Abstract
Purpose of Review:
Cardiovascular disease is a leading cause of morbidity and mortality in both men and women, although there are notable differences in presentation between men and women. Atherosclerosis remains the predominant driver of coronary heart disease in both sexes; however, sex differences in atherosclerosis should be investigated further to understand clinical manifestations between men and women.
Recent Findings:
There are sex differences in the prevalence, progression, and prognostic impact of atherosclerosis. Furthermore, developing evidence demonstrates unique differences in atherosclerotic plaque characteristics between men and women on both non-invasive and invasive imaging modalities. Coronary microvascular dysfunction may be present even if no obstructive lesions are found. Most importantly, non-obstructive coronary artery disease is associated with a heightened risk of future adverse cardiovascular events and should not be ignored.
Summary:
The distinct plaque signature in women should be recognized, and optimal preventive strategies should be performed for both sexes.
Keywords: plaque, atherosclerosis, coronary disease, sex differences
Introduction
Cardiovascular disease (CVD) remains the leading cause of death in the United States (US) with coronary heart disease (CHD) at the top, resulting in >365,000 deaths per year[1]. More so, an estimated 18.2 million Americans ≥20 years of age have CHD. Interestingly, while the overall prevalence of CHD is greater in men than in women (7.4 vs 6.2%), the frequency of angina, which is the most common physical manifestation of CHD, is comparable among men and women (3.5 vs 3.7%)[1].
The leading mechanism behind ischemic heart disease is atherosclerosis, although it should be acknowledged that endothelial and microvascular dysfunction can also result in ischemia, with a greater predilection in women. Atherosclerosis occurs following vascular injury and dysfunction, often in the setting of risk factors such as hypertension, diabetes mellitus, dyslipidemia and smoking, with a resultant inflammatory response causing plaque formation. In the acute setting, plaque rupture and thrombosis can result in myocardial infarction (MI).
While some of these traditional cardiovascular risk factors, such as smoking, diabetes mellitus and hypertension, are more prevalent in men, these factors are associated with greater cardiovascular risk in women than men[1]. For instance, smoking confers a relative risk of 2-5 in women but 2-fold in men, while diabetes mellitus has a higher adjusted hazard ratio (HR) for fatal coronary artery disease (CAD) in women compared to men (HR 14.7 (95% CI 6.2-35.3) vs 3.8 (2.5-5.7))[2-4]. Recently, there has also been growing evidence of sex differences in atherosclerotic plaque characteristics. Sex-related variations in plaque composition may explain the discrepancy between overall CHD and symptomatic CHD in women and men, and shed light on important diagnostic and therapeutic implications.
In this review, we will explore recent evidence supporting the unique plaque signature in women using primarily non-invasive imaging tools, such as the coronary artery calcium (CAC) score from non-contrast computed tomography (CT), the presence of stenosis and plaque characteristics by coronary CT angiography (CCTA), as well as invasive methods such as coronary angiography. The key findings are also briefly summarized in Table 1.
Table 1.
Sex-specific differences in coronary artery plaque
| What Is Known | Clinical Implications | |
|---|---|---|
| Coronary artery calcium (CAC) |
|
|
| Coronary computed tomography angiography (CCTA) |
|
|
| Invasive coronary angiography (ICA) |
|
|
Coronary artery calcium
One tool that has provided much needed insight into sex differences in plaque is the CAC score. The CAC score is measured using non-contrast cardiac CT and is prognostic of incident atherosclerotic cardiovascular disease (ASCVD) events and long-term cardiovascular mortality[5,6]. Although the CAC score only detects calcified plaque, it is a useful surrogate marker of total atherosclerotic burden[7]. The CAC score has been shown to be a superior predictor of CHD events compared to traditional risk factors and also when compared to other subclinical imaging and biomarkers[8,9]. In the Multi-Ethnic Study of Atherosclerosis (MESA), the CAC score was shown to be strongly associated with 10-year risk of incident ASCVD in a graded fashion, independent of traditional CVD risk factors, and predicted risk similarly among different age, sex, and race/ethnic groups[5]. The presence of CAC can further refine a given estimated CVD risk upwards that would favor the initiation or intensification of preventive therapies such as statins[10]. Conversely, the absence of CAC (i.e. CAC=0) is associated with a low event rate (<5%) over 10-years, in a range where statin therapy may be deferred if desired[5,10,11]. Therefore, the 2019 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline for Primary Prevention has endorsed CAC scoring as a useful tool to refine risk estimations when there is uncertainty about risk and guide shared decision making for preventive therapies such as statins and aspirin[12].
At a given-age, women on average have a lower prevalence of CAC compared to men[6,13,14]. Nevertheless, the CAC score has been shown to predict ASCVD risk in women[5,6,14,15], even among women at low estimated ASCVD risk using standard risk estimator tools like the Pooled Cohort Equations[16,17]. A meta-analysis of the literature published between 2003 and 2006 of 17,850 men and 17,779 women revealed that CAC screening has similar prognostic value regardless of sex, an important finding given that women have smaller coronary arteries and a lower prevalence of obstructive luminal disease[18].
Nevertheless, whether the magnitude of excess risk conferred by higher CAC scores is modified by sex remains a subject of debate. While some studies suggest CAC has similar risk by sex[5,15,18,19], other studies have found that a given CAC score portends greater relative risk in women[6,14,17,20].
Mehta et al. pooled data from MESA and the Dallas Heart Study and found no sex or race based interactions for CAC presence or burden for the prediction of ASCVD, CHD, or stroke events; although notably CAC was a better predictor of CHD than for stroke for all sex and race groups[15]. Budoff et al. also looked at the entire MESA cohort, including statin-treated patients over a median study period of 11.1 years. Their study further validated an increase in ASCVD rates, including stroke, across CAC categories regardless of sex[5]. Nakanashni et al. studied 13,092 asymptomatic adults and found CAC was more prevalent among men across various age groups and at any level of number of risk factors[19]. Adults with more risk factors had higher CAC burden, but this trend that was more significant in women than in men. Among adults with no risk factors, CAC was more likely to be present in women than in men. Importantly, CAC presence and different burden level equally predicted mortality risk among men and women. CAC had an independent prognostic value in predicting long-term mortality over traditional risk factors, a trend that was significant in men and of incremental value in women.
In contrast, other studies have found excess risks associated with CAC in women compared to men. One early observational study demonstrated that asymptomatic women had a higher rate of death from all causes than men at any level of CAC measured on electron beam CT. This higher rate persisted after adjustment for traditional risk factors, exposing the failure of traditional risk factors alone in capturing risk in women[14]. At the time, this was attributed to decreased work-up in women, delay in diagnosis, and under-treatment. More recent studies have corroborated the previous results but have also shed light on pathophysiologic mechanisms underlying this difference. Kelkar et al. looked at a cohort of 9,715 asymptomatic men and women with 15 years of follow-up[17]. In that cohort, women were more likely to have traditional risk factors overall, but dyslipidemia was more prevalent among men. Low-to-intermediate risk women were also nearly a decade older than their male counterparts. Women had a greater prevalence and extent of CAC, as well as greater 15-year mortality at any CAC level compared to men. Examining the Net Reclassification Improvement, using CAC resulted in 6.2% correct reclassification of low-risk women compared to 3.9% of men, further supporting the failure of current global risk scores to accurately risk stratify low-risk patients, and that women may particularly benefit from CAC-assessment to refine their risk.
Furthermore, the CAC Consortium evaluated sex differences in the CAC prevalence and prognosis among adults referred for clinically indicated CAC scores[6]. A CAC score of 0 conferred similar risk of CVD mortality in men vs. women. On the other hand, CAC score >0 conferred greater risk of CVD mortality in women [HR 1.26 (1.08-1.48)], and this excess hazard in women was also seen for CAC scores 100-399 and >400, and with 2 or 3 calcified vessels. This study also found sex-specific differences in the risk conferred by atherosclerotic plaque types, with larger calcified plaque size and more numerous CAC lesions conferring more than 2-fold higher CVD mortality in women than in men; whereas CAC density was predictive of CVD mortality in men but not in women[6]. This study brings attention to the potential for a sex-specific signature in calcified plaque risk and suggests that examining the distribution and patterns of CAC lesions, beyond just the total CAC score, may further refine risk and guide prevention strategies. However, it should be noted though that there were some underlying differences in medication use and risk factors by sex which may be potential confounders[6].
Normally CAC scoring is not typically recommended in patients with diabetes since these patients are already recommended for at least a moderate intensity statin for prevention[12]. Nevertheless, this excess risk for CVD and all-cause mortality for greater CAC scores for women compared to men was even seen among individuals with diabetes[20]. In the CAC Consortium cohort, among individuals with diabetes, women had an overall lower prevalence of subclinical CHD than men[20]. Additionally, women with CAC had similar lesion size as men but fewer CAC lesions, fewer calcified vessels, and lower volume score. This again suggests that the pattern of plaque is sex-specific even among individuals with diabetes. In both men and women with diabetes, a CAC score of 0 conferred low mortality rates from CHD and CVD of <1 per 1,000 person-years, confirming prior work that the presence of diabetes alone is not a CHD-risk equivalent. On the other hand, the presence of CAC was associated with an elevated risk, a relationship that was stronger among women. Compared to CAC=0, CAC scores of 101-400 and >400 was associated with HRs of 1.63 (95% CI 0.64-4.14) and 3.48 (1.44-8.37) in men but 3.67 (1.30-10.38) and 6.27 (2.27-17.28) in women, p-interaction by sex=0.04. Risks of CVD mortality were also greater for women vs. men for having calcium volume and lesion size above the median and for having ≥3 calcified vessels.
Not all low risk women have an indication for a CAC score. For many low risk women (<5% 10-year ASCVD risk), reinforcement of healthy lifestyle measures may be sufficient to protect women from adverse CV events. However, CAC screening may be particularly important in women who may be considered at lower risk by traditional risk factors but have female-specific risk enhancing factors such as a history of polycystic ovarian syndrome[21], preeclamptic pregnancy[22], premature menopause[23], or autoimmune disease[24,25]. In these women, identification of subclinical coronary atherosclerosis can help facilitate earlier initiation of preventive therapies to reduce their lifetime risk. Breast arterial calcium (BAC) identified on routine mammography is also emerging as a potential marker of future CVD risk in women. The finding of BAC should trigger intensification of lifestyle changes and may also prompt consideration of CAC score for further risk assessment, if decisions for preventive therapies like statins are uncertain[26].
The integrated above data lead to the concept that numerous factors impair our current ASCVD risk assessment in women and urges for greater atherosclerosis screening in women. The Society of Cardiovascular Computed Tomography (SCCT) expert consensus statement for women suggests that CAC scanning be considered in women at intermediate risk or for those women whose risk score is inconsistent with perceived clinical risk and request additional information through shared decision making[27]. Recent ACC/AHA[12], European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS)[28], ESC/European Association for the Study of Diabetes (EASD)[29], and National Lipid Association (NLA) guidelines[30] have also all incorporated the use of CAC for risk stratification and guidance of statin therapy.
Non-obstructive CAD in women and plaque type
CAC scores are generally performed among patients who are asymptomatic for risk stratification. However it estimated that there are over 9.4 million adults in the US living with angina[1]. Among patients with stable chest pain symptoms, women are more likely to have non-obstructive CAD, compared to men who are more likely to have obstructive CAD[31]. Notably non-obstructive CAD can be a nidus for plaque erosion with subsequent thrombus formation leading to MI. While historically women presenting with ischemia on functional testing who were found to have non-obstructive CAD were labeled as having a “false positive test,” modern understanding is that coronary microvascular dysfunction is frequently present even in the absence of obstructive CAD. There are multiple mechanisms that can contribute to ischemia with non-obstructive coronary arteries (INOCA) such as endothelial dysfunction, impaired vasodilation, and coronary microvasospasm[32]. It is important to note that even in INOCA, atherosclerosis is highly prevalent. One case series of women with stable chest pain in the absence of obstructive CAD reported that nearly 80% had atherosclerosis detectable by intravascular ultrasound and furthermore, that risk factors did correlate with percent atheroma volume[33]. Non-obstructive CAD should not be ignored as it is associated with a heightened risk for adverse outcomes, and ultimately CAD should be approached as a chronic disease with long-term management targeted[34,35].
In the CONFIRM registry of individuals who underwent a clinically indicated CCTA, the presence of non-obstructive CAD conferred an approximate 2-fold increased risk of subsequent major adverse cardiovascular events (MACE) compared to patients with normal coronary arteries over an average 2.3 year follow-up[36]. Non-obstructive CAD conferred similar risk for both women and men after accounting for age and risk factors[36].
The PROMISE trial of patients with stable chest pain compared two testing strategies – an anatomical approach with CCTA vs a functional approach with stress testing[37]. In the PROMISE trial, among patients randomized to the CCTA arm, the presence of high risk plaque (e.g. positive coronary remodeling, low CT attenuation, or napkin-ring sign) conferred a greater risk of MACE in women [adjusted HR 2.41 (95% CI 1.25-4.64) than in men [1.40 (0.81-2.39)][38]. Additionally, data from the CONFIRM registry showed that the presence of non-obstructive disease in the left main coronary artery (compared to no disease) conferred a greater 5-year risk of MACE in women [adjusted HR 1.48 (1.21-1.75)] compared to men [0.98 (0.81-1.18)]; p-interaction by sex=0.001[39]. These studies suggest that although women have a lower burden of plaque overall, there may be differential risk in women compared to men depending on the location and degree of obstruction.
Men are more likely than women to have coronary plaque progression on serial CCTA scans[40]. However, the composition of plaque progression may differ by sex. In one study of patients who had serial CCTAs, women had less total and non-calcified plaque volume (PV) at baseline, and there was similar total PV progression rates by sex in multivariable analysis. However women had greater calcified PV progression, but slower non-calcified PV progression and less development of high-risk plaques than in men[41]. The authors concluded that comprehensive plaque evaluation may help further refining of risk stratification according to sex. Of course, as with all observational analyses, there can be issues with residual confounding. As mentioned above, it should be acknowledged that differences in medication use and underlying risk factors may be potential confounders here contributing to sex differences.
Computational analysis of CCTA has evaluated the ratio of total epicardial coronary arterial lumen volume to left ventricular mass ratio (V/M) that have provided insights regarding sex based differences in coronary anatomy and flow. Women have been found to have smaller coronary arteries and may be more adversely affected by a given plaque load then men. A lower V/M ratio has been associated with microvascular disease coupled with increased symptoms in the face of lower plaque burden. In a recent analysis of the ADVANCE (Assessing Diagnostic Value of Noninvasive FFRCT in Coronary Care Registry), women are less likely to have anatomically obstructive CAD that correlates to higher FFRCT (>0.80) compared to men. At the same time, women with reduced FFRCT (<0.80) were less likely to have obstructive (>50%) stenosis and underwent less revascularization when compared to men. Sex differences in the coronary V/M ratio may provide a physiologic explanation for these sex-based differences in FFRCT and revascularization patterns, although the impact of unconscious bias is unclear from this registry data[42].
In the PROMISE trial, which compared CCTA vs stress testing for patients with stable chest pain, it is important to note that in women the event rates of MACE with a negative CCTA were similar to that of a negative stress test, but women with a positive CCTA had a significantly higher event rate than women with a positive stress test[43]. However, in men the event rates were similar for a positive test result between the 2 testing modalities. These findings suggest that a CCTA-approach for evaluation of stable chest pain may have particular prognostic value in women. These findings should be viewed with the understanding that contemporary CCTA radiation exposure is now very low, and certainly much lower than that conferred by nuclear stress testing or PET.
Importantly, “seeing is believing” and the detection of non-obstructive plaque can help facilitate initiation and adherence to risk-reducing preventive therapies. This was most clearly demonstrated in the SCOT-Heart trial of patients with stable chest pain (41% women), which compared a CCTA-guided approach vs a usual care approach and found a 41% reduction in MACE at 5 years [HR 0.59 (0.41-0.84)] which was largely driven by the uptake of more preventive therapies in the CCTA group compared to usual care [OR 1.40 (1.19-1.65)][44]. Notably there was no evidence of effect modification by sex (p-interaction=0.57) meaning the CCTA-guided approach for management of stable chest pain was equally efficacious in women compared to men[44]. The advantage of CCTA over functional testing in women suggest that perhaps CCTA should be the first line test for the evaluation of chest pain of intermediate probability for CAD[43].
The Initial Invasive or Conservative Strategy for Stable Coronary Disease (ISCHEMIA) trial randomized patients with stable chest pain to a revascularization approach vs medical management, after CCTA excluded high risk anatomy (i.e. left main disease)[45]. A secondary analysis of the ISCHEMIA trial by sex found that women had more frequent angina than men, independently of having less extensive CAD and less severe ischemia than men[46]. In other words, extent of myocardial ischemia was not correlated with angina severity in women. These findings implicate that the relationships between angina, ischemia, and atherosclerosis are complex and there are inherent sex differences. The overall ISCHEMIA trial results showing no difference in MACE between the 2 arms does not support the need for early revascularization in patients with stable chest pain[45]. Most stable CAD patients can be managed more effectively and safely with aggressive medical therapy rather than revascularization. This has implications in the approach to initial testing in women with stable chest pain symptoms[35].
Given the known elevated risk of MACE with non-obstructive CAD, it is important that these women are aggressively treated for atherosclerosis such as with high intensity statin, angiotensin converting enzyme inhibitors (ACEi) /angiotensin receptor blockers (ARB), consideration of low dose aspirin, as well as lifestyle changes and smoking cessation. The absence of obstructive CAD does not indicate “false positive” stress testing and conversely, the presence of plaque detection should prompt initiation of intensive preventive therapies to slow the progression of atherosclerosis, confer plaque stabilization, and prevent acute vascular events.
Atherosclerosis by invasive coronary angiography
Since the 1980s there has been growing recognition of the discrepancy in the extent of CAD by invasive coronary angiography (ICA) and the clinical presentation and outcomes in women. A clear pattern has emerged of less extensive CAD in women without any significant difference in associated morbidity and mortality compared to men.
An early example comes from the CASS (Coronary Artery Surgery Study) registry in which investigators studied the prevalence of severe CAD in patient subsets defined by definite angina, probable angina and non-specific chest pain[47]. This analysis included 2,810 women among 8,157 total participants (approximately 34%) and showed, as expected, that patients with more typical symptoms were more likely to have CAD, including left main disease and three-vessel disease. In those with “definite angina”, the prevalence of any obstructive CAD was 72% in women compared to 93% in men. The prevalence of obstructive CAD in women with “definite angina” was in fact more comparable to men with “probable angina” in whom the prevalence of obstructive CAD was 66%. Furthermore, among women with “probable angina” the prevalence of obstructive CAD was significantly lower at only 36%. Obstructive CAD prevalence increased with age across groups in both sexes, but in the “probable angina” group, men over the age of 70 had a prevalence of nearly 100% compared to just over 50% in women of the same age. Notably, this study included primarily patients with chronic stable angina[47].
By comparison, the TIMI (Thrombolysis in Myocardial Infarction) IIIB trial showed similar findings in the setting of ACS. TIMI IIB studied the efficacy of thrombolytics and an early invasive strategy in ACS. It included 1,473 individuals (including 467 women, approximately 33%) presenting with ACS. As with CASS, investigators found that women tended to have less extensive CAD by ICA defined by fewer critical stenoses (>70% stenosis) and fewer vessels with plaque. Despite less extensive CAD, investigators found that death from a cardiovascular cause, recurrent MI and stroke were no different between men and women[48].
The differences in plaque between women and men have been further characterized with the use of intravascular ultrasound (IVUS). This is highlighted by an analysis from the PROSPECT study, where investigators used multimodality invasive imaging (quantitative ICA, IVUS, and virtual histology) to evaluate the characteristics of non-culprit lesions in the coronary tree of patients who presented with ACS and were treated with percutaneous coronary intervention (PCI). Consistent with prior reports, women who presented with ACS were older and more likely to have diabetes. Women had fewer total non-culprit lesions and fewer vessels with non-culprit lesions compared to men. In women, there were fewer plaque ruptures and lesions tended to be shorter in length and narrower. The plaque morphology also varied between men and women. Coronary plaque in women was characterized by a smaller necrotic core and higher risk features including a lower fibrous volume, less calcium, and a smaller mean luminal area. There was also a trend, though not meeting the statistical threshold, of women having greater thin cap fibroatheromas and fewer thick cap fibroatheromas compared to men. As shown in other reports, there was no difference in CVD death, recurrent MI and stroke at up to 3 years, but over this time period, women were twice as likely to be re-hospitalized for angina[49].
This paradox of less extensive obstructive CAD in women with similar cardiovascular outcomes as men exemplified in the CASS, TIMI IIIB and PROSPECT reports has been consistent across multiple contemporary studies in both stable ischemic heart disease and ACS. Some key lessons can be taken away from these studies: despite less extensive CAD, women who undergo ICA for evaluation of angina or MI tend to be on average 10 years older than male counterparts, tend to have a higher burden of traditional risk factors such as a diabetes and obesity, tend to have more persistent angina and have a higher rate of rehospitalization for angina[50-54].
The difference in age in particular has been raised as a possible explanation for these paradoxical findings. Bjerking and colleagues evaluated this possibility with a matched cohort study in which 250 randomly selected female cases of acute MI were matched to male cases based on age and availability of a catheterization lab and found no difference in mortality. Moreover, despite a lack of difference in troponin level or possible contraindications to ICA, women were 18% less likely to undergo ICA at 60 days[55]. Disparate use of ICA for women presenting with chest pain, acute MI and even sudden cardiac arrest has been well documented in multiple reports and is more pronounced in the extremes of age in women. The reason for this disparity is likely multifactorial and previously attributed to older age in women at presentation, higher rates of renal insufficiency, atypical presentation, and likely some residual perception that this is a “man’s disease”[56-61].
Taken together, these findings support the notion that the mechanism of ischemia and infarction in women extends beyond just flow-limiting obstructive lesions and may more frequently be due to endothelial dysfunction, microvascular disease and plaque erosion. For instance, plaque erosion has been reported in 27% of cases of coronary thrombosis resulting in ACS, and tends to occur more often in younger premenopausal women[62-64]. The disparate use of ICA in the evaluation of ischemia in women may lead to the underdiagnoses of these possible mechanisms and may be hindering opportunities for medical intervention.
Conclusions
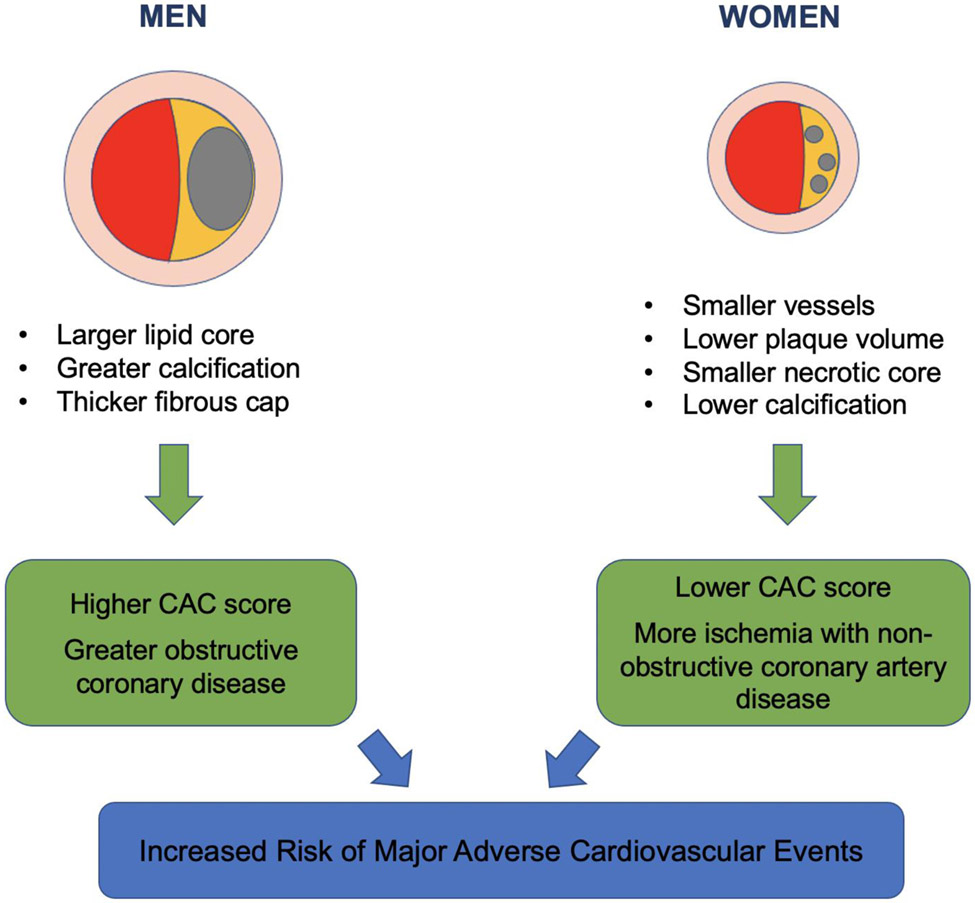
In summary, there may be a sex-specific plaque signature (Figure 1); however, the presence of plaque confers elevated ASCVD risk in both sexes and optimal prevention strategies should be employed[65]. Women have been associated to have a lower burden of coronary plaque on average compared to similarly-aged men; however, several studies have shown that a greater atherosclerotic plaque burden assessed by CAC score confers even higher prognostic risk in women than in men. Among stable chest pain patients, women benefit from CCTA due to high normalcy rate (which translates to fewer 1-year downstream testing) and offers the opportunity to initiative preventive therapies if non-obstructive CAD is found. Notable, coronary microvascular dysfunction may be present if no obstructive lesions found[49].
Figure 1.
Differences in plaque characteristics between men and women
In INOCA, atherosclerotic plaque is prevalent and should be treated with aggressive medical therapy (i.e. statin, low dose aspirin, ACEi/ARB). Women with non-obstructive CAD have higher MACE than those with no CAD, especially non-obstructive left main disease. Compared to men, women are less likely to be offered a statin for prevention and women are more likely to decline or discontinue statin therapy[66]. However, knowledge of one’ CAC score or the detection of plaque by CCTA can potentially confer better adherence to lifestyle changes and preventive medications such as statins[67]. Additionally, women are more likely to have recurrent angina post admission for ACS, and should be placed on appropriate anti-anginal therapies if indicated[49]. We recommend intensification of primary and secondary preventive care for all at-risk women with demonstrable atherosclerosis.
Funding:
Dr. Minhas is supported by NHLBI Research Training Grant T32HL007024. Dr. Michos is supported by the Amato Fund for Women’s Cardiovascular Health Research at Johns Hopkins University.
Footnotes
Conflict of Interest
No conflicts of interest related to this work. Outside of the submitted work, Dr. Zadeh reports grants from Canon Medical Systems, and Dr. Choi reports other from Cleerly, Inc. and grants from GW Heart and Vascular Institute.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- •1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation [Internet]. 2020. [cited 2020 Mar 18];141. Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000757 This annual update from the American Heart Association provides sex-disaggregated statistics on U.S trends in the burden of cardiovascular disease and its risk factors.
- 2.Njølstad I, Arnesen E, Lund-Larsen PG. Smoking, Serum Lipids, Blood Pressure, and Sex Differences in Myocardial Infarction: A 12-Year Follow-up of the Finnmark Study. Circulation. 1996;93:450–6. [DOI] [PubMed] [Google Scholar]
- 3.Juutilainen A, Kortelainen S, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Gender Difference in the Impact of Type 2 Diabetes on Coronary Heart Disease Risk. Diabetes Care. 2004;27:2898–904. [DOI] [PubMed] [Google Scholar]
- 4.Jónsdóttir LS, Sigfússon N, Gudnason V, Sigvaldason H, Thorgeirsson G. Do lipids, blood pressure, diabetes, and smoking confer equal risk of myocardial infarction in women as in men? The Reykjavik Study. J Cardiovasc Risk. 2002;9:67–76. [PubMed] [Google Scholar]
- 5.Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). European Heart Journal. 2018;39:2401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••6. Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. European Heart Journal. 2018;39:3727–35. This large registry of over 60,000 individuals who underwent a coronary artery calcium (CAC) score test, linked with their mortality data, provides important insights in the prognostic value of CAC in women vs men.
- 7.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary Artery Calcium Area by Electron-Beam Computed Tomography and Coronary Atherosclerotic Plaque Area: A Histopathologic Correlative Study. Circulation. 1995;92:2157–62. [DOI] [PubMed] [Google Scholar]
- 8.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, et al. Comparison of Novel Risk Markers for Improvement in Cardiovascular Risk Assessment in Intermediate-Risk Individuals. JAMA. 2012;308:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, et al. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. Journal of the American College of Cardiology. 2016;67:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michos ED, Blaha MJ, Blumenthal RS. Use of the Coronary Artery Calcium Score in Discussion of Initiation of Statin Therapy in Primary Prevention. Mayo Clinic Proceedings. 2017;92:1831–41. [DOI] [PubMed] [Google Scholar]
- 11.Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, et al. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••12. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation [Internet]. 2019. [cited 2020 May 8];140. Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000678 This recently updated prevention Guideline put forth by the ACC/AHA acknowledged unique female specific factors of pre-eclampsia and early menopause as "risk enhancing" factors that would elevated a woman into a higher risk category where statin therapy may be considered for primary prevention. Moreover this Guideline gave CAC scoring a IIa recommendation to be used as a risk decision tool to guide shared decision making about statin therapy in cases where ASCVD risk was otherwise uncertain.
- 13.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N Engl J Med. 2008;358:1336–45. [DOI] [PubMed] [Google Scholar]
- 14.Raggi P, Shaw LJ, Berman DS, Callister TQ. Gender-based differences in the prognostic value of coronary calcification. J Womens Health (Larchmt). 2004;13:273–83. [DOI] [PubMed] [Google Scholar]
- •15. Mehta A, Pandey A, Ayers CR, Khera A, Sperling LS, Szklo MS, et al. Predictive Value of Coronary Artery Calcium Score Categories for Coronary Events Versus Strokes: Impact of Sex and Race: MESA and DHS. Circ: Cardiovascular Imaging [Internet]. 2020. [cited 2020 Nov 28];13. Available from: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.119.010153 This study pooled data from two large community-based observational cohorts and found that CAC scoring predicted risk for both coronary heart disease and stroke, similarly for both men and women. This was in contrast to other studies that suggested CAC conferred relatively greater risk in women compared to men.
- 16.Lakoski SG. Coronary Artery Calcium Scores and Risk for Cardiovascular Events in Women Classified as “Low Risk” Based on Framingham Risk Score: The Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2007;167:2437. [DOI] [PubMed] [Google Scholar]
- 17.Kelkar AA, Schultz WM, Khosa F, Schulman-Marcus J, O’Hartaigh BWJ, Gransar H, et al. Long-Term Prognosis After Coronary Artery Calcium Scoring Among Low-Intermediate Risk Women and Men. Circ Cardiovasc Imaging [Internet]. 2016. [cited 2020 Nov 28];9. Available from: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.115.003742 [DOI] [PubMed] [Google Scholar]
- 18.Bellasi A, Lacey C, Taylor AJ, Raggi P, Wilson PWF, Budoff MJ, et al. Comparison of Prognostic Usefulness of Coronary Artery Calcium in Men Versus Women (Results from a Meta- and Pooled Analysis Estimating All-Cause Mortality and Coronary Heart Disease Death or Myocardial Infarction). The American Journal of Cardiology. 2007;100:409–14. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi R, Li D, Blaha MJ, Whelton SP, Darabian S, Flores FR, et al. All-cause mortality by age and gender based on coronary artery calcium scores. Eur Heart J Cardiovasc Imaging. 2016;17:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong ND, Cordola Hsu AR, Rozanski A, Shaw LJ, Whelton SP, Budoff MJ, et al. Sex Differences in Coronary Artery Calcium and Mortality From Coronary Heart Disease, Cardiovascular Disease, and All Causes in Adults With Diabetes: The Coronary Calcium Consortium. Dia Care. 2020;43:2597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends in Cardiovascular Medicine. 2020;30:399–404. [DOI] [PubMed] [Google Scholar]
- 22.Benschop L, Brouwers L, Zoet GA, Meun C, Boersma E, Budde RPJ, et al. Early Onset of Coronary Artery Calcification in Women With Previous Preeclampsia. Circ: Cardiovascular Imaging [Internet]. 2020. [cited 2020 Nov 28];13. Available from: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.119.010340 [DOI] [PubMed] [Google Scholar]
- 23.Chu J, Michos ED, Ouyang P, Vaidya D, Blumenthal RS, Budoff MJ, et al. Abstract 13298: The Association Between Coronary Artery Calcium and Atherosclerotic Cardiovascular Disease Risk in Women With Early Menopause: The Multi-Ethnic Study of Atherosclerosis. Circulation [Internet]. 2020. [cited 2020 Nov 28];142. Available from: https://www.ahajournals.org/doi/10.1161/circ.142.suppl_3.13298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52:3045–53. [DOI] [PubMed] [Google Scholar]
- 25.Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, et al. Premature Coronary-Artery Atherosclerosis in Systemic Lupus Erythematosus. N Engl J Med. 2003;349:2407–15. [DOI] [PubMed] [Google Scholar]
- 26.Quispe R, Al-Rifai M, Di Carlo PA, Michos ED, Amin NP, Kianoush S, et al. Breast Arterial Calcium. JACC: Cardiovascular Imaging. 2019;12:2538–48. [DOI] [PubMed] [Google Scholar]
- ••27. Truong QA, Rinehart S, Abbara S, Achenbach S, Berman DS, Bullock-Palmer R, et al. Coronary computed tomographic imaging in women: An expert consensus statement from the Society of Cardiovascular Computed Tomography. Journal of Cardiovascular Computed Tomography. 2018;12:451–66. This expert statement put forth by the Society of Cardiovascular Computed Tomography (SCCT) synthesized the literature and provided guidance on the use of CT imaging for both diagnosis and risk stratification of coronary artery disease in women.
- 28.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal. 2020;41:111–88. [DOI] [PubMed] [Google Scholar]
- 29.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. European Heart Journal. 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 30.Orringer CE, Blaha MJ, Blankstein R, Budoff MJ, Goldberg RB, Gill EA, et al. The National Lipid Association Scientific Statement on Coronary Artery Calcium Scoring to Guide Preventive Strategies for ASCVD Risk Reduction. Journal of Clinical Lipidology. 2020;S1933287420303421. [DOI] [PubMed] [Google Scholar]
- 31.Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, et al. Emergence of Nonobstructive Coronary Artery Disease. Journal of the American College of Cardiology. 2015;66:1918–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathews L, Subramanya V, Zhao D, Ouyang P, Vaidya D, Guallar E, et al. Endogenous Sex Hormones and Endothelial Function in Postmenopausal Women and Men: The Multi-Ethnic Study of Atherosclerosis. J Womens Health (Larchmt). 2019;28:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, et al. An Intravascular Ultrasound Analysis in Women Experiencing Chest Pain in the Absence of Obstructive Coronary Artery Disease: A Substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE): INTRAVASCULAR ULTRASOUND IN WOMEN WITH CHEST PAIN. Journal of Interventional Cardiology. 2010;23:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arbab-Zadeh A, Fuster V. From Detecting the Vulnerable Plaque to Managing the Vulnerable Patient: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:1582–93. [DOI] [PubMed] [Google Scholar]
- ••35. Ferraro R, Latina JM, Alfaddagh A, Michos ED, Blaha MJ, Jones SR, et al. Evaluation and Management of Patients With Stable Angina: Beyond the Ischemia Paradigm. Journal of the American College of Cardiology. 2020;76:2252–66. This recent state of the art review discusses the rationale why coronary CT angiography should be used as the first line diagnostic test for evaluation of stable chest pain in patients at intermediate risk for coronary artery disease. Notably this review highlights that an imaging based approach (as opposed to a functional approach) has the potential to improve uptake of preventive therapies like statins, if plaque is detected to be present.
- 36.Leipsic J, Taylor CM, Gransar H, Shaw LJ, Ahmadi A, Thompson A, et al. Sex-based prognostic implications of nonobstructive coronary artery disease: results from the international multicenter CONFIRM study. Radiology. 2014;273:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease. N Engl J Med. 2015;372:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••38. Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT, et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol. 2018;3:144. This was a secondary analysis of individuals who underwent a coronary CT angiography in the PROMISE trial and found that the presence of high risk plaque conferred a greater risk for future major adverse cardiovascular events in women compared to men.
- •39. Xie JX, Eshtehardi P, Varghese T, Goyal A, Mehta PK, Kang W, et al. Prognostic Significance of Nonobstructive Left Main Coronary Artery Disease in Women Versus Men: Long-Term Outcomes From the CONFIRM (Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter) Registry. Circ Cardiovasc Imaging [Internet]. 2017. [cited 2020 Nov 28];10. Available from: https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.117.006246 This analysis from the CONFIRM registry of patients who were referred for a clinically indicated coronary CT angiography examined sex specific differences in non-obstructive plaque. The authors found that non-obstructive left main disease conferred greater cardiovascular risk in women than in men.
- •40. Gu H, Gao Y, Wang H, Hou Z, Han L, Wang X, et al. Sex differences in coronary atherosclerosis progression and major adverse cardiac events in patients with suspected coronary artery disease. Journal of Cardiovascular Computed Tomography. 2017;11:367–72. This registry study found that men are more likely to have coronary atherosclerosis progression between serial coronary CT angiography scans, compared to women.
- •41. Lee S-E, Sung JM, Andreini D, Al-Mallah MH, Budoff MJ, Cademartiri F, et al. Sex Differences in Compositional Plaque Volume Progression in Patients With Coronary Artery Disease. JACC: Cardiovascular Imaging. 2020;13:2386–96. This analysis from the CONFIRM registry of patients referred for a clinically indicated coronary CT angiography who had more than 1 scan found that men and women patients experienced differences in the type (composition) of plaque progression.
- 42.Fairbairn TA, Dobson R, Hurwitz-Koweek L, Matsuo H, Norgaard BL, Rønnow Sand NP, et al. Sex Differences in Coronary Computed Tomography Angiography–Derived Fractional Flow Reserve. JACC: Cardiovascular Imaging. 2020;S1936878X20306124. [DOI] [PubMed] [Google Scholar]
- ••43. Pagidipati NJ, Hemal K, Coles A, Mark DB, Dolor RJ, Pellikka PA, et al. Sex Differences in Functional and CT Angiography Testing in Patients With Suspected Coronary Artery Disease. Journal of the American College of Cardiology. 2016;67:2607–16. In this secondary analysis of the PROMISE trial examining two testing strategies for the evaluation of stable chest pain, stratified by sex, the authors suggest that an imaging based approach with coronary CT angiography may have greater prognostic value in women than a functional (stress) testing approach.
- 44.The SCOT-HEART Investigators. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med. 2018;379:924–33. [DOI] [PubMed] [Google Scholar]
- 45.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••46. Reynolds HR, Shaw LJ, Min JK, Spertus JA, Chaitman BR, Berman DS, et al. Association of Sex With Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients With Moderate or Severe Ischemia: Secondary Analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol. 2020;5:773. This was a secondary analysis of the ISCHEMIA trial stratified by sex which highlighted that women had more angina than men. However despite their greater symptom burden, women had less extensive coronary artery disease and less ischemia than men.
- 47.Chaitman BR, Bourassa MG, Davis K, Rogers WJ, Tyras DH, Berger R, et al. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS). Circulation. 1981;64:360–7. [DOI] [PubMed] [Google Scholar]
- 48.Hochman JS, McCabe CH, Stone PH, Becker RC, Cannon CP, DeFeo-Fraulini T, et al. Outcome and profile of women and men presenting with acute coronary syndromes: a report from TIMI IIIB. TIMI Investigators. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1997;30:141–8. [DOI] [PubMed] [Google Scholar]
- 49.Lansky AJ, Ng VG, Maehara A, Weisz G, Lerman A, Mintz GS, et al. Gender and the Extent of Coronary Atherosclerosis, Plaque Composition, and Clinical Outcomes in Acute Coronary Syndromes. JACC: Cardiovascular Imaging. 2012;5:S62–72. [DOI] [PubMed] [Google Scholar]
- 50.Ouellette ML, Löffler AI, Beller GA, Workman VK, Holland E, Bourque JM. Clinical Characteristics, Sex Differences, and Outcomes in Patients With Normal or Near-Normal Coronary Arteries, Non-Obstructive or Obstructive Coronary Artery Disease. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Acharjee S, Teo KK, Jacobs AK, Hartigan PM, Barn K, Gosselin G, et al. Optimal medical therapy with or without percutaneous coronary intervention in women with stable coronary disease: A pre-specified subset analysis of the Clinical Outcomes Utilizing Revascularization and Aggressive druG Evaluation (COURAGE) trial. Am Heart J. 2016;173:108–17. [DOI] [PubMed] [Google Scholar]
- 52.Anand SS, Xie CC, Mehta S, Franzosi MG, Joyner C, Chrolavicius S, et al. Differences in the management and prognosis of women and men who suffer from acute coronary syndromes. J Am Coll Cardiol. 2005;46:1845–51. [DOI] [PubMed] [Google Scholar]
- 53.Chiha J, Mitchell P, Gopinath B, Plant AJH, Kovoor P, Thiagalingam A. Gender differences in the severity and extent of coronary artery disease. Int J Cardiol Heart Vasc. 2015;8:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamis-Holland JE, Lu J, Korytkowski M, Magee M, Rogers WJ, Lopes N, et al. Sex differences in presentation and outcome among patients with type 2 diabetes and coronary artery disease treated with contemporary medical therapy with or without prompt revascularization: a report from the BARI 2D Trial (Bypass Angioplasty Revascularization Investigation 2 Diabetes). J Am Coll Cardiol. 2013;61:1767–76. [DOI] [PubMed] [Google Scholar]
- 55.Bjerking LH, Hansen KW, Madsen M, Jensen JS, Madsen JK, Sørensen R, et al. Use of diagnostic coronary angiography in women and men presenting with acute myocardial infarction: a matched cohort study. BMC Cardiovasc Disord. 2016;16:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •56. Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, et al. Twenty Year Trends and Sex Differences in Young Adults Hospitalized With Acute Myocardial Infarction. Circulation. 2019;139:1047–56. This community-based study found there has been an increase in myocardial infarction among young women from 1995 to 2014, which highlights that more intensive preventive efforts are needed in this population.
- 57.Davis M, Diamond J, Montgomery D, Krishnan S, Eagle K, Jackson E. Acute coronary syndrome in young women under 55 years of age: clinical characteristics, treatment, and outcomes. Clin Res Cardiol. 2015;104:648–55. [DOI] [PubMed] [Google Scholar]
- 58.Ferrari R, Abergel H, Ford I, Fox KM, Greenlaw N, Steg PG, et al. Gender- and age-related differences in clinical presentation and management of outpatients with stable coronary artery disease. Int J Cardiol. 2013;167:2938–43. [DOI] [PubMed] [Google Scholar]
- 59.Zandecki L, Sadowski M, Janion M, Gierlotka M, Gasior M, Polonski L. Trends in sex differences in clinical characteristics, treatment strategies, and mortality in patients with ST-elevation myocardial infarction in Poland from 2005 to 2011. Coron Artery Dis. 2017;28:417–25. [DOI] [PubMed] [Google Scholar]
- 60.Sulzgruber P, Koller L, Pavo N, El-Hamid F, Rothgerber D-J, Forster S, et al. Gender-related differences in elderly patients with myocardial infarction in a European Centre. Eur J Clin Invest. 2016;46:60–9. [DOI] [PubMed] [Google Scholar]
- 61.Skelding KA, Boga G, Sartorius J, Wood GC, Berger PB, Mascarenhas VH, et al. Frequency of coronary angiography and revascularization among men and women with myocardial infarction and their relationship to mortality at one year: an analysis of the Geisinger myocardial infarction cohort. J Interv Cardiol. 2013;26:14–21. [DOI] [PubMed] [Google Scholar]
- 62.Stone GW, Narula J. Emergence of Plaque Erosion as an Important Clinical Entity. JACC: Cardiovascular Imaging. 2015;8:623–5. [DOI] [PubMed] [Google Scholar]
- 63.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of Risk Factors on the Mechanism of Acute Thrombosis and Sudden Coronary Death in Women. Circulation. 1998;97:2110–6. [DOI] [PubMed] [Google Scholar]
- 64.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. European Heart Journal. 2013;34:719–28. [DOI] [PubMed] [Google Scholar]
- 65.Choi AD, Parwani P, Michos ED, Lee J, Singh V, Fentanes E, et al. The global social media response to the 14th annual Society of Cardiovascular Computed Tomography scientific sessions. J Cardiovasc Comput Tomogr. 2020;14:124–30. [DOI] [PubMed] [Google Scholar]
- 66.Bradley CK, Wang TY, Li S, Robinson JG, Roger VL, Goldberg AC, et al. Patient-Reported Reasons for Declining or Discontinuing Statin Therapy: Insights From the PALM Registry. JAHA [Internet]. 2019. [cited 2020 Nov 28];8. Available from: https://www.ahajournals.org/doi/10.1161/JAHA.118.011765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mamudu HM, Paul TK, Veeranki SP, Budoff M. The effects of coronary artery calcium screening on behavioral modification, risk perception, and medication adherence among asymptomatic adults: A systematic review. Atherosclerosis. 2014;236:338–50. [DOI] [PubMed] [Google Scholar]