Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathways mediate the signaling of multiple cytokines for a myriad of leukocyte functions.1 Somatic gain-of-function (GOF) mutations in STAT5B, especially N642H, are described in multiple neoplasms, in particular leukemia and lymphoma, and can be associated with significant eosinophilia.2–5 A different phenotype for somatic STAT5B N642H mutations has recently been observed whereby a nonmalignant process affects multiple hematopoietic lineages and presents with earlyonset, extreme hypereosinophilia with urticaria, dermatitis, and diarrhea.6 We describe the responses of 2 patients to targeted JAK inhibition.
Patient A is a newly identified 2-year-old boy who presented with severe dermatitis, chronic diarrhea, and failure to thrive. He had leukocytosis (57,000 cells/μL) and hypereosinophilia (22,000 cells/μL) at age 3 months (Fig 1). He had lymphadenopathy, splenomegaly, hepatomegaly, anemia, and elevated liver transferase enzymes (alanine transferase, 71 U/L; aspartate transaminase, 74 U/L).
FIGURE 1.
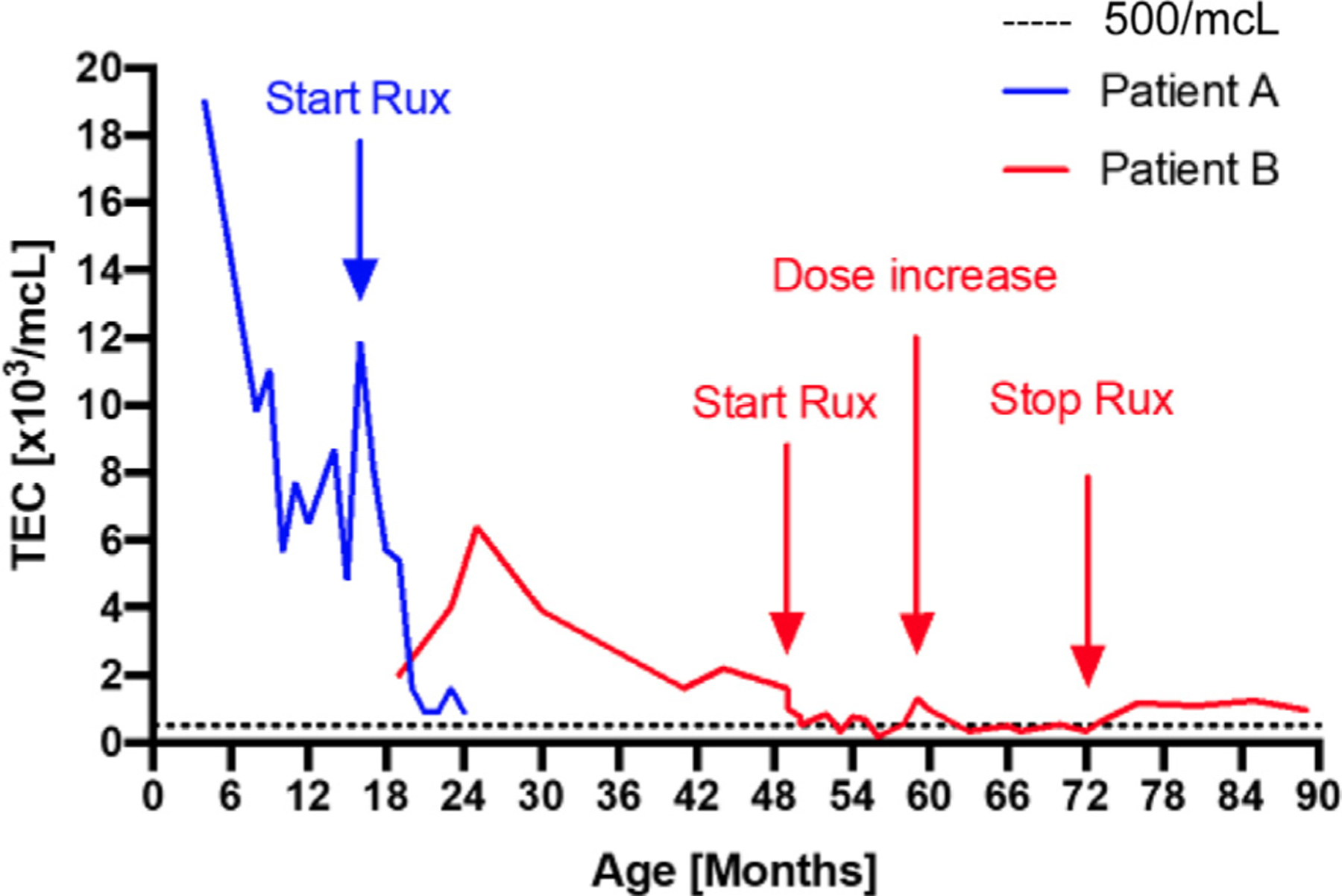
Total blood eosinophil count (TEC) over time in patients A and B.
His infectious history was notable for recurrent viral upper respiratory illnesses, viral gastrointestinal illnesses, methicillin-resistant Staphylococcus aureus skin infections, and otitis media. He experienced monthly episodes of wheezing in the setting of viral illnesses that responded to corticosteroids and beta agonists. He had difficulty swallowing liquid and solid foods with prominent abdominal distention and emesis. Atopic manifestations included urticarial rash, atopic dermatitis, and food allergy with sensitization to multiple food antigens. The patient is 1 of 8 children, and there was no family history of immunodeficiency, malignancy, unexplained deaths, or consanguinity.
At age 1 year, he had profound eosinophilia (22,000 cells/μL, 39%), lymphocytosis (17,600 cells/μL), and elevated IgE (4,385 IU/mL). Testing for FIP1L1-PDGFRA, JAK2, V617F, and other mutations associated with myeloid neoplasms was negative. There was no evidence of clonal T- or B-cell receptor rearrangement. Bone marrow biopsy showed 90% to 95% normocellular marrow with increased, nondysmorphic eosinophils and normal cytogenetics. Endoscopy and colonoscopy revealed eosinophilia throughout the gastrointestinal tract. Chest computed tomography revealed left lingular traction bronchiectasis. Bronchoscopy cultures were negative, and cytology lacked eosinophilia, but was notable for 50% lipid-laden macrophages, concerning for aspiration secondary to eosinophilic gastrointestinal disease. Immune evaluation showed normal lymphocyte immunophenotyping with appropriate ratio of naive, memory, and regulatory T cells. PHA and concanavalin A— induced lymphocyte proliferation was normal. He had normal B-cell immunophenotyping, normal immunoglobulins, and appropriate antibody responses to immunization. Live vaccines were withheld.
Next-generation sequencing revealed a de novo STAT5B N642H missense mutation. A custom droplet digital PCR assay (PrimePCR, BioRad, Hercules, Calif) targeting the STAT5B N642H mutation revealed variable fractions of the mutant allele, suggesting an acquired postzygotic somatic mosaicism. The mutant allele was present in nearly 100% of eosinophils and natural killer cells, 75% of T cells, and less than 50% in B cells and dendritic cells (Figure 2). In addition, there was exaggerated STAT5 phosphorylation of T cells on stimulation with IL-6, IL-21, and IFN-α. Baseline STAT5 phosphorylation was not increased in the patient compared with parental controls (see Figure E1 in this article’s Online Repository at www.jaci-inpractice.org).
FIGURE 2.
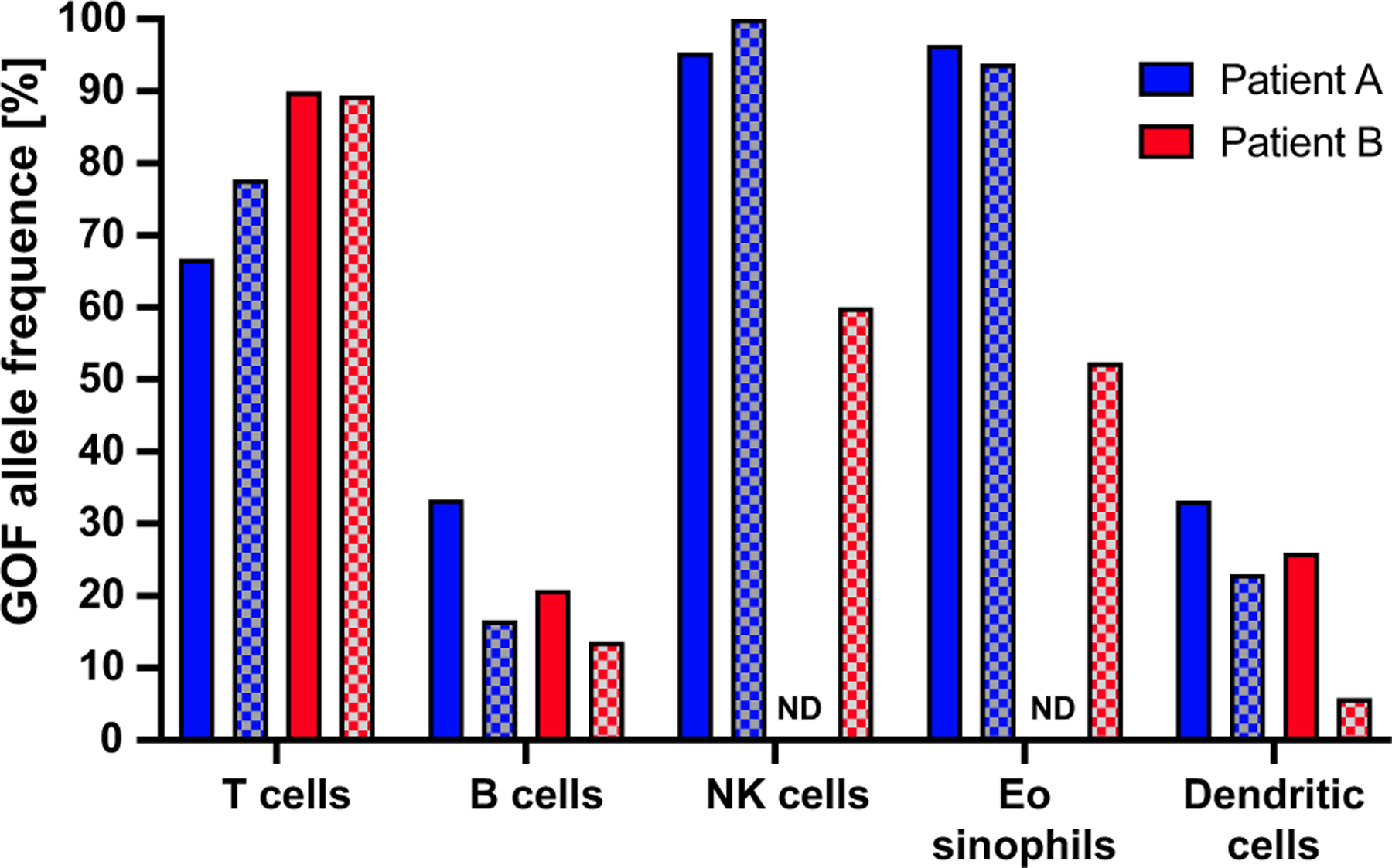
Percentage of cells expressing STAT5B N642H genomic DNA determined by targeted droplet digital PCR (ddPCR) in sorted leukocyte lineages before and after ruxolitinib in patients A and B. Percentages of cells expressing the variant are calculated as measured allelic frequency observed × 2. NK, Natural killer.
Oral corticosteroids did not ameliorate symptoms or laboratory findings. A 6-food elimination diet somewhat improved his weight gain and dermatitis, suggesting a component of reactivity. With a molecular diagnosis made, a targeted approach with ruxolitinib JAK inhibition was initiated. Ruxolitinib, titrated to a dose of 50 mg/m2/dose divided twice daily, was used on the basis of its mode of action and previous success in controlling GOF disease in STAT1, STAT3, as well as JAK1—which has substantial phenotypic overlap with STAT5B GOF.7–9 Infectious serology was monitored, and he was on acyclovir prophylaxis. Within several weeks, there was a marked clinical improvement with decreased diarrhea, increased weight gain, and improved respiratory symptoms. He showed improvement in laboratory parameters with normalized liver transferase enzymes, peripheral eosinophilia (900 cells/μL), leukocytosis (12,000 cells/μL), and IgE (840 IU/mL) in addition to improvement of his anemia. His hepatomegaly (12.1 cm to 9.8 cm), splenomegaly (8.5 cm to 6.7 cm), diffuse lymphadenopathy, and bronchiectasis were significantly improved. He was able to reintroduce all food protein antigens, his dysphagia resolved, and his weight improved further to the 75th percentile. Although his infection frequency decreased, he still experienced several upper respiratory illness and 1 episode of cellulitis. Nearing 1 year on therapy he continues to display a sustained response. Despite the improvement, the proportion of subsets carrying N642H in peripheral blood was not altered (Figure 2).
Patient B was previously described and presented at 4 months of life with annular migratory urticaria and persistent eosinophilia.6
Ruxolitinib (1 mg/kg divided twice a day) was initiated at age 4 years with a prompt decline in her eosinophil count (Figure 1). The family reported an improvement in her gastrointestinal symptoms and energy level. There was a nonsustained improvement in her arthralgia. The patient’s alopecia improved slightly with regrowth of wispy hairs. She did not suffer any significant opportunistic infections on treatment. STAT5 hyperphosphorylation in response to IL-21 was alleviated by ruxolitinib treatment (see Figure E2 in this article’s Online Repository at www.jaci-inpractice.org). Similar to patient A, ruxolitinib treatment did not alter the frequency of cells bearing the N642H variant (Figure 2). Ruxolitinib was discontinued after her sixth birthday with no evidence of disease flare. However, her eosinophilia has rebounded (Figure 1).
Patient A’s presentation complements the phenotype of the previously published cases of early-onset somatic STAT5B GOF, with marked peripheral and gastrointestinal eosinophilia, elevated IgE, atopy, recurrent viral respiratory infections, and failure to thrive.6 In addition, we report for the first time the response to targeted therapy with ruxolitinib in this disease. JAKs are associated with cytokine receptors that on stimulation lead to phosphorylation and transport of STAT proteins to the nucleus, culminating in gene expression. Ruxolitinib, a JAK 1/2 inhibitor, broadly dampens STAT5b phosphorylation with activation. Patient A had a remarkable response with improvement in eosinophilia, weight gain, lymphoproliferation, bronchiectasis, and infections, with cessation of clinical food allergy. Patient B’s symptoms were less florid, and consequently the response to treatment was less pronounced. In both patients, ruxolitinib therapy was accompanied by a fall in the peripheral eosinophil count.
What is yet to be determined is the durability of response to JAK inhibition, the ever-present concern for eventual malignant transformation, and when to consider bone marrow transplantation. Nonclonal STAT5B GOF is a newly recognized somatic monogenic atopic disorder, now with demonstrable sustained response to targeted therapy.
Supplementary Material
FIGURE E1. STAT5 phosphorylation in patient A’s basal and stimulated T cells compared with a healthy control. In the presence of IL-21 and IFN-α, patient A’s T cells had significantly increased phosphorylated STAT5 levels compared with the healthy control. Baseline STAT5B phosphorylation was not increased in the patient compared with healthy controls. stim, Stimulated; unstim, unstimulated.
FIGURE E2. STAT5 phosphorylation in patient B’s basal and stimulated cells compared with a healthy control. In the presence of IL-21, patient B’s cells had increased phosphorylated STAT5 levels compared with the healthy control. Baseline STAT5B phosphorylation was not increased in the patient compared with healthy controls. Levels of phosphorylated STAT5 in the presence of IL-21 are significantly decreased when the patient is on treatment with ruxolitinib. NK, Natural killer.
Clinical Implications.
Early-onset somatic signal transducer and activator of transcription 5B(STAT5B) gain-of-function is a newly recognized monogenic atopic disorder, now with demonstrable sustained response to targeted therapy.
Acknowledgments
F.G. was supported by the Deutsche Forschungsgemeinschaft (grant no. GO2955/1–1). S. H. received funding by the Wellcome Trust (Investigator Award no. 207556/Z/17/Z). This work was supported by the intramural program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The patients were enrolled on an institutional review board–approved protocol and were provided informed consent.
Footnotes
Conflicts of interest: All authors contributed equally to this letter, and there are no conflicts of interest to disclose for any of the authors.
REFERENCES
- 1.Dorritie KA, McCubrey JA, Johnson DE. STAT transcription factors in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia 2014;28:248–57. [DOI] [PubMed] [Google Scholar]
- 2.Bandapalli OR, Schuessele S, Kunz JB, Rausch T, Stutz AM, Tal N, et al. The activating STAT5B N642H mutation is a common abnormality in pediatric T-cell acute lymphoblastic leukemia and confers a higher risk of relapse. Haematologica 2014;99:e188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady A, Gibson S, Rybicki L, Hsi E, Saunthararajah Y, Sekeres MA, et al. Expression of phosphorylated signal transducer and activator of transcription 5 is associated with an increased risk of death in acute myeloid leukemia. Eur J Haematol 2012;89:288–93. [DOI] [PubMed] [Google Scholar]
- 4.Kucuk C, Jiang B, Hu X, Zhang W, Chan JK, Xiao W, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat Commun 2015;6:6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross NCP, Hoade Y, Tapper WJ, Carreno-Tarragona G, Fanelli T, Jawhar M, et al. Recurrent activating STAT5B N642H mutation in myeloid neoplasms with eosinophilia. Leukemia 2019;33:415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma CA, Xi L, Cauff B, DeZure A, Freeman AF, Hambleton S, et al. Somatic STAT5b gain-of-function mutations in early onset nonclonal eosinophilia, urticaria, dermatitis, and diarrhea. Blood 2017;129:650–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Bel KL, Ragotte RJ, Saferali A, Lee S, Vercauteren SM, Mostafavi SA, et al. JAK1 gain-of-function causes an autosomal dominant immune dysregulatory and hypereosinophilic syndrome. J Allergy Clin Immunol 2017;139:2016–2020.e5. [DOI] [PubMed] [Google Scholar]
- 8.Forbes LR, Vogel TP, Cooper MA, Castro-Wagner J, Schussler E, Weinacht KG, et al. Jakinibs for the treatment of immune dysregulation in patients with gain-of-function signal transducer and activator of transcription 1 (STAT1) or STAT3 mutations. J Allergy Clin Immunol 2018;142:1665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loh ΜL, Tasian SK, Rabin KR, Brown P, Magoon D, Reid JM, et al. A phase 1 dosing study of ruxolitinib in children with relapsed or refractory solid tumors, leukemias, or myeloproliferative neoplasms: a Children’s Oncology Group phase 1 consortium study (ADVL1011). Pediatr Blood Cancer 2015; 62:1717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE E1. STAT5 phosphorylation in patient A’s basal and stimulated T cells compared with a healthy control. In the presence of IL-21 and IFN-α, patient A’s T cells had significantly increased phosphorylated STAT5 levels compared with the healthy control. Baseline STAT5B phosphorylation was not increased in the patient compared with healthy controls. stim, Stimulated; unstim, unstimulated.
FIGURE E2. STAT5 phosphorylation in patient B’s basal and stimulated cells compared with a healthy control. In the presence of IL-21, patient B’s cells had increased phosphorylated STAT5 levels compared with the healthy control. Baseline STAT5B phosphorylation was not increased in the patient compared with healthy controls. Levels of phosphorylated STAT5 in the presence of IL-21 are significantly decreased when the patient is on treatment with ruxolitinib. NK, Natural killer.


