Abstract
Medullary thyroid carcinoma (MTC) is a rare malignancy comprising 1–2% of all thyroid cancers in the United States. Approximately 20% of cases are familial, secondary to a germline RET mutation, while the remaining 80% are sporadic and also harbour a somatic RET mutation in more than half of all cases. Up to 15–20% of patients will present with distant metastatic disease, and retrospective series report a 10-year survival of 10–40% from time of first metastasis. Historically, systemic therapies for metastatic MTC have been limited, and cytotoxic chemotherapy has demonstrated poor objective response rates. However, in the last decade, targeted therapies, particularly multitargeted tyrosine kinase inhibitors (TKIs), have demonstrated prolonged progression-free survival in advanced and progressive MTC. Both cabozantinib and vandetanib have been approved as first-line treatment options in many countries; nevertheless, their use is limited by high toxicity rates and dose reductions are often necessary. New generation TKIs, such as selpercatinib or pralsetinib, that exhibit selective activity against RET, have recently been approved as a second-line treatment option, and they exhibit a more favourable side-effect profile. Peptide receptor radionuclide therapy or immune checkpoint inhibitors may also constitute potential therapeutic options in specific clinical settings. In this review, we aim to present all current therapeutic options available for patients with progressive MTC, as well as new or as yet experimental treatments.
Keywords: medullary thyroid cancer, treatment, tyrosine kinase inhibitors, selpercatinib, pralsetinib, PRRT, immunotherapy
Introduction
Medullary thyroid carcinoma (MTC) is a malignant neuroendocrine tumour originating from the parafollicular or C-cells of the thyroid, capable of secreting calcitonin and carcinoembryonic antigen (CEA) (Ceolin et al. 2019). It is a rare malignancy comprising 1–2% of all thyroid cancers in the United States (Tuttle et al. 2014, Lim et al. 2017). Approximately 20% of cases are familial, secondary to a germline rearranged during transfection (RET) mutation, while the remaining 80% are sporadic (Bai et al. 2020) and also harbour a somatic RET mutation in more than half of all cases, with RET M918T being the most frequent genetic alteration encountered in 30–50% of aggressive sporadic MTC (Dvorakova et al. 2008, Taccaliti et al. 2011).
Initial therapeutic approach to MTC
The standard treatment for MTC is total thyroidectomy and dissection of cervical lymph node compartments, as surgery is the only curative treatment (Wells et al. 2015). Patients with persistent or recurrent MTC localised to the neck are candidates for repeated neck explorations. However, in the presence of widespread regional or metastatic disease, extensive surgery is not associated with a higher cure rate or survival benefit and should be considered mainly for local symptom control (Wells et al. 2015, Randle et al. 2017).
Post-operative biochemical remission of serum calcitonin is significantly correlated with an improved 5-year recurrence rate but not 5-year survival (Jung et al. 2016). The 5-year and 10-year survival rates are 25 and 8%, respectively, when the doubling time of calcitonin is less than 6 months, and 92 and 37%, respectively, and when the doubling time ranges from 6 months to 2 years (Wells et al. 2015). Currently, a short calcitonin and CEA doubling time (<6 months) are considered the best available indicators to assess tumour behaviour, MTC recurrence and cancer mortality (Barbet et al. 2005, Laure Giraudet et al. 2008). Post-operative levels of serum calcitonin > 150 ng/L generally indicate distant metastases (Laure Giraudet et al. 2008).
Neck and/or chest computerised tomography (CT), hepatic magnetic resonance imaging (MRI) and bone scintigraphy are the main imaging modalities utilised for MTC staging and follow-up (Maia et al. 2014, Wells et al. 2015). Although several radionuclide imaging modalities are available, positron emission tomography (PET) CT using 18F-fluorodeoxyglucose (18F-FDG), 18F-DOPA and 68Ga-DOTA-somatostatin analogues (68Ga-SSA) offer higher sensitivity compared with conventional imaging (CT/MRI). Of interest, a study evaluating the performance of 68Ga-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-octreotate (DOTATATE)-PET/CT in detecting MTC lesions suggested that it is highly sensitive in identifying bone metastases and could be a substitute for bone scans, and importantly, as a theranostic, it indicates a potential therapeutic tool in the presence of considerable uptake (Castroneves et al. 2018, Hayes et al. 2021).
Metastatic disease
Up to 15–20% of patients will present with distant metastatic disease at diagnosis, and retrospective series report a 10-year survival of 10–40% from time of first metastasis (Roman et al. 2006, Chiacchiarini et al. 2021).
Cytotoxic chemotherapy, as well as local treatments such as external beam radiotherapy, has only a limited and short-term benefit (Wells et al. 2015). Currently available systemic treatments include the use of multi-tyrosine kinase inhibitors (TKIs), which partially inhibit multiple kinases including RET, and often impair multiple signalling pathways (Filetti et al. 2019). TKIs have shown prolonged progression-free survival in patients with tumour progression, although at a cost of considerable toxicity (Elisei et al. 2013). Although the Food and Drug Administration (FDA) and European Medicines Agency (EMA) have approved several TKIs for treating advanced MTC, novel therapeutic modalities are still required to treat patients who fail to respond to TKIs or experience unacceptable toxicity. New-generation targeted therapies such as immunotherapy and peptide receptor radionuclide therapy (PRRT) may be promising but require prospective randomised trials.
In this review, we aim to present all current therapeutic options available for patients with progressive MTC, as well as the new or as yet experimental treatments based on the individualised molecular profile of each patient.
Therapeutic strategies for metastatic MTC
When evaluating a patient with advanced MTC, various factors need to be taken into account. Is the patient symptomatic or asymptomatic? Is the locoregional disease controlled? Is there any calcitonin-related secretory syndrome or ectopic adrenocorticotropic hormone (ACTH) syndrome? Are there lesions that require intervention due to an imminent risk or associated symptoms? What is the rate of progression (calcitonin doubling-time and imaging findings based on Response Evaluation Criteria in Solid Tumors (RECIST) criteria)?
Tyrosine kinase inhibitors
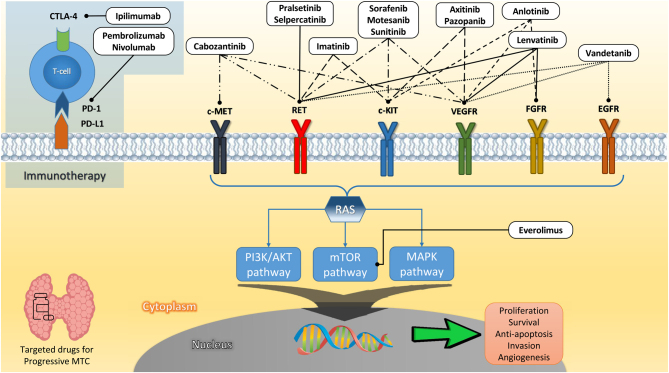
Advances in understanding the molecular mechanisms and intracellular signalling pathways involved in MTC pathogenesis have allowed for the development of targeted therapies, offering new perspectives on effective therapies for advanced MTC. Generally, these therapies are based on the blockade of the MAPK and RAS pathways, which promote MTC development. Many compounds have been developed to inhibit the activity of these pathways (Fig. 1).
Figure 1.
Regulating pathways of MTC and MTC-targeted therapy (potential and confirmed agents) with their corresponding targets and receptors.
Multikinase inhibitors
Tyrosine kinase inhibitors (TKIs) provide a therapeutic benefit in cancer by blocking tyrosine kinase-dependent oncogenic pathways. Several multitargeted TKIs have been tested in MTC, including motesanib (Schlumberger et al. 2009), sorafenib (de Castroneves et al. 2016), sunitinib (Carr et al. 2010), axitinib (Cohen et al. 2008, 2014), imatinib (de Groot et al. 2007), pazopanib (Bible et al. 2014), anlotinib (Sun et al. 2018), lenvatinib (Haugen et al. 2016), vandetanib (Wells et al. 2012) and cabozantinib (Elisei et al. 2013) (Table 1). Of these various TKIs, only vandetanib and cabozantinib are currently FDA and EMA approved for treating unresectable and progressive advanced MTC (Wells et al. 2015, Schlumberger et al. 2017). Because patients with progressive MTC may follow a relatively indolent disease course and can experience prolonged survival even without therapy, prospective trials should preferably mandate RECIST progression on trial enrolment and use progression-free survival (PFS) along with overall survival (OS) to evaluate drug efficacy (Hadoux & Schlumberger 2017).
Table 1.
Clinical trials of multikinase inhibitors in medullary thyroid cancer.
| TKIs | Phase | Population (n) | Studied groups | Target of action | Median PFS, (HR (95% CI), months),P | Median OS (HR (95% CI), months), P | AEs | FDA approval |
|---|---|---|---|---|---|---|---|---|
| Vandetanib (ZETA study) (NCT00410761) | 3 | Advanced or metastatic MTC (n = 331) | Vandetanib-treated MTC patients (n = 231) vs placebo (n = 100) | VEGFR, KIT, RET, EGFR | 30.5 months, HR: 0.46 (0.31–0.69), P < 0.001 | HR: 0.89 (0.48–1.65) | Diarrhoea (56%), rash (45%), nausea (33%), hypertension (32%) and headache (26%) | 2011 |
| Cabozantinib (EXAM study) (NCT00704730) | 3 | Advanced or metastatic MTC (n = 330) | Cabozantinib-treated MTC patients (n = 220) vs placebo (n = 110) | VEGFR2, KIT, FLT3, AXL, MET, RET | 11.2 months, HR: 0.28 (0.19–0.40), P < 0.0001 | 44.3 months, HR: 0.85 (0.64–1.12), P = 0.24, 44.3 months, RET M918T + HR: 0.60 (0.38–0.94), P = 0.03 | Fatigue, diarrhoea, decreased appetite, nausea, weight loss and palmar-plantar erythrodysaesthesia, no cardiological AEs | 2012 |
| Lenvatinib (NCT01728623) | 2 | Advanced or metastatic MTC (n = 9) | Lenvatinib-treated MTC patients (n = 9) | VEGFR, KIT, RET, PDGFR, FGFR | 9.2 months (1.8–NR) | 12.1 months (3.8–NR) | Hypertension (89%), palmar-plantar erythrodysaesthesia (89%), diarrhoea (89%) | No |
| Motesanib (NCT00121628) | 2 | Advanced MTC (n = 9) | Motesanib-treated MTC patients (n = 91) | VEGFR1,2,3, PDGFR, KIT | 4 months, HR:13 (13–13.2) | NA, at 12 months: 75% of patients survived | Diarrhoea (41%), fatigue (41%), hypothyroid (29%), hypertension (27%) and anorexia (27%) | No |
| Sunitinib (NCT00519896) | 2 | Iodine-refractory WDTC or MTC (n = 7) (total thyroid cancer, n = 33) | Sunitinib-treated MTC patients (n = 7) | VEGFR1,2, PDGFR, c-KIT, FLT3, RET | 12.8 months (for all thyroid cancers) | Not reached | Fatigue (11%), neutropaenia (34%), palmar-plantar erythrodysaesthesia (17%), diarrhoea (17%), leukopenia (31%) | No |
| Imatinib (with dacarbazine and capecitabine) (NCT00354523) | 2 | Advanced or metastatic MTC (n = 15) | Imatinib-treated MTC patients (n = 15) | Bcr-Abl, (PDGFR)α, PDGFRβ, c-Fms, c-Kit, RET | 7/15 pts: 3 months (2–8) | NA | Hypothyroid (33%), rash, malaise, and laryngeal mucosal swelling (12%), serious haematological toxicity (grade 3) (6%) | No |
| Anlotinib (ALTER 01031)(NCT02586350) | 2B | Advanced or metastatic MTC not previously treated with antiangiogenetic target therapy (n = 62) | Anlotinib-treated MTC patients (n = 62) vs placebo (n = 29) | VEGFR, FGFR, PDGFR, c-Kit | 20.67 (14.03–34.63) vs 11.07 (5.82–14.32), (HR: 0.53, P = 0.0289) | ND | Palmar-plantar erythrodysaesthesia, hypertension, hypertriglyceridaemia and diarrhoea | No |
| Pazopanib (NCT00625846) (completed 2020) | 2 | Advanced thyroid cancer (MTC, n = 35) | Pazopanib-treated MTC patients (n = 35) | VEGF1,2, PDGFa,b, FGF, ITK | 6 months, 0.686 (0.548–0.858) | NA | Weight loss (11.4%), depression (8.6%), abdominal pain (5.7%), skin rash (5.7%) | No |
| Sorafenib (NCT00390325) (ongoing) | Systematic review of 8 studies/ongoing trial | Advanced MTC n = 101/MTC n = 21 (active but no recruitment) | Sorafenib-treated MTC patients (n = 101)/not completely | Raf serine/threonine kinases, VEGFR1,2,3 (PDGFRβ) | 14.5 (12.4–16.3)/no data yet | ND | Palmar-plantar erythrodysaesthesia (69%), diarrhea (49%), hypertension (35%), skin rash (39%), fatigue (39%) | No |
| Selpercatinib (LOXO-29) (NCT03157128) | 1/2 | RET-mutant or not MTC patients (n = 143) | RET-mutant MTC patients treated (n = 55) vs not previously treated (n = 88) with vandetanib or cabozantinib | - ATP-competitive small molecule RET inhibitor - Inhibit different RET alterations, including the V804M mutation - Low activity on VEGFR |
1-year-PFS: 82% (69–90) in the RET-mutant MTC pre-treated and 92% (82–97) in not previously treated patients | ND | Hypertension (43%), diarrhoea (38%), fatigue (38%) | 2020 |
| Pralsetinib (BLU-667) ARROW trial (NCT03037385) | 1/2 | RET-mutant MTC patients (n = 84) | RET-mutant MTC patients treated (n = 61) vs not previously treated (n = 23) with vandetanib or cabozantinib) | - 10× potency over other MKIs against RET variants and resistance mutants - No VEGFR inhibition |
- Median PFS: not reached - Estimated 1-year PFS: 75% (63–86) in RET-mutant pre-treated and 81% (63–98) in not previously treated patients |
- Median OS : not reached - Estimated 1-year OS: 89% (81–97) in RET-mutant pretreated and 91% (78–100) in not previously treated patients |
Grade 3: hypertension (40%), diarrhoea (38%), fatigue (35%) and anaemia, lymphopaenia, neutropaenia | 2020 |
| Axitinib (NCT00094055) | 2 | Advanced or metastatic thyroid cancer (total n = 60; MTC, n = 11) | Axitinib-treated MTC patients (n = 11) | VEGFR1,2,3 | - 18.1 months (for all thyroid cancers)a - MTC patients: 2/11 had PR, 3/11 had SD |
Not reached | Grade > 3: hypertension (n = 7; 12%) | No |
aNo separated data for MTC.
AE, adverse events; EGFR, epithelial growth factor; FLT3, FMS/KIT-like gene; HR, hazard ratio; KIT, proto-oncogene, receptor tyrosine kinase; MTC, medullary thyroid cancer; NA, not available; ND, no data; NR, not reported; OS, overall survival; PDGFR, podocyte growth factor receptor; PFS, progression-free survival; PR, partial response; RET, rearranged during transfection; SD, stable disease; TKI, tyrosine kinase inhibitors; VEGFR, vascular epithelial growth factor; WDTC, well differentiated thyroid cancer.
The first approved TKI, vandetanib, selectively targets RET, vascular epithelial growth factor receptor (VEGFR) and EGF receptors (EGFR) (Wedge et al. 2002, Wells et al. 2012). The efficacy of vandetanib was evaluated in 331 individuals with progressive MTC (123 had tumour progression, either locoregional or distant metastases) randomised 2:1 to receive vandetanib (300 mg/day) or placebo (Zactima Efficacy in Thyroid Cancer Assessment(ZETA) study) (Wells et al. 2012). Inclusion criteria included measurable, unresectable locally advanced or metastatic, hereditary or sporadic MTC. The results showed a statistically significant increase in the PFS in the vandetanib-treated group compared to placebo (30.5 vs 19.3 months, P < 0.001), as well as a higher objective response rate. Vandetanib has also been successfully used to treat children with MEN2B (Fox et al. 2013).
The second approved TKI, cabozantinib (140 mg/day), is a c-MET, VEGFR2 and RET inhibitor. A randomised (2:1) study of 330 individuals demonstrated a significant increase in PFS in the cabozantinib-treated group compared to placebo (11.2 vs 4.0 months, P < 0.001) in the advanced MTC (EXAM) study (Elisei et al. 2013). In this trial, all patients had documented radiological disease progression according to RECIST (40% had received previous anti-cancer therapies, including other TKIs). The effect of vandetanib or cabozantinib on the overall survival of MTC patients remains unknown, but interim analyses have not revealed any difference between the experimental and placebo arms (Wells et al. 2012, Elisei et al. 2013). This could potentially be due to the cross-over design in the ZETA study, but patients receiving placebo were not allowed to cross-over in the EXAM study. In vivo data have shown that both drugs inhibit angiogenesis in a dose-dependent manner, although cabozantinib showed more potent anti-angiogenic activity than vandetanib (Carra et al. 2021). In a recent retrospective study including MTC patients (n = 48) with locally advanced disease and/or evidence of distant metastases treated with vandetanib and/or cabozantinib (as first-line and later-line treatment), the median PFS for first-line vandetanib- and cabozantinib-treated patients was 12 and 9 months, respectively. The median total OS (for the total duration of treatment, first-line and later-line settings) was 54 and 24 months, respectively (Koehler et al. 2021); however, these results are difficult to interpret given that significantly more patients received vandetanib in the first-line setting (85%) compared with cabozantinib (15%), and this likely accounts for the poorer prognosis of cabozantinib-treated patients. In vandetanib-treated patients, the PFS and OS were significantly longer in patients aged ≤60 years at TKI initiation and in patients with ≥5 treatment-related adverse events. In addition, the PFS was prolonged in those without bone metastases (Koehler et al. 2021).
The genetic signature is crucial for predicting responsiveness to therapy in progressive MTC. Phase 3 trials have helped identify predictors of response to both vandetanib and cabozantinib. The subgroup of patients in the ZETA study with the RET M918T mutation showed a higher response rate to vandetanib than RET M918-negative patients (54.5% vs 32%), but these data were based on a small sample size (Wells et al. 2012). In a larger phase 3 trial, cabozantinib appeared to prolong PFS vs placebo in the RET mutation-positive subgroup of patients with radiologically documented progressive MTC (HR: 0.23; 95% CI: 0.14–0.38; P < 0.0001), in the RET mutation-unknown subgroup (HR: 0.30; 95% CI: 0.16–0.57; P = 0.0001) and in the RAS mutation-positive subgroup (HR: 0.15; 95% CI: 0.02–1.10; P = 0.0317). The RET M918T subgroup achieved the greatest observed PFS from cabozantinib compared to placebo (HR: 0.15; 95% CI: 0.08–0.28; P < 0.0001). Furthermore, the PFS following cabozantinib was prolonged in patients with RET M918T (61 vs 17 weeks) or RAS mutations compared to patients with other mutations (Sherman et al. 2016). A microRNA expression analysis in MTC has shown increased expression of miR-375 and decreased protein levels of the miR-375 target SEC23A in MTC, both of which conferred increased sensitivity to vandetanib. In vitro studies have shown that RET codon 804 and 806 mutations confer resistance to vandetanib therapy (Carlomagno et al. 2009).
Common adverse effects of TKIs include hypertension, palmar–plantar erythrodysaesthesia, proteinuria and QTc interval prolongation, with the most prominent being fatigue, lethargy, anorexia and gastrointestinal disturbances; however, all TKIs have a unique adverse event profile (Table 1). Approximately 35% of the patients treated with vandetanib had a QTc prolongation of more than 60 ms, which is considered a grade 4 adverse event requiring dosage reduction, but this was not encountered in the phase 3 cabozantinib trial (Schlumberger et al. 2017). Fistulas in the gastrointestinal tract are rare (<1%), although extremely dangerous, adverse events associated with cabozantinib. Practical guidelines on the management of the more common adverse events have been developed (Rao & Cabanillas 2018, Cabanillas & Takahashi 2019). Patients need to be carefully assessed, focusing on their performance status along with the burden and location of metastases. After treatment commencement, regular review is required to allow for the early and aggressive management of potential adverse events. In cases of less serious adverse events (grade 1–2), dose modification, by lowering the administered dose, should be considered in parallel with symptomatic treatment; however, in cases of serious adverse events (grades 3–4), treatment should be interrupted or suspended.
In the case of progression on an initial TKI, treatment with another TKI, besides those currently approved, may be considered when accessible. Lenvatinib is a TKI inhibiting VEGFR-1, 2, and 3, fibroblast growth factor receptor (FGFR)-1–4, platelet-derived growth factor receptor (PDGFR), RET and KIT signalling pathways, that has been approved for the treatment of differentiated thyroid cancer, but only scanty data exist on its use in MTC. In a phase 2 study including 59 patients with unresectable progressive MTC, disease control was achieved in 80% (95% CI: 67–89%), 44% of whom had stable disease (Schlumberger et al. 2016). Median PFS was 9.0 months (95% CI: 7.0 – not evaluable). Similarly, in a smaller but more recent phase 2 trial of 51 patients with all histological types of progressive thyroid cancer, including 9 patients with MTC, the median PFS for MTC patients was 9.2 months, with a 22% response rate (Takahashi et al. 2019). Lenvatinib can induce posterior reversible encephalopathy syndrome, which in combination with Takotsubo cardiomyopathy resulted in a fatal outcome in one case (Chae et al. 2018). Other rarer adverse events include thrombocytopaenia, fistula formation and heart failure (Cabanillas & Takahashi 2019).
Sorafenib is a dual inhibitor, targeting Raf pathways as well as tyrosine kinase pathways including VEGFR-1, VEGFR-2, VEGFR-3 and PDGFR-β. In two meta-analyses including 101 and 99 progressive MTC patients, respectively, from 8 studies using sorafenib as the single TKI treatment (previous treatments included surgery, chemotherapy or radiotherapy), sorafenib showed an intermediate efficacy. In the first meta-analysis, an overall partial response and stable disease were found in 21 and 58% of MTC patients, respectively (Vuong et al. 2019), while in the other, an objective response (including complete and partial responses) was found in 27.6% of patients with a PFS of 12.4 months (95% CI: 8.4–16). However, this was associated with a high discontinuation rate of up to 32.3% (95% CI: 24.3–40) of patients due to toxicity (Vuong et al. 2019, Efstathiadou et al. 2021). Responses to sorafenib were not durable, with resistance developing, usually within 1–2 years (Vuong et al. 2019).
Anlotinib is a TKI that inhibits both tumour angiogenesis and tumour cell proliferation by blocking VEGFR, FGFR, PDGFR and c-Kit simultaneously (Shen et al. 2018, Li et al. 2021). In a single-arm phase 2 study conducted in China, 58 patients with progressive MTC (unresectable, locally advanced or metastatic MTC) treated with anlotinib exhibited an objective response rate (ORR) of 56.9% (Sun et al. 2018), with a PFS rate at 48 weeks of 85.5%. The most common adverse events were palmar-plantar erythrodysaesthesia, hypertriglyceridaemia, cholesterol elevation, fatigue and proteinuria.
In a recent meta-analysis including 33 studies (23 prospective, 9 retrospective, 1 observational) with 99 metastatic MTC patients and 16 cohorts showing progressive disease ab initio, overall stable disease was observed in 46.2% of TKI-treated patients and progressive disease in 22.9% (Efstathiadou et al. 2021). In particular, the use of TKIs conferred a PFS of 23.3 months (95% CI: 21.07–25.5). The meta-analysis included patients treated with various TKIs including axitinib, cabozantinib, dovinitib, imatinib, lenvatinib, motesanib, pazopanib, sorafenib, sunitinib, vandetanib and combinations of these treatments. In particular, progressive disease occurred in 23.7% of patients treated with vandetanib, in 22.6% treated with cabozantinib and in 19.3% treated with sorafenib. Grade 3 or greater adverse events occurred in 48.5% of TKI-treated patients and drug discontinuation was reported in 44.7%. Vandetanib induced an objective response in 33.8% and cabozantinib in 27.7% of MTC patients (Efstathiadou et al. 2021).
A limitation of TKI treatment is that the tumour cells eventually develop resistance, associated with tumour progression, independent of the type of TKI used or molecular tumour profile. Secondary mutations in the kinase domains that inhibit the binding of TKIs, usually downstream from the TKI target, resulting in a mechanism that bypasses the action of the drug (Viola et al. 2016). In MTC patients, acquired RET V804M ‘gatekeeper’ resistance mutations have been described in vandetanib-treated patients (Subbiah et al. 2018a ). In such cases, a second TKI such as cabozantinib that acts via more than one molecular pathway might be considered in order to counteract such resistance (Priya et al. 2017). Of note, the discontinuation of TKI treatment could lead to a rapid increase in tumour growth and disease progression (Resteghini et al. 2017), presumably because the TKI blockade was being partially (but only partially) reversed by the activation of alternative signalling pathways. In such a situation, combination drug use may be considered, although this approach is investigational (see below).
Specific inhibitors of RET oncogene alterations
In 2020, two new RET-selective inhibitors were FDA-approved: selpercatinib (LOXO-292) and pralsetinib (BLU-667), with the former now also having been approved by the EMA.
Recently, selpercatinib (LOXO-292), which selectively targets oncogenic RET alterations, was approved by the FDA in the United States for the treatment of RET-mutant MTC and RET fusion thyroid cancers (Wirth et al. 2020); it has recently also been approved in the United Kingdom in patients resistant or intolerant of the multikinase inhibitors. In 55 RET-mutant MTC patients who had previously progressed through or not tolerated vandetanib and/or cabozantinib, the objective response rate was 69% (95% CI: 55–81), and the 1-year PFS was 82% (95% CI: 69–90). In 88 RET-mutant MTC patients who had not previously received vandetanib or cabozantinib, the response rate was 73% (95% CI: 62–82), and the 1-year PFS 92% (95% CI: 82–97). The most common adverse events of grade 3 or greater were hypertension in 21% of patients, increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in 11 and 9% respectively, hyponatraemia in 8% and diarrhoea in 6%. Similar response rates were observed in 3 out of 11 patients (27%) who harboured the RETV804M mutation, which confers acquired resistance to vandetanib, showing clinical and radiological responses with a maximum change in tumour size ≥60% after receiving selpercatinib, although resistance eventually developed (Wirth et al. 2020).
Pralsetinib (BLU-667) is also a highly selective RET inhibitor (Subbiah et al. 2018a ). Pralsetinib potently inhibits the growth of thyroid cancer xenografts driven by various RET mutations and fusions, without inhibiting VEGFR-2. In a phase 2 study (NCT03037385) including MTC patients with documented radiological progression, 122 patients with RET-mutant MTC (61 patients pre-treated with cabozantinib or vandetanib, or both, and 23 treatment-naïve patients) showed an overall objective radiological response rate of 71% (95% CI: 48–89) by RECIST criteria in treatment-naive patients and 60% (95% CI: 46–73) in pre-treated patients (Subbiah et al. 2018b ). The median PFS and OS were not reached, but the estimated one-year PFS after a median follow-up of 15 months was 75% (95% CI: 63–86) in pre-treated patients and 81% (95%CI: 63–98) in treatment-naïve patients. Overall, pralsetinib appeared to be well-tolerated. The most common adverse event was grade 1 constipation (23%). Grade 3 adverse events included hypertension (8%) and neutropaenia (4%), but there were no grade 4/5 adverse events (Subbiah et al. 2018b ).
In 2020, both the new generation TKIs, selpercatinib and pralsetinib, were granted FDA approval in patients with advanced RET-mutant MTC who require systemic therapy and indeed they are considered a highly potent treatment in patients with RET-driven MTCs (Subbiah et al. 2018a ). However, there is concern that these highly selective inhibitors may not be as ‘durable’ as the multikinase inhibitors, with resistance being seen to occur more rapidly as follow-up studies progress (Lin et al. 2020, Lin & Gainor 2021).
The vast majority of MTC cases resistant to selpercatinib or pralsetinib are RET-mutant negative; further investigation of RET-independent resistance mechanisms has demonstrated that the resistance to selective RET inhibition may be driven by acquired MET or KRAS amplifications (Lin et al. 2020). In the cases of resistance in which the RET pathway is involved, RET solvent front (G810) mutations as well as hinge region (Y806) mutations confer selpercatinib resistance through steric hindrance with drug binding (Lin et al. 2020, Solomon et al. 2020, Subbiah et al. 2021). Moreover, using X-ray crystal structures of RET–selpercatinib and RET–pralsetinib complexes, these inhibitors are not susceptible to RET gatekeeper (V804) mutations because of their mode of binding, while remaining susceptible to non-gatekeeper mutations (Subbiah et al. 2021). In addition, gatekeeper mutations have been more readily identified after progression on approved TKIs in EGFR-mutant or ALK fusion-positive non-small-cell lung cancer (NSCLC) as compared with RET fusion-positive NSCLC (Lin et al. 2020). It will be necessary to develop RET inhibitors with maintained potency against RET resistance mutations, and it may be that combination strategies will be needed to effectively overcome resistance in these patients. As an example, TPX-0046 is a potent RET/SRC inhibitor which has demonstrated preclinical potency against RET G810 solvent front mutations and is currently in phase 1 testing in patients with advanced RET-altered solid tumours (NCT04161391).
When to initiate TKIs and what TKI to choose
The initiation of systemic treatment with TKIs is recommended in the setting of progressive or extensive distant metastases. The current recommendation (Wells et al. 2015, Haddad et al. 2018) is to consider treatment for tumours >1–2 cm in diameter growing >20% per year, or for symptomatic control: clearly depending on availability, one could use the selective RET-antagonists for germline or somatic RET-mutation-positive tumours and vandetaninib or cabozantinib when these are absent. However, in the United Kingdom, the more selective RET inhibitors are currently only funded when patients progress through the multikinase inhibitors or when these cannot be tolerated, even after dose reduction. It may be that the superior adverse-event profile of pralsetinib and selpercatinib will determine their first-line use, availability and expense allowing, but as yet there is a question mark over their response durability, and longer trial follow-up is awaited. It is also unclear as to whether the presence of a RET mutation, either germline or somatic, is mandatory before the use of either the multikinase or the RET selective inhibitors. TKIs in MTC patients with progressive disease are associated with a moderate therapeutic benefit, including disease stability or partial responses in up to 73% of cases, decreasing the 10-year disease-specific mortality rate from 38 to 13.5%, mainly seen in patients harbouring RET M918T mutations (Dvorakova et al. 2008, Taccaliti et al. 2011, Kuo et al. 2018). While the toxicity of these drugs is not negligible, it is usually manageable.
Investigational therapy
Combined targeted therapies
Certain small molecule inhibitors possess efficacy against MTC in vivo in combination with other drugs (Priya et al. 2017). Sunitinib and cisplatin cooperatively interfere with the autophagic lysosomal pathway, and this combination appears to be active in metastatic or progressive MTC (Lopergolo et al. 2014). Acquired resistance to targeted therapy could possibly be overcome by employing a combination of targets such as everolimus plus 5-aza-2′-deoxycytidine (AZA), as this combination was found to have a synergistic effect even in everolimus-resistant cell lines (Vitale et al. 2017). In other neuroendocrine tumours, combination therapies may show synergistic effects, at least in vitro (Aristizabal Prada et al. 2018).
In a recent meta-analysis (Funakoshi et al. 2014), the combination of cytotoxic chemotherapy and various TKIs (vandetanib, cabozantinib, sorafenib and sunitinib) exhibited a prolonged PFS (HR: 0.82, 95% CI: 0.76–0.89) but had no effect on OS (HR: 0.99, 95% CI: 0.95–1.03) compared to chemotherapy alone in a variety of malignancies, including MTC. However, compared with chemotherapy alone, the addition of a TKI significantly increased the risk of any AEs (RR: 1.63, 95% CI: 1.32–2.01), treatment discontinuation (RR: 1.80, 95% CI: 1.58–2.06) and any severe AEs (RR: 1.25, 95% CI: 1.16–1.36).
Monotherapy agents and epigenetic therapy
Nelfinavir (NFV), a heat shock protein (HSP)-90 chaperone, is required for the folding and stability of RET mutants (Alfano et al. 2010). HSP-90 overexpression is found in a significant proportion of MTCs and may correlate with metastatic potential and RET mutations. HSP90 is a molecular target for the HIV-protease inhibitor NFV. It has been shown to have a wide spectrum of activity in vivo against MTC cells and may emerge as an important therapeutic option (Valea & Georgescu 2016).
mTOR inhibitors
Given that RET and RAS activate the PI3K/AKT/mTOR pathway, the anti-proliferative activity of everolimus, an inhibitor of mechanistic target of rapamycin (mTOR) approved for the treatment of neuroendocrine tumours and renal cell carcinoma, was demonstrated in an MTC human cell line (Grozinsky-Glasberg et al. 2010) and some activity in a single patient with an MTC (Druce et al. 2012). In a small phase 2 trial, seven patients with progressive metastatic or inoperable MTC (Schneider et al. 2015) were treated with everolimus. The median PFS was 33 weeks, but no objective response was observed (Schneider et al. 2015). Similar findings were encountered in another phase 2 trial that included nine MTC patients treated with everolimus (Lim et al. 2013). The median PFS was not reached (after a median follow-up of 11 months), indicating that everolimus may have activity against MTC and that larger prospective studies are warranted. Biochemical response, defined as calcitonin and CEA levels ≥50% lower than baseline, was observed in three (30%) and four (44%) patients, respectively. The most common treatment-related AEs were mucositis, anorexia and AST/ALT elevation, and were in general mild to moderate (Lim et al. 2013).
Nevertheless, promising experimental data have been reported in MTC-1.1 cells exposed to combination treatment including everolimus and sorafenib or sunitinib (Lin et al. 2012). Everolimus increased the anti-proliferative effects of sunitinib and sorafenib by 24 and 27%, respectively, in MTC-1.1 cells and by 20 and 23%, respectively, in thyroid papillary (TT) cells (Lin et al. 2012).
In another report, everolimus was prescribed in addition to vandetanib in a patient who developed disease progression, resulting in a 25% reduction of tumour volume for 8 months (Heilmann et al. 2016). The combination of RET kinase inhibitors and mTOR inhibitors might be an interesting dual-targeting strategy, but further evaluation in larger clinical trials is mandatory for establishing the most appropriate therapeutic regimen. Most importantly, such combinations, while theoretically attractive and soundly-based, may lead to a compounding of adverse effects rendering them clinically impractical.
Immunotherapy
Over the past few years, immunotherapy has been established as an oncological treatment for several types of malignancies, and preclinical studies on MTC have revealed potential benefit (Naoum et al. 2018). Data on clinical studies in MTC are scarce, but in a single phase 1 study including a patient with MTC, blocking the PD-1/PD-L1 interaction using nivolumab, an anti-PD-1 agent, resulted in a partial response (Yamamoto et al. 2017). Another case report also described a patient with MTC already treated with sunitinib and, having undergone a 3-month trial with the GI-6207 cancer vaccine, was enrolled in a phase 1 trial with the PD-L1 inhibitor avelumab (NCT01772004). Following treatment, he developed stable disease along with a >40% decrease in his calcitonin level (Del Rivero et al. 2020). Very preliminary results of a phase 2 trial (NCT03246958) evaluating nivolumab plus ipilimumab in seven patients with progressive MTC and prior TKI failure showed a lack of objective response in all seven patients, although without providing any further specific detail (Lorch et al. 2020).
It is well known that high tissue tumour mutational burden (TMB) is a useful marker to recognise patients with recurrent or metastatic advanced solid tumours who may show a better therapeutic benefit from immune checkpoint inhibitors. Therefore, the NCCN guidelines for MTC management suggest that pembrolizumab should be considered in patients with symptomatic, progressive MTC with high TMB ≥ 10 mutations/megabase (NCCN 2021). MTC is reported to show low PD-L1 expression in both tumour cells and tumour-infiltrating immune cells and no microsatellite instability (Bongiovanni et al. 2017, Bi et al. 2019), irrespective of the presence or absence of either desmoplasia, lymph node metastases and/or RET mutation (Bai et al. 2019, 2020, Shi et al. 2019, Ingenwerth et al. 2020). Nevertheless, a cohort of 201 consecutive Chinese patients with MTC (Shi et al. 2019) demonstrated that PD-L1 positivity was associated with more aggressive clinico-pathologic features, such as larger tumour size, number of lymph node metastases and advanced TNM staging and was independently predictive of morphological and/or biochemical recurrence or persistent disease (Sørlie et al. 2003). Similarly, PD-L1 positivity found in approximately 20% of surgical specimens (although very faint or moderate staining) significantly correlated with distant metastases at surgery (Bi et al. 2019). The correlation between PD-1/PD-L1 status and prognosis suggests that immunotherapy targeting PD-L1/PD-1 might indeed be effective in treating advanced MTC under some circumstances.
Five registered clinical trials are currently active studying immune-checkpoint inhibitors in progressive MTC patients (Table 2). Pembrolizumab (NCT03072160), nivolumab and ipilimumab (NCT03246958) are currently being evaluated in phase 2 trials for the treatment of recurrent or metastatic MTC. Interestingly, among the registered studies, there are also trials evaluating the combination of TKI agents together with immunotherapy or chemotherapy as a novel therapeutic option in the treatment of advanced MTC, although the combination of immunotherapy and chemotherapy is well established in other areas of oncology (Table 2).
Table 2.
Registered (clinicaltrials.gov) clinical trials for the treatment of advanced and /or metastatic MTC.
| Clinical trial | ID | Molecule tested | Status of the study | Type of the study (phase) |
|---|---|---|---|---|
| MKIs | ||||
| Phase 1 and 2 | ||||
| - A Study of LOXO-292 in Participants With Advanced Solid Tumours, RET Fusion-Positive Solid Tumours and MTC | NCT03157128 | Selpercatinib (LOXO-292) | Recruiting | 1 and 2 |
| - Study of TPX-0046, a RET/SRC Inhibitor in Adult Subjects With Advanced Solid Tumours Harbouring RET Fusions or Mutations | NCT03647657 | TPX-0046 (third generation, highly selective TKI) | Recruiting | 1 and 2 |
| - A Study of HA121-28 Tablets in Patients With MTC | NCT04787328 | HA121-28 | Recruiting | 2 |
| - A Study Using Regorafenib as Second or Third-Line Therapy in Metastatic MTC | NCT02657551 | Regorafenib | Recruiting | 2 |
| - A Study Using Regorafenib as Second or Third-Line Therapy in Metastatic MTC | NCT02657551 | Regorafenib | Recruiting | 2 |
| - Selpercatinib Before Surgery for the Treatment of RET-Altered Thyroid Cancer | NCT04759911 | Selpercatinib (LOXO-292) | Recruiting | 2 |
| - Sorafenib Tosylate in Treating Patients With Metastatic, Locally Advanced or Recurrent MTC | NCT00390325 | Sorafenib tosylate | Active recruitment completed | 2 |
| - Sunitinib Malate in Treating Patients With Thyroid Cancer That Did Not Respond to Iodine I 131 and Cannot Be Removed by Surgery | NCT00381641 | Sunitinib malate | Active not recruited yet | 2 |
| - A Study of Selpercatinib (LY3527723) in Participants With Advanced Solid Tumours Including RET Fusion-positive Solid Tumours, MTC and Other Tumours With RET Activation | NCT04280081 | Selpercatinib | Active not recruited yet | 2 |
| - Cabozantinib-S-Malate in Treating Younger Patients With Recurrent, Refractory, or Newly Diagnosed Sarcomas, Wilms Tumour, or Other Rare Tumours | NCT02867592 | Cabozantinib-S-malate | Active not recruited yet | 2 |
| - Pazopanib Hydrochloride in Treating Patients With Advanced Thyroid Cancer | NCT00625846 | Pazopanib hydrochloride | Completed (2020) results pending | 2 |
| - Safety, Efficacy and Tolerability of BOS172738 in Patients With Advanced Rearranged During Transfection (RET) Gene-Altered Tumours | NCT03780517 | BOS172738 (selective RET inhibitor) | 2 | |
| - Cabozantinib-S-Malate in Treating Younger Patients With Recurrent, Refractory, or Newly Diagnosed Sarcomas, Wilms Tumour, or Other Rare Tumours | NCT02867592 | Cabozantinib-S-malate | Active not recruited yet | 2 |
| Phase 3 and 4 | ||||
| - A Study of Selpercatinib (LY3527723) in Participants With RET-Mutant MTC | NCT04211337 | Selpercatinib, cabozantinib, vandetanib | Recruiting | 3 |
| - A Study of Two Different Doses of Cabozantinib (XL184) in Progressive, Metastatic MTC | NCT01896479 | Cabozantinib (XL184) 140 mg vs cabozantinib (XL184) 60 mg vs placebo | Active not recruited yet | 4 |
| - A Study of Pralsetinib Versus Standard of Care (SOC) for Treatment of RET-Mutated MTC | NCT04760288 | Pralsetinib, cabozantinib, vandetanib | Active, not yet recruiting | 3 |
| - To Compare The Effects Of Two Doses Of Vandetanib In Patients With Advanced MTC | NCT01496313 | Vandetanib 150 vs 300 mg | Active not recruited yet | 4 |
| - An Efficacy Study Comparing ZD6474 to Placebo in MTC | NCT00410761 | ZD6474 (vandetanib) | Active not recruited yet | 3 |
| - A Study of Two Different Doses of Cabozantinib (XL184) in Progressive, Metastatic MTC | NCT01896479 | Cabozantinib 60 vs 140mg | Active not recruited yet | 4 |
| ICIs (all phase 1 and 2) | ||||
| - A Phase II Study of Durvalumab (MEDI4736) Plus Tremelimumab for the Treatment of Patients With Progressive, Refractory Advanced Thyroid Carcinoma – The DUTHY Trial | NCT03753919 | Durvalumab | Recruiting | 2 |
| - A Phase 2 Study of Nivolumab Plus | NCT03246958 | Nivolumab+ipilimumab | Active not recruiting | 2 |
| - Ipilimumab in RAI Refractory, Aggressive Thyroid Cancer With Exploratory Cohorts in Medullary and Anaplastic Thyroid Cancer | ||||
| - Pilot Trial of Nivolumab Plus Cabozantinib for Advanced Solid Tumours in Patients With HIV Infection | NCT04514484 | Nivolumab | Recruiting | 1 |
| - Phase II Trial of Pembrolizumab in Recurrent or Metastatic MTC | NCT03072160 | Pembrolizumab | Completed (results submitted) | 2 |
| - Secured Access to Pembrolizumab for Patients With Selected Rare Cancer Types | NCT03012620 | Pembrolizumab | Recruiting | 2 |
| PRRT (all phase 1 and 2) | ||||
| - Indium In 111 pentetreotid in treating patients with refractory cancer | NCT00002947 | 111In pentetreotid | Terminated | 1 |
| - 177Lu-PP-F11N in Combination With Sacubitril for Receptor-Targeted Therapy and Imaging of Metastatic Thyroid Cancer | NCT03647657 | 177Lu-PP-F11N | Recruiting | 1 |
| - 177Lu-PP-F11N for Receptor-Targeted Therapy and Imaging of Metastatic Thyroid Cancer (Lumed) | NCT02088645 | 177Lu-PP-F11N | Recruiting | 1 |
| - Radioactive Drug (177Lu-DOTATE) for the Treatment of Locally Advanced metastatic or Unresectable Rare Endocrine Cancers | NCT04106843 | 177Lu Dotatate | Recruiting | 2 |
| Others | ||||
| - GFRα4 CAR T Cells in MTC Patients | NCT04877613 | Single dose of CART-GFRa4 cells vs fludarabine vs cyclophosphamide | Recruiting | 1 |
| - Thermal Ablation of Cervical Metastases From Thyroid Carcinoma | NCT04522570 | Laser ablation, cryoablation, radiofrequency ablation | Recruiting | n/a |
| - QUILT-3.006 for Recurrent MTC (vaccine) | NCT01856920 | GI-6207 (recombinant Saccharomyces cerevisiae-CEA (610D)) | Active not recruited yet | 2 |
Inhibitors, 177Lu-PP-F11N, 177Lutetium-labelled minigastrin analogue; ICI, immune-check point inhibitors; 111In, 111Indium; MKI, multikinase inhibitors; MTC, medullary thyroid cancer; PRRT, peptide receptor radionuclide therapy.
Peptide receptor radionuclide therapy (PRRT)
Several studies targeting somatostatin receptors (SSTRs) with radionuclides have been reported in patients with progressive, inoperable or advanced metastatic MTC (Grossrubatscher et al. 2020), using radiopharmaceuticals such as 90Yttrium (90Y), 177Lutetium (177Lu) or 111Indium (111In) (Salavati et al. 2016, Parghane et al. 2020, Satapathy et al. 2020). In a recent review including 11 studies and 117 MTC patients treated with 90Y, 177Lu or 111In, radiological response data showed that 37.6% of patients developed progressive disease, 54.7% stable disease, 5.1% partial response and 2.6% complete response; however, response criteria across the studies were heterogeneous (Grossrubatscher et al. 2020). Besides radiological evaluation, biochemical responses based on calcitonin plasma concentrations showed that 37% of the patients had a more than 50% calcitonin decrease (Grossrubatscher et al. 2020). In a prospective phase II study (n = 31 patients ), a prolongation of calcitonin doubling-time levels in 58% of patients post-90Y-DOTATOC was reported (Iten et al. 2007). In general, patients with partial responses, disease stability and a biochemical response showed prolonged survival (Iten et al. 2007, Salavati et al. 2016, Parghane et al. 2020). After 17 years of experience following treatment with 177Lu-DOTATATE (Beukhof et al. 2019), albeit in a very small group of patients, it was concluded that this treatment could be considered in patients with high uptake on an 111In-DTPA-octreotide scan and positive somatostatin receptor-2a (SSTR2a) expression in tumour samples (Beukhof et al. 2019). Twenty-one patients with advanced MTC treated with PRRT (90Y-DOTATATE, 90Y-DOTATOC, 177Lu-DOTATATE or 177Lu-DOTATOC) achieved a median time-to-treatment-failure interval of 14 months (95% CI: 8–25) and a median OS of 63 months (95% CI: 21, not reached). Predictors of poorer OS included a short calcitonin doubling-time (≤24 months), strong 18F-FDG avidity and age ≥60 years (Hayes et al. 2021). A larger retrospective series of 43 patients all treated with 177Lu-DOTATATE showed a RECIST partial response in 4%, stable disease in 58% and a median PFS of 24 months (Parghane et al. 2020).
PRRT is often well tolerated but is not free of adverse effects such as nausea, asthenia and elevation of liver enzyme levels, although they are usually mild. Serious complications, such as radiation nephrotoxicity and treatment-related myeloid neoplasms, are relatively rare especially with the more frequently used 177Lu-labelled radiopeptides (Brabander et al. 2017, Parghane et al. 2020, Sonbol et al. 2020). Adverse events of all grades of nephrotoxicity were reported in 0–1.3% of patients, with grade 3–4 in 0.8%, and grade 3–4 haematological toxicity in up to 6.5% of cases (Grossrubatscher et al. 2020, Parghane et al. 2020). Proximal tubular reabsorption of the radiopeptide leads to glomerular fibrosis, which is particularly observed after treatment with 90Y-DOTATOC (Parghane et al. 2020), and thus the 177Lu isotope is usually preferred over 90Y. Cumulative and per-cycle renal uptake dose, age, presence of hypertension, diabetes mellitus and previous chemotherapy with nephrotoxic agents could potentially accelerate the decrease in renal function after PRRT. Considering these risk factors, one can modify the treatment plan or change the choice of radiopeptide based on the overall tumour burden (Rottenburger et al. 2020).
The use of a concomitant radiosensitising agent, such as low-dose oral capecitabine, with 177Lu-DOTATATE, might be also beneficial, as suggested by recent studies reporting up to an 86% disease control rate (Grossrubatscher et al. 2020, Satapathy et al. 2020). However, the only prospective, randomised study (CONTROL NETs), though non-comparative, of PRRT with radiosensitising chemotherapy (capecitabine/temozolomide) in metastatic midgut NETs showed a similar PFS rate at 15 months compared to PRRT alone (Pavlakis et al. 2020). The objective response rate was numerically higher in the combination arm but at the expense of greater G3/4 toxicity.
There is recently in vitro and in vivo evidence that somatostatin receptor subtype-2 (SSTR2) antagonists are better tools to target neuroendocrine tumours than SSTR agonists (Reubi et al. 2017). Using SSTR2 in vitro autoradiography on MTC tissue sections, Reubi et al. found that 125I-JR11 was associated with a significantly higher labelling intensity compared to the agonist 125I-Tyr3-octreotide. A small pilot study including four patients with advanced neuroendocrine tumours found that 177Lu-DOTA-JR11 delivers tumour doses that are approximately 3.5 times higher than those obtained with 177Lu-DOTATATE (Wild et al. 2014).
While meta-iodobenzylguanidine (MIBG) labelled with 123Iodine is most effectively used in diagnostic scintigraphy, radio-labelled 131I attached to MIBG is mainly used as a therapeutic, with uptake found in up to 35% MTC (Pasieka et al. 2004). Concerning the efficacy of 131I-MIBG treatment in MTC, data are limited to case reports and evidence from a prospective randomised trial is lacking (Mukherjee et al. 2001, Sze et al. 2013). This radionuclide may in general be more myelotoxic (Sze et al. 2013). CEA targeting using murine or chimeric anti-CEA bispecific antibodies and pre-targeted hapten-peptides labelled with 111In or 131I has been also developed for radioimmunotherapy in relapsed MTC (Bodet-Milin et al. 2016) (Table 2).
The cholecystokinin-2 receptor (CCK2R or gastrin receptor) is over-expressed in several tumour types, including MTC. CCK2R targeting shows promise for MTC imaging and represents, together with SSTR ligands, potential theranostic approaches in selected cases (Refardt et al. 2021). Many peptide-based radiopharmaceuticals for targeting CCK2R have been developed (Behr & Béhé 2002). In a recent study, the 177Lu-labelled minigastrin analogue 177Lu-PP-F11N showed promising results in six patients with advanced MTC (Rottenburger et al. 2020). Recently, a new highly metabolically stable minigastrin analogue (DOTA-MGS5) has shown excellent targeting properties in preclinical studies, supporting its possible translation into clinical trials (Klingler et al. 2019).
Other systemic or local therapies
Chemotherapy
The objective response rate of MTC patients to classical cytotoxic chemotherapy such as dacarbazine and 5-fluorouracil is 10–20%, with most studies performed on limited numbers of patients and without robust evaluation criteria such as RECIST (Hadoux & Schlumberger 2017). Long-term responses are uncommon.
External beam radiotherapy
External beam radiotherapy (EBRT) may be recommended to improve loco-regional control in patients at high risk of relapse in the neck. The usual indication for EBRT is macroscopically unresectable tumour in a patient older than 45 years of age. EBRT is indicated to improve the local control of disease in cases of local recurrence or loco-regional lymph node metastases, or as palliative therapy to reduce pain from bone metastases or to treat brain metastases (Wells et al. 2015). A few studies have shown an improvement in locoregional control but no survival benefit, confirming that although neck disease can be controlled in high-risk patients, the progression of distant disease is still a significant problem (Schwartz et al. 2008, Martinez et al. 2010, Brierley & Sherman 2012, Call et al. 2013, Tsoukalas et al. 2017). Localised EBRT and/or bisphosphonate administration should be considered for painful bone metastases or for the prevention of skeletal-related events (Farooki et al. 2012). Recommendations from a group with extensive clinical experience suggest quarterly zoledronic acid (Haugen et al. 2016). According to ATA guidelines for MTC, adjuvant EBRT can be considered in patients with a high risk of recurrence or with incompletely resected MTC (Haugen et al. 2016). However, EBRT was not associated with improved overall or disease-specific survival in a recent study of patients with MTC (Jin et al. 2021).
Embolisation or cryoablation
Embolisation or cryoablation as a palliative therapy may be beneficial in select cases to decrease tumour burden, pain or refractory diarrhoea associated with liver metastases (Fromigué et al. 2006). Radiofrequency thermo-ablation is frequently applicable to bone, liver and lung to treat single metastases or a single progressive and symptomatic lesion in the context of stable disease (Eisele 2016). Another local treatment is the conventional transarterial chemoembolisation (TACE) or radioembolisation (TARE), sometimes used in advanced cases of liver metastatic disease, especially when liver metastases are smaller than 3 cm and liver involvement is less than 30% (Roche et al. 2003).
Hormonal syndromes secondary to MTC
MTC has also been associated with the ectopic production of bioactive compounds, peptides or amines, leading to distinct clinical syndromes (Kaltsas et al. 2010). The most commonly described ectopic syndrome is ACTH leading to Cushing’s syndrome that in some cases can be severe. Other compounds include serotonin, somatostatin, pro-opiomelanocortin, vasoactive intestinal peptide, chromogranin A, amyloid, gastrin-releasing peptide and histamine (Wells et al. 2015). Patients with advanced MTC may experience debilitating diarrhoea and flushing due to hypersecretion of calcitonin, VIP, or increases in intestinal motility (Cox et al. 1979, Rambaud et al. 1988). Antimotility agents (loperamide or codeine) may be initially used for symptom relief. Further options for refractory cases include somatostatin analogues, although tachyphylaxis typically occurs over time (Zatelli et al. 2006). For patients with extensive liver metastases, surgical resection, percutaneous radiofrequency ablation or arterial (chemo)embolisation may be considered in an attempt to reduce the calcitonin levels (Fromigué et al. 2006).
Cushing’s syndrome due to ectopic ACTH secretion is found in 0.7% of MTC patients, accounting for 2.2–7.5% of all ectopic ACTH-Cushing’s syndrome cases (Barbosa et al. 2005). Fifty cases of MTC-related Cushing’s syndrome have been reported so far. In those cases, the tumour cells are positive for calcitonin but mostly display negative immunostaining for ACTH, presumably due to the high secretion rate (Shahani et al. 2010). Ectopic Cushing’s syndrome from MTC is associated with significant morbidity and mortality, as secondary complications of hypercortisolism account for 50% of the mortality in these patients (Hayes & Grossman 2018). In the past, management of hypercortisolism was limited to decreasing metastatic tumour burden and using anti-adrenal therapies such as ketoconazole, mitotane and/or metyrapone. Surgical intervention with bilateral adrenalectomy is another option available to some patients (Hayes & Grossman 2018). However, systemic therapy with tyrosine kinase inhibitors such as vandetanib offers a further management strategy for disease control of metastatic disease and might now be considered a first-line therapy for ectopic Cushing’s syndrome, following control of the excess cortisol, in the setting of unresectable disease or progressive, metastatic disease, as well as for controlling secretory Cushing’s syndrome (Wells et al. 2012, Paepegaey et al. 2017).
Future perspectives
In a subset of MTC, octreotide and pasireotide inhibit cell proliferation and invasiveness, especially when the expression of SSRT2 and SSRT5 is high, supporting their potential use in the control of tumour growth. On the contrary, MTC with high SSRT3 expression was unresponsive to both octreotide and pasireotide (Zatelli et al. 2006). Calcitonin secretion was not affected by octreotide or by pasireotide in primary cultured MTC cells, regardless of their responsiveness to the SSA’s anti-proliferative effects. These data confirm a dissociation between the anti-mitotic and anti-secretory effects of SSAs in primary cultured MTC cells (Zatelli et al. 2006).
Activating transcription factor 4 (ATF4) is a negative regulator of the RET tyrosine kinase receptor in MTC. Low ATF4 protein or ATF4 loss alone had a significant negative impact on median survival compared to high protein expression or diploid ATF4. The combination of somatic RET expression and low ATF4 protein levels further decreased overall survival. These data suggest that ATF4 may predict response to tyrosine kinase inhibitors, serve as a prognostic marker for personalised care and represent a therapeutic target in MTC (Williams et al. 2021). The small-molecule ONC201 caused apoptotic MTC cell death, decreased transcription of RET, VEGFR2, IGFBP2 and increased mRNA levels of ATF4 representing a promising strategy for the treatment of all patients with MTC, including those with TKI-refractory disease and other cancers with RET abnormalities (Bagheri-Yarmand et al. 2021).
Many novel therapeutic strategies are being pursued to develop personalised treatment approaches in MTC. Inhibitors of alternative targets and pathways, such as aurora kinase inhibitors, mTOR inhibitors, farnesyltransferase inhibitors and exploiting microRNA levels, seem promising (Gild et al. 2013, Joo et al. 2019). The potential for administering these drugs in a neoadjuvant setting to facilitate surgery and aiming for surgical cure are also on the horizon (Cabanillas et al. 2018, Wang et al. 2019). Recently, new targets have been highlighted, such as the glucose-dependent insulinotropic polypeptide receptor (GIPR) (Gourni et al. 2014) and minigastrin, a ligand targeting cholecystokinin-2 (CCK2) receptors, as noted previously. Ongoing studies (under recruitment or active) are presented in Table 2.
Conclusions
The introduction of the multikinase inhibitors, together with the recent more selective RET inhibitors, has opened a new phase in our treatment of these rare but fascinating tumours. While current response durations are relatively limited, they do suggest the prospect of a gradual improvement in therapies over time, with increasing durability as our understanding of the molecular biology develops. Nevertheless, their efficacy should be further investigated by larger trials with robust methods. In time, we would anticipate a more personalised therapeutic stratagem based on individualised patient tumour profiling and selective signalling pathway targeting. Treatments reducing tumour bulk, such as additional surgery and (chemo)embolisation, as well as external beam radiotherapy, are also utilised, but, generally, the results are not especially encouraging and are best considered as palliative therapies to control symptoms.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- Alfano L, Guida T, Provitera L, Vecchio G, Billaud M, Santoro M, Carlomagno F.2010RET is a heat shock protein 90 (HSP90) client protein and is knocked down upon HSP90 pharmacological block. Journal of Clinical Endocrinology and Metabolism 953552–3557. ( 10.1210/jc.2009-2315) [DOI] [PubMed] [Google Scholar]
- Aristizabal Prada ET, Spöttl G, Maurer J, Lauseker M, Koziolek EJ, Schrader J, Grossman A, Pacak K, Beuschlein F, Auernhammer CJet al. 2018The role of GSK3 and its reversal with GSK3 antagonism in everolimus resistance. Endocrine-Related Cancer 25893–908. ( 10.1530/ERC-18-0159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri-Yarmand R, Dadu R, Ye L, Shiny Jebaraj Y, Martinez JA, Ma J, Tarapore RS, Allen JE, Sherman SI, Williams MDet al. 2021ONC201 shows potent anticancer activity against medullary thyroid cancer via transcriptional inhibition of RET, VEGFR2, and IGFBP2. Molecular Cancer Therapeutics 20665–675. ( 10.1158/1535-7163.MCT-20-0386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Li JY, Li J, Zhang B, Liu YH, Zhang BY, Jing J.2019Risk of venous and arterial thromboembolic events associated with tyrosine kinase inhibitors in advanced thyroid cancer: a meta-analysis and systematic review. Oncotarget 104205–4212. ( 10.18632/oncotarget.24599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Niu D, Yao Q, Lin D, Kakudo K.2020Updates in the advances of sporadic medullary thyroid carcinoma: from the molecules to the clinic. Gland Surgery 91847–1856. ( 10.21037/gs-2019-catp-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet J, Campion L, Kraeber-Bodéré F, Chatal JF. & GTE Study Group 2005Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 906077–6084. ( 10.1210/jc.2005-0044) [DOI] [PubMed] [Google Scholar]
- Barbosa SL-S, Rodien P, Leboulleux S, Niccoli-Sire P, Kraimps JL, Caron P, Archambeaud-Mouveroux F, Conte-Devolx B, Rohmer V. & Groupe d’Etude des Tumeurs Endocrines 2005Ectopic adrenocorticotropic hormone-syndrome in medullary carcinoma of the thyroid: a retrospective analysis and review of the literature. Thyroid 15618–623. ( 10.1089/thy.2005.15.618) [DOI] [PubMed] [Google Scholar]
- Behr TM, Béhé MP.2002Cholecystokinin-B/gastrin receptor-targeting peptides for staging and therapy of medullary thyroid cancer and other cholecystokinin-B receptor-expressing malignancies. Seminars in Nuclear Medicine 3297–109. ( 10.1053/snuc.2002.31028) [DOI] [PubMed] [Google Scholar]
- Beukhof CM, Brabander T, van Nederveen FH, van Velthuysen M-LF, de Rijke YB, Hofland LJ, Franssen GJH, Fröberg LAC, Kam BLR, Visser WEet al. 2019Peptide receptor radionuclide therapy in patients with medullary thyroid carcinoma: predictors and pitfalls. BMC Cancer 19 325. ( 10.1186/s12885-019-5540-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Ren X, Bai X, Meng Y, Luo Y, Cao J, Zhang Y, Liang Z.2019PD-1/PD-L1 expressions in medullary thyroid carcinoma: clinicopathologic and prognostic analysis of Chinese population. European Journal of Surgical Oncology 45353–358. ( 10.1016/j.ejso.2018.10.060) [DOI] [PubMed] [Google Scholar]
- Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Karlin N, Sideras K, Morris JCet al. 2014A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. Journal of Clinical Endocrinology and Metabolism 991687–1693. ( 10.1210/jc.2013-3713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodet-Milin C, Faivre-Chauvet A, Carlier T, Rauscher A, Bourgeois M, Cerato E, Rohmer V, Couturier O, Drui D, Goldenberg DMet al. 2016Immuno-PET using anticarcinoembryonic antigen bispecific antibody and 68 Ga-labeled peptide in metastatic medullary thyroid carcinoma: clinical optimization of the pretargeting parameters in a first-in-human trial. Journal of Nuclear Medicine 571505–1511. ( 10.2967/jnumed.116.172221) [DOI] [PubMed] [Google Scholar]
- Bongiovanni M, Rebecchini C, Saglietti C, Bulliard JL, Marino L, de Leval L, Sykiotis GP.2017Very low expression of PD-L1 in medullary thyroid carcinoma. Endocrine-Related Cancer 24L35–L38. ( 10.1530/ERC-17-0104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, van Eijck CHJ, Franssen GJH, Krenning EP, Kwekkeboom DJ.2017Long-term efficacy, survival, and safety of [177Lu-DOTA 0,Tyr 3] octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clinical Cancer Research 234617–4624. ( 10.1158/1078-0432.CCR-16-2743) [DOI] [PubMed] [Google Scholar]
- Brierley J, Sherman E.2012The role of external beam radiation and targeted therapy in thyroid cancer. Seminars in Radiation Oncology 22254–262. ( 10.1016/j.semradonc.2012.03.010) [DOI] [PubMed] [Google Scholar]
- Cabanillas ME, Takahashi S.2019Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. Seminars in Oncology 4657–64. ( 10.1053/j.seminoncol.2018.11.004) [DOI] [PubMed] [Google Scholar]
- Cabanillas ME, Ferrarotto R, Garden AS, Ahmed S, Busaidy NL, Dadu R, Williams MD, Skinner H, Gunn GB, Grosu Het al. 2018Neoadjuvant BRAF- and immune-directed therapy for anaplastic thyroid carcinoma. Thyroid 28945–951. ( 10.1089/thy.2018.0060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call JA, Caudill JS, McIver B, Foote RL.2013A role for radiotherapy in the management of advanced medullary thyroid carcinoma: the Mayo Clinic experience. Rare Tumors 5e37. ( 10.4081/rt.2013.e37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlomagno F, Guida T, Anaganti S, Provitera L, Kjaer S, McDonald NQ, Ryan AJ, Santoro M.2009Identification of tyrosine 806 as a molecular determinant of RET kinase sensitivity to ZD6474. Endocrine-Related Cancer 16233–241. ( 10.1677/ERC-08-0213) [DOI] [PubMed] [Google Scholar]
- Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, Bauman JE, Martins RG.2010Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clinical Cancer Research 165260–5268. ( 10.1158/1078-0432.CCR-10-0994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carra S, Gaudenzi G, Dicitore A, Saronni D, Cantone MC, Plebani A, Ghilardi A, Borghi MO, Hofland LJ, Persani Let al. 2021Vandetanib versus cabozantinib in medullary thyroid carcinoma: a focus on anti-angiogenic effects in zebrafish model. International Journal of Molecular Sciences 22 3031. ( 10.3390/ijms22063031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castroneves LA, Coura Filho G, de Freitas RMC, Salles R, Moyses RA, Lopez RVM, Pereira MAA, Tavares MR, Jorge AAL, Buchpiguel CAet al. 2018Comparison of 68Ga PET/CT to other imaging studies in medullary thyroid cancer: superiority in detecting bone metastases. Journal of Clinical Endocrinology and Metabolism 1033250–3259. ( 10.1210/jc.2018-00193) [DOI] [PubMed] [Google Scholar]
- Ceolin L, Duval MADS, Benini AF, Ferreira CV, Maia AL.2019Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocrine-Related Cancer 26R499–R518. ( 10.1530/ERC-18-0574) [DOI] [PubMed] [Google Scholar]
- Chae YK, Chiec L, Adney SK, Waitzman J, Costa R, Carneiro B, Matsangou M, Agulnik M, Kopp P, Giles F.2018Posterior reversible encephalopathy syndrome and takotsubo cardiomyopathy associated with lenvatinib therapy for thyroid cancer: a case report and review. Oncotarget 928281–28289. ( 10.18632/oncotarget.25606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiacchiarini M, Trocchianesi S, Besharat ZM, Po A, Ferretti E.2021Role of tissue and circulating microRNAs and DNA as biomarkers in medullary thyroid cancer. Pharmacology and Therapeutics 219107708. ( 10.1016/j.pharmthera.2020.107708) [DOI] [PubMed] [Google Scholar]
- Cohen EEW, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott Pet al. 2008Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. Journal of Clinical Oncology 264708–4713. ( 10.1200/JCO.2007.15.9566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EEW, Tortorici M, Kim S, Ingrosso A, Pithavala YK, Bycott P.2014A phase II trial of axitinib in patients with various histologic subtypes of advanced thyroid cancer: long-term outcomes and pharmacokinetic/pharmacodynamic analyses. Cancer Chemotherapy and Pharmacology 741261–1270. ( 10.1007/s00280-014-2604-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TM, Fagan EA, Hillyard CJ, Allison DJ, Chadwick VS.1979Role of calcitonin in diarrhoea associated with medullary carcinoma of the thyroid. Gut 20629–633. ( 10.1136/gut.20.7.629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castroneves LA, Negrão MV, de Freitas RMC, Papadia C, Lima JV, Fukushima JT, Simão EF, Kulcsar MAV, Tavares MR, Jorge AAde Let al. 2016Sorafenib for the treatment of progressive metastatic medullary thyroid cancer: efficacy and safety analysis. Thyroid 26414–419. ( 10.1089/thy.2015.0334) [DOI] [PubMed] [Google Scholar]
- de Groot JWB, Zonnenberg BA, van Ufford-Mannesse PQ, de Vries MM, Links TP, Lips CJM, Voest EE.2007A phase II trial of imatinib therapy for metastatic medullary thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 923466–3469. ( 10.1210/jc.2007-0649) [DOI] [PubMed] [Google Scholar]
- Del Rivero J, Donahue RN, Marté JL, Gramza AW, Bilusic M, Rauckhorst M, Cordes L, Merino MJ, Dahut WL, Schlom Jet al. 2020A case report of sequential use of a yeast-CEA therapeutic cancer vaccine and anti-PD-L1 inhibitor in metastatic medullary thyroid cancer. Frontiers in Endocrinology 11490. ( 10.3389/fendo.2020.00490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druce M, Chung TT, Grozinsky-Glasberg S, Gross DJ, Grossman AB.2012Preliminary report of the use of everolimus in a patient with progressive medullary thyroid carcinoma. Clinical Endocrinology 77154–155. ( 10.1111/j.1365-2265.2011.04296.x) [DOI] [PubMed] [Google Scholar]
- Dvorakova S, Vaclavikova E, Sykorova V, Vcelak J, Novak Z, Duskova J, Ryska A, Laco J, Cap J, Kodetova Det al. 2008Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinomas. Molecular and Cellular Endocrinology 28421–27. ( 10.1016/j.mce.2007.12.016) [DOI] [PubMed] [Google Scholar]
- Efstathiadou ZA, Tsentidis C, Bargiota A, Daraki V, Kotsa K, Ntali G, Papanastasiou L, Tigas S, Toulis K, Pazaitou-Panayiotou Ket al. 2021Benefits and limitations of TKIs in patients with medullary thyroid cancer: a systematic review and meta-analysis. European Thyroid Journal 10125–139. ( 10.1159/000509457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele RM.2016Advances in local ablation of malignant liver lesions. World Journal of Gastroenterology 22 3885–3891. ( 10.3748/wjg.v22.i15.3885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MCet al. 2013Cabozantinib in progressive medullary thyroid cancer. Journal of Clinical Oncology 313639–3646. ( 10.1200/JCO.2012.48.4659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooki A, Leung V, Tala H, Tuttle RM.2012Skeletal-related events due to bone metastases from differentiated thyroid cancer. Journal of Clinical Endocrinology and Metabolism 972433–2439. ( 10.1210/jc.2012-1169) [DOI] [PubMed] [Google Scholar]
- Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, Papotti MG, Berruti A.2019Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 301856–1883. ( 10.1093/annonc/mdz400) [DOI] [PubMed] [Google Scholar]
- Fox E, Widemann BC, Chuk MK, Marcus L, Aikin A, Whitcomb PO, Merino MJ, Lodish M, Dombi E, Steinberg SMet al. 2013Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clinical Cancer Research 194239–4248. ( 10.1158/1078-0432.CCR-13-0071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromigué J, De Baere T, Baudin E, Dromain C, Leboulleux S, Schlumberger M.2006Chemoembolization for liver metastases from medullary thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 912496–2499. ( 10.1210/jc.2005-2401) [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Latif A, Galsky MD.2014Safety and efficacy of addition of VEGFR and EGFR-family oral small-molecule tyrosine kinase inhibitors to cytotoxic chemotherapy in solid cancers: a systematic review and meta-analysis of randomized controlled trials. Cancer Treatment Reviews 40636–647. ( 10.1016/j.ctrv.2014.02.004) [DOI] [PubMed] [Google Scholar]
- Gild ML, Landa I, Ryder M, Ghossein RA, Knauf JA, Fagin JA.2013Targeting mTOR in RET mutant medullary and differentiated thyroid cancer cells. Endocrine-Related Cancer 20659–667. ( 10.1530/ERC-13-0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourni E, Waser B, Clerc P, Fourmy D, Reubi JC, Maecke HR.2014The glucose-dependent insulinotropic polypeptide receptor: a novel target for neuroendocrine tumor imaging-first preclinical studies. Journal of Nuclear Medicine 55976–982. ( 10.2967/jnumed.113.133744) [DOI] [PubMed] [Google Scholar]
- Grossrubatscher E, Fanciulli G, Pes L, Sesti F, Dolci C, de Cicco F, Colao A, Faggiano A. & NIKE Group 2020Advances in the management of medullary thyroid carcinoma: focus on peptide receptor radionuclide therapy. Journal of Clinical Medicine 9 3507. ( 10.3390/jcm9113507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozinsky-Glasberg S, Rubinfeld H, Nordenberg Y, Gorshtein A, Praiss M, Kendler E, Feinmesser R, Grossman AB, Shimon I.2010The rapamycin-derivative RAD001 (everolimus) inhibits cell viability and interacts with the Akt–mTOR–p70s6K pathway in human medullary thyroid carcinoma cells. Molecular and Cellular Endocrinology 31587–94. ( 10.1016/j.mce.2009.09.027) [DOI] [PubMed] [Google Scholar]
- Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, Dickson P, Duh QY, Ehya H, Goldner Wet al. 2018NCCN Guidelines Insights: thyroid carcinoma, version 2.2018. Journal of the National Comprehensive Cancer Network 161429–1440. ( 10.6004/jnccn.2018.0089) [DOI] [PubMed] [Google Scholar]
- Hadoux J, Schlumberger M.2017Chemotherapy and tyrosine-kinase inhibitors for medullary thyroid cancer. Best Practice and Research: Clinical Endocrinology and Metabolism 31335–347. ( 10.1016/j.beem.2017.04.009) [DOI] [PubMed] [Google Scholar]
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger Met al. 20162015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 261–133. ( 10.1089/thy.2015.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AR, Grossman AB.2018The ectopic adrenocorticotropic hormone syndrome: rarely easy, always challenging. Endocrinology and Metabolism Clinics of North America 47409–425. ( 10.1016/j.ecl.2018.01.005) [DOI] [PubMed] [Google Scholar]
- Hayes AR, Crawford A, Al Riyami K, Tang C, Bomanji J, Baldeweg SE, Wild D, Morganstein D, Harry A, Grozinsky-Glasberg Set al. 2021Metastatic medullary thyroid cancer: the role of 68Gallium-DOTA-Somatostatin analogue PET/CT and peptide receptor radionuclide therapy. Journal of Clinical Endocrinology and Metabolism 106e4903–e4916. ( 10.1210/clinem/dgab588) [DOI] [PubMed] [Google Scholar]
- Heilmann AM, Subbiah V, Wang K, Sun JX, Elvin JA, Chmielecki J, Sherman SI, Murthy R, Busaidy NL, Subbiah Iet al. 2016Comprehensive genomic profiling of clinically advanced medullary thyroid carcinoma. Oncology 90339–346. ( 10.1159/000445978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingenwerth M, Goetz M, Schmid KW, Theurer S.2020The mismatch repair system is not affected in medullary thyroid carcinoma independent of stromal desmoplasia or RET proto-oncogene mutation. Annals of Diagnostic Pathology 44151445. ( 10.1016/j.anndiagpath.2019.151445) [DOI] [PubMed] [Google Scholar]
- Iten F, Müller B, Schindler C, Rochlitz C, Oertli D, Mäcke HR, Müller-Brand J, Walter MA.2007Response to [90 yttrium-DOTA]-TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: a phase II clinical trial. Clinical Cancer Research 136696–6702. ( 10.1158/1078-0432.CCR-07-0935) [DOI] [PubMed] [Google Scholar]
- Jin M, Megwalu UC, Noel JE.2021External beam radiotherapy for medullary thyroid cancer following total or near-total thyroidectomy. Otolaryngology: Head and Neck Surgery 16497–103. ( 10.1177/0194599820947696) [DOI] [PubMed] [Google Scholar]
- Joo LJS, Weiss J, Gill AJ, Clifton-Bligh R, Brahmbhatt H, MacDiarmid JA, Gild ML, Robinson BG, Zhao JT, Sidhu SB.2019RET kinase-regulated microRNA-153-3p improves therapeutic efficacy in medullary thyroid carcinoma. Thyroid 29830–844. ( 10.1089/thy.2018.0525) [DOI] [PubMed] [Google Scholar]
- Jung KY, Kim SM, Yoo WS, Kim BW, Lee YS, Kim KW, Lee KE, Jeong JJ, Nam KH, Lee SHet al. 2016Postoperative biochemical remission of serum calcitonin is the best predictive factor for recurrence-free survival of medullary thyroid cancer: a large-scale retrospective analysis over 30 years. Clinical Endocrinology 84587–597. ( 10.1111/cen.12852) [DOI] [PubMed] [Google Scholar]
- Kaltsas G, Androulakis II, de Herder WW, Grossman AB.2010Paraneoplastic syndromes secondary to neuroendocrine tumours. Endocrine-Related Cancer 17R173–R193. ( 10.1677/ERC-10-0024) [DOI] [PubMed] [Google Scholar]
- Klingler M, Summer D, Rangger C, Haubner R, Foster J, Sosabowski J, Decristoforo C, Virgolini I, von Guggenberg E.2019DOTA-MGS5, a new cholecystokinin-2 receptor-targeting peptide analog with an optimized targeting profile for theranostic use. Journal of Nuclear Medicine 601010–1016. ( 10.2967/jnumed.118.221283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler VF, Adam P, Frank-Raue K, Raue F, Berg E, Hoster E, Allelein S, Schott M, Kroiss M, Spitzweg C.2021Real-world efficacy and safety of cabozantinib and vandetanib in advanced medullary thyroid cancer. Thyroid 31459–469. ( 10.1089/thy.2020.0206) [DOI] [PubMed] [Google Scholar]
- Kuo EJ, Sho S, Li N, Zanocco KA, Yeh MW, Livhits MJ.2018Risk factors associated With reoperation and disease-specific mortality in patients with medullary thyroid carcinoma. JAMA Surgery 153 52–59. ( 10.1001/jamasurg.2017.3555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laure Giraudet A, Al Ghulzan A, Aupérin A, Leboulleux S, Chehboun A, Troalen F, Dromain C, Lumbroso J, Baudin E, Schlumberger M.2008Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. European Journal of Endocrinology 158239–246. ( 10.1530/EJE-07-0667) [DOI] [PubMed] [Google Scholar]
- Li D, Chi Y, Chen X, Ge M, Zhang Y, Guo Z, Wang J, Chen J, Zhang J, Cheng Yet al. 2021Anlotinib in locally advanced or metastatic medullary thyroid carcinoma: a randomized, double-blind phase IIB trial. Clinical Cancer Research 273567–3575. ( 10.1158/1078-0432.CCR-20-2950) [DOI] [PubMed] [Google Scholar]
- Lim SM, Chang H, Yoon MJ, Hong YK, Kim H, Chung WY, Park CS, Nam KH, Kang SW, Kim MKet al. 2013A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Annals of Oncology 243089–3094. ( 10.1093/annonc/mdt379) [DOI] [PubMed] [Google Scholar]
- Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM.2017Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 317 1338–1348. ( 10.1001/jama.2017.2719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ, Gainor JF.2021An early look at selective RET inhibitor resistance: new challenges and opportunities. British Journal of Cancer 1241757–1758. ( 10.1038/s41416-021-01344-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CI, Whang EE, Lorch JH, Ruan DT.2012Autophagic activation potentiates the antiproliferative effects of tyrosine kinase inhibitors in medullary thyroid cancer. Surgery 1521142–1149. ( 10.1016/j.surg.2012.08.016) [DOI] [PubMed] [Google Scholar]
- Lin JJ, Liu SV, McCoach CE, Zhu VW, Tan AC, Yoda S, Peterson J, Do A, Prutisto-Chang K, Dagogo-Jack Iet al. 2020Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion-positive non-small-cell lung cancer. Annals of Oncology 311725–1733. ( 10.1016/j.annonc.2020.09.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopergolo A, Nicolini V, Favini E, Dal Bo L, Tortoreto M, Cominetti D, Folini M, Perego P, Castiglioni V, Scanziani Eet al. 2014Synergistic cooperation between sunitinib and cisplatin promotes apoptotic cell death in human medullary thyroid cancer. Journal of Clinical Endocrinology and Metabolism 99498–509. ( 10.1210/jc.2013-2574) [DOI] [PubMed] [Google Scholar]
- Lorch JH, Barletta JA, Nehs M, Uppaluri R, Alexander EK, Haddad RI, Hanna GJ, Margalit DN, Tishler RB, Schoenfeld JDet al. 2020A phase II study of nivolumab (N) plus ipilimumab (I) in radioidine refractory differentiated thyroid cancer (RAIR DTC) with exploratory cohorts in anaplastic (ATC) and medullary thyroid cancer (MTC). Journal of Clinical Oncology 386513–6513. ( 10.1200/JCO.2020.38.15_suppl.6513) [DOI] [Google Scholar]
- Maia AL, Siqueira DR, Kulcsar MAV, Tincani AJ, Mazeto GMFS, Maciel LMZ.2014Diagnosis, treatment, and follow-up of medullary thyroid carcinoma: recommendations by the Thyroid Department of the Brazilian Society of Endocrinology and Metabolism. Arquivos Brasileiros de Endocrinologia e Metabologia 58667–700. ( 10.1590/0004-2730000003427) [DOI] [PubMed] [Google Scholar]
- Martinez SR, Beal SH, Chen A, Chen SL, Schneider PD.2010Adjuvant external beam radiation for medullary thyroid carcinoma. Journal of Surgical Oncology 102175–178. ( 10.1002/jso.21557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee JJ, Kaltsas GA, Islam N, Plowman PN, Foley R, Hikmat J, Britton KE, Jenkins PJ, Chew SL, Monson JPet al. 2001Treatment of metastatic carcinoid tumours, phaeochromocytoma, paraganglioma and medullary carcinoma of the thyroid with 131 I-meta-iodobenzylguanidine (131 I-mIBG). Clinical Endocrinology 5547–60. ( 10.1046/j.1365-2265.2001.01309.x) [DOI] [PubMed] [Google Scholar]
- Naoum GE, Morkos M, Kim B, Arafat W.2018Novel targeted therapies and immunotherapy for advanced thyroid cancers. Molecular Cancer 17 51. ( 10.1186/s12943-018-0786-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network 2021NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma. Plymouth Meeting, PA, USA: Plymouth Meeting: (available at: (https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf) [Google Scholar]
- Paepegaey AC, Cochand-Priollet B, Louiset E, Sarfati PO, Alifano M, Burnichon N, Bienvenu-Perrard M, Lahlou N, Bricaire L, Groussin L.2017Long-term control of hypercortisolism by vandetanib in a case of medullary thyroid carcinoma with a somatic RET mutation. Thyroid 27587–590. ( 10.1089/thy.2016.0334) [DOI] [PubMed] [Google Scholar]
- Parghane RV, Naik C, Talole S, Desmukh A, Chaukar D, Banerjee S, Basu S.2020Clinical utility of 177 Lu‐DOTATATE PRRT in somatostatin receptor‐positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head and Neck 42401–416. ( 10.1002/hed.26024) [DOI] [PubMed] [Google Scholar]
- Pasieka JL, McEwan AJB, Rorstad O.2004The palliative role of 131I-MIBG and 111In-octreotide therapy in patients with metastatic progressive neuroendocrine neoplasms. Surgery 1361218–1226. ( 10.1016/j.surg.2004.06.050) [DOI] [PubMed] [Google Scholar]
- Pavlakis N, Ransom DT, Wyld D, Sjoquist KM, Asher R, Gebski V, Wilson K, Kiberu AD, Burge ME, Macdonald Wet al. 2020First results for Australasian Gastrointestinal Trials Group (AGITG) control net study: phase II study of 177Lu-octreotate peptide receptor radionuclide therapy (LuTate PRRT) +/- capecitabine, temozolomide (CAPTEM) for midgut neuroendocrine tumors (mNETs). Journal of Clinical Oncology 38604–604. ( 10.1200/JCO.2020.38.4_suppl.604) [DOI] [Google Scholar]
- Priya SR, Dravid CS, Digumarti R, Dandekar M.2017Targeted therapy for medullary thyroid cancer: a review. Frontiers in Oncology 7238. ( 10.3389/fonc.2017.00238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaud JC, Jian R, Flourie B, Hautefeuille M, Salmeron M, Thuillier F, Ruskone A, Florent C, Chaoui F, Bernier JJ.1988Pathophysiological study of diarrhoea in a patient with medullary thyroid carcinoma. Evidence against a secretory mechanism and for the role of shortened colonic transit time. Gut 29537–543. ( 10.1136/gut.29.4.537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle RW, Balentine CJ, Leverson GE, Havlena JA, Sippel RS, Schneider DF, Pitt SC.2017Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery 161137–146. ( 10.1016/j.surg.2016.04.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SN, Cabanillas ME.2018Navigating systemic therapy in advanced thyroid carcinoma: from standard of care to personalized therapy and beyond. Journal of the Endocrine Society 21109–1130. ( 10.1210/js.2018-00180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refardt J, Hofland J, Kwadwo A, Nicolas GP, Rottenburger C, Fani M, Wild D, Christ E.2021Theranostics in neuroendocrine tumors: an overview of current approaches and future challenges. Reviews in Endocrine and Metabolic Disorders 22581–594. ( 10.1007/s11154-020-09552-x) [DOI] [PubMed] [Google Scholar]
- Resteghini C, Cavalieri S, Galbiati D, Granata R, Alfieri S, Bergamini C, Bossi P, Licitra L, Locati LD.2017Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best Practice and Research: Clinical Endocrinology and Metabolism 31349–361. ( 10.1016/j.beem.2017.04.012) [DOI] [PubMed] [Google Scholar]
- Reubi JC, Waser B, Mäcke H, Rivier J.2017Highly increased 125 I-JR11 antagonist binding in vitro reveals novel indications for sst 2 targeting in human cancers. Journal of Nuclear Medicine 58300–306. ( 10.2967/jnumed.116.177733) [DOI] [PubMed] [Google Scholar]
- Roche A, Girish BV, de Baère T, Baudin E, Boige V, Elias D, Lasser P, Schlumberger M, Ducreux M.2003Trans-catheter arterial chemoembolization as first-line treatment for hepatic metastases from endocrine tumors. European Radiology 13136–140. ( 10.1007/s00330-002-1558-0) [DOI] [PubMed] [Google Scholar]
- Roman S, Lin R, Sosa JA.2006Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 1072134–2142. ( 10.1002/cncr.22244) [DOI] [PubMed] [Google Scholar]
- Rottenburger C, Nicolas GP, McDougall L, Kaul F, Cachovan M, Vija AH, Schibli R, Geistlich S, Schumann A, Rau Tet al. 2020Cholecystokinin 2 receptor agonist 177 Lu-PP-F11N for radionuclide therapy of medullary thyroid carcinoma: results of the lumed phase 0a study. Journal of Nuclear Medicine 61520–526. ( 10.2967/jnumed.119.233031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salavati A, Puranik A, Kulkarni HR, Budiawan H, Baum RP.2016Peptide receptor radionuclide therapy (PRRT) of medullary and nonmedullary thyroid cancer using radiolabeled somatostatin analogues. Seminars in Nuclear Medicine 46215–224. ( 10.1053/j.semnuclmed.2016.01.010) [DOI] [PubMed] [Google Scholar]
- Satapathy S, Mittal BR, Sood A, Verma R, Panda N.2020Efficacy and safety of concomitant 177Lu-DOTATATE and low-dose capecitabine in advanced medullary thyroid carcinoma: a single-centre experience. Nuclear Medicine Communications 41629–635. ( 10.1097/MNM.0000000000001205) [DOI] [PubMed] [Google Scholar]
- Schlumberger MJ, Elisei R, Bastholt L, Wirth LJ, Martins RG, Locati LD, Jarzab B, Pacini F, Daumerie C, Droz JPet al. 2009Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. Journal of Clinical Oncology 273794–3801. ( 10.1200/JCO.2008.18.7815) [DOI] [PubMed] [Google Scholar]
- Schlumberger M, Jarzab B, Cabanillas ME, Robinson B, Pacini F, Ball DW, McCaffrey J, Newbold K, Allison R, Martins RGet al. 2016A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer. Clinical Cancer Research 2244–53. ( 10.1158/1078-0432.CCR-15-1127) [DOI] [PubMed] [Google Scholar]
- Schlumberger M, Elisei R, Müller S, Schöffski P, Brose M, Shah M, Licitra L, Krajewska J, Kreissl MC, Niederle Bet al. 2017Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Annals of Oncology 282813–2819. ( 10.1093/annonc/mdx479) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider TC, de Wit D, Links TP, van Erp NP, van der Hoeven JJM, Gelderblom H, van Wezel T, van Eijk R, Morreau H, Guchelaar HJet al. 2015Beneficial effects of the mTOR inhibitor everolimus in patients with advanced medullary thyroid carcinoma: subgroup results of a phase II trial. International Journal of Endocrinology 2015348124. ( 10.1155/2015/348124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DL, Rana V, Shaw S, Yazbeck C, Ang KK, Morrison WH, Rosenthal DI, Hoff A, Evans DB, Clayman GLet al. 2008Postoperative radiotherapy for advanced medullary thyroid cancer-local disease control in the modern era. Head and Neck 30883–888. ( 10.1002/hed.20791) [DOI] [PubMed] [Google Scholar]
- Shahani S, Nudelman RJ, Nalini R, Kim HS, Samson SL.2010Ectopic corticotropin-releasing hormone (CRH) syndrome from metastatic small cell carcinoma: a case report and review of the literature. Diagnostic Pathology 5 56. ( 10.1186/1746-1596-5-56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, Zhao F, Ahmad R, Zhao J.2018Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. Journal of Hematology and Oncology 11 120. ( 10.1186/s13045-018-0664-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SI, Clary DO, Elisei R, Schlumberger MJ, Cohen EEW, Schöffski P, Wirth LJ, Mangeshkar M, Aftab DT, Brose MS.2016Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer 1223856–3864. ( 10.1002/cncr.30252) [DOI] [PubMed] [Google Scholar]
- Shi X, Yu PC, Lei BW, Li CW, Zhang Y, Tan LC, Shi RL, Wang J, Ma B, Xu WBet al. 2019Association between programmed death-ligand 1 expression and clinicopathological characteristics, structural recurrence, and biochemical recurrence/persistent disease in medullary thyroid carcinoma. Thyroid 291269–1278. ( 10.1089/thy.2019.0079) [DOI] [PubMed] [Google Scholar]
- Solomon BJ, Tan L, Lin JJ, Wong SQ, Hollizeck S, Ebata K, Tuch BB, Yoda S, Gainor JF, Sequist LVet al. 2020RET solvent front mutations mediate acquired resistance to selective RET inhibition in RET-driven malignancies. Journal of Thoracic Oncology 15541–549. ( 10.1016/j.jtho.2020.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonbol MB, Halfdanarson TR, Hilal T.2020Assessment of therapy-related myeloid neoplasms in patients with neuroendocrine tumors after peptide receptor radionuclide therapy: a systematic review. JAMA Oncology 6 1086–1092. ( 10.1001/jamaoncol.2020.0078) [DOI] [PubMed] [Google Scholar]
- Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler Set al. 2003Repeated observation of breast tumor subtypes in independent gene expression data sets. PNAS 1008418–8423. ( 10.1073/pnas.0932692100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, Hu W, Cao Q, Sheets MP, Wilson Det al. 2018aPrecision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discovery 8836–849. ( 10.1158/2159-8290.CD-18-0338) [DOI] [PubMed] [Google Scholar]
- Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, Wirth LJ, Stock S, Smith S, Lauriault Vet al. 2018bSelective RET kinase inhibition for patients with RET-altered cancers. Annals of Oncology 291869–1876. ( 10.1093/annonc/mdy137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah V, Shen T, Terzyan SS, Liu X, Hu X, Patel KP, Hu M, Cabanillas M, Behrang A, Meric-Bernstam Fet al. 2021Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Annals of Oncology 32261–268. ( 10.1016/j.annonc.2020.10.599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Du F, Gao M, Ji Q, Li Z, Zhang Y, Guo Z, Wang J, Chen X, Wang Jet al. 2018Anlotinib for the treatment of patients with locally advanced or metastatic medullary thyroid cancer. Thyroid 281455–1461. ( 10.1089/thy.2018.0022) [DOI] [PubMed] [Google Scholar]
- Sze WCC, Grossman AB, Goddard I, Amendra D, Shieh SCC, Plowman PN, Drake WM, Akker SA, Druce MR.2013Sequelae and survivorship in patients treated with 131I-MIBG therapy. British Journal of Cancer 109565–572. ( 10.1038/bjc.2013.365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccaliti A, Silvetti F, Palmonella G, Boscaro M.2011Genetic alterations in medullary thyroid cancer: diagnostic and prognostic markers. Current Genomics 12618–625. ( 10.2174/138920211798120835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Yet al. 2019A phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncology 15717–726. ( 10.2217/fon-2018-0557) [DOI] [PubMed] [Google Scholar]
- Tsoukalas N, Chatzellis E, Rontogianni D, Alexandraki KI, Boutzios G, Angelousi A, Kaltsas G.2017Pancreatic carcinoids (serotonin-producing pancreatic neuroendocrine neoplasms) report of 5 cases and review of the literature. Medicine 96e6201. ( 10.1097/MD.0000000000006201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle RM, Haddad RI, Ball DW, Byrd D, Dickson P, Duh QY, Ehya H, Haymart M, Hoh C, Hunt JPet al. 2014Thyroid carcinoma, version 2.2014. Journal of the National Comprehensive Cancer Network 121671–1680. ( 10.6004/jnccn.2014.0169) [DOI] [PubMed] [Google Scholar]
- Valea A, Georgescu CE.2016A perspective on the current medical approach of advanced medullary thyroid carcinoma. In Thyroid Cancer – Advances in Diagnosis and Therapy. London, UK: InTech. ( 10.5772/63650) [DOI] [Google Scholar]
- Viola D, Valerio L, Molinaro E, Agate L, Bottici V, Biagini A, Lorusso L, Cappagli V, Pieruzzi L, Giani Cet al. 2016Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocrine-Related Cancer 23R185–R205. ( 10.1530/ERC-15-0555) [DOI] [PubMed] [Google Scholar]
- Vitale G, Dicitore A, Pepe D, Gentilini D, Grassi ES, Borghi MO, Gelmini G, Cantone MC, Gaudenzi G, Misso Get al. 2017Synergistic activity of everolimus and 5-aza-2′-deoxycytidine in medullary thyroid carcinoma cell lines. Molecular Oncology 111007–1022. ( 10.1002/1878-0261.12070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HG, Ho ATN, Tran TTK, Capdevila J, Benekli M, Nakazawa T, Katoh R, Kondo T.2019Efficacy and toxicity of sorafenib in the treatment of advanced medullary thyroid carcinoma: a systematic review and meta‐analysis. Head and Neck 412823–2829. ( 10.1002/hed.25832) [DOI] [PubMed] [Google Scholar]
- Wang JR, Zafereo ME, Dadu R, Ferrarotto R, Busaidy NL, Lu C, Ahmed S, Gule-Monroe MK, Williams MD, Sturgis EMet al. 2019Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAF V600E – mutated anaplastic thyroid carcinoma. Thyroid 291036–1043. ( 10.1089/thy.2019.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HLet al. 2002ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Research 624645–4655. [PubMed] [Google Scholar]
- Wells SA, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JRet al. 2012Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. Journal of Clinical Oncology 30134–141. ( 10.1200/JCO.2011.35.5040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini Fet al. 2015Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25567–610. ( 10.1089/thy.2014.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild D, Fani M, Fischer R, Del Pozzo L, Kaul F, Krebs S, Fischer R, Rivier JEF, Reubi JC, Maecke HRet al. 2014Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. Journal of Nuclear Medicine 551248–1252. ( 10.2967/jnumed.114.138834) [DOI] [PubMed] [Google Scholar]
- Williams MD, Ma J, Grubbs EG, Gagel RF, Bagheri-Yarmand R.2021ATF4 loss of heterozygosity is associated with poor overall survival in medullary thyroid carcinoma. American Journal of Cancer Research 113227–3239. [PMC free article] [PubMed] [Google Scholar]
- Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, Worden F, Brose M, Patel J, Leboulleux Set al. 2020Efficacy of selpercatinib in RET – altered thyroid cancers. New England Journal of Medicine 383825–835. ( 10.1056/NEJMoa2005651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Nokihara H, Yamada Y, Shibata T, Tamura Y, Seki Y, Honda K, Tanabe Y, Wakui H, Tamura T.2017Phase I study of nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Investigational New Drugs 35207–216. ( 10.1007/s10637-016-0411-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatelli MC, Piccin D, Tagliati F, Bottoni A, Luchin A, Vignali C, Margutti A, Bondanelli M, Pansini GC, Pelizzo MRet al. 2006Selective activation of somatostatin receptor subtypes differentially modulates secretion and viability in human medullary thyroid carcinoma primary cultures: potential clinical perspectives. Journal of Clinical Endocrinology and Metabolism 912218–2224. ( 10.1210/jc.2006-0334) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a