Abstract
Human peripheral blood mononuclear cells (PBMCs) originate from hematopoietic stem cells in the bone marrow, which mainly includes lymphocytes (T cells, B cells, and natural killer cells) and monocytes. Cryopreserved PBMCs providing biobank resources are crucial for clinical application or scientific research. Here, we used flow cytometry to explore the influence of long-term cryopreservation on the quality of PBMCs with the aim of providing important evidence for the effective utilization of biobank resources. The PBMCs were isolated from the peripheral blood, which was collected from volunteers in the hospital. After long-term cryopreservation in liquid nitrogen, we analyzed the changes in cell numbers, viability, and multiple subtypes of PBMCs and studied the apoptosis, proliferation, activation, function, and status of T cells in comparison with freshly isolated PBMCs by flow cytometry, and then further tracked the effects of long-term cryopreservation on the same sample. Although the different cell types in the PBMCs dynamically changed compared with those in the freshly isolated samples, PBMC recovery and viability remained stable after long-term cryopreservation, and the number of most innate immune cells (e.g., monocytes and B cells) was significantly reduced compared to that of the freshly isolated PBMCs or long-term cryopreserved PBMCs; more importantly, the proportion of T cell subtypes, apoptosis, proliferation, and functional T cells, except for Tregs, were not affected by long-term cryopreservation. However, the proportions of activated T, naïve T, central memory T, effector T, and effector memory T cells dynamically changed after long-term cryopreservation. This article provides important evidence for the effective utilization of biobank resources. Long-term cryopreserved PBMCs can be partly used as biological resources for clinical research or basic studies, but the effect of cryopreservation on PBMCs should be considered when selecting cell samples, especially in research relating to activating or inhibiting function.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12865-022-00505-4.
Keywords: PBMCs, Cryopreservation, Adaptive immune cells, Innate immune cells, T cells
Introduction
Human peripheral blood mononuclear cells (PBMCs) are important biological resources that aid in improving our understanding of immunological disease and play a crucial role in immunotherapy. To address the limitations of freshly isolated PBMCs, cryopreserved PBMCs are widely used in basic studies and clinical trials. Several clinical trials necessitate the high quality of functional T cell products and stable cell subtypes; consequently, large numbers of cryopreserved PBMCs that were collected for years need to be evaluated in a suitable manner.
PBMCs are mixtures of multiple immune cells, which can be roughly divided into two immune cell subtypes, including innate immune cells (natural killer [NK] cells and monocytes) and adaptive immune cells (e.g., B cells and T cells). Furthermore, the discovery of innate lymphoid cells (ILCs) is critical for maintaining immune homeostasis or in response to inflammation [1]. T cells can be classified into CD4+ T (T helper cells) and CD8+ T (cytotoxic T cells), which can activate other immune cells and fight pathogens, respectively. Memory cells in CD4+ T cells respond rapidly to previously encountered antigens [2–5]. CD4+ T cells can also be divided into four functional subtypes: T helper (Th) 1 (interferon [IFN]-γ+), Th2 (interleukin [IL]-4+), Th17 (IL-17+), and regulatory T cells (Tregs), which express intracellular forkhead box P3 (FOXP3) and exhibit a strong suppressive function [6, 7]. Cytokines produced by Th1, such as IFN-α, IFN-γ, IL-2, granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor (TNF)-α, participate in cell immunity against pathogens and bacteria [8]. T helper 2 cell cytokines, including IL-4, IL-5, IL-6, and transforming growth factor (TGF)-β, enhance humoral immunity from allergens, bacteria, or toxins. T helper 17 cells secrete IL-17 and IL-23, which are involved in inflammatory responses or infection defense [9–11]. However, the cryopreservation process and storage time may affect the PBMC subtypes; thus, it is important to evaluate the quality of long-term cryopreserved PBMCs.
As a proven immunological assay, multi-parameter flow cytometry is widely used in cytobiology, immunology, and clinical medicine, and can rapidly and effectively analyze the diversity of PBMC subpopulations; for example, flow cytometry (FCM) can be used for disease diagnosis i.e., detecting the number of CD4+ T cells in the peripheral blood and monitoring the disease progression of human immunodeficiency virus (HIV) infection [12]. The human leukocyte antigen (HLA), HLA-B27, can also be detected by FCM for the diagnosis of ankylosing spondylitis [13]. Flow cytometry can also be used to measure the proportion of lymphocyte subtypes and observe the differences between healthy individuals and patients with autoimmune diseases [14].
At present, the available literature focuses on the cell viability of fresh or frozen PBMCs from different samples in biobanks [15]. Moreover, published studies have usually focused on the cellular immunological status of certain PBMC subpopulations after cryopreservation, rather than conducting a comprehensive analysis of the cell samples. Thus, in this study, we aimed to analyze the total numbers of PBMCs and the different changes in the cell subtypes at different cryopreservation time points for the same sample and to comprehensively evaluate the impact of cryopreservation on immune status by tracing the total cell number, cell viability, and markers of each subtype of immune cells in the same sample to provide important evidence for the effective utilization of biobank resources.
Materials and methods
Study sample preparation
From 2019 to 2020, a total of 91 blood samples were collected and tested from healthy adults. Four milliliters of peripheral blood was drawn from individuals using BD Vacutainer ethylenediaminetetraacetic acid (EDTA) tubes, which were then kept at 25 °C until the PBMCs were isolated within 4 h.
PBMC isolation and cryopreservation
First, the peripheral blood was diluted and mixed with RPIM1640 (Gibco, Germany) at a ratio of 1:1, and then gently layered on top of 4 mL of Ficoll-Hypaque 10,771 (Sigma, USA). The tubes were centrifugated at 1600 rpm for 30 min at 25 °C. Then, the PBMC layer was aspirated and transferred into a new fresh 15 mL conical centrifuge tube. The PBMCs were mixed with RPIM1640 to wash twice at 1300 rpm for 10 min. After cell counting, fresh PBMCs were stained with different FCM antibodies and tested using a BD Canto II Flow Cytometry System. The remaining PBMCs were resuspended and cryopreserved with freezing media, including 10% dimethysulfoxide (DMSO) and 90% fetal bovine serum. The cell number was generally at least 5 × 106 per tube. Lastly, the frozen PBMC samples were transported into a liquid nitrogen container for long-time preservation after being stored in the freezing container at − 80 °C overnight. 5–10 samples were processed at a time and the subsequent freez thaw process followed every step of the protocol strictly.
Thawing the PBMCs
The cryopreserved PBMCs were placed in a 37 °C water bath for rapid thawing, and then transferred into a 15 mL conical centrifuge tube filled with pre-warmed RPIM1640. The cells were washed at 1300 rpm for 10 min, resuspended in culture medium, and incubated overnight.
Cell proliferation assay
The carboxyfluorescein diacetate succinimidyl ester (CFSE, Biolegend, USA) was used to label the proliferation of the PBMCs. The cells were resuspended with 5 μM CFSE working fluid and incubated in a carbon dioxide cell incubator for 20 min. Next, a five-time volume of the RPIM1640 medium containing 10% fetal bovine serum was added to terminate the reaction, and then washed three times by RPIM1640 medium. After labeling with CFSE, the cells were incubated with human CD3/CD28 T cell activator (Stem Cell, Canada) at a dose of 25 µL/mL and IL-2 (Pepro Tech, USA) at a dose of 50 ng/mL for up to 3 d, and then analyzed by fluorescence-activated cell sorting (FACS).
Cell stimulation assay
The cell suspension (1 × 106) was placed into an Ultra-Low Attachment 24-well plate (Corning, USA), and 2 µL of the cell stimulating agent containing phorbol 12-myristate 13-acetate/ionomycin/Golgi body inhibitor was added to stimulate cell activation rapidly, and the plate was incubated at 37 °C in 5% CO2 for 4 h before the follow-up experiments.
Inductive and supressive assays of tregs
Inductive assay: The CFSE labeled PBMCs were stimulated and induced by human CD3/CD28 T cell activator (Stem Cell, Canada) at a dose of 25 µL/mL, IL-2 (Pepro Tech, USA) at a dose of 150 ng/mL and TGF-β (Pepro Tech, USA) at a dose of 5 ng/mL for up to 3 d in 37 °C, 5% CO2 incubator, and then analyzed by fluorescence-activated cell sorting (FACS).
Supressive assays: The CFSE labeled PBMCs were cocultured with the Tregs isolated by Regulatory T Cell Isolation Kit II(Miltenyi Biotec, Germany) at the ratio of 1:0, 2:1 respectively and stimulated with human CD3/CD28 T cell activator (Stem Cell, Canada) at a dose of 25 µL/mL for 4 d in 37 °C, 5% CO2 incubator, and then analyzed by fluorescence-activated cell sorting (FACS).
Flow cytometry
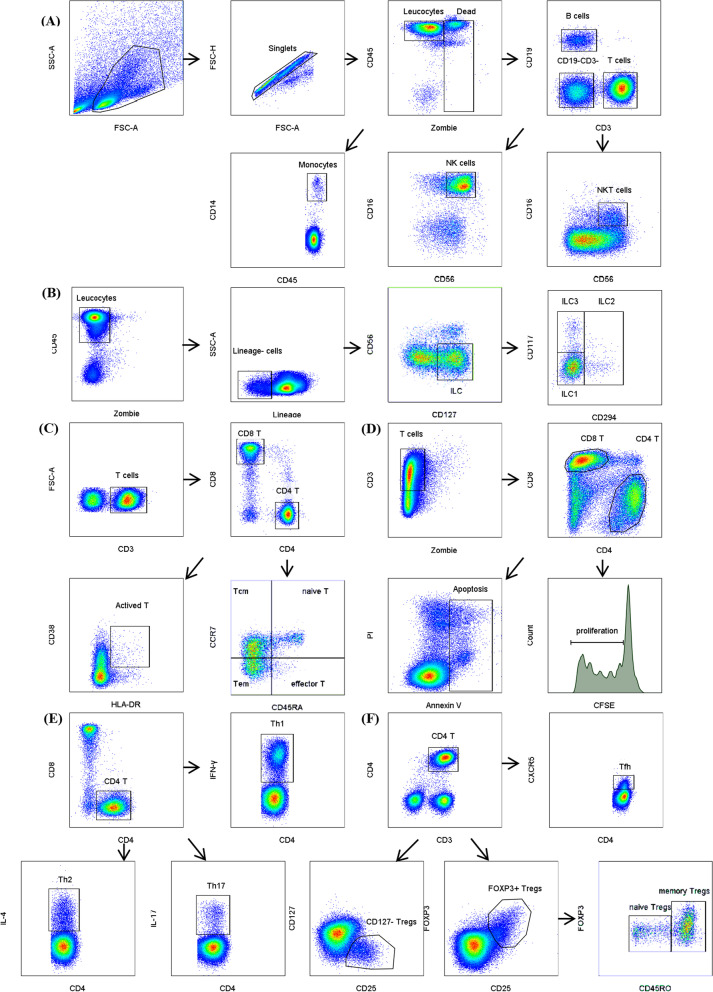
The PBMCs were washed twice and resuspended in 100 μL of phosphate-buffered saline (PBS), and then stained with LIVE/DEAD Fixable Aqua Stain (Biolegend, USA) for the cell viability testing, and then incubated with surface markers in the dark for at least 30 min.Then, the PBMCs were fixed and permeabilized after surface marker staining and stained with intracellular cytokines such as IFN-r, IL-4, IL-17, or the transcription factor FOXP3. The cell gating strategies are shown in Fig. 1, and the color scheme and antibody information are shown in Additional file 1: Table S1. Data were acquired by BD Canto II Flow Cytometry and analyzed using the BD FACSDiva software or FlowJo.v10 software (BD, USA).
Fig. 1.
Gating strategies by flow cytometry. A, B Gating strategies for PBMCs subtypes. C Gating strategies for Th, Tc and Tcm, naive T, Tem, effector T. D Gating strategies for T cell apoptosis and proliferation. E Gating strategies for functional T cell subtypes-Th1, Th2, Th17. F Gating strategies for Tfh, Tregs and naïve Tregs,memory Tregs
Statistical analysis
The total cell numbers were calculated through multiple comparison analysis testing in two-way analysis of variance (ANOVA), and the other data were calculated using one-way ANOVA multiple comparisons, wherein P < 0.05 was considered statistically significant. Statistical analysis of the results was performed using GraphPad Prism 8.0 (GraphPad Software Inc., USA).
Results
PBMC recovery and viability remained stable after long-term cryopreservation
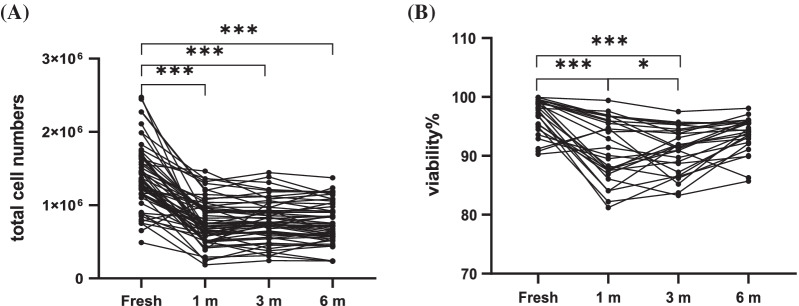
Fifty-seven peripheral blood samples were randomly obtained from the Xijing Hospital. After obtaining PBMCs by density gradient centrifugation, the cells of each patient were divided into five tubes, the fresh sample was used for subsequent experiments, and the remaining four samples were frozen for preservation to determine whether frozen time could affect the quality of PBMCs. The total cell numbers were detected using a Bio-Rad automatic cell counter, and the total number of frozen PBMCs was significantly reduced compared with that in fresh samples (P < 0.0001). However, there was no significant change after cryopreservation for 1, 3, or 6 months, and the statistical results showed 1 m versus 3 m, P = 0.48; 1 m versus 6 m, P = 0.84; 3 m versus 6 m, P = 0.11 (Fig. 2A). The viability of the PBMCs was also reduced significantly after cryopreservation (P < 0.0001) but remained stable during the different cryopreservation times, the statistical results showed that 1 m versus 3 m, P = 0.99; 1 m versus 6 m, P = 0.10; 3 m versus 6 m, P = 0.05 (Fig. 2B), indicating that although cryopreservation affects the cell recovery efficiency and viability of PBMCs, PBMCs still maintain a stable state during long-term cryopreservation.
Fig. 2.
PBMCs recovery and viability. A total cells of PBMCs after cryopreservation(n = 57) and B proportions of live PBMCs (n = 20) in fresh isolated and cryopreserved (1, 3 and 6 mo, respectively) PBMCs
T cell subtypes in the pbmcs were not susceptible to long-term cryopreservation
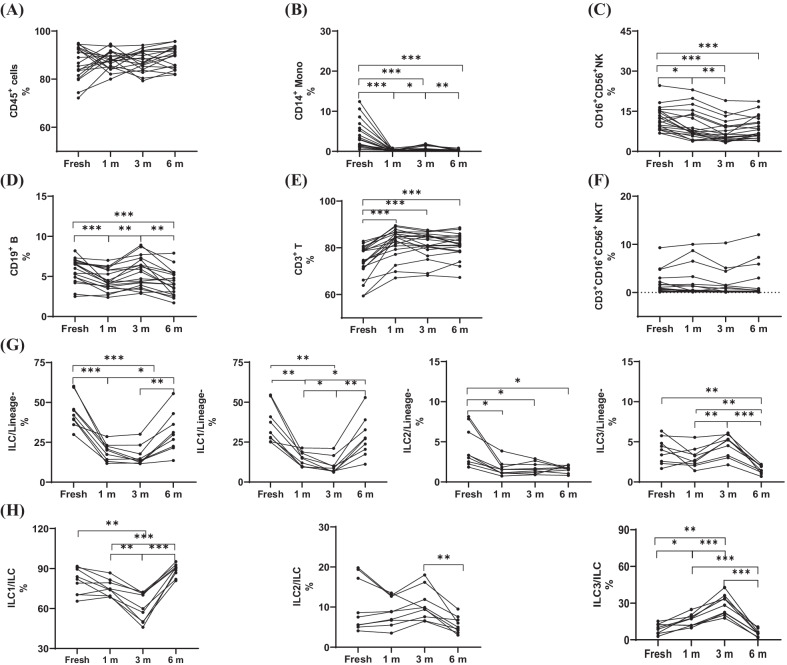
Peripheral blood mononuclear cells contain two kinds of immune cell subtypes, namely, innate immune cells and adaptive immune cells. To further elucidate the changes in the PBMC subtypes after cryopreservation, the expression of the immune cell markers between freshly isolated and cryopreserved PBMCs were detected by flow cytometry. Firstly, the proportion of total leukocytes showed no significant difference during long-term cryopreservation. Then, the phenotypes of the innate immune cells were observed(Fig. 3A). Compared with the freshly isolated PBMCs, the number of monocytes and ILC were not only reduced significantly after cryopreservation (P < 0.01 for monocytes; P < 0.01 for ILC) but also changed dynamically during long-term cryopreservation (1 m vs. 3 m, 3 m vs. 6 m, P < 0.05, P < 0.05 for monocytes; 1 m vs. 6 m, 3 m vs. 6 m, P < 0.05, P < 0.01 for ILC) (Fig. 3B, G). Further analysis of the ILC subtypes showed that ILC1 and ILC3 were affected by long-term cryopreservation (1 m vs. 3 m, 1 m vs. 6 m, 3 m vs. 6 m, P < 0.05, P < 0.05, P < 0.01 for ILC1; 1 m vs. 3 m, 1 m vs. 6 m, 3 m vs. 6 m, P < 0.01, P < 0.01, P < 0.01 for ILC3), except for ILC2 (Fig. 3G), and the proportions of these subtypes in ILC were changed either during long-term cryopreservation or in freshly isolated PBMCs(Fig. 3H). Although the number of natural killer (NK) cells decreased significantly compared with that of the freshly isolated cells (Table 1), the NK cells remained stable during long-term cryopreservation (Fig. 3C). The phenotypes of the adaptive immune cells were observed, and there was a significant change in the percentages of T cells and B cells between the fresh and cryopreserved adaptive immune cells (Fig. 3D, Table 1). However, there was no difference in the percentages of T cells and natural killer T (NKT) cells between the PBMCs cryopreserved at different times (Fig. 3E, F).
Fig. 3.
Effects of long-term cryopreservation on PBMCs subtypes. A The proportions of leucocytes. B The proportions of CD14+ Monocytes in CD45+ gate. C The proportions of CD16+CD56+ NK in CD45+ gate. D The proportions CD19+ B in CD45+ gate. E The proportions CD3+ T in CD45+ gate. F The proportions CD3+CD16+CD56+ NKT in CD45+ gate. The tested samples are fresh isolated and cryopreserved(1, 3 and 6 mo, respectively) PBMCs. G The proportions of total ILC and the subtypes ILC1,ILC2,ILC3 in Lineage−lymphocytes or H the proportions of the subtypes ILC1,ILC2,ILC3 in total ILC.*P < 0.05, **P < 0.01,***P < 0.001
Table 1.
The effect of fresh isolation and cryopreservation on innate and adaptive immune cells
| Fresh (%) | 1 m (%) | 3 m (%) | 6 m (%) | ||||
|---|---|---|---|---|---|---|---|
| Mean (range) | Mean (range) | Fresh versus 1 m P |
Mean (range) | Fresh versus 3 m P |
Mean (range) | Fresh versus 6 m P |
|
| Lymphocytes | 86.6 (72.2–94.9) | 87.8 (80–94.6) | ns | 87.5 (79.3–94.1) | ns | 89.3 (81.9–95.7) | ns |
| T | 73.9 (59.4–80.7) | 82.2 (67.1–89.5) | < 0.0001 | 81.3 (68.2–87.6) | < 0.0001 | 81.0 (67.3–88.6) | < 0.0001 |
| B | 5.77 (2.5–7.3) | 4.56 (2.4–7.0) | 0.0004 | 5.51 (3.0–7.7) | ns | 4.19 (1.7–7.9) | 0.0004 |
| NK | 12.3 (6.8–24.6) | 10.0 (4.1–23.0) | 0.0136 | 7.6 (3.2–19.0) | < 0.0001 | 8.9 (3.9–18.7) | < 0.0001 |
| NKT | 1.7 (0.1–9.3) | 1.8 (0.1–10) | ns | 1.3 (0.1–10.3) | ns | 1.6 (0.1–12) | ns |
| Monocyte | 3.9 (0.5–12.4) | 0.2 (0.0–0.8) | 0.0004 | 0.6 (0.0–1.9) | 0.0008 | 0.2 (0.0–0.8) | 0.0004 |
| ILC | 44.1 (29.9–60.2) | 19.1 (11.8–28.6) | 0.0002 | 16.6 (11.6–30.1) | 0.0004 | 31.1 (13.6–55.6) | ns |
| ILC1 | 36.0 (25.1–54.6) | 14.4 (9.4–21.4) | 0.0013 | 10.3 (6.2–21.1) | 0.0012 | 28.0 (11.1–53.0) | ns |
| ILC2 | 4.3 (1.8–8.1) | 1.7 (0.7–3.7) | 0.0176 | 1.7 (0.9–2.9) | 0.0148 | 1.6 (0.8–2.1) | 0.0308 |
| ILC3 | 3.9 (1.7–6.3) | 3.0 (1.4–5.6) | ns | 4.5 (2.1–6.1) | ns | 1.5 (1.0–2.2) | 0.0088 |
T cell proportion, apoptosis, and proliferation were not affected by long-term cryopreservation
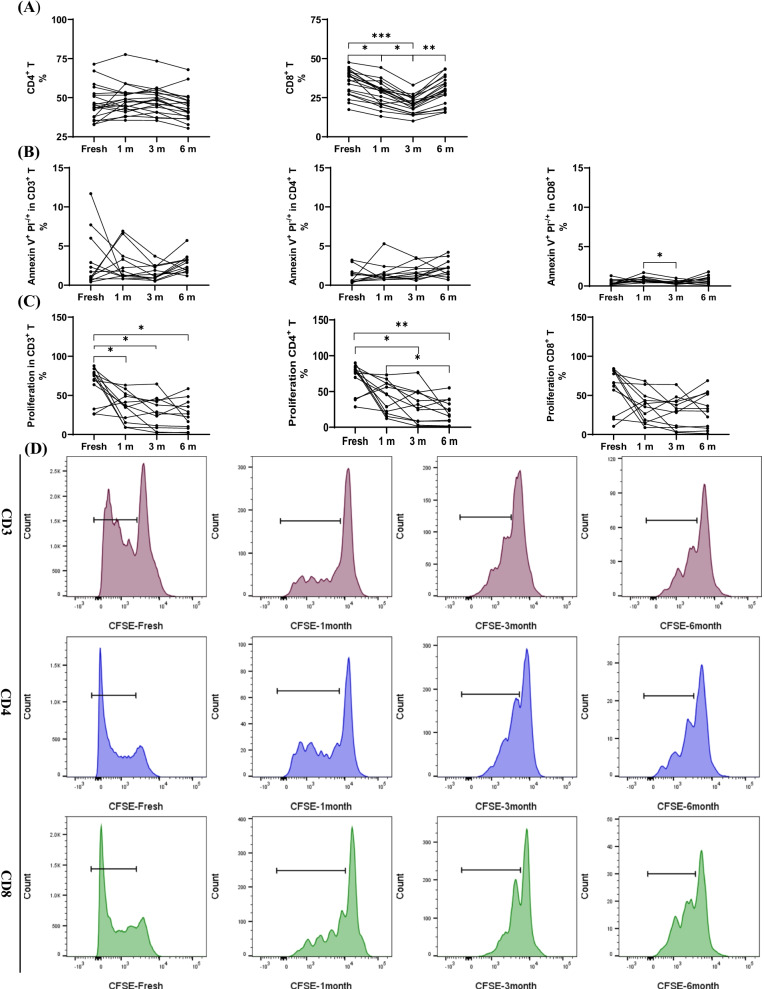
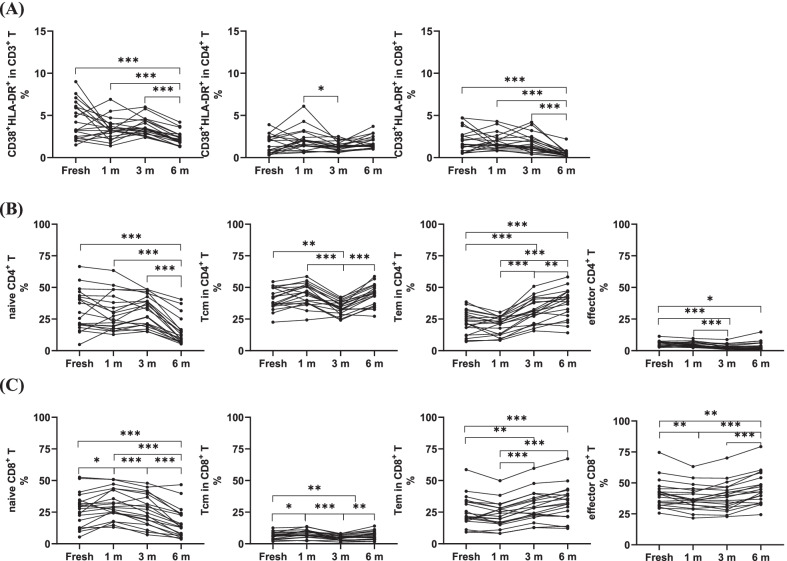
T cell response is an important part of cellular immunity and is involved in various types of biological functions against diseases and infections. Previous results have already confirmed the stability of T cell proportion after cryopreservation for 1, 3, and 6 months, respectively. T cells can be divided into CD4+ helper T cells and CD8+ cytotoxic T cells. The percentages of CD4+ T had no difference between the freshly isolated and cryopreserved PBMCs. Interestingly, the percentages of CD8+ T cells in the cryopreserved PBMCs were significantly reduced when compared with that in freshly isolated PBMCs (fresh vs. 1 m, 3 m, 6 m, P < 0.0001, P < 0.0001, P < 0.001; 1 m vs. 3 m, 6 m, P < 0.0001, P < 0.0001), indicating that the number of T cells decreased after cryopreservation which was mainly influenced by CD8+ T cells (Fig. 4A).
Fig. 4.
Effects of long-term cryopreservation on Tc, Th, apoptosis and proliferation (n = 20, 12, 12 respectively). A The proportion of Th and Tc in total T cells and B the proportion of apoptosis and C proliferation in fresh isolated and cryopreserved (1, 3 and 6 mo, respectively) PBMCs. D The proliferation results of flow cytometry in fresh isolated and cryopreserved (1, 3 and 6 mo, respectively) PBMCs.*P < 0.05, **P < 0.01,***P < 0.001
Although the viability of PBMCs have been proven to remain stable during cryopreservation in previous studies, cryopreservation may disrupt the integrity of the cell membrane, change the mitochondrial membrane potential, and cause cell apoptosis. The results indicated that the apoptosis of CD4+ T and CD8+ T cells were not affected by cryopreservation (Fig. 4B). On the other hand, proliferation of CFSE-labeled T cells was assessed, and the proliferation capacity between the freshly isolated and cryopreserved PBMCs changed significantly after stimulation with T cell activator and IL-2 (fresh vs. 1 m, 3 m, 6 m, P < 0.05, P < 0.05, P < 0.05), but no significant change was observed after prolonging the freezing time. This change in T cells was mainly caused by the CD4+ T cells, and the proliferation of CD8+ T cells was unchanged between the freshly isolated and cryopreserved PBMCs during cryopreservation (Fig. 4C). Although the proliferation ratio of the T cells was not affected by the extension of freezing time, it seemed that the passages of the T cells were changed, and after 72 h of stimulation under the same conditions, the number of proliferating cells cryopreserved for 3 or 6 months was significantly less than that of the cells cryopreserved for 1 month (Fig. 4D). In the experiment, we also found that sufficient cell numbers had a great influence on the results of proliferation (data not shown).
Proportions of the activated T cells, naïve T cells, central memory T cells, effector T cells, and effector memory T cells were dynamically changed after long-term cryopreservation
Further study is necessary as there are several T cell subtypes according to their status and activation. Proportion of activated CD3+ T cells decreased significantly not only between the freshly isolated and cryopreserved PBMCs but also in the different cryopreservation times (fresh vs. 6 m, P < 0.01; 1 m vs. 3 m, 3 m vs. 6 m, P < 0.01, P < 0.0001). This change was mainly caused by CD8+ T cells (Fig. 5A, Table 2).
Fig. 5.
Effects of long-term cryopreservation on actived T cells and the T cell subtyes expressing CCR7 and CD45RA(n = 20). AThe proportion of CD38+HLA-DR+ T cells,Th and Tc. B The proportion of Th Naïve(CD45RA+CCR7+)Tcm(CD45−CCR7+)Tem(CCR7−CD45RA−)effector T(CD45RA+CCR−). C The proportion of Tc Naïve(CD45RA+CCR7+)Tcm(CD45−CCR7+)Tem(CCR7−CD45RA−)effector T(CD45RA+CCR−) in fresh isolated and cryopreserved (1, 3 and 6 mo, respectively) PBMCs. *P < 0.05, **P < 0.01,***P < 0.001
Table 2.
Effect of long-term cryopreservation on different stage of T cells
| Fresh (%) | 1 m (%) | 3 m (%) | 6 m (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (range) | Mean (range) | Fresh versus 1 m P |
Mean (range) | Fresh versus 3 m P |
1 m versus 3 m P |
Mean (range) | Fresh versus 6 m P |
1 m versus 6 m P |
3 m versus 6 m P |
|
| CD4 | ||||||||||
| Naïve | 31.9 (4.9–66.5) | 30.1 (12.8–63.4) | ns | 31.6 (15.0–48.4) | ns | ns | 14.8 (5.4–40.5) | < 0.0001 | < 0.0001 | < 0.0001 |
| Central memory | 41.0 (22.5–54.5) | 44.3 (24.3–58.6) | ns | 34.1 (24.1–42.3) | 0.0013 | < 0.0001 | 44.0 (27.2–58.7) | ns | ns | < 0.0001 |
| Effector | 4.8 (2.5–11.4) | 4.8 (2.4–9.7) | ns | 2.9 (0.9–8.8) | < 0.0001 | < 0.0001 | 3.8 (0.8–14.8) | 0.0264 | ns | ns |
| Effector memory | 22.4 (7.2–38.5) | 20.2 (8.2–30.4) | ns | 31.6 (15.7–50.0) | < 0.0001 | < 0.0001 | 72.4 (14.1–58.3) | < 0.0001 | < 0.0001 | 0.0014 |
| Actived | 1.5 (0.3–3.9) | 2.1 (0.6–6.1) | ns | 1.4 (0.6–2.5) | ns | 0.0467 | 1.8 (1.0–3.7) | ns | ns | ns |
| CD8 | ||||||||||
| Naïve | 27.1 (5.4–52.6) | 31.3 (13.1–50.9) | 0.0449 | 26.7 (7.1–47.9) | ns | 0.0003 | 16.6 (3.7–46.7) | < 0.0001 | < 0.0001 | < 0.0001 |
| Central memory | 6.5 (1.9–12.5) | 8.1 (2.3–13.6) | 0.0178 | 4.7 (1.3–8.0) | 0.0073 | 0.0001 | 6.2 (1.7–14.0) | ns | ns | 0.0091 |
| Effector | 41.0 (25.6–74.6) | 37.9 (21.6–63.2) | 0.0065 | 39.1 (22.8–70.1) | ns | ns | 45.1 (24.2–79.3) | 0.0053 | < 0.0001 | < 0.0001 |
| Effector memory | 25.3 (25.6–58.2) | 23.2 (21.6–63.2) | ns | 29.5 (22.8–70.1) | 0.0024 | < 0.0001 | 32.1 (24.4–79.3) | < 0.0001 | < 0.0001 | ns |
| Actived | 2.0 (0.5–4.7) | 1.9 (0.8–4.3) | ns | 1.7 (0.4–4.2) | ns | ns | 0.5 (0.1–2.2) | < 0.0001 | < 0.0001 | < 0.0001 |
T cells, whether expressing C–C chemokine receptor type 7 (CCR7) and CD45RA or not, can be divided into naïve T cells, central memory T cells, effector T cells, and effector memory T cells, and can be further divided into CD4+ T or CD8+ T cells. Compared with that in freshly isolated cells, the proportions of naïve CD4+ T cells and naïve CD8+ T cells both decreased after cryopreservation for 6 months, and these changed dynamically at different freezing times. Although the proportion of CD4+ T central memory (Tcm) and CD8+ Tcm decreased significantly at 3 months, there was no significant change in 6 months as compared to that in freshly isolated cells. The proportions of effector memory cells (Tem) in both the CD4+ or CD8+ T cells, which decreased slightly after 1 month of cryopreservation, increased significantly with the extension of the freezing time (Fig. 5B, C, Table 2). The effector CD4+ and CD8+ T cells also tended to increase after a decrease in the cryopreservation time (Fig. 5B, C, Table 2). These results suggest that cryopreservation resulted in a significant reduction in the proportion of naïve T cells and that other subtype T cells increased after long-term cryopreservation but still differed from that in freshly isolated samples.
Functional T cells remained stable after long-term cryopreservation, expect for tregs
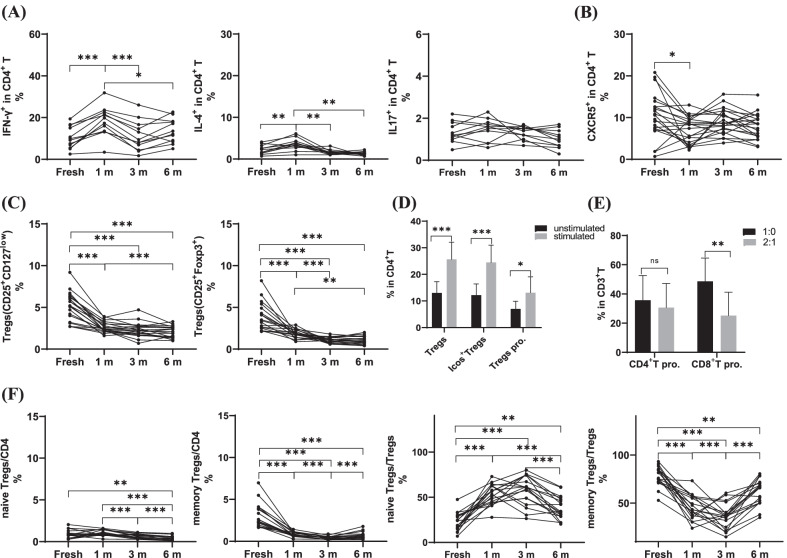
To determine whether functional T cells were affected by long-term cryopreservation, the Th cells were marked with IFN-γ, IL-4, and IL-17, the T follicular helper cells (Tfh) were marked with CD45RO and C-X-C motif chemokine receptor 5 (CXCR5), and the Tregs were marked with CD25, CD127, and FOXP3. As expected, the functional T cells remained stable after long-term cryopreservation, and compared with that in freshly isolated PBMCs, the percentage of inflammatory T cells Th17 remained stable both before and after cryopreservation, Th1 and Th2 increased after 1 month of cryopreservation, and then remained stable after longer cryopreservation (Fig. 6A, Table 3). The number of Tfhs decreased slightly after cryopreservation for 1 month and remained unchanged after 3 months or longer (Fig. 6B, Table 3). The CD4+ T cells, which express the surface marker CD25 together with intracellular FOXP3, are Tregs. Down-modulated IL-7 receptor CD127 is also used to identify Tregs. Percentages of the CD25+CD127low Tregs reduced significantly after cryopreservation, but there was no noticeable change after long-term cryopreservation. The results of CD25+FOXP3+Tregs led to the same conclusion (Fig. 6C, Table 3). Moreover, the function of cryopreserved Tregs were tested. It was showed that the frequency of Tregs, actived Tregs, proliferated Tregs increased significantly when 1 year cryopreserved PBMCs were induced into Tregs.The result of suppressive experiment also indicated that cryopreserved Tregs maintained suppressive function after long-term cryopreservation, especially the capacity of suppressing the proliferation of CD8+T. (Fig. 6D, E, Additional file 4: Fig. S3)The percentages of naïve Tregs and memory Tregs in the CD4+ T cells that were identified with CD45RO decreased significantly after cryopreservation and remained unchanged with increasing freezing time, and the proportions of naïve Tregs and memory Tregs in the Tregs dramatically changed (Fig. 6F, Table 3).
Fig. 6.
Effects of long-term cryopreservation on functional T cells-Th1, Th2, Th17(n = 12)and Tfh, Tregs(n = 18).The proportions of IFN-γ+Th1,IL-4+Th2,IL-17+Th17 (A) and CXCR5+Tfh (B) in fresh isolated and cryopreserved (1, 3 and 6 mo, respectively) PBMCs. C The proportions of CD4+CD25+Tregs expressing CD127− or Foxp3+. D The frequency of Tregs, actived Tregs, proliferated Tregs after long-term cryopreservation. E Suppressive experiment using the CFSE label PBMCs cocultured with cryopreserved Tregs. F CD45RO−Naïve Tregs,CD45RO+Tregs in total CD4+T cells or in Tregs in fresh isolated and cryopreserved (1, 3 and 6 mo, respectively) PBMCs. *P < 0.05, **P < 0.01,***P < 0.001
Table 3.
Effect of long-term cryopreservation on functional T cells
| Fresh (%) | 1 m (%) | 3 m (%) | 6 m (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (range) | Mean (range) | Fresh versus 1 m P |
Mean (range) | Fresh versus 3 m P |
1 m versus 3 m P |
Mean (range) | Fresh versus 6 m P |
1 m versus 6 m P |
3 m versus 6 m P |
|
| Th1 | 10.4 (5.4–19.4) | 18.1 (3.4–31.9) | 0.0003 | 11.1 (1.7–26.0) | ns | < 0.0001 | 13.7 (5.1–22.7) | ns | 0.0293 | ns |
| Th2 | 2.2 (0.7–4.1) | 3.5 (1.0–6.1) | 0.0048 | 1.6 (1.0–3.1) | ns | 0.0057 | 1.3 (0.7–2.2) | ns | 0.0010 | ns |
| Th17 | 1.3 (0.5–2.2) | 1.5 (0.6–2.3) | ns | 1.3 (0.7–1.7) | ns | ns | 1.0 (0.7–1.7) | ns | ns | ns |
| Tfh | 10.4 (1.9–20.8) | 7.1 (2.2–13.0) | 0.0491 | 9.0 (5.0–15.6) | ns | ns | 7.9 (3.0–15.4) | ns | ns | ns |
| Tregs(CD25+CD127low) | 5.1 (2.7–9.2) | 2.6 (1.6–3.9) | < 0.0001 | 2.3 (0.7–4.7) | < 0.0001 | ns | 2.1 (1.0–3.3) | < 0.0001 | < 0.0001 | ns |
| Tregs(CD25+Foxp3+) | 3.8 (2.1–8.2) | 1.8 (0.9–2.9) | 0.0006 | 1.1 (0.6–1.8) | < 0.0001 | 0.0006 | 1.0 (0.4–2.0) | < 0.0001 | 0.0006 | ns |
| naïve Tregs/CD4 | 0.9 (0.3–2.0) | 1.0 (0.3–1.6) | ns | 0.6 (0.3–1.1) | ns | 0.0004 | 0.4 (0.1–1.0) | 0.0026 | < 0.0001 | 0.0003 |
| memory Tregs/CD4 | 3.1 (1.7–7.0) | 0.9 (0.3–1.4) | < 0.0001 | 0.4 (0.2–0.7) | < 0.0001 | < 0.0001 | 0.7 (0.2–1.8) | < 0.0001 | ns | 0.0099 |
Discussion
Peripheral blood mononuclear cells, which contain various immune cell types, play a crucial role in peripheral circulation; therefore, the quality of PBMCs needs to be controlled for clinical application or scientific research to obtain large numbers of samples to assess changes in the immune microenvironment during disease progression; that is, PBMCs should be cryopreserved for long periods of time [16]. Several reports have suggested that both isolation techniques and temperature fluctuations may affect cell viability and lymphocyte subtypes [17–19]. Cryopreservation is a relatively violent process in which freezing and thawing methods may cause physical and chemical stress on cells, resulting in changes in the cell surface markers [5]. In this study, autologous serum was used for cryopreservation, and cell numbers, viability, and subpopulations divided by different antibodies were assessed between freshly isolated and long-term cryopreserved PBMCs. Compared with freshly isolated PBMCs, both the number and viability of PBMCs were reduced after cryopreservation, but there was no significant difference during long-term cryopreservation; this result was consistent with the research that confirmed that PBMCs frozen from 2006 to 2015 maintained a high quality after recovery [15].
Peripheral blood mononuclear cells contain multiple immune cell types and perform different functions in the body. Although, in the research mentioned above, the PBMCs lasted a long period of time, the cryopreservation efficiency of the PBMC subtypes were not assessed further. For this purpose, we assessed innate immune cells and adaptive immune cells in PBMCs and concluded that the T, NK, and NKT cells did not change after long-term cryopreservation, while B cells, monocytes, and ILC changed significantly. In agreement with this, a previous study found that the number of B cells decreased and induced a higher total immunoglobulin (Ig) G production after 12 months of storage [20]. Although we confirmed that the proportion of T cells was not reduced during cryopreservation, the proportions of T cell subtypes and their status or functions may be affected by long-term cryopreservation, and the process of ice recrystallization or changes in osmotic pressure may damage the cytoskeleton and cell membrane [21]. The results of this study were similar to those of previous studies, which showed that T cells, especially CD4+ T cells, were not significantly affected by cryopreservation, regardless of cell proportion, active station, apoptosis, or proliferation [7, 22, 23].
Naive T cells, effector T cells, and Tem cells both in the CD4+ T and CD8+ T cells, which were not only compared with freshly isolated PBMCs but also in different cryopreserved times, were affected by cryopreservation. Some studies have reported that the number of Tcm increases and that of Tem decreases after 12 months of cryopreservation, and it could be concluded that these types of T cells seem to be more sensitive to the length of cryopreservation [7, 24, 25]. T follicular helper cells that express CXCR5 participate in information transmission or activation of B cells and maintain the humoral immune response for a long period of time [26]. Although our results showed significant changes in the B cells, Tfh was not affected by long-term cryopreservation after a slight decrease compared with fresh isolated PBMCs. Some studies have reported that the freeze–thaw process can result in a three to five-fold reduction of malaria antigen-specific IFN-γ-producing CD3+CD4+ effector populations, and others have suggested that cryopreservation in general leads to increased cytokine and chemokine responses, which is expected for IFN-γ in cultured PBMC supernatants [5, 27]. A recent report also suggested that there were no significant differences in the ratio of IFN-γ, IL-4, and IL-17 positive T cells between cell preparation tubes (CPT) and barrier-based tube option-Lymphoprep (LP) [28]. In our study, cytokines stimulated with phorbol 12-myristate 13-acetate remained stable after long-term cryopreservation, regardless of pro-inflammatory IFN-γ and IL-17 or anti-inflammatory IL-4. Several reports noted that cryopreservation was detrimental for the suppressive function and downregulated the key molecular features of Tregs; this was determined by single-cell RNA-sequencing [29, 30]. Our study found that both CD127low Tregs and Foxp3+ Tregs were sensitive, and the ratio of cells rapidly decreased after initial cryopreservation. Then, the percentage of Tregs remained stable at a lower level after long-term cryopreservation, which agreed with the results of previous studies [31, 32]. Further studies confirmed that different states of Tregs, such as naïve Tregs and memory Tregs, significantly decreased in the CD4+ T cells and also dramatically changed in the Treg subpopulations after long-term cryopreservation. Although cryopreservation has a greater impact on Tregs, Tregs still maintain suppressive function after long-term cryopreservation. Notably, althougt Foxp3 is a highly specific marker for Treg cells, some effector CD4+ T cell can upregulate the expression of Foxp3 but have no regulatory/suppressive function[33]. The heterogeneity of human Foxp3+ regulatory T cells is an enssential and worthy issue in subsequent researches.
Finally, we truly considered the certain cell population loss may effect the frequency of other subsets during the experiment. So we counted absolute cell numbers and the results showed the similar trends to the frequency of cell subsets (Additional file 2: Fig. S1; Additional file 3: Fig. S2), which futher illustrated the reliability of the results in this manuscript.
Conclusions
In the present study, we comprehensively evaluated the quality of long-term cryopreserved PBMCs and explored changes in the cell numbers, viability, and subtypes of PBMCs before and after cryopreservation. Particularly, changes in T cell apoptosis, proliferation, activation, and function were investigated and the effects of long-term cryopreservation on the same sample were analyzed. The results showed that long-term cryopreservation affected the activation of T cells and the function of Tregs, which are essential to the occurrence and development of immune diseases, and the imbalance of the ratio of effector T cells and Tregs will cause abnormal immune regulation in the body [34, 35]. Overall, the long-term cryopreservation of PBMCs can be partly used as a biological resource for clinical research or basic studies, but the effect of cryopreservation on PBMCs should be considered when selecting cell samples, especially in studying the function of activation or suppression. This study emphasized a comprehensive assessment of PBMC status after long-term cryopreservation without further exploring the effects of sample collection, including the concentration of cryopreserved cells, cryopreservation method (such as the concentration of cryoprotectant DMSO or fetal bovine serum), warming process, and even the use of anticoagulants. It is well known that the standardized management of PBMC biobanks is essential for the cell-based immune therapy in worldwide clinical trials; this study motivated us to pay more attention to the profound influence of cryopreservation itself on PBMCs in scientific research or clinical trials while improving the standard of biobank establishment.
Supplementary Information
Additional file 1. Table S1: Antibody information
Additional file 2. Figure S1: The absolute cell count of long-term cryopreserved PBMCs subtypes. (A)leucocytes (B)CD14+ Monocytes in CD45+ gate (C)CD16+CD56+ NK in CD45+ gate (D)ILC in Lineage- lymphocytes (E)CD19+ B in CD45+ gate (F) CD3+ T in CD45+ gate (G)CD4+ Th (H) CD8+ Tc in fresh isolated and cryopreserved(1,3 and 6 mo,respectively) PBMCs.*P<0.05, **P<0.01,***P<0.001
Additional file 3. Figure S2: (A) The absolute cell count and proportions of ILC in cryopresered(6 and 12 month) PBMCs. *P<0.05, **P<0.01,***P<0.001
Additional file 4. Figure S3: Suppressive experiment using the CFSE label PBMCs cocultured with cryopreserved Tregs (A) The histogram results to show CD4-CFSE in the absence and presence of Tregs (B) The histogram results to show CD8-CFSE in the absence and presence of Tregs. The numbers represent the percentages of proliferative cells
Acknowledgements
The authors are thankful to all the volunteers who participated in this study. The authors are grateful to the National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases for providing high-quality scientific research platform. The authors would also like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- PBMCs
Peripheral blood mononuclear cells
- HSCs
Hematopoietic stem cells
- NK
Natural killer cells
- ILC
Innate lymphoid cells
- Th
T helper cells
- IFN-γ
Interferon-γ
- IL-2
Interleukin-2
- IL-4
Interleukin-4
- IL-17
Interleukin-17
- Tregs
Regulatory T cells
- FOXP3
Intracellular forkhead box P3
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- TNF
Tumor necrosis factor
- TGF-β
Transforming growth factor-β
- FCM
Flow cytometry
- HIV
Humanodeficiency virus
- HLA
Human leukocyte antigen
- EDTA
Ethylenediaminetetraacetic acid
- DMSO
Dimethysulfoxide
- CFSE
Carboxyfluorescein diacetate succinimidyl ester
- FACS
Fluorescence-activated cell sorting
- CCR7
C–C chemokine receptor type 7
- Tcm
T central memory cells
- Tem
T effector memory cells
- CXCR5
C-X-C motif chemokine receptor 5
- Tfh
T follicular helper cells
Author contributions
BL and CY performed data collection, analysis, and wrote the manuscript. GJ, YL and NW provided technical support. FY and RS provided academic advice to the study. YH and YS designed the study and revised the manuscript. The order of co-first authors appearing in the manuscript was decided by discussions among the two first authors and the corresponding author. All authors read and approved the final manuscript.
Funding
This study was supported by the National Key R&D Program of China(No. 2020YFA0710803), International Cooperation and Exchange of the National Natural Science Foundation of China (No. 81820108005), China Postdoctoral Science Foundation (No. 2018M643871).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional file.
Declarations
Ethics approval and consent to participate
The Institutional Research Ethics Committee of Xijing Hospital of Digestive Diseases approved this study (KY20173316-1). The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bo Li and Chunmei Yang contributed equally to this work and share first authorship
Contributor Information
Yulong Shang, Email: shangyul870222@163.com.
Ying Han, Email: hanying1@fmmu.edu.cn.
References
- 1.Shin SB, McNagny KM. ILC-You in the thymus: a fresh look at innate lymphoid cell development. Front Immunol. 2021;12:681110. doi: 10.3389/fimmu.2021.681110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16(4):343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkander J, Hojyo S, Tokoyoda K. Vaccination to gain humoral immune memory. Clin Transl Immunology. 2016;5(12):e120. doi: 10.1038/cti.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson J, Toh ZQ, Reitsma A, Do LAH, Nathanielsz J, Licciardi PV. Effect of peripheral blood mononuclear cell cryopreservation on innate and adaptive immune responses. J Immunol Methods. 2019;465:61–66. doi: 10.1016/j.jim.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25+ regulatory cells from human peripheral blood express very high levels of CD25 ex vivo. Novartis Found Symp. 2003;252:67–88. doi: 10.1002/0470871628.ch6. [DOI] [PubMed] [Google Scholar]
- 7.Tompa A, Nilsson-Bowers A, Faresjö M. Subsets of CD4(+), CD8(+), and CD25(hi) lymphocytes are in general not influenced by isolation and long-term cryopreservation. J Immunol. 2018;201(6):1799–1809. doi: 10.4049/jimmunol.1701409. [DOI] [PubMed] [Google Scholar]
- 8.Crawford MP, Sinha S, Renavikar PS, Borcherding N, Karandikar NJ. CD4 T cell-intrinsic role for the T helper 17 signature cytokine IL-17: effector resistance to immune suppression. Proc Natl Acad Sci USA. 2020;117(32):19408–19414. doi: 10.1073/pnas.2005010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhammad Yusoff F, Wong KK, Mohd RN. Th1, Th2, and Th17 cytokines in systemic lupus erythematosus. Autoimmunity. 2020;53(1):8–20. doi: 10.1080/08916934.2019.1693545. [DOI] [PubMed] [Google Scholar]
- 10.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43(3):402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64(2):477–485. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Ciuceis C, Rossini C, Airò P, Scarsi M, Tincani A, Tiberio GA, et al. Relationship between different subpopulations of circulating CD4+ T-lymphocytes and microvascular structural alterations in humans. Am J Hypertens. 2017;30(1):51–60. doi: 10.1093/ajh/hpw102. [DOI] [PubMed] [Google Scholar]
- 13.Colbert RA, Navid F, Gill T. The role of HLA-B*27 in spondyloarthritis. Best Pract Res Clin Rheumatol. 2017;31(6):797–815. doi: 10.1016/j.berh.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Boldt A, Borte S, Fricke S, Kentouche K, Emmrich F, Borte M, et al. Eight-color immunophenotyping of T-, B-, and NK-cell subpopulations for characterization of chronic immunodeficiencies. Cytometry B Clin Cytom. 2014;86(3):191–206. doi: 10.1002/cytob.21162. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Gao M, Li K, Qin L, Sun H, Qiao G, et al. Quality of cryopreserved peripheral blood mononuclear cells recovered from the hepatitis/AIDS biobank. Biopreserv Biobanking. 2018;16(6):397–401. doi: 10.1089/bio.2018.0050. [DOI] [PubMed] [Google Scholar]
- 16.Mallone R, Mannering SI, Brooks-Worrell BM, Durinovic-Belló I, Cilio CM, Wong FS, et al. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-cell workshop committee of the immunology of diabetes society. Clin Exp Immunol. 2011;163(1):33–49. doi: 10.1111/j.1365-2249.2010.04272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Germann A, Oh YJ, Schmidt T, Schön U, Zimmermann H, von Briesen H. Temperature fluctuations during deep temperature cryopreservation reduce PBMC recovery, viability and T-cell function. Cryobiology. 2013;67(2):193–200. doi: 10.1016/j.cryobiol.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Lockmann A, Seitz CS, Schön MP, Mößner R. Creeping eruption and eosinophilic folliculitis: atypical cutaneous larva migrans. J Dtsch Dermatol Ges. 2018;16(2):202–204. doi: 10.1111/ddg.13414. [DOI] [PubMed] [Google Scholar]
- 19.Grievink HW, Luisman T, Kluft C, Moerland M, Malone KE. Comparison of three isolation techniques for human peripheral blood mononuclear cells: cell recovery and viability, population composition, and cell functionality. Biopreserv Biobanking. 2016;14(5):410–415. doi: 10.1089/bio.2015.0104. [DOI] [PubMed] [Google Scholar]
- 20.Ticha O, Moos L, Bekeredjian-Ding I. Effects of long-term cryopreservation of PBMC on recovery of B cell subpopulations. J Immunol Methods. 2021;495:113081. doi: 10.1016/j.jim.2021.113081. [DOI] [PubMed] [Google Scholar]
- 21.Bischof JC, Rubinsky B. Large ice crystals in the nucleus of rapidly frozen liver cells. Cryobiology. 1993;30(6):597–603. doi: 10.1006/cryo.1993.1062. [DOI] [PubMed] [Google Scholar]
- 22.Merlin E, Hannani D, Veyrat-Masson R, Chassagne J, Gabert F, Berger M, et al. Cryopreservation of mononuclear cells before extracorporeal photochemotherapy does not impair their anti-proliferative capabilities. Cytotherapy. 2011;13(2):248–255. doi: 10.3109/14653249.2010.501787. [DOI] [PubMed] [Google Scholar]
- 23.Juhl M, Christensen JP, Pedersen AE, Kastrup J, Ekblond A. Cryopreservation of peripheral blood mononuclear cells for use in proliferation assays: first step towards potency assays. J Immunol Methods. 2021;488:112897. doi: 10.1016/j.jim.2020.112897. [DOI] [PubMed] [Google Scholar]
- 24.Owen RE, Sinclair E, Emu B, Heitman JW, Hirschkorn DF, Epling CL, et al. Loss of T cell responses following long-term cryopreservation. J Immunol Methods. 2007;326(1–2):93–115. doi: 10.1016/j.jim.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costantini A, Mancini S, Giuliodoro S, Butini L, Regnery CM, Silvestri G, et al. Effects of cryopreservation on lymphocyte immunophenotype and function. J Immunol Methods. 2003;278(1–2):145–155. doi: 10.1016/S0022-1759(03)00202-3. [DOI] [PubMed] [Google Scholar]
- 26.Olatunde AC, Hale JS, Lamb TJ. Cytokine-skewed Tfh cells: functional consequences for B cell help. Trends Immunol. 2021;42(6):536–550. doi: 10.1016/j.it.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford T, Wenden C, Mbekeani A, Dally L, Cox JH, Morin M, et al. Cryopreservation-related loss of antigen-specific IFNγ producing CD4(+) T-cells can skew immunogenicity data in vaccine trials: lessons from a malaria vaccine trial substudy. Vaccine. 2017;35(15):1898–1906. doi: 10.1016/j.vaccine.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Schürch CM, Noble K, Kim K, Krutzik PO, O'Donnell E, et al. Functional comparison of PBMCs isolated by cell preparation tubes (CPT) vs. Lymphoprep Tubes BMC Immunol. 2020;21(1):15. doi: 10.1186/s12865-020-00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JS, Yi K, Ju YS, Shin EC. Effects of cryopreservation and thawing on single-cell transcriptomes of human T cells. Immune Netw. 2020;20(4):e34. doi: 10.4110/in.2020.20.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sattui S, de la Flor C, Sanchez C, Lewis D, Lopez G, Rizo-Patrón E, et al. Cryopreservation modulates the detection of regulatory T cell markers. Cytometry B Clin Cytom. 2012;82(1):54–58. doi: 10.1002/cyto.b.20621. [DOI] [PubMed] [Google Scholar]
- 31.Ulbar F, Montemurro T, Jofra T, Capri M, Comai G, Bertuzzo V, et al. Regulatory T cells from patients with end-stage organ disease can be isolated, expanded and cryopreserved according good manufacturing practice improving their function. J Transl Med. 2019;17(1):250. doi: 10.1186/s12967-019-2004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Nilles TL, Johnson JR, Margolick JB. The effect of cellular isolation and cryopreservation on the expression of markers identifying subsets of regulatory T cells. J Immunol Methods. 2016;431:31–37. doi: 10.1016/j.jim.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wing JB, Tanaka A, Sakaguchi S. Human FOXP3+ regulatory T Cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50(2):302–316. doi: 10.1016/j.immuni.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Horst AK, Wegscheid C, Schaefers C, Schiller B, Neumann K, Lunemann S, et al. Carcinoembryonic antigen-related cell adhesion molecule 1 controls IL-2-dependent regulatory T-cell induction in immune-mediated hepatitis in mice. Hepatology. 2018;68(1):200–214. doi: 10.1002/hep.29812. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Hou X, Jia H, Cui G, Wu Z, Wang L, et al. Regulatory T cells with a defect in inhibition on co-stimulation deteriorated primary biliary cholangitis. Oncotarget. 2017;8(65):108406–108417. doi: 10.18632/oncotarget.22658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1: Antibody information
Additional file 2. Figure S1: The absolute cell count of long-term cryopreserved PBMCs subtypes. (A)leucocytes (B)CD14+ Monocytes in CD45+ gate (C)CD16+CD56+ NK in CD45+ gate (D)ILC in Lineage- lymphocytes (E)CD19+ B in CD45+ gate (F) CD3+ T in CD45+ gate (G)CD4+ Th (H) CD8+ Tc in fresh isolated and cryopreserved(1,3 and 6 mo,respectively) PBMCs.*P<0.05, **P<0.01,***P<0.001
Additional file 3. Figure S2: (A) The absolute cell count and proportions of ILC in cryopresered(6 and 12 month) PBMCs. *P<0.05, **P<0.01,***P<0.001
Additional file 4. Figure S3: Suppressive experiment using the CFSE label PBMCs cocultured with cryopreserved Tregs (A) The histogram results to show CD4-CFSE in the absence and presence of Tregs (B) The histogram results to show CD8-CFSE in the absence and presence of Tregs. The numbers represent the percentages of proliferative cells
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional file.