Abstract
An alkane-degrading, sulfate-reducing bacterial strain, AK-01, isolated from a petroleum-contaminated sediment was studied to elucidate its mechanism of alkane metabolism. Total cellular fatty acids of AK-01 were predominantly C even when it was grown on C-even alkanes and were predominantly C odd when grown on C-odd alkanes, suggesting that the bacterium anaerobically oxidizes alkanes to fatty acids. Among these fatty acids, some 2-, 4-, and 6-methylated fatty acids were specifically found only when AK-01 was grown on alkanes, and their chain lengths always correlated with those of the alkanes. When [1,2-13C2]hexadecane or perdeuterated pentadecane was used as the growth substrate, 13C-labeled 2-Me-16:0, 4-Me-18:0, and 6-Me-20:0 fatty acids or deuterated 2-Me-15:0, 4-Me-17:0, and 6-Me-19:0 fatty acids were recovered, respectively, confirming that these monomethylated fatty acids were alkane derived. Examination of the 13C-labeled 2-, 4-, and 6-methylated fatty acids by mass spectrometry showed that each of them contained two 13C atoms, located at the methyl group and the adjacent carbon, thus indicating that the methyl group was the original terminal carbon of the [1,2-13C2]hexadecane. For perdeuterated pentadecane, the presence of three deuterium atoms, on the methyl group and its adjacent carbon, in each of the deuterated 2-, 4-, and 6-methylated fatty acids further supported the hypothesis that the methyl group was the terminal carbon of the alkane. Thus, exogenous carbon appears to be initially added to an alkane subterminally at the C-2 position such that the original terminal carbon of the alkane becomes a methyl group on the subsequently formed fatty acid. The carbon addition reaction, however, does not appear to be a direct carboxylation of inorganic bicarbonate. A pathway for anaerobic metabolism of alkanes by strain AK-01 is proposed.
Alkanes are major components of petroleum fuels commonly found in polluted environments, and numerous studies of their biodegradability by microorganisms have been conducted. Most of the earlier studies focused on processes that take place under aerobic conditions (6), in which alkanes are initially oxidized by oxygen to alcohols by monooxygenases, with detailed accounts of the biochemistry and genetics (37, 38). In contrast, while alkane degradation under anaerobic conditions has been reported in early as well as recent literature (2, 7, 9, 27, 32), much less is understood about the mechanism(s) of degradation in the absence of oxygen. Some reports indicated that alkanes could be enzymatically dehydrogenated to alkenes (8, 19), and the reaction may have served as an initial reaction for anaerobic alkane degradation (29). These observed activities, however, could not be shown to support growth under anaerobic conditions (17).
In more recent studies, Aeckersberg et al. isolated and characterized a sulfate-reducing, alkane-degrading strain, Hxd3 (2). We have also recently reported the isolation and characterization of an alkane-degrading strain, AK-01, which is different from Hxd3 phylogenetically (35). Hxd3 appears to anaerobically oxidize alkanes to fatty acids, as evidenced by the influence of alkane carbon chain lengths on its cellular fatty acid composition (3). Other observations also suggest that an odd-numbered carbon atom(s) may be initially added to or removed from the alkane chain terminus before the fatty acids are formed. On the other hand, evidence suggests that desaturation of the alkane to the corresponding 1-alkene, a hypothetical initial step previously proposed in the literature (29), does not occur during anaerobic alkane oxidation by Hxd3 (1, 3). Another alkane-degrading strain, Pnd3, reported by the same authors (3) also appears to anaerobically oxidize alkanes to fatty acids, though seemingly through a different initial reaction(s) (3, 14). These initial reactions are not yet understood.
In this study, we investigated the initial reaction(s) of alkane degradation by strain AK-01. This strain, which was isolated from a petroleum-contaminated estuarine sediment, is able to oxidize alkanes completely to carbon dioxide, with coupling to sulfate reduction (35). Early evidence also suggests that it anaerobically oxidize alkanes to fatty acids. By using stable- isotope-labeled alkanes as growth substrates, we clearly documented that alkanes are oxidized to fatty acids by this organism. Structural characterizations of the isotope-labeled fatty acids derived from the labeled alkanes demonstrated that exogenous carbon is initially added to the alkane chain subterminally at the C-2 position, with subsequent formation of fatty acids.
MATERIALS AND METHODS
Source of organism and culture conditions.
The alkane-degrading bacterial strain AK-01 was isolated from a petroleum-contaminated estuarine sediment collected from the Arthur Kill in New York (35). The medium used for maintaining AK-01 was the same as that described previously (35) except that no yeast extract was added. Liquid alkanes and 1-alkenes used as the substrates were sterilized separately by either autoclaving or filtration (0.22 μm pore size) before use. Long-chain fatty acids (as sodium salts) were prepared anoxically as concentrated stock solutions and were autoclaved before being added (while still melted) to the medium. Solid 1-alkanols were autoclaved together with the medium. In most cases, subcultures of AK-01 grown on alkanes for about a month were used as the inocula, which differed in size with the experiment (see details for each experiment below). All cultures were incubated in the dark without shaking.
Effect of alkanes on total cellular fatty acid composition.
Bottles containing 100 ml of medium were amended with 20 μl of tetradecane, pentadecane, hexadecane, or heptadecane (99%; Sigma Chemical Co., St. Louis, Mo.) and inoculated with cultures of AK-01 maintained on hexadecane (5%, vol/vol). For each alkane, two experimental cultures and two sterile controls were set up. After growing for about a month, cultures were subcultured again in medium supplemented with the same alkane. After incubation for 38 to 51 days to reach the stationary phase (growth rates differed depending on the substrate), all cultures were sacrificed for extraction and analysis of total cellular fatty acids.
Effect of other substrates on total cellular fatty acid composition.
Bottles containing 100 ml of medium were amended with the following substrates: 1-pentadecene (20 μl), 1-hexadecene (20 μl), 1-pentadecanol (20 mg), 1-hexadecanol (20 mg), pentadecanoate (0.5 mM), and hexadecanoate (0.5 mM). The media were then inoculated with cultures of AK-01 maintained on hexadecane (5%, vol/vol). For each substrate, two experimental cultures and two autoclaved controls were prepared. After incubation for 14 to 51 days to reach stationary phase, the cultures were sacrificed for extraction and analysis of total cellular fatty acids.
Degradation of [1,2-13C2]hexadecane.
Cultures were prepared by adding 20 μl of [1,2-13C2]hexadecane (isotopic enrichment, 99%; Isotec Inc., Miamisburg, Ohio) to 100 ml of medium and inoculating with a culture pregrown on pentadecane (5%, vol/vol). Two experimental cultures and one sterile control were prepared. For the sterile control, the inoculum was autoclaved before use. All cultures were incubated in the dark without shaking for 47 days and sacrificed for extraction and analysis of total cellular fatty acids.
Degradation of perdeuterated pentadecane.
Cultures were prepared by adding 20 μl of perdeuterated pentadecane (C15D32; isotopic enrichment, 98%; Cambridge Isotope Laboratories, Inc., Andover, Mass.) to 100 ml of medium and inoculating with a culture pregrown on hexadecane (5%, vol/vol). Similar to the study described above, two experimental cultures and one sterile control were prepared for the experiment, and the inoculum was autoclaved before use as the sterile control. All cultures were incubated in the dark without shaking for 39 to 47 days and then sacrificed for extraction and analysis of total cellular fatty acids.
Alkane degradation in the presence of [13C]bicarbonate.
The mineral salt medium was prepared with NaH13CO3 (isotopic enrichment, 99%; Cambridge Isotope Laboratories) instead of unlabeled sodium bicarbonate for growth of the cultures in this experiment. Each bottle containing 50 ml of medium was amended with 20 μl of pentadecane or hexadecane and then inoculated with a hexadecane-grown culture (1%, vol/vol; a small inoculum size was used to minimize [12C]bicarbonate contamination from the inoculum). Duplicates of active cultures were prepared for each alkane. Cultures were incubated for 45 to 67 days and then sacrificed for extraction and analysis of total cellular fatty acids.
Extraction of total cellular fatty acids.
Each culture was sacrificed by acidification with HCl (0.05 N final concentration), and cells were collected by filtering them through a Teflon filter (0.5 μm pore size, 47 mm diameter; Gelman Sciences, Inc., Ann Arbor, Mich.). The collected cells were transferred to a screw-capped glass test tube with a Teflon-faced septum. A method to extract the total cellular fatty acids from the cells was adapted from a protocol for fatty acid analysis (Sherlock Microbial Identification System: MIDI, Inc., Newark, Del.). To each tube with cells on the filter was added 2 ml of a NaOH-methanol solution (45 g of NaOH in 150 ml of methanol plus 150 ml of deionized water); the tubes were then capped and heated at 100°C for 30 min to release the cellular fatty acids by saponification and cooled. Then 4 ml of an HCl-methanol solution (325 ml of 6 N HCl and 275 ml of methanol) was added to each tube, and the tubes were heated at 80°C for 10 min to esterify the fatty acids. When the tube contents had cooled down, 1.25 ml of hexane-methyl tert-butyl ether (1:1) was added to extract the fatty acid methyl esters. The solvent extract was then washed with an aqueous NaOH solution (1.2%, wt/vol) to remove any unesterified fatty acids. Samples were stored in glass vials for subsequent analysis by gas chromatography-mass spectrometry (GC-MS).
Analysis of fatty acid methyl esters.
Samples of fatty acid methyl esters were initially analyzed by GC-flame ionization detection (FID) with a gas chromatograph (model 5890, series II; Hewlett-Packard, Wilmington, Del.) equipped with a flame ionization detector and a DB-WAX column (30 m by 0.25 mm; film thickness, 0.25 μm; J&W Scientific, Folsom, Calif.). The column temperature was initially set at 50°C for 2 min, then increased to 140°C by 15°C per min and, finally, to 220°C by 4°C per min. The injector and detector temperatures were 250 and 300°C, respectively. The n-saturated and monounsaturated fatty acids were identified by comparing their retention times with those of commercially available authentic standards (Sigma Chemical Co.).
Samples were also analyzed by GC-MS with a 5890 series II gas chromatograph (Hewlett-Packard) equipped with a DB-5ms column (30 m by 0.25 mm; film thickness, 0.25 μm; J&W Scientific) and a 5971 series mass-selective detector (Hewlett-Packard). The temperature program was as follows: 60°C for 1 min, increased by 20°C per min to 200°C, and then increased by 4°C per min to 280°C. The temperature for the injector and detector was 280°C. Identities of most n-saturated and unsaturated fatty acids were determined by comparing their retention times and mass spectra with those of commercially available authentic standards (Sigma Chemical Co.). Since no authentic standards were available for them, the various methylated and isotope-labeled fatty acids were identified by interpretation of their mass spectra based on information reported in the literature (11, 16, 26, 28, 33). Specific locations of 13C or deuterium atoms on the isotope-labeled fatty acids were determined by examining the diagnostically important ion fragments whose structural compositions had been previously determined (10, 12, 26, 37a). Each fatty acid in a sample was quantified based on its peak area in the total-ion chromatogram and was expressed as a percentage of the total fatty acids recovered. Preliminary analytical studies of a standard mixture of fatty acid methyl esters on the GC-MS instrument showed that those with chain lengths of C12 to C24 had similar response ratios based on the total mass of each compound injected.
Nomenclature for fatty acids.
The fatty acid nomenclature recommended by the IUPAC-IUB (20) was adopted in this study. An n-saturated hexadecanoic acid is designated as 16:0, with the first number representing the number of carbons in the acyl group and the second number representing the number of double bonds present. The branched fatty acid 2-methylhexadecanoic acid, for example, is designated as 2-Me-16:0.
RESULTS
Effect of alkanes on total cellular fatty acid composition.
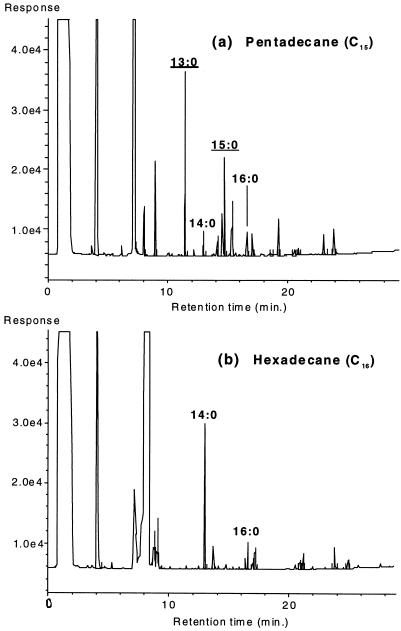
Figure 1 shows the fatty acid profiles of strain AK-01 grown on pentadecane (Fig. 1a) and hexadecane (Fig. 1b) as determined by GC-FID. The effect of the chain lengths of the alkane substrates on the total cellular fatty acid composition is apparent. When pentadecane was used as the growth substrate, the 13:0 and 15:0 fatty acids, both C odd, predominated over the C-even fatty acids. In contrast, when hexadecane was used, the 14:0 and 16:0 fatty acids became dominant while the C-odd fatty acids were virtually absent.
FIG. 1.
Gas chromatograms (GC-FID) showing the fatty acid profiles of strain AK-01 grown on pentadecane (a) and hexadecane (b). The n-saturated fatty acids identified by their retention times are annotated. C-odd fatty acids are underlined.
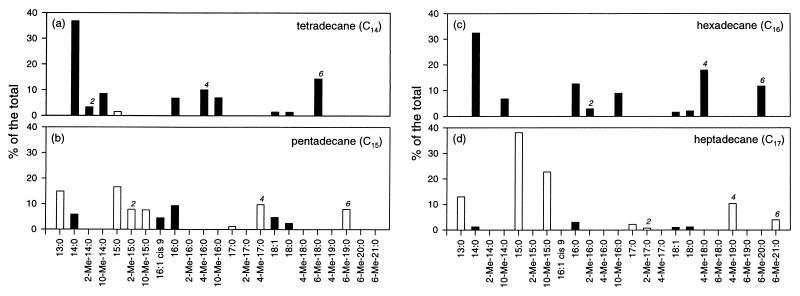
The total cellular fatty acid composition of AK-01 upon growth on different alkanes (C14 to C17) was further studied in detail by using GC-MS. As summarized in Fig. 2, when tetradecane (Fig. 2a) and hexadecane (Fig. 2c), both C-even alkanes, were used as growth substrates, the cellular fatty acids of AK-01 were predominantly C even (comprising 89.3 and 97.1% of the total, respectively). On the other hand, when pentadecane (Fig. 2b) and heptadecane (Fig. 2d) (both C odd) were used, C-odd fatty acids were dominant (65.6 and 91.1% of the total, respectively). No abiotic formation of these fatty acids was observed for any of the sterile controls. The carbon numbers of other aliphatic compounds, such as 1-alkenes (C15 and C16), 1-alkanols (C15 and C16), and fatty acids (C15 and C16), also had the same effect on the fatty acid composition of AK-01 when these compounds were used as growth substrates (data not shown). The results, therefore, support the hypothesis that alkanes (and the other aliphatic compounds) are oxidized to fatty acids by strain AK-01.
FIG. 2.
Relative abundance of the total cellular fatty acids of strain AK-01 grown on tetradecane (a), pentadecane (b), hexadecane (c), and heptadecane (d). Only those which can be identified and comprise more than 1% of the total are shown. C-even fatty acids are represented by black bars, and C-odd fatty acids are represented by white bars. The 2-methyl, 4-methyl, and 6-methyl fatty acids are denoted by the numbers 2, 4, and 6, respectively. (The 2-Me-17:0 fatty acid is also shown, though it constitutes only 0.7% of the total.) No fatty acid was observed in the sterile controls.
It should be noted that some monomethylated fatty acids with methyl groups located at the C-2, C-4, or C-6 position (as indicated in Fig. 2) were only found when the organism is grown on alkanes, not found when it was grown on 1-alkenes, 1-alkanols, or fatty acids. In addition, the chain lengths of these monomethylated fatty acids also specifically correlated with those of the alkane substrates. As shown in Fig. 2a, the 2-Me-14:0, 4-Me-16:0, and 6-Me-18:0 fatty acids were found only when tetradecane (C14) was used as the substrate. The 2-Me-15:0, 4-Me-17:0, and 6-Me-19:0 fatty acids were found only when pentadecane (C15) was used as the substrate (Fig. 2b). The same pattern was also observed in cultures grown on hexadecane or heptadecane (Fig. 2c and d). Furthermore, these monomethylated fatty acids were uniquely found in strain AK-01 only when it was grown on alkanes, and they have not been reported to occur in other sulfate-reducing bacteria (36). C10-methylated fatty acids, however, were found in strain AK-01 when it was grown on the other aliphatic substrates as well as alkanes. In addition, 10-methylated fatty acids can also be found in other sulfate-reducing bacteria incapable of alkane degradation, and thus they are not unique to strain AK-01 (36).
The fact that these 2-, 4-, and 6-methylated fatty acids were only observed when AK-01 was grown on alkanes is a strong indication that their formation is specifically related to the initial reactions of anaerobic oxidation of alkanes. Conversely, the absence of these methylated fatty acids when AK-01 is grown on 1-alkenes and 1-alkanols also suggests that the latter two classes of compounds are probably not intermediates in anaerobic alkane metabolism.
Analyses of [1,2-13C2]hexadecane-derived fatty acids.
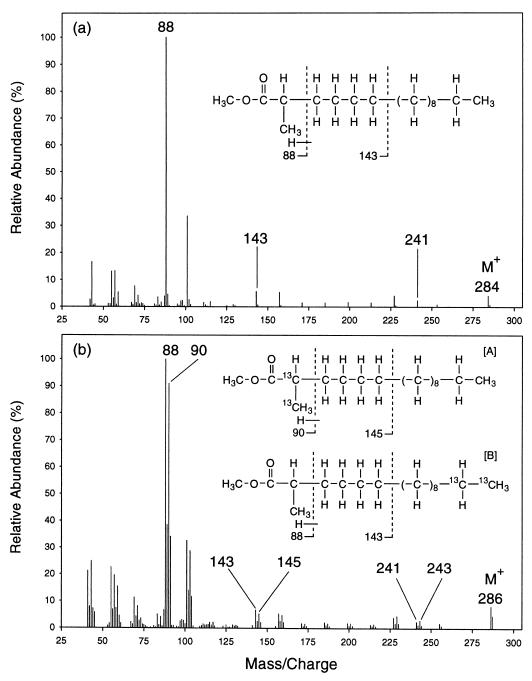
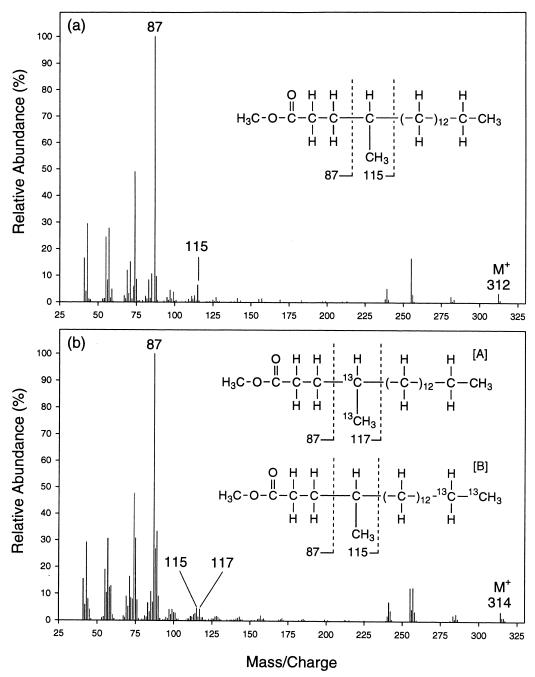
Mass spectral analyses of the 2-, 4-, and 6-methylated fatty acids recovered from cultures of AK-01 grown on [1,2-13C2]hexadecane confirmed that these fatty acids were derived from the alkane. Figure 3 shows the mass spectra of the methyl esters of the unlabeled (Fig. 3a) and 13C-labeled (Fig. 3b) 2-Me-16:0 fatty acids recovered from AK-01 cultures grown on unlabeled hexadecane and [1,2-13C2]hexadecane, respectively. As shown in Fig. 3a, the molecular ion peak for the unlabeled 2-Me-16:0 fatty acid appears at m/z = 284. The molecular ion peak for the same fatty acid from the [1,2-13C2]hexadecane-grown cultures is shifted up by 2 mass units to m/z = 286 (Fig. 3b), reflecting the presence of two 13C atoms in the molecule.
FIG. 3.
Mass spectra of the methyl esters of the unlabeled (a) and 13C-labeled (b) 2-Me-16:0 fatty acids recovered from cultures of strain AK-01 grown on unlabeled hexadecane and [1,2-13C2]hexadecane, respectively. Chemical structures of the fatty acids represented by the mass spectra are shown as insets. The key diagnostic ion peaks are annotated with their m/z values. Structural compositions of the ion fragments represented by the annotated peaks are delineated. Intersection with a dotted line indicates a point of bond cleavage, and the ion fragment formed contains only the part of the molecule on the left side of the dotted line. (Note: the mass spectrum for the 13C-labeled 2-Me-16:0 fatty acid indicates the coexistence of two isotopmers.)
A detailed examination of the mass spectra shows that the initial attack can occur at either the labeled or unlabeled end of the [1,2-13C2]hexadecane. As depicted in Fig. 3a, the ion fragment represented by the ion peak at m/z = 88 includes C-1, C-2, and the 2-methyl group of the unlabeled 2-Me-16:0 fatty acid (along with the ester-linked methyl group). This fragment is formed by a process, known as the McLafferty rearrangement, which specifically involves the migration of a hydrogen from C-4 to the carbonyl oxygen and the cleavage of the C-2–C-3 bond (26, 33). Furthermore, the ion fragment at m/z = 143 includes C-1 to C-6 (and the 2-methyl group) of the unlabeled fatty acid and is formed by the cleavage of the C-6–C-7 bond (12, 26, 33).
On the mass spectrum for the 13C-labeled 2-Me-16:0 fatty acid (Fig. 3b), peaks of comparable intensity appear at m/z = 88 and 90. Thus, this ion fragment, which includes C-1, C-2, and the 2-methyl group of the fatty acid, occurs in two forms that are present in comparable abundance but that contain different numbers of 13C atoms. The one at m/z = 90 contains two 13C atoms, while the one at m/z = 88 contains none at all. It appears, therefore, that the 13C-labeled 2-Me-16:0 fatty acid occurs as two isotopmers of comparable abundance. One isotopmer contains two 13C atoms among the C-1, C-2, and the 2-methyl group carbons (isotopmer A in Fig. 3b), while the other has the two labels located at the other end of the molecule (isotopmer B). Isotopmers are versions of the same molecule with different specific isotopic labelling patterns (33a).
Further analysis of the mass spectrum of the 13C-labeled 2-Me-16:0 fatty acid showed that isotopmer B contained two 13C atoms between C-7 and C-16. As shown in Fig. 3b, two peaks of comparable intensity appear at m/z = 143 and 145, indicating again the occurrence of two isotopmers. One (isotopmer A) contains two 13C atoms between C-1 and C-6 (thus, m/z = 145), while the other (isotopmer B) has the two labels located between C-7 and C-16 (thus, m/z = 143). These two labels in isotopmer B are most likely located at C-15 and C-16, their original locations on the [1,2-13C2]hexadecane. The presence of two 13C atoms at either end of the fatty acid, therefore, indicates that the initial attack occurs with the same likelihood at the labeled and the unlabeled termini of the hexadecane.
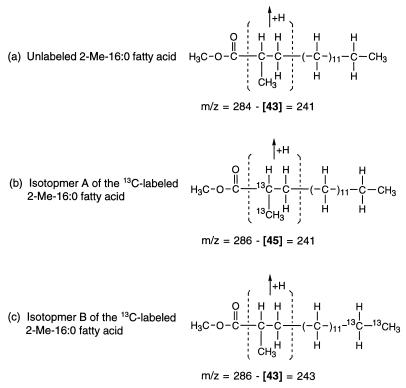
In addition, further examination of the mass spectra indicated that the two 13C atoms in isotopmer A of the 2-Me-16:0 fatty acid are located at C-2 and the 2-methyl group. As illustrated in Fig. 4a, the ion peak at m/z = 241 for the unlabeled fatty acid (indicated in the mass spectrum in Fig. 3a) is formed by the elimination of the segment of the molecule containing C-2, C-3, and the 2-methyl group (and an additional hydrogen, yielding a combined mass of 43) followed by the rearrangement of the remaining parts. This fragmentation mechanism is documented in the literature (12, 33, 37a). The corresponding ion peak in the mass spectrum of the 13C-labeled fatty acid appears as a doublet of peaks of comparable intensity at m/z = 241 and 243 (Fig. 3b), indicating that isotopmer A contains two 13C atoms in the eliminated segment (Fig. 4b) while isotopmer B does not (Fig. 4c). The results show that the two 13C atoms in isotopmer A are located at C-2, C-3, and/or the 2-methyl group, not at C-1 or elsewhere (Fig. 4b). Furthermore, as shown earlier by examination of the doublet at m/z = 88 and 90, none of the two 13C carbons on isotopmer A is located at C-3. Thus, they are located at C-2 and the 2-methyl group of isotopmer A.
FIG. 4.
Ion fragments represented by the peaks at m/z = 241 for the unlabeled 2-Me-16:0 fatty acid methyl ester (a) (see Fig. 3a) and m/z = 241 (b) and m/z = 243 (c) for the 13C-labeled 2-Me-16:0 fatty acid methyl ester (see Fig. 3b). These ion fragments are formed by the elimination of the bracketed segment of the molecule and a hydrogen, followed by the rearrangement of the remaining parts (12, 33, 37a).
Based on the results described above, we conclude that the 2-methyl group of the 2-Me-16:0 fatty acid is the original terminal carbon of the [1,2-13C2]hexadecane. This suggests that exogenous carbon is initially added onto the [1,2-13C2]hexadecane subterminally at the C-2 position such that the original terminal carbon of the alkane then becomes the methyl group of the resulting 2-Me-16:0 fatty acid.
Mass spectral data on the 4-Me-18:0 fatty acid from the [1,2-13C2]hexadecane-grown cultures also indicate that the 4-methyl group is the original terminal carbon of the alkane. Figure 5 shows the mass spectra of the unlabeled (Fig. 5a) and 13C-labeled (Fig. 5b) 4-Me-18:0 fatty acid recovered from cultures grown on unlabeled hexadecane and [1,2-13C2]hexadecane, respectively. The presence of two 13C atoms in the latter fatty acid is indicated by the molecular ion at m/z = 314 (Fig. 5b).
FIG. 5.
Mass spectra of the methyl esters of the unlabeled (a) and 13C-labeled (b) 4-Me-18:0 fatty acids recovered from cultures of strain AK-01 grown on unlabeled hexadecane and [1,2-13C2]hexadecane, respectively. Chemical structures of the fatty acids represented by the mass spectra are shown as insets. (Note: the mass spectrum for the 13C-labeled 4-Me-18:0 fatty acid indicates the coexistence of two isotopmers.)
The 13C-labeled 4-Me-18:0 fatty acid also occurs as two isotopmers, one with the two 13C atoms located at C-4 and the 4-methyl group. As shown in Fig. 5a, the ion fragment at m/z = 87 is formed by cleavage of the C-3–C-4 bond and includes C-1 to C-3 of the unlabeled 4-Me-18:0 fatty acid. In addition, the ion fragment at m/z = 115 is formed by cleavage of the C-4–C-5 bond and includes C-1 to C-4 and the 4-methyl group of the molecule (12, 26, 33). The difference between the m/z values of these two ion peaks, which amounts to 28 mass units, represents the combined mass of C-4 plus the attached hydrogen atom and the 4-methyl group [—CH(CH3)—].
On the mass spectrum for the 13C-labeled 4-Me-18:0 fatty acid (Fig. 5b), the ion peak corresponding to that at m/z = 87 for the unlabeled fatty acid (Fig. 5a) remains the same, indicating the absence of 13C label at C-1 to C-3. The peak corresponding to m/z = 115, however, appears as a doublet of peaks at m/z = 115 and 117 of comparable intensity. Thus, the 13C-labeled 4-Me-18:0 fatty acid occurs as two isotopmers of comparable abundance, one labeled with two 13C atoms, at C-4 and the 4-methyl group (isotopmer A in Fig. 5b), and the other with no isotope label on these carbons. The two 13C atoms in the latter case are most likely located at C-17 and C-18 (isotopmer B in Fig. 5b). These results shows that the 4-methyl group is the original terminal carbon of the [1,2-13C2]hexadecane and further underscore the point that exogenous carbon is added onto the alkane subterminally at the C-2 position. It also appears that the 4-Me-18:0 fatty acid is formed after elongation of the 2-Me-16:0 fatty acid by two carbon units through fatty acid chain elongation reactions (31).
Table 1 summarize the characteristics of the 2-, 4-, and 6-methylated fatty acids derived from the [1,2-13C2]hexadecane. For each fatty acid, the positions of the 13C atoms on each isotopmer and the relative abundance of the two different isotopmers are shown. None of these 13C-labeled fatty acids was found in the sterile control. Like the 2-Me-16:0 and 4-Me-18:0 fatty acids, the 13C-labeled 6-Me-20:0 fatty acid contains two 13C atoms, at C-6 and the 6-methyl group, in one isotopmer (mass spectrum not shown). Thus, it is a result of a similar carbon addition mechanism followed by chain elongation. Other C-even fatty acids (14:0, 10-Me-14:0, 16:0, and 10-Me-16:0), which are also 13C labeled, appear to be formed after further metabolism of the 2-Me-16:0 fatty acid (data not shown).
TABLE 1.
Characteristics of the 13C-labeled 2-, 4-, and 6-methylated fatty acids recovered from cultures of strain AK-01 grown on [1,2-13C2]hexadecane
| Cellular fatty acida | Chemical structure and location of 13C label for the fatty acid isotopmerb:
|
Relative abundance of isotopmers A and Bc | |
|---|---|---|---|
| A | B | ||
| 2-Me-16:0 | 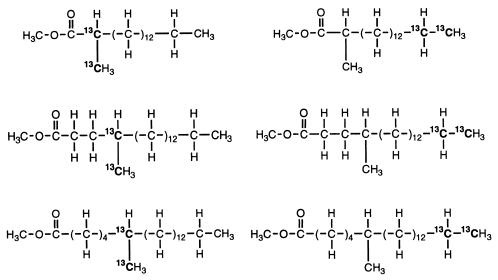 |
94:100 | |
| 4-Me-18:0 | 91:100 | ||
| 6-Me-20:0 | 100:81 | ||
Other recovered 13C-labeled fatty acids, namely 14:0, 10-Me-14:0, 16:0, and 10-Me-16:0, are not shown here. None of these fatty acids was found in the sterile control.
Determined by interpretation of the mass spectrum for each fatty acid.
Isotopmer A/B ratios. Results are estimations based on the relative intensities of the doublet of diagnostic ion peaks for each fatty acid.
Analyses of perdeuterated pentadecane-derived fatty acids.
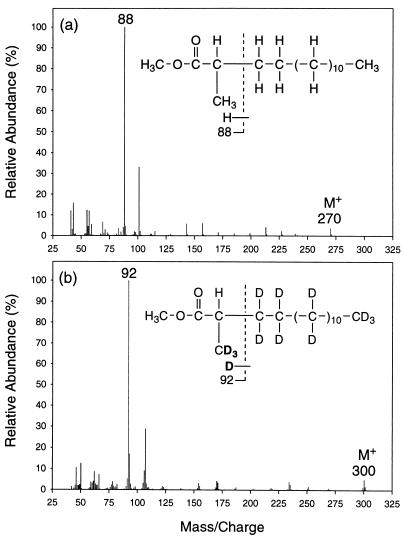
Mass spectral analyses of the deuterated fatty acids also indicate that exogenous carbon is added onto the alkane chain subterminally to form a monomethylated fatty acid. Figure 6 shows the mass spectra of the methyl esters of the unlabeled (Fig. 6a) and deuterated (Fig. 6b) 2-Me-15:0 fatty acid recovered from cultures grown on unlabeled and perdeuterated pentadecane, respectively. As shown in Fig. 6a, the ion fragment, m/z = 88, of the unlabeled 2-Me-15:0 fatty acid is formed by the McLafferty rearrangement as described earlier (26, 33). The corresponding peak for the deuterated fatty acid (Fig. 6b) is shifted up by 4 mass units (m/z = 92), indicating the presence of four deuterium atoms in the ion fragment. Since the methanol used for the fatty acid derivatization was not deuterated and one deuterium would have been contributed by the McLafferty rearrangement (12), we deduce that three deuterium atoms are located at C-2 and the 2-methyl group. Similar to the study with the [1,2-13C2]hexadecane, the results here indicate that the 2-methyl group of the deuterated 2-Me-15:0 fatty acid is the original terminal carbon of the perdeuterated pentadecane. Thus, the 2-Me-15:0 fatty acid is the result of subterminal carbon addition to the perdeuterated pentadecane at the C-2 position.
FIG. 6.
Mass spectra of the methyl esters of the unlabeled (a) and deuterated (b) 2-Me-15:0 fatty acids recovered from cultures of strain AK-01 grown on unlabeled and perdeuterated pentadecane, respectively. Chemical structures of the fatty acids represented by the mass spectra are shown as insets. Interpretation of the mass spectra shows that there are three deuterium atoms, located on C-2 and the 2-methyl group of the deuterated 2-Me-15:0 fatty acid. The locations of all three deuterium atoms on the 2-methyl group as shown in Fig. 6b represent one possible arrangement.
Mass spectral analyses of the deuterated 4-Me-17:0 and 6-Me-19:0 fatty acids from cultures grown on perdeuterated pentadecane also indicated the occurrence of subterminal carbon addition. As shown in Table 2, the 2-, 4-, and 6-methylated fatty acids all contain three deuterium atoms, located on their methyl groups and the adjacent carbons, indicating that the methyl groups also contain the original terminal carbon of the perdeuterated pentadecane and that exogenous carbon is added to the deuterated pentadecane subterminally at C-2. This thus shows that the deuterated 4-Me-17:0 and 6-Me-19:0 fatty acids are formed by chain elongation of the 2-Me-15:0 fatty acid. Deuterium atoms are also found on the carbons between the carboxyl groups and methyl groups of the 4-Me-17:0 and 6-Me-19:0 fatty acids (Table 2). This may be effected by the initial carbon addition reaction in that the carbon compound being added to the perdeuterated pentadecane is also directly derived from the labeled alkane and thus still carries deuterium atoms. Other C-odd deuterated fatty acids, namely 15:0 and 10-Me-15:0, are also recovered and are likely formed by further metabolism of the 2-Me-15:0 fatty acid. The lack of deuterium atoms in the 10-methyl group of the deuterated 10-Me-15:0 (data not shown) indicates that this C-10 methyl group is exogenously added, in contrast to those of the 2-, 4-, and 6-methylated fatty acids.
TABLE 2.
Characteristics of the deuterated, 2-, 4-, and 6-methylated fatty acids recovered from cultures of strain AK-01 grown on perdeuterated pentadecane
| Deuterated fatty acida | Chemical structure and distribution of deuterium labelb |
|---|---|
| 2-Me-15:0 | 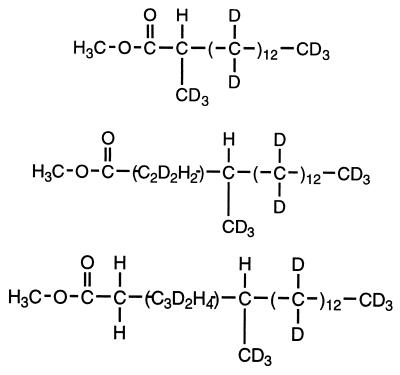 |
| 4-Me-17:0 | |
| 6-Me-19:0 |
Other recovered deuterated fatty acids, namely 15:0 and 10-Me-15:0, are not shown here. No deuterated fatty acids were found in the sterile control.
Determined by interpretation of the mass spectrum for each fatty acid.
Fatty acids formed in the presence of [13C]bicarbonate.
The 2-methylated fatty acids recovered from cultures of AK-01 grown on unlabeled pentadecane and hexadecane in the presence of [13C]bicarbonate were characterized by mass spectrometry. The mass spectra of the 2-Me-15:0 and 2-Me-16:0 fatty acids formed in the presence of [13C]bicarbonate appeared the same as those formed in the presence of unlabeled bicarbonate. The results indicate that the carboxyl groups of the 2-methylated fatty acids are not 13C labeled. Hence, in AK-01, the carbon addition at C-2 of the alkane chain does not appear to occur through the addition of an inorganic carboxyl group.
DISCUSSION
The alkane-degrading strain AK-01 was isolated from petroleum-contaminated estuarine sediments. It grows anaerobically on alkanes (C13 to C18) and is able to oxidize them to carbon dioxide coupled to sulfate reduction (35). In the present study, the mechanism for anaerobic alkane degradation by the strain was determined. Although 1-alkene and 1-alkanol have been suggested as intermediates (29) and strain AK-01 is able to utilize both for growth (35), neither compound was found in the alkane-grown cultures in our experiments. That they are unlikely to be intermediates is also supported by data from studies of strain Hxd3 (1, 3).
Although no free fatty acid was extractable from whole cultures of AK-01 grown on alkanes, the impact of the alkane substrates’ carbon numbers on the total cellular fatty acid composition (Fig. 2) is highly suggestive of alkanes being oxidized to fatty acids by the organism. This has been observed with the other alkane-utilizing sulfate reducers (3, 14) and also for microorganisms capable of aerobic alkane utilization (13, 21). The fatty acids formed after alkane oxidation can be directly incorporated into the cellular lipids of the alkane-degrading organisms (5, 18, 22).
The recovery of deuterated and 13C-labeled fatty acids in cultures of AK-01 grown on the perdeuterated and 13C-labeled alkanes, respectively, clearly confirms that alkanes are anaerobically oxidized to fatty acids. In addition, the carbon numbers of the labeled fatty acids also correlate with those of the alkane substrates. When [1,2-13C2]hexadecane was used as a growth substrate, the recovered 13C-labeled fatty acids were all C even (Table 1). When perdeuterated pentadecane was used, the recovered deuterated fatty acids were all C odd (Table 2). The results show that the fatty acids derived from the alkanes can be β-oxidized and mineralized to CO2 (24, 35) or further transformed through chain elongation and C-10 methylation (31) by AK-01.
Evidence presented here for a proposed mechanism of alkane oxidation is derived from mass spectral analyses of the resulting stable-isotope-labeled fatty acids. The mass spectra of the methyl esters of many unlabeled and deuterated fatty acids have been reported (11, 16, 26, 28, 33). The compositions and fragmentation mechanisms of various ion fragments, which contain different parts of the fatty acid molecule, are also well studied (10, 12, 26). Based on this information, many fatty acids can be identified and the locations of 13C and deuterium atoms on the isotope-labeled fatty acids can be determined.
Three lines of evidence support a mechanism by which exogenous carbon is added to an alkane subterminally as an initial step in anaerobic alkane metabolism. First, the 2-Me-16:0, 4-Me-18:0, and 6-Me-20 fatty acids derived from the [1,2-13C2]hexadecane each contains two 13C atoms, located at the methyl group and the adjacent carbon (Table 1). The results indicate that exogenous carbon is added subterminally to the hexadecane at the C-2 position, such that the terminal carbon of the alkane becomes the 2-, 4-, and 6-methyl groups of the fatty acids formed subsequently.
Second, the 2-Me-15:0, 4-Me-17:0, and 6-Me-19 fatty acids derived from the perdeuterated pentadecane each contains three deuterium atoms, located on the methyl group and the adjacent carbon (Table 2). This also supports the same mechanism, namely subterminal carbon addition to the perdeuterated pentadecane at C-2, resulting in the original terminal carbon of the alkane forming the 2-, 4-, and 6-methyl groups of these fatty acids.
Third, the 2-, 4-, and 6-methylated fatty acids are found only in cultures grown on alkanes, and their chain lengths always correlate with those of the alkane substrates (Fig. 2). These uncommon monomethylated fatty acids are not observed when AK-01 is grown on other aliphatic substrates (1-alkenes, 1-alkanols, and fatty acids), and they are not observed in other sulfate-reducing bacteria (36). Therefore, these fatty acids are formed specifically by the initial reactions of alkane oxidation. Furthermore, it appears that the 2-methylated fatty acids are initially formed from the alkanes and can be subsequently elongated by two carbon units to form the 4-methylated and 6-methylated fatty acids through the fatty acid elongation reactions (Tables 1 and 2) (31). The nature of the carbon compound being added onto the alkane chain, however, remains unknown at this time. The absence of the 2-, 4-, and 6-methylated fatty acids in AK-01 cultures grown on 1-alkenes also indicates that carbon addition is not mediated by the formation of a terminal double bond in the alkane.
Although carboxylation has been demonstrated as an initial reaction of aromatic hydrocarbon metabolism under anaerobic conditions (30, 39), results from our experiment with [13C]bicarbonate show that the subterminal carbon addition is probably not due to a direct carboxylation. On the other hand, it has been demonstrated that toluene is anaerobically metabolized by addition of fumarate to form benzyl succinate and then benzoyl coenzyme A (4). Hence, we cannot discount the possibility that a compound with more than one carbon is added to an alkane to form the first intermediate, which is then transformed into a 2-methylated fatty acid.
The anaerobic attack on alkanes by AK-01 involves carbon addition at the C-2 position. Such selectivity for the subterminal carbon may be caused by the conformational structure of the degrading enzyme. Studies with model systems support the hypothesis that the terminal carbons of alkanes are selectively hydroxylated by cytochrome P-450 because of steric hindrance at its active site (23). The degrading enzyme of AK-01 may have a less sterically hindered active site, such that the subterminal carbon at C-2 can be attacked. Furthermore, as a secondary carbon, its C—H bond is weaker than that of a terminal, primary carbon (bond dissociation energy, 94.5 versus 98 kcal/mol for propane, for example) (15) and thus should be more readily broken.
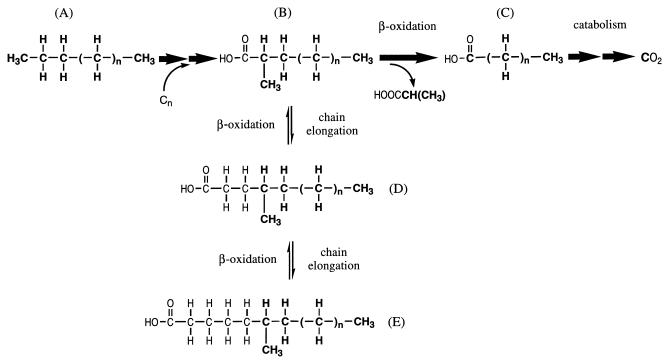
Based on the collective observations in this study, a proposed pathway for anaerobic oxidation of alkanes by strain AK-01 is summarized in Fig. 7. An alkane (A) is initially attacked subterminally by the addition of a carbon compound at the C-2 position to form a 2-methylated fatty acid (B). This fatty acid then undergoes β-oxidation (24, 25, 34) to form an n-saturated fatty acid (C) which can be further β-oxidized and eventually mineralized to CO2 (35). In addition, the initially formed 2-methylated fatty acid (B) can also be elongated to form the 4-methylated (D) and 6-methylated (E) fatty acids.
FIG. 7.
Proposed pathway for anaerobic alkane metabolism by strain AK-01. (The original alkane atoms are boldfaced, and the major pathway is indicated by bold arrows.)
In summary, we provide direct evidence that alkanes are oxidized to fatty acids by strain AK-01 under strictly anaerobic conditions. Subterminal addition of a carbon(s) to the hydrocarbon chain as an initial reaction in anaerobic alkane degradation is also demonstrated through a detailed characterization of these fatty acid metabolites. This carbon addition reaction represents a novel mechanism by which alkanes can be activated without oxygen, in contrast to the well-known hydroxylation reaction mediated by monooxygenases in aerobic organisms.
ACKNOWLEDGMENTS
We thank Max Häggblom, Xiaoming Zhang, Mike Logan, Craig Phelps, and Alfred Boyle for technical advice and editorial help, Ingeborg Bossert and Andreas Naef for translation of German literature, and Maria Rivera and Brian Donovan for technical assistance.
This work was supported in part by the Office of Naval Research and the Defense Advanced Research Project Agency.
REFERENCES
- 1.Aeckersberg F. Anaerober Abbau von Alkanen und 1-Alkenen durch sulfatreduzierende Bakterien. Ph.D. dissertation. Bremen, Germany: University of Bremen; 1994. . Verlag Mainz, Mainz, Germany. [Google Scholar]
- 2.Aeckersberg F, Bak F, Widdel F. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch Microbiol. 1991;156:5–14. [Google Scholar]
- 3.Aeckersberg F, Rainey F A, Widdel F. Growth, natural relationships, cellular fatty acids and metabolic adaptation of sulfate-reducing bacteria that utilize long-chain alkanes under anoxic conditions. Arch Microbiol. 1998;170:361–369. doi: 10.1007/s002030050654. [DOI] [PubMed] [Google Scholar]
- 4.Biegert T, Fuchs G, Heider J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur J Biochem. 1996;238:661–668. doi: 10.1111/j.1432-1033.1996.0661w.x. [DOI] [PubMed] [Google Scholar]
- 5.Blasig R, Huth J, Franke P, Borneleit P, Schunck W-H, Müller H-G. Degradation of long-chain n-alkanes by yeast Candida maltosa. III. Effect of solid n-alkanes on cellular fatty acid composition. Appl Microbiol Biotechnol. 1989;31:571–576. [Google Scholar]
- 6.Britton L N. Microbial degradation of aliphatic hydrocarbons. In: Gibson D T, editor. Microbial degradation of organic compounds. New York, N.Y: Marcel Dekker, Inc.; 1984. pp. 89–129. [Google Scholar]
- 7.Caldwell M E, Garrett R M, Prince R C, Suflita J M. Anaerobic biodegradation of long-chain n-alkanes under sulfate-reducing conditions. Environ Sci Technol. 1998;32:2191–2195. [Google Scholar]
- 8.Chouteau J, Azoulay E, Senez J C. Anaerobic formation of n-hept-1-ene from n-heptane by resting cells of Pseudomonas aeruginosa. Nature. 1962;194:576–578. [Google Scholar]
- 9.Coates J D, Woodward J, Allen J, Philp P, Lovley D R. Anaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments. Appl Environ Microbiol. 1997;63:3589–3593. doi: 10.1128/aem.63.9.3589-3593.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Đinh-Nguyên N. Contribution à l’étude de la spectrométrie de masse: utilisation des esters méthyliques de monoacides à longue chaîne normale marqués au deutérium et au carbone-13. Ark Kemi. 1968;28:289–362. [Google Scholar]
- 11.Đinh-Nguyên N, Raal A, Stenhagen E. Perdeuteriated organic compounds. I. Normal long-chain saturated deuteriocarbons, monocarboxylic acids and methyl esters. Chem Scr. 1972;2:171–178. [Google Scholar]
- 12.Đinh-Nguyên N, Ryhage R, Ställberg-Stenhagen S, Stenhagen E. Mass spectrometric studies. VIII. A study of the fragmentation of normal long-chain methyl esters and hydrocarbons under electron impact with the aid of deuterium substituted compounds. Ark Kemi. 1961;18:393–399. [Google Scholar]
- 13.Dunlap K R, Perry J J. Effect of substrate on the fatty acid composition of hydrocarbon-utilizing microorganisms. J Bacteriol. 1967;94:1919–1923. doi: 10.1128/jb.94.6.1919-1923.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrenreich P. Anaerobes Wachstum neuartiger sulfatreduzierender und nitratreduzierender Bakterien auf n-Alkanen und Erdöl. Ph.D. dissertation. Bremen, Germany: University of Bremen; 1996. [Google Scholar]
- 15.Fessenden R J, Fessenden J S. Organic chemistry. Boston, Mass: PWS Publishers; 1982. [Google Scholar]
- 16.Graff G, Szczepanik P, Klein P D, Chipault J R, Holman R T. Identification and characterization of fully deuterated fatty acids isolated from Scenedesmus obliquus cultured in deuterium oxide. Lipids. 1970;5:786–792. doi: 10.1007/BF02531394. [DOI] [PubMed] [Google Scholar]
- 17.Griffin W M, Traxler R W. Some aspects of hydrocarbon metabolism by Pseudomonas. Dev Ind Microbiol. 1981;22:425–435. [Google Scholar]
- 18.Hamilton J T G, McRoberts W C, Larkin M J, Harper D B. Long-chain haloalkanes are incorporated into fatty acids by Rhodococcus rhodochrous NCIMB 13064. Microbiology. 1995;141:2611–2617. [Google Scholar]
- 19.Iizuka H, Iida M, Fujita S. Formation of n-decene-1 from n-decane by resting cells of Candida rugosa. Z Allg Mikrobiol. 1969;9:223–226. [PubMed] [Google Scholar]
- 20.IUPAC-IUB Commission on Biochemical Nomenclature. The nomenclature of lipids. Biochem J. 1978;171:21–35. doi: 10.1042/bj1710021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones D F, Howe R. Microbiological oxidation of long-chain aliphatic compounds. Part I. Alkanes and alk-1-enes. J Chem Soc. 1968;1968:2801–2808. doi: 10.1039/j39680002801. [DOI] [PubMed] [Google Scholar]
- 22.King D H, Perry J J. The origin of fatty acids in the hydrocarbon-utilizing microorganism Mycobacterium vaccae. Can J Microbiol. 1975;21:85–89. doi: 10.1139/m75-012. [DOI] [PubMed] [Google Scholar]
- 23.Krevor J V C. Alkane oxidation: the oxidation of alkanes by group VIII metal ions. In: Davis J A, Watson P L, Liebman J F, Greenberg A, editors. Selective hydrocarbon activation. New York, N.Y: VCH Publishers; 1990. pp. 19–42. [Google Scholar]
- 24.Kunau W-H, Dommes V, Schulz H. β-Oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog Lipid Res. 1995;34:267–342. doi: 10.1016/0163-7827(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 25.Mao L F, Chu C, Luo M J, Simon A, Abbas A S, Schulz H. Mitochondrial β-oxidation of 2-methyl fatty acids in rat liver. Arch Biochem Biophys. 1995;321:221–228. doi: 10.1006/abbi.1995.1389. [DOI] [PubMed] [Google Scholar]
- 26.McCloskey J A. Mass spectrometry of fatty acid derivatives. In: Gunstone F D, editor. Topics in lipid chemistry. I. New York, N.Y: John Wiley and Sons, Inc.; 1970. pp. 369–440. [Google Scholar]
- 27.Muller F M. On methane fermentation of higher alkanes. Antonie Leeuwenhoek. 1957;23:369–384. doi: 10.1007/BF02545890. [DOI] [PubMed] [Google Scholar]
- 28.Oldfield E. Gas chromatography-mass spectrometry of biosynthetic 1H-2H hybrid fatty acid methyl esters. J Chem Soc Chem Commun. 1972;1972:719–720. [Google Scholar]
- 29.Parekh V R, Traxler R W, Sobek J M. n-Alkane oxidation enzymes of a pseudomonad. Appl Environ Microbiol. 1977;33:881–884. doi: 10.1128/aem.33.4.881-884.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabus R, Heider J. Initial reactions of anaerobic metabolism of alkylbenzenes in denitrifying and sulfate-reducing bacteria. Arch Microbiol. 1998;170:377–384. [Google Scholar]
- 31.Rock C O, Jackowski S, Cronan J E., Jr . Lipid metabolism in prokaryotes. In: Vance D E, Vance J E, editors. Biochemistry of lipids, lipoproteins and membranes. Amsterdam, The Netherlands: Elsevier Science B.V.; 1996. pp. 35–74. [Google Scholar]
- 32.Rosenfeld W D. Anaerobic oxidation of hydrocarbons by sulfate-reducing bacteria. J Bacteriol. 1947;54:664–665. [PMC free article] [PubMed] [Google Scholar]
- 33.Ryhage R, Stenhagen E. Mass spectrometric studies. IV. Esters of monomethyl-substituted long chain carboxylic acids. Ark Kemi. 1960;15:291–304. [Google Scholar]
- 33a.Schmidt K, Carlsen M, Nielsen J, Villadsen J. Modeling isotopmer distribution in biochemical networks uising isotopmer mapping matrices. Biotechnol Bioengin. 1997;55:831–840. doi: 10.1002/(SICI)1097-0290(19970920)55:6<831::AID-BIT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 34.Singh H, Brogan M, Johnson D, Poulos A. Peroxisomal β-oxidation of branched chain fatty acid in human skin fibroblasts. J Lipid Res. 1992;33:1597–1605. [PubMed] [Google Scholar]
- 35.So C M, Young L Y. Isolation and characterization of a sulfate-reducing bacterium that anaerobically degrades alkanes. Appl Environ Microbiol. 1999;65:2969–2976. doi: 10.1128/aem.65.7.2969-2976.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vainshtein M, Hippe H, Kroppenstedt R M. Cellular fatty acid composition of Desulfovibrio species and its use in classification of sulfate-reducing bacteria. Syst Appl Microbiol. 1992;15:554–566. [Google Scholar]
- 37.van Beilen J B, Wubbolts M G, Witholt B. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation. 1994;5:161–174. doi: 10.1007/BF00696457. [DOI] [PubMed] [Google Scholar]
- 37a.Vidavsky I, Chorush R A, Longevialle P, McLafferty F W. Functional group migration in ionized long-chain compounds. J Am Chem Soc. 1994;116:5865–5872. [Google Scholar]
- 38.Watkinson R J, Morgan P. Physiology of aliphatic hydrocarbon-degrading microorganisms. Biodegradation. 1990;1:79–92. doi: 10.1007/BF00058828. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Young L Y. Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and phenanthrene by sulfidogenic consortia. Appl Environ Microbiol. 1997;63:4759–4764. doi: 10.1128/aem.63.12.4759-4764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]