene deletion syndromes, including the 10p13-10p14 region contributed to the DiGeorge critical region 2 (DGCR2) syndrome, [13] the 10p14 region containing GATA3 gene that responsible for hypoparathyroidism, deafness, and renal anomalies (HDR Syndrome), [14] and 10p15.3 microdeletion syndrome [15, 16]. Thus, we believe that the large deletion in chromosome 10p may ascribe to the miscarriage in our study. Since the aborted tissues of the first three pregnancies are not available, the chromosomal abnormality types were unknown. However, a previous study also delineated a fetus who harbored trisomy 10p and monosomy 10q with stillborn after delivery. [9]
In the present study, none of the relevant clinical phenotypes were observed due to early spontaneous abortion. Despite that, the OMIM genes in the 10p monosomy involving CACNB2, [17] KLF6, [18] MLLT10, [19] RAB18, [20] WDR37, [21] ZEB1 [22] have been reported to be essential for embryonic critical organs development or neuronal development and migration, which may cause pregnancy loss. While, more work must be done to investigate the critical genes for embryonic development in this region.
In conclusion, we presented a recombinant chromosome l0 resulting from the paternal pericentric inversion inv(10)(p11.21q26.13), which was the first reported case of a large deletion of chromosome 10p associated with 10q trisomy that resulted in spontaneous abortion in the first trimester. Moreover, our study provides more information on the chromosome aberration of 10p monosomy and 10q trisomy and also provides valuable data for prenatal genetic consultation.
Acknowledgements
We would like to thank the patients who participated in this study. We would also like to express our appreciation for the support provided by the Fujian Provincial Health Commission.
Abbreviations
- SNP array
Single nucleotide polymorphism array
- Chr
Chromosome
- NT
Nuchal translucency
- dup
Duplication
- inv
Inversion
- rec
Recombinant
- RSA
Recurrent spontaneous abortion
- OMIM
Online Mendelian inheritance in man
Author contributions
JZ designed the study; CC and JZ wrote the article; JZ and SZ performed routine chromosome analysis; RH and QL conducted the parental recruitment and data analysis; YX, YJ and YW modified and proofread the paper. All authors have approved the final article.
Funding
This research was supported by the Fujian Provincial Health Commission Youth Science and Technology Project (2020QNB045).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author by reasonable request.
Declarations
Ethics approval and consent to participate
Ethics Committee approval was obtained from the Institutional Ethics Committee of Quanzhou Women’s and Children’s Hospital for the commencement of this study (2020No.31). We received informed consent from the study participants, and they agreed to the publication of a report on the study.
Consent for publication
We confirm that all subjects who participated in this study signed written informed consent for publication of their own and their children’s genetic data and other relevant information.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianlong Zhuang and Chunnuan Chen contributed equally to this work.
Contributor Information
Jianlong Zhuang, Email: 415913261@qq.com.
Yingjun Xie, Email: xieyjun@mail2.sysu.edu.cn.
References
- 1.Blue NR, Page JM, Silver RM. Genetic abnormalities and pregnancy loss. Semin Perinatol. 2019;43(2):66–73. doi: 10.1053/j.semperi.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tunç E, Tanrıverdi N, Demirhan O, Süleymanova D, Çetinel N. Chromosomal analyses of 1510 couples who have experienced recurrent spontaneous abortions. Reprod Biomed Online. 2016;32(4):414–419. doi: 10.1016/j.rbmo.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Rajcan-Separovic E. Chromosome microarrays in human reproduction. Hum Reprod Update. 2012;18(5):555–567. doi: 10.1093/humupd/dms023. [DOI] [PubMed] [Google Scholar]
- 4.Dhillon RK, Hillman SC, Morris RK, McMullan D, Williams D, Coomarasamy A, et al. Additional information from chromosomal microarray analysis (CMA) over conventional karyotyping when diagnosing chromosomal abnormalities in miscarriage: a systematic review and meta-analysis. BJOG. 2014;121(1):11–21. doi: 10.1111/1471-0528.12382. [DOI] [PubMed] [Google Scholar]
- 5.Ciuladaite Z, Preiksaitiene E, Utkus A, Kučinskas V. Relatives with opposite chromosome constitutions, rec(10)dup(10p)inv(10)(p15 1q26 12) and rec(10)dup(10q)inv(10)(p15 1q26 12), due to a familial pericentric inversion. Cytogenet Genome Res. 2014;144(2):109–113. doi: 10.1159/000368863. [DOI] [PubMed] [Google Scholar]
- 6.Dutrillaux B, Laurent C, Robert JM, Lejeune J. Inversion péricentrique, inv(10), chez la mére et aneusomie de recombinaison, inv(10), rEC(10), chez son fils [Pericentric inversion, inv(10), in a mother and aneusomy by recombination, inv(10), rec(10), in her son (author's transl)] Cytogenet Cell Genet. 1973;12(4):245–253. doi: 10.1159/000130460. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez MT, Martin MJ, Abrisqueta JA. Familial pericentric inversion (10) and its effect on two offspring. J Med Genet. 1984;21(4):317–319. doi: 10.1136/jmg.21.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohba K, Ohdo S, Sonoda T. Trisomy 10p syndrome owing to maternal pericentric inversion. J Med Genet. 1990;27(4):264–266. doi: 10.1136/jmg.27.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulharya AS, Schneider NR, Wilson GN. Three cases of dup(10p)/del(10q) syndrome resulting from maternal pericentric inversion. Am J Med Genet. 1993;47(6):817–819. doi: 10.1002/ajmg.1320470604. [DOI] [PubMed] [Google Scholar]
- 10.Kozma C, Meck JM. Familial 10p trisomy resulting from a maternal pericentric inversion. Am J Med Genet. 1994;49(3):281–287. doi: 10.1002/ajmg.1320490308. [DOI] [PubMed] [Google Scholar]
- 11.Chen CP, Ko TM, Su YN, Wang LK, Chern SR, Wu PS, et al. Prenatal diagnosis and molecular cytogenetic characterization of rec(10)dup(10p)inv(10)(p11 2q26 3) in a fetus associated with paternal pericentric inversion. Taiwan J Obstet Gynecol. 2016;55(5):733–737. doi: 10.1016/j.tjog.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Davies J, Jaffe A, Bush A. Distal 10q trisomy syndrome with unusual cardiac and pulmonary abnormalities. J Med Genet. 1998;35(1):72–74. doi: 10.1136/jmg.35.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuffenhauer S, Lichtner P, Peykar-Derakhshandeh P, Murken J, Haas OA, Back E, Wolff G, Zabel B, Barisic I, Rauch A, Borochowitz Z, Dallapiccola B, Ross M, Meitinger T. Deletion mapping on chromosome 10p and definition of a critical region for the second DiGeorge syndrome locus (DGS2) Eur J Hum Genet. 1998;6(3):213–225. doi: 10.1038/sj.ejhg.5200183. [DOI] [PubMed] [Google Scholar]
- 14.Melis D, Genesio R, Boemio P, Del Giudice E, Cappuccio G, Mormile A, Ronga V, Conti A, Imperati F, Nitsch L, Andria G. Clinical description of a patient carrying the smallest reported deletion involving 10p14 region. Am J Med Genet A. 2012;158A(4):832–835. doi: 10.1002/ajmg.a.34133. [DOI] [PubMed] [Google Scholar]
- 15.Poluha A, Bernaciak J, Jaszczuk I, Kędzior M, Nowakowska BA. Molecular and clinical characterization of new patient with 1,08 Mb deletion in 10p15 3 region. Mol Cytogenet. 2017;10:34. doi: 10.1186/s13039-017-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vargiami E, Ververi A, Kyriazi M, Papathanasiou E, Gioula G, Gerou S, Al-Mutawa H, Kambouris M, Zafeiriou DI. Severe clinical presentation in monozygotic twins with 10p15 3 microdeletion syndrome. Am J Med Genet A. 2014;164A(3):764–768. doi: 10.1002/ajmg.a.36329. [DOI] [PubMed] [Google Scholar]
- 17.Weissgerber P, Held B, Bloch W, Kaestner L, Chien KR, Fleischmann BK, et al. Reduced cardiac L-type Ca2+ current in Ca(V)beta2-/- embryos impairs cardiac development and contraction with secondary defects in vascular maturation. Circ Res. 2006;99(7):749–757. doi: 10.1161/01.RES.0000243978.15182.c1. [DOI] [PubMed] [Google Scholar]
- 18.Zhao X, Monson C, Gao C, Gouon-Evans V, Matsumoto N, Sadler KC, et al. Klf6/copeb is required for hepatic outgrowth in zebrafish and for hepatocyte specification in mouse ES cells. Dev Biol. 2010;344(1):79–93. doi: 10.1016/j.ydbio.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogoh H, Yamagata K, Nakao T, Sandell LL, Yamamoto A, Yamashita A, et al. Mllt10 knockout mouse model reveals critical role of Af10-dependent H3K79 methylation in midfacial development. Sci Rep. 2017;7(1):11922. doi: 10.1038/s41598-017-11745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bem D, Yoshimura S, Nunes-Bastos R, Bond FC, Kurian MA, Rahman F, et al. Loss-of-function mutations in RAB18 cause Warburg micro syndrome. Am J Hum Genet. 2011;88(4):499–507. doi: 10.1016/j.ajhg.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanca O, Andrews JC, Lee PT, Patel C, Braddock SR, Slavotinek AM, et al. De novo variants in WDR37 are associated with epilepsy, colobomas, dysmorphism, developmental delay, intellectual disability, and cerebellar hypoplasia. Am J Hum Genet. 2019;105(2):413–424. doi: 10.1016/j.ajhg.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Xiao Z, Zheng J, Wu J, Hu XL, Yang X, et al. ZEB1 represses neural differentiation and cooperates with CTBP2 to dynamically regulate cell migration during neocortex development. Cell Rep. 2019;27(8):2335–2353.e6. doi: 10.1016/j.celrep.2019.04.081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author by reasonable request.