Abstract
Objective
Type 1 diabetes and Hashimoto’s thyroiditis frequently cluster in individuals and in families, indicating shared origins. The objective of this study was to investigate familial co-aggregation of these diseases and to quantify shared genetic and environmental factors.
Design
This study is a twin cohort study.
Methods
National health registers were used to identify cases among 110 814 Swedish twins. Co-aggregation was calculated as risk ratios for type 1 diabetes among co-twins of individuals with Hashimoto’s thyroiditis, and vice-versa. Variance explained by genetics (i.e. heritability), and the proportions thereof shared between the diseases, was estimated by contrasting associations in monozygotic and dizygotic twins using structural equation models.
Results
Individuals with one disease were at a high risk for the other disease (adjusted risk ratio: 11.4 (95% CI: 8.5–15.3)). Co-aggregation was more common in monozygotic than in dizygotic pairs, with adjusted risk ratios of 7.0 (95% CI: 3.2–15.1) and 1.7 (95% CI: 0.7–4.1), respectively. Genetic effects shared across diseases accounted for 11% of the variance for type 1 diabetes and 9% of the variance for Hashimoto’s thyroiditis, while environmental factors unique to individual twins, but shared across diseases, accounted for 10% of the variance for type 1 diabetes and 18% of the variance for Hashimoto’s thyroiditis.
Conclusions
Both genes and environment unique to individual twins contribute to considerable etiologic overlap between type 1 diabetes and Hashimoto’s thyroiditis. These findings add to the current knowledge on the mechanisms behind autoimmune disease clustering and could guide future research aimed at identifying pathophysiological mechanisms and intervention targets.
Introduction
Type 1 diabetes and hypothyroidism due to Hashimoto’s thyroiditis are two of the most common autoimmune diseases (1, 2). Co-occurrence of both diseases in the same individual is common, with up to one in four patients with type 1 diabetes harboring autoantibodies predisposing to thyroid disease (3) and more than one in ten eventually developing overt Hashimoto’s thyroiditis (4). Vice versa, the risk of type 1 diabetes is also increased in patients with Hashimoto’s thyroiditis, most notably in early onset disease (5). The combination of type 1 diabetes and Hashimoto’s thyroiditis in the same patient is in fact the most common autoimmune polyendocrinopathy, sometimes referred to as a variant of the autoimmune polyglandular syndrome type 3 (APS3). Moreover, familial studies have repeatedly demonstrated that relatives of individuals with one disorder are at increased risk of the other disorder, further supporting a common origin (6, 7, 8).
Both type 1 diabetes and Hashimoto’s thyroiditis are complex autoimmune disorders, with multiple genetic and non-genetic factors contributing to disease. Twin studies have established that genetic components (i.e. heritability) explain most of the observed variance for both diseases (9, 10, 11), and molecular genetic studies have identified genetic polymorphisms linked to both disorders (12). Still, monozygotic twin pairs affected by either disease are usually discordant, with only one twin affected by the disease, underscoring the importance of environmental factors in triggering autoimmunity (13). Hence, the magnitude and make-up of the shared origin of these diseases are still largely unknown.
The aim of this study was to assess shared familial risk across type 1 diabetes and Hashimoto’s thyroiditis and to quantify common genetic and environmental origins of disease using a large, population-based cohort of twins.
Subjects and methods
Data sources and study population
The personal identity number, a unique identifier assigned to all Swedish residents (>99.9%) at birth or upon immigration, is used for all health care contacts. It thus allows for cross-register linkages with essentially perfect coverage (14).
The Swedish Twin Registry (STR) contains information on twins born in Sweden from the late 19th century onwards, with a coverage of approximately 95% for birth years relevant to this study. Zygosity has been determined according to a validated intra-pair similarity algorithm, DNA, and opposite sex, in approximately 90% of twin pairs born before 1959 and in 70–80% of most, but not all, later birth-cohorts (15). The study population encompassed all twins of known zygosity, born up until December 31, 2006, with information on both twins in a pair present in the STR. To maximize power, information on both same-sex and opposite-sex dizygotic twin pairs was included, and to improve coverage and diagnostic precision, both twins in a pair were required to be alive (or not yet born) in 1976 for inclusion. A total of 120 286 individuals from complete twin pairs were identified in the STR. Of these, 3966 were excluded because of unknown zygosity. A further 3453 individuals deceased before 1976 were excluded along with 2049 co-twins of deceased twins. Two twin pairs with ambiguous birth data were also excluded, yielding a final sample of 110 814 twins.
The National Diabetes Register, which holds data on nearly all individuals with diabetes in Sweden, was used to identify diagnoses of type 1 diabetes through 2015. Type 1 diabetes is defined in the register on the basis of treatment with insulin only and a diagnosis at age 30 years or younger, and has been validated as accurate, with a positive predictive value of 97% (16).
The National Patient Register (NPR) (17) and the Prescribed Drug Register (PDR) (18) were used to identify cases of overt Hashimoto’s thyroiditis. The NPR, found in 1964, holds inpatient discharge records, coded according to the International Statistical Classification of Diseases. The NPR reached nationwide coverage (>99%) in 1987. As of 2001, records for hospital-associated outpatient care are also included in the register. The PDR, in operation since 2005, collects data on all prescribed drugs dispensed in Sweden, coded according to the Anatomical Therapeutic Chemical Classification System (ATC).
In brief, Hashimoto’s thyroiditis was defined as an ICD diagnosis of Hashimoto’s thyroiditis, without records suggesting congenital, drug-induced, infectious, postprocedural, or iodine-deficient hypothyroidism. For patients alive in 2006, multiple (≥2) dispensations of levothyroxine (ATC H03AA) were required to validate an ICD diagnosis of Hashimoto’s thyroiditis. Importantly, in individuals with type 1 diabetes, multiple (≥2) dispensations of levothyroxine were considered sufficient for a diagnosis of Hashimoto’s thyroiditis in the absence of ICD diagnoses indicating other thyroid disorders, as Hashimoto’s thyroiditis is sometimes not ICD-coded when co-occurring with type 1 diabetes (diagnostic criteria are detailed in Supplementary Table 1, see section on supplementary materials given at the end of this article).
Statistical analysis
Type 1 diabetes and Hashimoto’s thyroiditis were defined as binary variables indicating the presence or absence of disease prior to end of follow-up. In a subanalysis, we also examined the APS3 phenotype.
Association and (co-)aggregation
Disease associations were estimated as risk ratios, that is the ratio of probabilities of Hashimoto’s thyroiditis among individuals with, compared with individuals without, type 1 diabetes. Familial aggregations were calculated as the risk of disease among individuals whose co-twin had the same disease compared with individuals whose co-twin did not have the same disease. We estimated familial co-aggregation as risk ratios for type 1 diabetes among individuals whose co-twin had Hashimoto’s thyroiditis compared to individuals whose co-twin did not have the disease (and vice versa). We estimated aggregation and co-aggregation separately by zygosity. All estimates were calculated as crude and adjusted for sex and birth cohort (<1940, 1940–1959, 1960–1979, and >1979). For these analyses, we used generalized estimating equations with log-link and standard errors clustered on twin pair ID.
Concordances and tetrachoric correlations
A twin pair is considered concordant affected for a trait when both twins are affected and discordant when only one twin is affected. A higher concordance in monozygotic than in dizygotic twins indicates a genetic contribution. Probandwise concordance rates were therefore calculated separately by zygosity to give an absolute measure of disease risk. We also calculated concordance rates across disorders, that is the proportion of individuals diagnosed with one disease whose co-twin had the other disease and vice versa.
Next, tetrachoric correlations, a measure of associations between dichotomous traits (e.g. disease or no disease), were estimated. For tetrachoric correlations, an un-observed normally distributed liability for the disease is assumed to exist. The disease becomes manifest when an individual exceeds a threshold on this assumed disease liability, and this threshold is estimated from the data. When assessing dichotomous disease in the two twins in a pair, the observed 2-by-2-table of disease vs no disease in one twin cross-tabulated with his/her co-twin is assumed to reflect a combination of the two normally distributed liabilities in the two twins (with the same thresholds as mentioned above). The distribution of concordance and discordance in the 2-by-2-table of disease across the twins allows the correlation between the liabilities to be inferred – this correlation is what is referred to as the tetrachoric correlation. A higher tetrachoric correlation in monozygotic than in dizygotic twin pairs is indicative of genetic influences on a trait. This measure is also the basis of subsequent calculations of heritability. We estimated tetrachoric correlations within individuals across disorders, referred to as phenotypic correlation, between twins in pairs within the same disorder, referred to as intra-class correlations (ICC), and between twins in pairs across disorders, referred to as cross-twin cross-trait correlations (CTCT). Tetrachoric correlations were estimated using structural equation models, unadjusted and adjusted for birth cohort and sex.
Quantitative genetic modeling
Quantitative genetic analyses were based on the classic twin assumptions that monozygotic twins are genetically identical, that dizygotic twins share on average half of their segregating alleles, and that monozygotic and dizygotic twins share environment to a similar degree. Bivariate quantitative genetic models were fitted in a structural equation modeling framework. In this model, the variance within disorders and covariance between disorders were modeled to result from additive genetic factors (A; the heritability), dominance deviations (D), shared environmental factors affecting both twins in a pair (C), and unique environmental factors not shared by twins (E). ACE (including A, C, and E), ADE, AE, and CE models were fitted by estimating A, D, C, and E contributions to each disorder, as well as the broad-sense heritability (H; the sum of A and D), when applicable. We also estimated the correlation between these sources of variance, that is the genetic correlation, rA, and rD, rC, and rE, as well as a broad sense genetic correlation, rH. Next, we calculated the inferred proportion of explained variance from A, D, C, and E, as well as H in type 1 diabetes shared with Hashimoto’s thyroiditis and vice versa. Model fit was compared using Akaike information criterion (AIC) and Bayesian information criterion (BIC), where a lower value indicates a better fitting model.
Subanalysis
We defined APS3 as present in individuals diagnosed with both type 1 diabetes and Hashimoto’s thyroiditis and absent in all other individuals. We estimated concordance rates and tetrachoric correlations and fitted quantitative genetic models for this variable.
For statistical analyses, we used R (R-Development-Core-Team, 2010) with packages drgee (19), and OpenMx (20).
This study was approved by the Regional Ethical Review Board in Stockholm, Sweden (Dnr: 2017/1546-32). Informed consent was waived by the ethics committee.
Results
Descriptive
Overall, 364 individuals (179 women, 185 men) had type 1 diabetes and 1683 individuals (1410 women, 273 men) fulfilled the diagnostic criteria for Hashimoto’s thyroiditis. Co-occurrence of both diseases was found in 48 individuals (37 females, 11 males), with 13.2% (48/364) of patients with type 1 diabetes also having Hashimoto’s thyroiditis, and 2.8% (48/1683) of patients with Hashimoto’s thyroiditis also diagnosed with type 1 diabetes. Disease prevalence according to year of birth, sex, and zygosity is presented in Table 1. Age at diagnosis and year of diagnosis are outlined in Supplementary Fig. 1.
Table 1.
Descriptive, number of individuals, and column percent. Data are presented as n (%).
| Total (%) | T1D (%)a | HT (%)a | T1D and HT (%)a | |
|---|---|---|---|---|
| Total | 110 814 (100.0) | 364 (0.3) | 1,683 (1.5) | 48 (<0.1) |
| Sex | ||||
| Males | 52 171 (47.1) | 185 (50.8) | 273 (16.2) | 11 (22.9) |
| Females | 58 643 (52.9) | 179 (49.2) | 1,410 (83.8) | 37 (77.1) |
| Birth year | ||||
| <1940 | 30 190 (27.2) | 27 (7.4) | 843 (50.1) | 3 (6.2) |
| 1940–1959 | 28 948 (26.1) | 118 (32.4) | 509 (30.2) | 13 (27.1) |
| 1960–1979 | 14 082 (12.7) | 101 (27.7) | 189 (11.2) | 17 (35.4) |
| >1979 | 37 594 (33.9) | 118 (32.4) | 142 (8.4) | 15 (31.2) |
| Zygosity | ||||
| Monozygotic | 35 990 (32.5) | 116 (31.9) | 553 (32.9) | 22 (45.8) |
| Dizygotic | 74 824 (67.5) | 248 (68.1) | 1,130 (67.1) | 26 (54.2) |
aCategories not mutually exclusive.
HT, Hashimoto’s thyroiditis; T1D, type 1 diabetes.
Association and (co-)aggregation
Results for association and (co-)aggregation are presented in Table 2. The adjusted risk ratio (aRR) for co-occurrence of both diseases (within individual association) was 11.4 (95% CI: 8.5–15.3). Disease aggregation (same disease in co-twins) was present in both monozygotic and dizygotic twins, with risk ratios higher for type 1 diabetes than for Hashimoto’s thyroiditis, possibly reflecting a lower population prevalence for type 1 diabetes. The highest relative risk was seen in monozygotic pairs, with aRR 131.3 (95% CI: 80.1–215.2) for type 1 diabetes and 14.5 (95% CI: 11.1–18.9) for Hashimoto’s thyroiditis, but aggregation was also statistically significant in dizygotic twin pairs (type 1 diabetes aRR: 11.8 (95% CI: 5.3–26.4); Hashimoto’s thyroiditis aRR 4.9 (95% CI: 3.8–6.4)). Co-aggregation, defined as risk ratios for Hashimoto’s thyroiditis among individuals whose co-twin had type 1 diabetes, was stronger in monozygotic (aRR: 7.0 (95% CI: 3.2–15.1)) than dizygotic twin pairs (aRR: 1.7 (95% CI: 0.7–4.1)). Results were similar when switching diseases in terms of exposure and outcome (Supplementary Table 2).
Table 2.
Association, familial aggregation, and familial co-aggregation of type 1 diabetes and Hashimoto’s thyroiditis in twins.
| RR (95% CI) | aRRa (95% CI) | |
|---|---|---|
| Within disorders | ||
| Risk of T1D when co-twin has T1D | ||
| Monozygotic twins | 175.5 (110.6–278.6) | 131.3 (80.1–215.2) |
| Dizygotic twins | 15.3 (6.9–34.1) | 11.8 (5.3–26.4) |
| Risk of HT when co-twin has HT | ||
| Monozygotic twins | 26.6 (21.1–33.4) | 14.5 (11.1–18.9) |
| Dizygotic twins | 7.3 (5.6–9.5) | 4.9 (3.8–6.4) |
| Between disordersb | ||
| Risk of HT in individuals with T1D | 8.9 (6.7–11.9) | 11.4 (8.5–15.3) |
| Risk of HT when co-twin has T1D | ||
| Monozygotic twins | 6.3 (3.0–13.0) | 7.0 (3.2–15.1) |
| Dizygotic twins | 1.3 (0.6–3.2) | 1.7 (0.7–4.1) |
aAdjusted for sex and birth cohort; bT1D was considered exposure and HT outcome. Results from analyses using HT as exposure and T1D as outcome are found in Supplementary Table 2.
HT, Hashimoto’s thyroiditis; T1D, type 1 diabetes;.
Concordances and tetrachoric correlations
Type 1 diabetes was present in 337 twin pairs and Hashimoto’s thyroiditis in 1545 pairs. 27 pairs were concordant for type 1 diabetes and 138 for Hashimoto’s thyroiditis with 16 twin pairs demonstrating cross-twin-cross-trait concordance (different diseases in co-twins). For all disease combinations, concordance rates were higher in monozygotic than in dizygotic pairs, resulting in higher tetrachoric correlations (Table 3).
Table 3.
Concordances, discordances, and tetrachoric correlations for type 1 diabetes and Hashimoto’s thyroiditis.
| Within disorders | |||||
| Concordant non-affected | Discordant | Concordant affected | Concordance rate | Tetrachoric correlationsa, adjustedb | |
| Type 1 diabetes | |||||
| MZ pairs | 17 900 | 74 | 21 | 0.36 (0.27–0.49) | 0.82 (0.75–0.89) |
| DZ pairs | 37 170 | 236 | 6 | 0.05 (0.02–0.10) | 0.37 (0.23–0.51) |
| Hashimoto’s thyroiditis | |||||
| MZ pairs | 17 523 | 391 | 81 | 0.29 (0.25–0.35) | 0.66 (0.61–0.71) |
| DZ pairs | 36 339 | 1016 | 57 | 0.10 (0.08–0.13) | 0.35 (0.29–0.42) |
| From type 1 diabetes to Hashimoto’s thyroiditis | |||||
| Neither disease | T1D only | T1D and HT | Proportion with T1D and HT | Tetrachoric correlationsa, adjustedb | |
| Individuals | 108 815 | 316 | 48 | 0.13 (0.10–0.17) | 0.45 (0.39–0.51) |
| MZ pairs | 35 332 | 105 | 11 | 0.09 (0.05–0.20) | 0.28 (0.17–0.39) |
| DZ pairs | 243 | 5 | 0.02 (0.01–0.05) | 0.08 (-0.03–0.20) | |
| From Hashimoto’s thyroiditis to type 1 diabetes | |||||
| Neither disease | HT only | T1D and HT | Proportion with T1D and HT | Tetrachoric correlationsa, adjustedb | |
| Individuals | 108 815 | 1635 | 48 | 0.03 (0.02–0.04) | 0.45 (0.39–0.51) |
| MZ pairs | 35 332 | 542 | 11 | 0.02 (0.01–0.04) | 0.28 (0.17–0.39) |
| DZ pairs | 73 451 | 1125 | 5 | 0.00 (0.00–0.01) | 0.08 (-0.03–0.20) |
aFor tetrachoric correlations across T1D and HT, the correlation is the phenotypic correlation, and no directionality exists, i.e. estimates are exactly the same in ‘From T1D to HT’ and ‘From HT to T1D’ sections; bAdjusted for sex and birth cohort.
HT, Hashimoto’s thyroiditis; T1D, type 1 diabetes;.
Quantitative genetic modelling
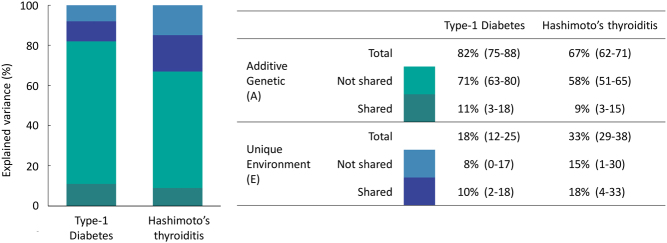
Overall, the AE model, with components of variance attributed to additive genetic factors (A) and unique environmental factors (E), provided the best fit according to both AIC and BIC, thus we present results from the adjusted AE model (Table 4), with results of the full models detailed in Supplementary Tables 3 and 4. Additive genetic factors accounted for 82% (95% CI: 75–88) of variance for type 1 diabetes and 67% (95% CI: 62–71) of variance for Hashimoto’s thyroiditis. The phenotypic correlation between the two diseases was 0.45 (95% CI: 0.39–0.50), with additive genetic sources (bivariate heritability) accounting for 59% (95% CI: 44–77) of the covariation and unique environment explaining 41% (95% CI: 23–59). The additive genetic (rA) and unique environmental correlations (rE) were 0.36 (95% CI: 0.23–0.48) and 0.74 (95% CI: 0.45–1.00), respectively (Table 4). Additive genetic factors shared between disorders explained 11% (95% CI, 3–18) of the variation in type 1 diabetes and 9% (95% CI, 3–15) in Hashimoto’s thyroiditis. The corresponding values for environmental factors shared between diseases but not between individuals was 10% (95% CI: 2–18) for type 1 diabetes and 18% (95% CI: 4–33) for Hashimoto’s thyroiditis (Fig. 1).
Table 4.
Adjusted results of the best fitting AE-modela.
| Univariate estimates | |
| Type 1 diabetes | |
| A | 82% (75–88) |
| E | 18% (12–25) |
| Hashimoto’s thyroiditis | |
| A | 67% (62–71) |
| E | 33% (29–38) |
| Bivariate estimates | |
| Phenotypic correlation | 0.45 (0.39–0.50) |
| rA | 0.36 (0.23–0.48) |
| rH | 0.36 (0.23–0.48) |
| rE | 0.74 (0.45–1.00) |
| Bivariate A | 0.59 (0.41–0.77) |
| Bivariate H | 0.59 (0.41–0.77) |
| Bivariate E | 0.41 (0.23–0.59) |
A, variance explained by additive genetics/narrow sense heritability; E, variance explained by individually unique environment; rA, correlation between additive genetic variance components for type 1 diabetes and Hashimoto’s thyroiditis (similar for rE); Bivariate A, explained phenotypic correlation by A sources of (co)variance (similar for Bivariate E). CIs are of Wald-type, thus they may span outside the parameter space but have been truncated at the parameter bound; aAdjusted for sex and birth cohort.
Figure 1.
A, additive genetic effects. E, environmental effects not shared by co-twins. A is equivalent to heritability. Estimates adjusted for sex and birth cohort.
Subanalysis
The APS3 phenotype was rare with a total of 48 twins (37 women, 11 men) fulfilling diagnostic criteria. All 26 dizygotic pairs were discordant for APS3. Among 18 monozygotic pairs affected, 4 were concordant and 14 discordant, yielding a monozygotic concordance rate of 0.36 (0.18–0.74) and an estimated heritability of 0.85 (0.71–0.98) according to the preferred AE-model (Supplementary Tables 5 and 6).
Conclusions
In this population-based study of Swedish twins, we found evidence of considerable etiologic overlap between type 1 diabetes and Hashimoto’s thyroiditis. Genetic effects were correlated across the diseases, with 11% (type 1 diabetes) and 9% (Hashimoto’s thyroiditis) of variance in each disease explained by additive genetic effects common to both disorders. Additionally, environmental factors unique to individual twins, but shared across diseases, accounted for 10% (type 1 diabetes) and 18% (Hashimoto’s thyroiditis) of variance. To the best of our knowledge, this is the first study to quantify shared etiologic fractions contributing to these disorders.
Co-occurrence of type 1 diabetes and Hashimoto’s thyroiditis was common among individual twins (aRR 11.4), and the prevalence of Hashimoto’s thyroiditis among patients diagnosed with type 1 diabetes was high at 13.2%, consistent with a prevalence of 9.8% reported in a recent meta-analysis (4). Conversely, type 1 diabetes was slightly more prevalent among individuals with Hashimoto’s thyroiditis (2.9%) than estimates of around 1% based on other European cohorts (8, 21). In line with previous studies, additive genetic factors (i.e. heritability) accounted for most of the phenotypic variance in both disorders, with unique environments explaining a smaller proportion (9, 10, 11, 22). Co-aggregation of diseases was also apparent, albeit statistically significant only among monozygotic twin pairs. This was reflected by a phenotypic correlation of 0.45, with substantial genetic correlation (0.36) and unique environment correlation (0.74) across diseases. In fact, the genetic coherence across diseases was similar in magnitude to the genetic overlap between Hashimoto’s thyroiditis and Graves’ disease, with 11% of variance across autoimmune thyroid diseases explained by common gene variants (22).
Several genetic polymorphisms have been implicated in both type 1 diabetes and Hashimoto’s thyroiditis (23), but results are inconsistent across different ethnicities, and varying definitions or thyroid autoimmunity, or lumping of Hashimoto’s thyroiditis and Graves’ disease into autoimmune thyroid disease, complicate interpretations (24). Focusing on genetic liability for type 1 diabetes and either overt Hashimoto’s thyroiditis or autoantibodies associated with Hashimoto’s thyroiditis in Caucasian populations narrows the list to only a few gene variants. These include HLA-DR3 and DR4 in association with DQ2 and DQ8 (25) and alleles of PTPN22 (24, 26, 27) and CTLA-4 (28, 29). Collectively, the identified polymorphisms explain only a small part of the genetic overlap between type-1 diabetes and Hashimoto’s thyroiditis reported in this study, suggesting other shared loci remain to be identified. There is also some evidence to support that APS3 represents a genetically distinct phenotype, with polymorphisms conferring risk for APS3 alone but not for isolated disease (30, 31). The APS3-variant was uncommon among our twins but demonstrated a high heritability of 85%, in line with estimates for organ-specific (single) autoimmune diseases (32).
The influence of unique environmental factors accounted for modest proportions of variance for type 1 diabetes and Hashimoto’s thyroiditis. Nevertheless, the environmental overlap was large, with 10–18% of the variance attributable to factors shared across the disorders but unique to individuals. Our findings contrast with results from a co-twin control analysis by Wang et al. (33) that did not find evidence of association between type 1 diabetes and thyroid peroxidase autoantibodies (TPOab) over and above shared factors in twin pairs (i.e. shared genetic and environmental influences). This may reflect differences in the etiology of TPOab vs overt HT, underlying differences between the examined populations or insufficient power to detect an association in the study by Wang due to a smaller twin sample.
In light of the dramatic increase in the incidence of autoimmune diseases in recent decades, including type 1 diabetes and Hashimoto’s thyroiditis (34, 35), environmental overlap should perhaps not come as a surprise. Rapid changes in disease incidences typically reflect environmental rather than genetic effects, and emergence of some environmental triggers shared across several autoimmune diseases, rather than a multitude of triggers unique to individual disorders, seems reasonable.
The hygiene hypothesis, stating that a lack of early life microbial exposure can increase susceptibility to immune-mediated diseases, is supported by studies on type 1 diabetes and early stages of thyroid autoimmunity. These demonstrate large differences in disease prevalence in genetically similar populations exposed to different environments (36, 37). However, with living conditions typically similar for co-twins, hygiene is best suited for explaining shared environmental components (C), which we did not detect. Nevertheless, some infections have been shown to increase the risk of type 1 diabetes and Hashimoto’s thyroiditis (38, 39), consistent with unique environmental exposure (E), but no single pathogen has been linked to both diseases. In fact, with the exception of smoking, which is known to reduce the risk of Hashimoto’s thyroiditis (39), and parental smoking during pregnancy, which has been linked to a reduced risk of type 1 diabetes in offspring (40), no unique environmental factors have been shown to alter the risk of both type 1 diabetes and Hashimoto’s thyroiditis in the same direction. Environmental triggers linked to type 1 diabetes are typically encountered early in life. These include high birth weight, early life dietary patterns, and enteroviral or respiratory infections at a young age (38). Interestingly, the observed overlap in unique environment suggests that early life or prenatal factors may be important triggers in Hashimoto’s thyroiditis as well. The influence of birth weight and prematurity on thyroid autoimmunity has indeed been studied, with mixed results (41, 42), while a small but significant increase in risk of Hashimoto’s thyroiditis in individuals born in summer, a potential proxy for exposure to seasonal infections early in life, has been demonstrated (43).
A major strength of this study was the use of nationwide registers with a high coverage, enabling us to examine a majority of Swedish twins, thus limiting selection bias. Moreover, the long observation period allowed us to detect most cases of two autoimmune diseases that typically debut decades apart. This was especially important for Hashimoto’s thyroiditis, lack of biochemical variables, most notably serologic data confirming autoimmune etiology, but also hormone levels, is a limitation to this study. However, the definition of type 1 diabetes in the National Diabetes Register has demonstrated high validity, with a positive predictive value of 97% (16). The validity of diagnostic records in the NPR for HT has not been evaluated, but a Danish study using virtually identical ICD and ATC codes reported a misclassification of <2% when compared with clinical records (44). With changes in register coverage over time, a first diagnostic record of Hashimoto’s thyroiditis may not reflect the date of diagnosis. We therefore refrained from running time-to-event analyses. We also lacked information on lifestyle factors including smoking and alcohol. Moreover, we used slightly different inclusion criteria for Hashimoto’s thyroiditis in subjects with type 1 diabetes compared to individuals without diabetes, which could potentially have inflated estimates of environments unique to the individual (E) but shared between the diseases. In addition, if within-individual co-occurrence of diseases was more likely to be detected; due to closer clinical surveillance in individuals with a prior diagnosis as compared to healthy individuals (i.e. screening for Hashimoto’s thyroiditis in patients with type 1 diabetes), this could have biased our results. The same would be true if monozygotic twins were more often screened for disease present in their co-twin, as compared to dizygotic twins. Finally, despite using a large twin sample, we did not have sufficient data to explore shared origins to type 1 diabetes and Graves’ disease, and based on previous findings that Graves’ disease and Hashimoto’s thyroiditis appear to be etiologically distinct, we chose not to cluster them into a combined autoimmune thyroid disease phenotype (22).
In summary, this is the first study to quantify the shared etiology between type 1 diabetes and Hashimoto’s thyroiditis. Our findings expand the current knowledge by demonstrating a considerable etiologic overlap, explained by genetic, but also individually unique environmental factors. This provides a foundation for future research aimed at characterizing the underlying biological mechanisms and modifiable risk factors.
Supplementary materials
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this study.
Funding
County Council of Värmland (J S), the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet (S B), Swedish Society for Medical Research (S B), Åke Wiberg Foundation (S B), The Swedish Research Council (O K), and Novo Nordisk Foundation (O K).
Author contribution statement
J S, R K-H, O K, and S B conceived the study. J S and R K-H designed the study and wrote the first draft of the manuscript. R K-H carried out statistical analysis. P M and S G acquired the necessary data. All authors were involved in the revision of the manuscript and approved the final version. J S and R K-H are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
The authors acknowledge The Swedish Twin Registry for access to data. The Swedish Twin Registry is managed by Karolinska Institutet and receives funding through Vetenskapsrådet under grant number 2017-00641.
References
- 1.Xu G, Liu B, Sun Y, Du Y, Snetselaar LG, Hu FB, Bao W. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ 2018362 k1497. ( 10.1136/bmj.k1497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, Okosieme OE. Global epidemiology of hyperthyroidism and hypothyroidism. Nature Reviews: Endocrinology 201814301–316. ( 10.1038/nrendo.2018.18) [DOI] [PubMed] [Google Scholar]
- 3.Triolo TM, Armstrong TK, McFann K, Yu L, Rewers MJ, Klingensmith GJ, Eisenbarth GS, Barker JM. Additional autoimmune disease found in 33% of patients at type 1 diabetes onset. Diabetes Care 2011341211–1213. ( 10.2337/dc10-1756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nederstigt C, Uitbeijerse BS, Janssen LGM, Corssmit EPM, de Koning EJP, Dekkers OM. Associated auto-immune disease in type 1 diabetes patients: a systematic review and meta-analysis. European Journal of Endocrinology 2019180135–144. ( 10.1530/EJE-18-0515) [DOI] [PubMed] [Google Scholar]
- 5.Ruggeri RM, Trimarchi F, Giuffrida G, Certo R, Cama E, Campenni A, Alibrandi A, De Luca F, Wasniewska M. Autoimmune comorbidities in Hashimoto’s thyroiditis: different patterns of association in adulthood and childhood/adolescence. European Journal of Endocrinology 2017176133–141. ( 10.1530/EJE-16-0737) [DOI] [PubMed] [Google Scholar]
- 6.Hemminki K, Li X, Sundquist J, Sundquist K. Familial association between type 1 diabetes and other autoimmune and related diseases. Diabetologia 2009521820–1828. ( 10.1007/s00125-009-1427-3) [DOI] [PubMed] [Google Scholar]
- 7.Anaya JM, Castiblanco J, Tobon GJ, Garcia J, Abad V, Cuervo H, Velasquez A, Angel ID, Vega P, Arango A. Familial clustering of autoimmune diseases in patients with type 1 diabetes mellitus. Journal of Autoimmunity 200626208–214. ( 10.1016/j.jaut.2006.01.001) [DOI] [PubMed] [Google Scholar]
- 8.Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, Heward JM, Manji N, Allahabadia A, Armitage M, Chatterjee KVet al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. American Journal of Medicine 2010123183.e1–183.e9. ( 10.1016/j.amjmed.2009.06.030) [DOI] [PubMed] [Google Scholar]
- 9.Skov J, Eriksson D, Kuja-Halkola R, Hoijer J, Gudbjornsdottir S, Svensson AM, Magnusson PKE, Ludvigsson JF, Kampe O, Bensing S. Co-aggregation and heritability of organ-specific autoimmunity: a population-based twin study. European Journal of Endocrinology 2020182473–480. ( 10.1530/EJE-20-0049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen PS, Brix TH, Iachine I, Kyvik KO, Hegedus L. The relative importance of genetic and environmental effects for the early stages of thyroid autoimmunity: a study of healthy Danish twins. European Journal of Endocrinology 200615429–38. ( 10.1530/eje.1.02060) [DOI] [PubMed] [Google Scholar]
- 11.Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes 2003521052–1055. ( 10.2337/diabetes.52.4.1052) [DOI] [PubMed] [Google Scholar]
- 12.Li L, Liu S, Yu J. Autoimmune thyroid disease and type 1 diabetes mellitus: same pathogenesis; new perspective? Therapeutic Advances in Endocrinology and Metabolism 2020112042018820958329. ( 10.1177/2042018820958329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogdanos DP, Smyk DS, Rigopoulou EI, Mytilinaiou MG, Heneghan MA, Selmi C, Gershwin ME. Twin studies in autoimmune disease: genetics, gender and environment. Journal of Autoimmunity 201238J156–J169. ( 10.1016/j.jaut.2011.11.003) [DOI] [PubMed] [Google Scholar]
- 14.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. European Journal of Epidemiology 200924659–667. ( 10.1007/s10654-009-9350-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnusson PK, Almqvist C, Rahman I, Ganna A, Viktorin A, Walum H, Halldner L, Lundstrom S, Ullen F, Langstrom Net al. The Swedish Twin Registry: establishment of a biobank and other recent developments. Twin Research and Human Genetics 201316317–329. ( 10.1017/thg.2012.104) [DOI] [PubMed] [Google Scholar]
- 16.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjornsdottir S, Eliasson B. Glycemic control and cardiovascular disease in 7,454 patients with type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes Care 2010331640–1646. ( 10.2337/dc10-0398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish National Inpatient Register. BMC Public Health 201111 450. ( 10.1186/1471-2458-11-450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, Persson I, Sundstrom A, Westerholm B, Rosen M. The new Swedish Prescribed Drug Register – opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiology and Drug Safety 200716726–735. ( 10.1002/pds.1294) [DOI] [PubMed] [Google Scholar]
- 19.Zetterqvist J, Sjölander A. Doubly Robust estimation with the R package drgee. Epidemiologic Methods 2015469–86. ( 10.1515/em-2014-0021) [DOI] [Google Scholar]
- 20.Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, Estabrook R, Bates TC, Maes HH, Boker SM. OpenMx 2.0: extended structural equation and statistical modeling. Psychometrika 201681535–549. ( 10.1007/s11336-014-9435-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giorda CB, Carna P, Romeo F, Costa G, Tartaglino B, Gnavi R. Prevalence, incidence and associated comorbidities of treated hypothyroidism: an update from a European population. European Journal of Endocrinology 2017176533–542. ( 10.1530/EJE-16-0559) [DOI] [PubMed] [Google Scholar]
- 22.Skov J, Calissendorff J, Eriksson D, Magnusson P, Kampe O, Bensing S, Kuja-Halkola R. Limited genetic overlap between overt Hashimoto’s thyroiditis and Graves’ disease in twins: a population-based study. Journal of Clinical Endocrinology and Metabolism 20211061101–1110. ( 10.1210/clinem/dgaa956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frommer L, Kahaly GJ. Type 1 diabetes and autoimmune thyroid disease-the genetic link. Frontiers in Endocrinology 202112618213. ( 10.3389/fendo.2021.618213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwangbo Y, Park YJ. Genome-wide association studies of autoimmune thyroid diseases, thyroid function, and thyroid cancer. Endocrinology and Metabolism 201833175–184. ( 10.3803/EnM.2018.33.2.175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gough SC, Simmonds MJ. The HLA region and autoimmune disease: associations and mechanisms of action. Current Genomics 20078453–465. ( 10.2174/138920207783591690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, Kern M, Moser KL, Begovich AB, Carlton VE, Li Wet al. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. American Journal of Human Genetics 200576561–571. ( 10.1086/429096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bottini N, Vang T, Cucca F, Mustelin T. Role of PTPN22 in type 1 diabetes and other autoimmune diseases. Seminars in Immunology 200618207–213. ( 10.1016/j.smim.2006.03.008) [DOI] [PubMed] [Google Scholar]
- 28.Si X, Zhang X, Luo Y, Tang W. Association between the CTLA-4 +49A/G polymorphism and type 1 diabetes: a meta-analysis. Genetic Testing and Molecular Biomarkers 2012161336–1342. ( 10.1089/gtmb.2012.0169) [DOI] [PubMed] [Google Scholar]
- 29.Hu Y, Xu K, Jiang L, Zhang L, Shi H, Cui D. Associations between three CTLA-4 polymorphisms and Hashimoto’s thyroiditis risk: an updated meta-analysis with trial sequential analysis. Genetic Testing and Molecular Biomarkers 201822224–236. ( 10.1089/gtmb.2017.0243) [DOI] [PubMed] [Google Scholar]
- 30.Dittmar M, Kahaly GJ. Genetics of the autoimmune polyglandular syndrome type 3 variant. Thyroid 201020737–743. ( 10.1089/thy.2010.1639) [DOI] [PubMed] [Google Scholar]
- 31.Tomer Y, Dolan LM, Kahaly G, Divers J, D’Agostino RB, Jr, Imperatore G, Dabelea D, Marcovina S, Black MH, Pihoker Cet al. Genome wide identification of new genes and pathways in patients with both autoimmune thyroiditis and type 1 diabetes. Journal of Autoimmunity 20156032–39. ( 10.1016/j.jaut.2015.03.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skov J, Hoijer J, Magnusson PKE, Ludvigsson JF, Kampe O, Bensing S. Heritability of Addison’s disease and prevalence of associated autoimmunity in a cohort of 112,100 Swedish twins. Endocrine 201758521–527. ( 10.1007/s12020-017-1441-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B, Hawa MI, Rijsdijk FV, Fain PR, Paschou SA, Boehm BO, Steck AK, Snieder H, Leslie RD. Heritability of thyroid peroxidase autoantibody levels in type 1 diabetes: evidence from discordant twin pairs. Diabetologia 2015582079–2086. ( 10.1007/s00125-015-3664-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine 201242252–265. ( 10.1007/s12020-012-9703-2) [DOI] [PubMed] [Google Scholar]
- 35.Norris JM, Johnson RK, Stene LC. Type 1 diabetes – early life origins and changing epidemiology. Lancet: Diabetes and Endocrinology 20208226–238. ( 10.1016/S2213-8587(1930412-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondrashova A, Viskari H, Haapala AM, Seiskari T, Kulmala P, Ilonen J, Knip M, Hyoty H. Serological evidence of thyroid autoimmunity among schoolchildren in two different socioeconomic environments. Journal of Clinical Endocrinology and Metabolism 200893729–734. ( 10.1210/jc.2007-1644) [DOI] [PubMed] [Google Scholar]
- 37.Kondrashova A, Reunanen A, Romanov A, Karvonen A, Viskari H, Vesikari T, Ilonen J, Knip M, Hyoty H. A six-fold gradient in the incidence of type 1 diabetes at the eastern border of Finland. Annals of Medicine 20053767–72. ( 10.1080/07853890410018952) [DOI] [PubMed] [Google Scholar]
- 38.Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet 20163872340–2348. ( 10.1016/S0140-6736(1630507-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ajjan RA, Weetman AP. The pathogenesis of Hashimoto’s thyroiditis: further developments in our understanding. Hormone and Metabolic Research 201547702–710. ( 10.1055/s-0035-1548832) [DOI] [PubMed] [Google Scholar]
- 40.Magnus MC, Tapia G, Olsen SF, Granstrom C, Marild K, Ueland PM, Midttun Ø, Svensson J, Johannesen J, Skrivarhaug Tet al. Parental smoking and risk of childhood-onset type 1 diabetes. Epidemiology 201829848–856. ( 10.1097/EDE.0000000000000911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brix TH, Hansen PS, Rudbeck AB, Hansen JB, Skytthe A, Kyvik KO, Hegedus L. Low birth weight is not associated with thyroid autoimmunity: a population-based twin study. Journal of Clinical Endocrinology and Metabolism 2006913499–3502. ( 10.1210/jc.2006-1348) [DOI] [PubMed] [Google Scholar]
- 42.Crump C, Winkleby MA, Sundquist J, Sundquist K. Preterm birth and risk of medically treated hypothyroidism in young adulthood. Clinical Endocrinology 201175255–260. ( 10.1111/j.1365-2265.2011.04034.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thvilum M, Brandt F, Brix TH, Hegedus L. Month of birth is associated with the subsequent diagnosis of autoimmune hypothyroidism. A nationwide Danish register-based study. Clinical Endocrinology 201787832–837. ( 10.1111/cen.13425) [DOI] [PubMed] [Google Scholar]
- 44.Vestergaard P, Mosekilde L. Fractures in patients with hyperthyroidism and hypothyroidism: a nationwide follow-up study in 16,249 patients. Thyroid 200212411–419. ( 10.1089/105072502760043503) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a