Abstract
Chemokines are a large family of small secreted proteins that have fundamental roles in organ development, normal physiology, and immune responses upon binding to their corresponding receptors. The primary functions of chemokines are to coordinate and recruit immune cells to and from tissues and to participate in regulating interactions between immune cells. In addition to the generally recognized antimicrobial immunity, the chemokine/chemokine receptor axis also exerts a tumorigenic function in many different cancer models and is involved in the formation of immunosuppressive and protective tumor microenvironment (TME), making them potential prognostic markers for various hematologic and solid tumors. In fact, apart from its vital role in tumors, almost all inflammatory diseases involve chemokines and their receptors in one way or another. Modulating the expression of chemokines and/or their corresponding receptors on tumor cells or immune cells provides the basis for the exploitation of new drugs for clinical evaluation in the treatment of related diseases. Here, we summarize recent advances of chemokine systems in protumor and antitumor immune responses and discuss the prevailing understanding of how the chemokine system operates in inflammatory diseases. In this review, we also emphatically highlight the complexity of the chemokine system and explore its potential to guide the treatment of cancer and inflammatory diseases.
Keywords: cancer progression, chemokine, chemokine receptor, inflammatory diseases, tumor microenvironment (TME)
Chemokines are a large family of small secreted proteins that coordinate and recruit immune cells into and out of tissues and to participate in regulating the interactions between immune cells. The chemokine/chemokine receptor axis is involved in the progression of multiple malignancy types and almost all inflammatory diseases. This review summarizes recent advances of chemokine system in antitumor and protumor immune responses and discuss the prevailing understanding of how the chemokine system operates in inflammatory diseases. Modulating the expression of chemokines and/or their corresponding receptors on tumor cells or immune cells provides the basis for the exploitation of new drugs for clinical evaluation in the treatment of related diseases.
1. INTRODUCTION
Chemokines (chemotactic cytokines or chemoattractant cytokines) are a complicated family of small secreted proteins that, when bound to the corresponding cell surface receptors, have a fundamental role in the development of human organs, physiological function, and homeostasis of the immune system. All chemokines are 8–12 kDa peptides that modulate cellular chemotactic migration, adhesion, localization, and cell–cell interactions through binding to the so‐called classical seven transmembrane receptors, that is, G protein‐coupled receptors (GPCRs). 1 , 2 Chemokines can also bind to atypical chemokine receptors (ACKRs) with high affinity, which are non‐G protein‐coupled seven‐transmembrane receptors that do not induce directional cell migration. Indeed, ACKRs mainly activate β‐arrestin‐dependent pathways that regulate the bioavailability of chemokines and modulate the expression of other typical chemokine receptors or downstream signaling pathways, thus playing a role in immune responses. 3 Chemokines possess a spectrum of characteristics and functions. Some chemokines are secreted without stimulation, which are called homeostatic chemokines and govern the chemotaxis of immune cells during immune surveillance, such as the induction of lymphocytes to lymph nodes. One function of the homeostatic chemokines is monitoring pathogen invasion by interacting with antigen‐presenting cells in tissues. 4 , 5 Other chemokines stimulate the formation of new blood vessels (angiogenesis), facilitate cells into tissues, and deliver specific signals for cell maturation, playing a major role in development. 6 There are also chemokines that can be released by various cells in response to viral or bacterial infections; such chemokines can also be produced in response to noninfectious stimuli, such as inhalation of silica and the presence of urinary stones, and thus exert important functions in the inflammatory response. 7 This group of inflammatory chemokines is released by many different types of cells and constitutes the bulk of the chemokine family. Inflammatory chemokines primarily act as chemotactic agents for leukocytes (e.g., monocytes and neutrophils), attracting leukocytes from the circulation to the site of infection or tissue damage, and activating cells to illicit an immune response or promoting wound healing. 8 Owing to their molecular stability and targeting specificity, chemokines are thought to be critical for leukocyte infiltration and subsequent elicitation of inflammatory responses, directing the natural and adaptive immune response of the host. 7
Given the diversity and dynamic regulation of chemokine ligand and chemokine receptor expression by tumor cells, stromal cells, and immune cells, the role of chemokines in tumor immunity is multifaceted. The tumor microenvironment (TME) is formed by cancer cells, tissue‐resident cells (like fibroblasts, endothelial cells), and infiltrating immune cells that express multiple chemokines and chemokine receptors. 9 , 10 , 11 In TME, chemokines act on tumor cells to regulate their proliferative, invasive, and stem cell properties; and in turn, chemokines generated by tumor cells appeal to leukocyte infiltration, regulate neurogenesis and fibrogenesis, and induce vascularization, thereby affecting the microenvironment. 12 However, the role of chemokines in regulating key aspects of immune cell activation, localized recruitment, phenotypic differentiation, and function within the TME during tumorigenesis is only beginning to be discovered. 13 To complicate matters further, the same chemokine system contributes to both protumor and antitumor immune responses. The stage of disease onset, the activation status of immune cells, and the expression of chemokine receptors on regulatory and effector target cells may all have an impact on the balance between the different functions. 13 , 14 Further studies of chemokine systems in malignant tumors will not only provide a better understanding of cancer biology, but more importantly will also suggest novel therapeutic strategies for cancer immunotherapy. The regulation of the immune system by chemokines and chemokine receptors is also involved in a variety of inflammatory diseases other than tumors, such as rheumatoid arthritis (RA), multiple sclerosis (MS), asthma, type 2 diabetes, and atherosclerosis. 15 , 16
We will attempt to make a brief summary of the classification and structure of chemokines, highlighting recent advances in the function of the eight most currently reported chemokine axes in cancer progression and cancer immune response. We also summarize the latest understanding of how these chemokine systems operate in inflammatory diseases. We focus on the intricacy of the chemokine system and explore its potential to guide the treatment of cancer and inflammation‐related diseases.
2. CHEMOKINE SYSTEMS
2.1. The structure of chemokines
The chemokine network is composed of nearly 50 chemokine ligands, 20 GPCRs, and four ACKRs, 17 which play significant roles in the body's immune homeostasis, inflammatory response, viral infection, and tumor progression (Figure 1). Most of the functional studies published in recent years have shown that the chemokine ligand axis conforms the classical GPCR activation paradigm. 18 , 19 , 20 Once the ligand is stimulated, the G protein dissociates from the corresponding receptor and initiates different signaling events downstream of ligand binding that can eventually lead to various responses, such as cell proliferation, survival, invasion, migration, and gene transcription. 2 , 8 , 17 All chemokines are small, with approximately 20–50% identical sequences between individual chemokines, suggesting homology in their gene sequences and amino acid sequences. 1 , 21 Typical chemokine proteins are synthesized as peptide precursors and during their secretion from cells, a signal peptide consisting of about 20 amino acids is split from the active part of the molecule. All chemokines possess conserved amino acid sequences, typically four cysteines that in most cases interact to form a Greek key shape, which is important for the formation of their three‐dimensional or tertiary structure. 22 The first two cysteines are close to the n‐terminus of the maturing protein, the third cysteine is located in the middle of the molecule, and the fourth cysteine is near the C‐terminus. After the first two cysteines, there is a ring of about 10 amino acids called the N ring. Chemokine receptors bind to G proteins and transmit cellular signals (Figure 2). When chemokines bind to the seven transmembrane GPCRs, they initiate the dissociation of G protein subunits α and βγ, which subsequently leads to the activation of phospholipase C (PLC). PLC acts by splitting the phosphatidylinositol bisphosphate (PIP2) molecule into two second messenger elements, inositol triphosphate (IP3) and diacylglycerol (DAG). DAG then activates protein kinase C, whereas IP3 triggers the release of intracellular calcium ions, thereby driving cell polarization, adhesion, and migration. These events also trigger multiple intracellular signaling cascades (e.g., PI3K/AKT and JAK/STAT pathways) that impel activated signaling molecules into the nucleus to initiate transcriptional processes. 8 , 17
FIGURE 1.
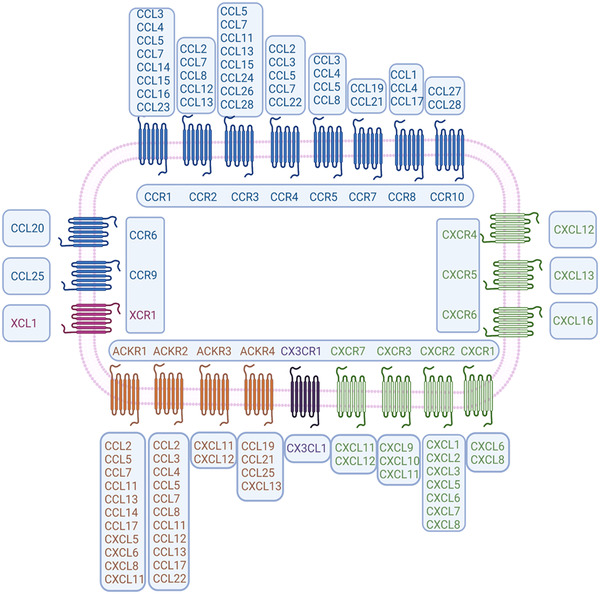
The ligand‐binding patterns of the seven‐transmembrane domain G‐protein‐coupled chemokine receptors. The receptors CCR1–CCR5, CCR7, CCR8, CCR10, CXCR1–CXCR3, and CXCR7 all bind to multiple chemokines. In contrast, CCR6, CCR9, CXCR4–CXCR6, CX3CR1, and XCR1 each bind only one ligand. Four molecules are included in the atypical chemokine receptor (ACKR) family and boast high affinity to CC‐/CXC‐ chemokines (the figure was created using biorender.com)
FIGURE 2.
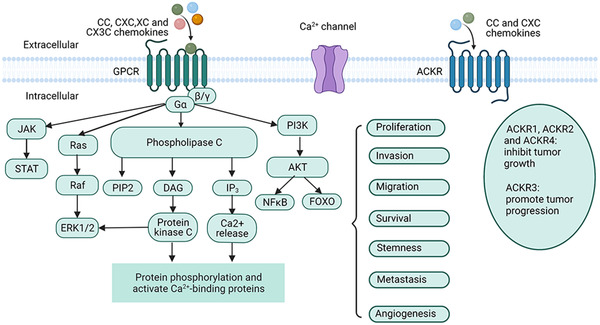
Chemokines/chemokine receptors signaling pathways. Chemokines transmit cellular signals by interacting with chemokine receptors, which are expressed on the cell surface as 7‐transmembrane proteins. Almost all types of chemokines bind to the classical G protein‐coupled receptor (GPCR), and activation of G proteins leads to subsequent activation of phospholipase C (PLC). PLC then cleaves a molecule called phosphatidylinositol‐bisphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (DAG); DAG activates protein kinase C, whereas IP3 triggers the intracellular release of stored calcium. Chemokines also activate the JAK/STAT, Ras/Raf/ERK, and PI3K/AKT signaling pathways through the GPCR signaling cascade. These events have an important role in cancer biology, involving tumor cell proliferation, invasion, metastasis, and angiogenesis. The atypical chemokine receptors (ACKRs) do not induce immune cell movement due to their structural inability to bind G proteins, rather their main function is to regulate the concentrations and bioavailability of chemokines on both sides of the cell membrane (the figure was created using biorender.com)
2.2. The classification of chemokines and their receptors
According to the number and location of the highly conserved N‐terminal cysteines, chemokines are grouped into four different subfamilies: CC, CXC, CX3C, XC, and the nomenclature of the receptors is essentially similar to that of corresponding chemokines, that is, CC chemokine (CCL) binds to CC chemokine receptor (CCR) and CX3C ligand binds to CX3C receptor (CX3CR) (Table 1). 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 Depending on their functions in the body, chemokines are also categorized into proinflammatory chemokines, homeostatic chemokines, or chemokines with both functions. Homeostatic chemokines, such as CCL17, CXCL14, and CXCL15, are produced constitutively in lymphocytes or other organs under normal biological conditions and are crucially important for immune surveillance because they primarily govern the homeostatic migration and homing of various immune cells. 71 Inflammatory chemokines are induced by infection and other proinflammatory stimuli and their main function is to rapidly attract leukocytes to the site of infection or injury to act as inflammatory mediators. 71 , 72 , 73 Chemokines can also be subdivided into the following categories according to the different cells on which they act. (1) monocyte/macrophage chemokines function as key chemokines to attract monocytes/macrophages to sites of inflammation, including CCL2–3, CCL5, CCL7–8, CCL13, CCL17, and CCL22. 74 , 75 , 76 , 77 (2) T lymphocyte chemokines include four chemokines implicated in the recruiting of T lymphocytes to sites of inflammation: CCL1, CCL2, CCL17, and CCL22. In addition, activated T cells induce the expression of CXCR3 and secretion of interferon (IFN)‐γ‐induced chemokines CXCL9–11 at sites of inflammation. 78 (3) Mast cell chemokines express multiple chemokine receptors on their surface including CCR1–5, CXCR2, and CXCR4. 79 , 80 CCL2 and CCL5, as ligands for these receptors, play a pivotal role in the recruitment and activation of lung mast cells. (4) Eosinophil chemokines direct the migration of eosinophils to different tissues chiefly involved in several chemokines of the CC subfamily: CCL3, CCL5, CCL7, CCL11, CCL13, CCL24, and CCL26. 81 , 82 Eosinophils are among the first immune cells to be recruited to the lesion, where the chemokines CCL5 and CCL11 act by binding to CCR3 that is expressed on the surface of eosinophils. 83 (5) Neutrophil chemokines are primarily CXC types of chemokines. For example, CXCL8 (interleukin [IL]‐8) in the TME is a chemotactic agent for neutrophils, inducing neutrophils into TME and activating their metabolism and degranulation. 84 , 85 , 86
TABLE 1.
The chemokine subfamilies and their receptors
| Class | Systemic name | Synonym | Receptor | Function |
|---|---|---|---|---|
| CXC (α subfamily) | CXCL1 23 | Growth‐regulated protein‐α (GROα), Gro‐oncogene 1 (GRO1), neutrophil‐activating protein‐3, and keratinocyte‐derived chemokine | CXCR2 | Inflammatory |
| CXCL2 24 | Macrophage inflammatory protein‐2a (MIP‐2α), GROβ, Gro2 | CXCR2 | Inflammatory | |
| CXCL3 25 | MIP‐2β, GROγ, Gro3 | CXCR2 | Inflammatory | |
| CXCL4 26 | Platelet factor 4 (PF4) | Unknown | Unknown | |
| CXCL5 27 | Epithelial‐derived neutrophil‐activating peptide 78 (ENA‐78) | CXCR2 and DARC | Inflammatory | |
| CXCL6 28 | Granulocyte chemotactic protein‐2 (GCP‐2) | CXCR1, CXCR2 | Inflammatory | |
| CXCL7 29 | Platelet basic protein (PBP), leukocyte‐derived growth factor (LDGF), macrophage‐derived growth factor (MDGF), Small‐inducible cytokine B7 | CXCR2 | Inflammatory | |
| CXCL8 30 | Interleukin‐8 (IL‐8), T‐cell chemotactic factor, lymphocyte derived neutrophil activating peptide (LYNAP), neutrophil activating peptide‐1 (NAP‐1) | CXCR1, CXCR2 | Inflammatory | |
| CXCL9 31 | Monokine induced by gamma interferon (MIG) | CXCR3 and CXCR3B | Inflammatory | |
| CXCL10 32 | Interferon (IFN)‐γ‐induced protein 10 (IP‐10), small inducible cytokine B10 | CXCR3 and CXCR3B | Dual: adaptive immunity (Th1 responses) | |
| CXCL11 33 | IP‐9, interferon‐inducible T‐cell α‐chemoattractant (I‐TAC) | CXCR3, CXCR3B, and CXCR7 | Inflammatory | |
| CXCL12 34 | Stromal cell‐derived factor‐1 (SDF‐1), SDF‐1α | CXCR4 and CXCR7 | Homeostatic | |
| CXCL13 35 | B‐lymphocyte chemoattractant (BLC) | CXCR5 and CCXCKR | Homeostatic | |
| CXCL14 36 | Breast‐ and kidney‐expressed chemokine | Unknown | Inflammatory: development of antigen‐presenting cells | |
| CXCL15 37 | Lungkine | CXCR2 | Unknown | |
| CXCL16 38 | CXCR6 | Inflammatory: T lymphopoiesis, extravasation | ||
| CXCL17 39 | Dendritic and monocyte chemokine‐like protein, vascular endothelial growth factor (VEGF)‐coregulated chemokine‐1 | Unknown | Unknown | |
| CC (β subfamily) | CCL1 40 | Inflammatory cytokine I‐309 | CCR8 | Inflammatory |
| CCL2 41 | Monocyte chemotactic protein‐1 (MCP‐1) and small inducible cytokine A2 | CCR2 | Inflammatory: innate and adaptive immunity | |
| CCL3 42 | MIP‐1α | CCR1 and CCR5 | Inflammatory | |
| CCL4 43 | MIP‐1β | CCR5 | Inflammatory | |
| CCL5 44 | Regulated on activation, normal T‐cell expressed and secreted (RANTES) | CCR1, CCR3, and CCR5 | Inflammatory | |
| CCL6 45 | Macrophage inflammatory protein‐related protein‐1 (MRP‐1) | CCR1 | Unknown | |
| CCL7 46 | MCP‐3 | CCR1, CCR2, and CCR3 | Inflammatory | |
| CCL8 47 , 52 | MCP‐2 | CCR1, CCR2, CCR3, and CCR5 | Inflammatory | |
| CCL9 48 | MIP‐1γ, MRP‐2 | CCR1 | Inflammatory | |
| CCL10 49 | Unknown | Unknown | Unknown | |
| CCL11 50 | Eotaxin | CCR3 | Inflammatory | |
| CCL12 51 | MCP‐5 | CCR2 | Inflammatory | |
| CCL13 52 | MCP‐4 | CCR1, CCR2, CCR3, and CCR5 | Inflammatory | |
| CCL14 53 | Hemofiltrate CC chemokine‐1 (HCC‐1) | CCR1 and CCR5 | Homeostatic | |
| CCL15 54 | Leukotactin‐1, MIP‐5, and HCC‐2 | CCR1 and CCR3 | Homeostatic | |
| CCL16 55 | Monotactin‐1, liver‐expressed chemokine, and HCC‐4 | CCR1, CCR2, CCR5, and CCR8 | Homeostatic | |
| CCL17 56 | Thymus‐ and activation‐regulated chemokine (TARC) | CCR4 | Dual | |
| CCL18 57 | MIP‐4, pulmonary‐ and activation‐regulated chemokine (PARC), alternative macrophage activation‐associated CC chemokine 1 (AMAC‐1) | Unknown | Homeostatic: T cell–dendritic cell interaction (spleen, lymph node) | |
| CCL19 58 | MIP‐3β, EBl1 ligand chemokine (ELC) exodus‐3 | CCR7 and CCXCKR | Homeostatic: T lymphopoiesis | |
| CCL20 52 , 59 | MIP‐3α, liver activation‐regulated chemokine (LARC), exodus‐1 | CCR6 | Dual: development of dendritic cells, adaptive immunity | |
| CCL21 60 | Secondary lymphoid tissue chemokine (SLTC), exodus‐2, TCA4 | CCR7 and CCXCKR | Dual: spleen and lymph node T cell homing | |
| CCL22 61 | Macrophage‐derived chemokine (MDC) | CCR4 | Dual: adaptive immunity (cutaneous T cells) | |
| CCL23 62 | MIP‐3, myeloid progenitor inhibitory factor‐1 (MPIF‐1) | CCR1 | Inflammatory | |
| CCL24 63 | Eotaxin‐2, MPIF‐2 | CCR3 | Inflammatory | |
| CCL25 64 | Thymus‐expressed chemokine (TECK) | CCR9 and CCXCKR | Dual: T lymphopoiesis, adaptive immunity, T cell and B cell trafficking in small intestine | |
| CCL26 65 | MIP‐4α, eotaxin‐3, thymic stroma chemokine‐1 | CCR3 | Inflammatory | |
| CCL27 66 | Cutaneous T‐cell‐attracting chemokine (CTACK), IL‐11 receptor a‐locus chemokine (ILC), embryonic stem cell chemokine | CCR10 | Homeostatic | |
| CCL28 67 | Mucosae‐associated epithelial chemokine (MEC) | CCR3 and CCR10 | Homeostatic | |
| XC (γ subfamily) | XCL1 68 | Lymphotactin α | XCR1 | Dual |
| XCL2 69 | Lymphotactin β | XCR2 | Dual | |
| CX3C (δ subfamily) | CX3CL1 70 | Fractalkine, neurotactin | CX3CR1 | Inflammatory: extravasation |
3. DIFFERENT CHEMOKINE AXES
Although a chemokine may bind to multiple receptors, when it binds to a specific receptor and exhibits excitatory effects on the binding site and antagonistic effects on other bindings, the chemokine and its receptor form a functional chemokine axis. 87 Below, we will summarize the characteristics of eight most reported chemokine axes, as well as recent advances in these chemokine axes in cancer and inflammatory diseases.
3.1. The CCL2/CCR2 axis
3.1.1. Brief introduction to the CCL2/CCR2 signaling axis
CCL2 belongs to the CC chemokine subfamily, also known as small inducible cytokine A2 and monocyte chemotactic protein‐1 (MCP‐1). As the most well‐studied chemokine, CCL2 was originally identified in 1989 from the culture supernatant of peripheral blood monocytes and tumor cell lines. 88 , 89 CCL2 was at first characterized as a “tumor‐derived chemokine” and has been documented to be a potent chemotactic agent for a couple of immune cells (e.g., monocytes, immature dendritic cells (DCs), memory T cells, and natural killer (NK) cells), thereby promoting inflammatory effects and neoangiogenesis. In addition, various stromal cells in the TME, including endothelial cells, DCs, fibroblasts, and adipocytes, are capable of producing CCL2 to promote tumor growth and progression. 90 , 91 , 92 CCL2 was the first CC chemokine to be identified and fully studied and has a high affinity for its receptor, CCR2. It has been revealed that CCR2 is widely expressed in a broad range of cell types, including monocytes, 93 endothelial cells, 94 DCs, 95 and various cancer cells and the upregulation of CCR2 is related to advanced cancer, metastasis, and relapse. 96 Once bound to its ligand CCL2, the activated signaling axis triggers various intracellular G protein‐mediated signaling cascades, such as mitogen‐activated protein kinase (MAPK)/p38, PI3K/AKT, and JAK/STAT3 pathways. 97 , 98 , 99 The CCL2/CCR2 axis is implied in the proliferation, invasion, and angiogenesis of tumor cells and recruitment of immunosuppressive cells. 100 , 101 Previous data also identified a regulatory role of the CCL2/CCR2 signaling axis on the nervous system. 102 Microglia are the resident immune cells of the central nervous system (CNS), which is phylogenetically related to monocytes and therefore also expresses CCR2. CCL2 can also be produced by microglia/macrophages or endothelial cells under basal and neuroinflammatory conditions. 103 , 104 Typically, CCL2 secreted by activated astrocytes (the major glial cells of the CNS) is thought to attract microglia to sites of neuronal infection or injury, where they engulf microbes or cellular debris. 105 The relationship between the CCL2/CCR2 signaling axis and the pathogenesis of autoimmune diseases has also been widely investigated, and a common pathological feature of these autoimmune diseases is the upregulation of CCL2 and/or CCR2 expression in the lesions. 106 , 107
3.1.2. Roles of the CCL2/CCR2 signaling axis in tumor progression
It has been demonstrated that CCL2 and its receptor CCR2 are implicated in the development and progression of various malignancies, such as prostate cancer, 108 breast cancer, 109 hepatocellular cancer, 110 lung cancer, 111 renal cancer, 112 pancreatic cancer, 113 and nasopharyngeal carcinoma. 114 The CCL2/CCR2 signaling axis is involved in different stages of tumorigenic progression, for example, maintaining the proliferation and stemness of tumor cells at the site of the primary tumor; and when malignant cells metastasize, promoting the invasion of cancer cells into surrounding tissues and circulatory system, and traveling down a specific chemotactic ladder to the site of metastasis (Figure 3). 96 After reaching a new organ and/or tissue, residual circulating tumor cells are able to successfully colonize and continue to grow through interactions with various components within the TME. 91 It has been suggested that CCL2 might act as an autocrine or paracrine chemokine to promote the growth of tumor cells, which can be partially abolished by CCR2 antagonists or PI3K inhibitors. 115 CCL2 can promote drug resistance in gastric cancer cells by inhibiting autophagy, and either knockdown of CCL2 or induction of autophagy successfully reversed drug resistance in tumor cells. 116 Similarly, in vitro experiments showed that downregulation of CCL2 decreased the viability of A549 cells and enhanced docetaxel (DTX)‐induced cytotoxicity, whereas upregulation of CCL2 protected A549 cells from DTX‐induced cytotoxicity. 117 The chemoresistance that occurs within lung cancer cells may be mediated by the stress response of CCL2‐expressing cells, implicating CCL2 as a possible target for augmenting the therapeutic efficacy of DTX on lung cancer. 117 CCL2 also attracts different immune cells to form an immunosuppressive microenvironment, which promotes the formation of tumor‐associated microvasculature and supports the growth and metastasis of tumor cells. In mouse melanoma and pancreatic cancer models, knockdown of CCL2 with siRNA or antibody neutralization effectively inhibited DC recruitment, reduced CD68+ macrophage infiltration, and decreased tumor growth and metastasis. 118 , 119 , 120 Furthermore, radiotherapy induces a significant increase in the recruitment of CCL2 and Ly6C+CCR2+ monocytes in pancreatic ductal adenocarcinoma (PDAC), thereby accelerating tumor proliferation and angiogenesis. Anti‐CCL2 antibodies selectively inhibit radiotherapy‐dependent monocyte/macrophage recruitment and retard tumor growth when used in combination with radiotherapy. 121 However, several studies have shown inconsistent results and thereby different conclusions. Fader et al. 122 included 37 patients with primary ovarian cancer to investigate the relationship between CCL2 expression in tumor specimens and patient response to chemotherapy and survival outcomes. The results suggested that increased expression of CCL2 in ovarian tumors was associated with better chemotherapy response and improved survival outcomes. Also, in vitro experiments illustrated that ovarian cancer cells with higher CCL2 expression were more sensitive to the traditional chemotherapeutic drugs paclitaxel and cisplatin. 122 Interestingly, recent evidence indicates that higher levels of CCL2 in patients with squamous lung cancer is related to favorable progression‐free survival (PFS) and overall survival (OS); however, lung adenocarcinoma patients with a high expression of CCL2 exhibited a shorter OS and PFS than those with a low expression. 123 Moreover, in vitro data from one study indicated that CCL2 could activate neutrophils and mediate the killing of breast cancer cells, whereas in mice breast cancer models, intranasal administration of CCL2 protein was able to increase the recruitment of CD4+ T cells in the lung, favoring tumor dissemination, and metastasis to the lung. 124
FIGURE 3.
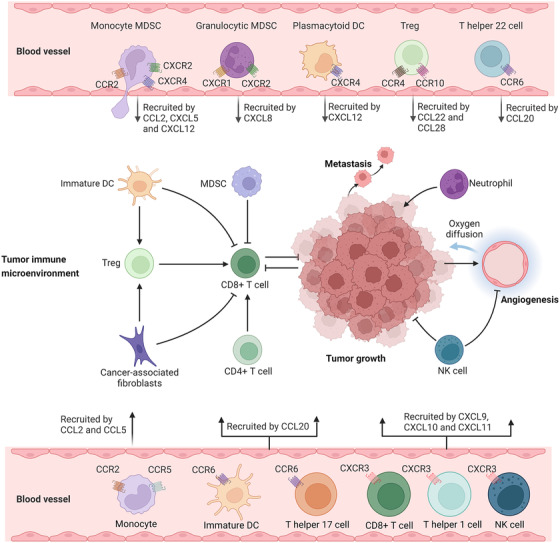
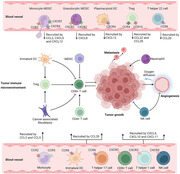
Chemokines/chemokine receptors in tumor immune microenvironment and their relevance in cancer immunotherapy. Immune cell populations, such as monocytic and granulocytic myeloid‐derived suppressor cells (MDSCs), plasmacytoid dendritic cells (DCs), regulatory T (Treg) cells, and IL‐22+CD4+ T helper 22 cells can promote tumor growth. Immune cells, such as T helper 17 cells, T helper 1 cells, CD8+ T cells, and natural killer cells (NK cells), have antitumor effects. These cells are recruited to the tumor, particularly the tumor immune microenvironment through chemokine/chemokine receptor signaling axes and are involved in almost all aspects of the tumor progression (e.g., tumor proliferation, angiogenesis, and metastasis) (the figure was created using biorender.com)
Taken together, the CCL2/CCR2 signaling axis is involved in a wide range of tumor cell activities, and regulation of CCL2 and/or CCR2 expression influences tumor progression. Deeper insight into the potential mechanisms of the CCL2/CCR2 axis in tumor progression and treatment will provide new directions for a better understanding of malignancies.
3.1.3. The CCL2/CCR2 axis in autoimmune diseases and neurological disorders
The pathology of autoimmune diseases is characterized by the infiltration of multiple lymphocytes in the tissues, which leads to inflammation and tissue damage. This process involves a complex network of immune cells in which chemokines act as signaling bridges. Many studies have elucidated monocytes as the molecular basis of immune cells recruitment in autoimmune disease, suggesting a major role for CCL2 and its cell surface receptor CCR2. 107 , 125 , 126 , 127 Psoriasis is a chronic skin disease caused by an imbalance between skin keratinocytes and infiltrating immune cells. In patients with psoriasis, keratinocytes secrete large amounts of CCL2, which, when combined with CCR2 on the surface of monocytes, can induce monocytes to differentiate into macrophages and migrate from the bloodstream to the site of inflammation. 128 CCL2 is an emerging novel target in systemic lupus erythematosus (SLE) and lupus nephritis, in which the CCL2/CCR2 axis mediates the infiltration of macrophages and T cells into the nephron in nephritis. 129 Moreover, a meta‐analysis covering 399 patients with lupus nephritis and 130 normal controls revealed that urinary CCL2 was markedly higher in patients with active lupus nephritis than those with inactive lupus nephritis and controls, suggesting that urinary CCL2 might serve as a biomarker for lupus nephritis. 130 Meanwhile, in vivo experimental studies have illustrated that blocking CCL2 was effective in inhibiting the progression of proliferative lupus nephritis. 131
Given the possibility that the CCL2/CCR2 axis may exert a range of immune regulation on the nervous system, its proinflammatory properties were subsequently thought to be a leading factor in the evolution of depression. Studies carried out by Stuart et al. 132 have shown that the CCL2/CCR2 axis might be involved in regulating the proliferation and differentiation of neural progenitor cells, modulating the infiltration and activation of central immune cells, and influencing the secretion of proinflammatory factors (e.g., IL‐1β and IL‐8). Many scientists and histologists have argued that elevated CCL2 levels lead to increased blood–brain barrier permeability by inducing macrophage recruitment, cytokine production, and directly altering endothelial cell tight junction protein expression, which are observed in a variety of pathological processes, such as neuroinflammation, 133 stroke, 134 MS, 135 and Alzheimer's disease (AD). 136
The infection of HIV causes chronic inflammation in the body, along with a dysregulated immune system, which further exacerbates the inflammatory response. 137 , 138 The interaction of chemokine receptors (for example, CCR2, CCR3, CCR5, and CXCR4) on the cell surface with external components of HIV and accessory proteins is an essential step in HIV infection of target cells. 139 , 140 Among them, CCR5 and CXCR4 are the primary coreceptors for HIV‐1 invasion into target cells, with CCR2 and CCR3 playing secondary synergistic roles. 141 In HIV infection, accentuated expression of CCL2 and/or CCR2 may contribute to HIV‐associated complications in multiple ways, depending on their role in leukocytes recruitment and maintenance of an inflammatory state. 142 Apart from its role in inflammation and cell‐directed migration, however, CCL2 has been proven to influence directly viral replication, as evidenced by studies performed in peripheral blood mononuclear cells, T lymphocytes, and macrophages. 143 , 144 , 145 Recently, several attempts have been made to investigate the relationship between high expression of CCL2 and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. 146 , 147 Conclusively, targeting the CCL2/CCR2 axis is considered to be an attractive target for the treatment of autoimmune diseases and viral infections. Therefore, more attention should be paid in the future to comprehensively investigate the potential mechanisms of action of the CCL2/CCR2 axis in these diseases mentioned above.
3.1.4. Clinical trials of drugs targeting the CCL2/CCR2 axis
Due to the pivotal role played by the CCL2/CCR2 signaling axis in the progression of multiple diseases, a host of clinical trials of drugs that modulate this axis have been continually launched (Table 2). Currently, clinical trials of human neutralizing antibodies against CCL2 are focused on carlumab and MLN1202 that can effectively block the differentiation of monocytes to macrophages and reduce immune cell recruitment after binding to CCL2, exhibiting broad anti‐tumor effects. In a phase I study evaluating the safety and antitumor activity of carlumab in advanced solid tumors (NCT00537368), 44 subjects were enrolled. Carlumab was shown to be well tolerated by all patients, as no carlumab‐related adverse events were observed. However, durable stable disease (SD) was observed in only four of 33 evaluable patients, and none achieved an objective response (OR). 148 In another completed phase II study of carlumab monotherapy for metastatic castration‐resistant prostate cancer (NCT00992186), none had an OR to carlumab treatment and only 34% of patients maintained SD for over 3 months. Notably, no patient generated antibodies to carlumab, although inhibition of free CCL2 serum concentrations was observed transiently after each dose, which seems unsustainable. 149 Similarly, in an open‐label, multicenter phase 1b study of carlumab in combination with chemotherapy for advanced solid tumors (NCT01204996), researchers found that carlumab could be safely used in combination with standard chemotherapy doses at 10 or 15 mg/kg and was well tolerated, but did not suppress serum CCL2 levels or produce a significant antitumor response over time, 150 suggesting that effects are not apparent and further clarification is a necessity. Meanwhile, the efficacy of MLN1202 in 44 patients with bone metastases was studied in a phase II clinical trial (NCT01015560). Of the 41 patients who completed treatment, 7.14% experienced serious adverse effects and fourteen patients experienced a decrease in urinary N‐terminal peptide values (an indicator of efficacy assessment), indicating a positive antitumor metastatic effect of MLN1202. MLN1202 was also used in randomized, double‐blind, placebo‐controlled studies of atherosclerotic cardiovascular disease and RA. The results showed that MLN1202 treatment was well tolerated and resulted in a significant reduction of high‐sensitivity C‐reactive protein levels in serum, 151 but did not lead to amelioration of synovial inflammation in active RA. 152
TABLE 2.
Overview of clinical trials targeting chemokines and their receptors
| Target | Drug name | Conditions | Phase | Status | Trial number |
|---|---|---|---|---|---|
| CCL2 | Carlumab | Metastatic castrate‐resistant prostate cancer | II | Completed | NCT00992186 |
| Carlumab | Solid tumors | I | Completed | NCT00537368 | |
| Carlumab | Combination with chemotherapy in patients with solid tumors | I | Completed | NCT01204996 | |
| CCR2 | MLN1202 | Cancer with bone metastases | II | Completed | NCT01015560 |
| MK‐0812 | Multiple sclerosis | Ⅱ | Terminated | NCT00239655 | |
| MK‐0812 | Rheumatoid arthritis | II | Completed | NCT00542022 | |
| CCX872‐B | Pancreatic adenocarcinoma | I | Not recruiting | NCT02345408 | |
| JNJ‐41443532 | Type 2 diabetes mellitus | II | Completed | NCT01230749 | |
| JNJ‐17166864 | Allergic rhinitis | II | Completed | NCT00604123 | |
| AZD2423 | Chronic obstructive pulmonary disease | II | Completed | NCT01215279 | |
| AZD2423 | Chronic obstructive pulmonary disease | II | Completed | NCT01153321 | |
| AZD2423 | Painful diabetic polyneuropathy | II | Completed | NCT01201317 | |
| AZD2423 | Posttraumatic neuralgia | II | Completed | NCT01200524 | |
| CCX872‐B | Combination with preoperative radiation therapy in pancreatic adenocarcinoma | I /Ⅱ | Withdrawn | NCT03778879 | |
| PF‐04136309 | Advanced pancreatic adenocarcinoma | I | Completed | NCT01413022 | |
| PF‐04136309 | Combination with gemcitabine and nab‐paclitaxel in metastatic pancreatic adenocarcinoma | Ⅱ | Terminated | NCT02732938 | |
| CCR5 | Maraviroc | Kaposi's sarcoma | Ⅱ | Completed | NCT01276236 |
| Maraviroc | Hematologic malignancy | Ⅱ | Completed | NCT01785810 | |
| Maraviroc | Colorectal cancer | I | Completed | NCT01736813 | |
| Maraviroc | Combination with pembrolizumab in metastatic colorectal cancer | I | Completed | NCT03274804 | |
| Maraviroc | Graft‐versus‐host disease | I/II | Completed | NCT00948753 | |
| Maraviroc | Hypertriglyceridemia | I | Completed | NCT01133210 | |
| Maraviroc | Combination with rehabilitation therapy in stroke | II/III | Recruiting | NCT03172026 | |
| Maraviroc | COVID‐19 | I | Completed | NCT04435522 | |
| Vicriviroc | Combination with pembrolizumab in colorectal neoplasms | Ⅱ | Active, not recruiting | NCT03631407 | |
| Vicriviroc | HIV infections, acquired immunodeficiency syndrome | III | Withdrawn | NCT00243568 | |
| Leronlimab | Solid tumors | III | Completed | NCT04504942 | |
| Leronlimab | Nonalcoholic steatohepatitis | II | Recruiting | NCT04521114 | |
| Leronlimab | COVID‐19 | II | Recruiting | NCT04347239 | |
| Leronlimab | Combination with carboplatin in CCR5+ triple negative breast neoplasm | I/II | Recruiting | NCT03838367 | |
| AZD5672 | Rheumatoid arthritis | II | Completed | NCT00713544 | |
| Lentivirus vector rHIV7‐shI‐TAR‐CCR5RZ‐transduced hematopoietic progenitor cells | Intermediate‐grade or high‐grade AIDS‐related lymphoma | I | Completed | NCT00569985 | |
| CCR2/ CCR5 | BMS‐813160 | Combination with GVAX for locally advanced pancreatic ductal adenocarcinoma (PDAC) | I/Ⅱ | Recruiting | NCT03767582 |
| BMS‐813160 | Combination with nivolumab, gemcitabine, and nab‐paclitaxel in borderline resectable and locally advanced PDAC | I/Ⅱ | Recruiting | NCT03496662 | |
| BMS‐813160 | Combination with chemotherapy or nivolumab in patients with pancreatic cancer | I/Ⅱ | Not recruiting | NCT03184870 | |
| BMS‐813160 | Advanced renal cell carcinoma | Ⅱ | Recruiting | NCT02996110 | |
| BMS‐813160 | Hepatocellular carcinoma | Ⅱ | Recruiting | NCT04123379 | |
| Cenicriviroc | COVID‐19 | Ⅱ | Recruiting | NCT04500418 | |
| Cenicriviroc | Nonalcoholic steatohepatitis | II | Completed | NCT03517540 | |
| Cenicriviroc | Nonalcoholic steatohepatitis | II | Completed | NCT02217475 | |
| Cenicriviroc | Liver insufficiency | I | Completed | NCT02120547 | |
| Cenicriviroc | Prediabetic state, nonalcoholic fatty liver disease, type 2 diabetes mellitus | II | Completed | NCT02330549 | |
| Cenicriviroc | Primary sclerosing cholangitis | II | Completed | NCT02653625 | |
| Cenicriviroc | Hepatic impairment | I | Completed | NCT03376841 | |
| CCL21 | CCL21 protein | Combination with GM.CD40L vaccine in stage IV lung adenocarcinoma | I/II | Completed | NCT01433172 |
| CCL21‐Gene‐modified dendritic cell vaccine | Combination with pembrolizumab stage IV nonsmall cell lung cancer | I | Recruiting | NCT03546361 | |
| Autologous dendritic cell‐adenovirus CCL21 vaccine | Melanoma (Skin) | I | Completed | NCT00798629 | |
| Autologous dendritic cell‐adenovirus CCL21 vaccine | Advanced or recurrent nonsmall cell lung cancer | I | Completed | NCT00601094 | |
| CCR7 | CD4 + CCR7 +T lymphocytes | Acute myeloblastic leukemia | Not Applicable | Completed | NCT03280290 |
| JBH492 | Relapsed/refractory chronic lymphocytic leukemia and non‐Hodgkin's lymphoma | I/Ib | Recruiting | NCT04240704 | |
| CCL20 | GSK3050002 | Ulcerative colitis | I | Completed | NCT01984047 |
| AZD0284 | Plaque psoriasis vulgaris | I | Terminated | NCT03310320 | |
| CXCL5 | Immunohistochemical expression of CXCL5 | Urinary bladder urothelial carcinoma | Not Applicable | Recruiting | NCT05139134 |
| Sunitinib malate | Kidney cancer | Not Applicable | Completed | NCT00943839 | |
| CXCR2 | AZD5069 | Neutrophil number and function study | I | Completed | NCT01480739 |
| AZD5069 | Combination with enzalutamide in metastatic castration resistant prostate cancer | I/II | Recruiting | NCT03177187 | |
| Navarixin | Combination with pembrolizumab advanced/metastatic solid tumors | II | Completed | NCT03473925 | |
| Navarixin | Psoriasis | II | Completed | NCT00684593 | |
| Navarixin | Allergen‐induced asthma | II | Completed | NCT00688467 | |
| Navarixin | Chronic obstructive pulmonary disease | II | Terminated | NCT01006616 | |
| CXCR2 ligands/CXCR2 | Biological axis in pancreatic cancer | Not Applicable | Completed | NCT00851955 | |
| RIST4721 | Inflammatory response | I | Completed | NCT04105959 | |
| CXCR2‐transduced autologous tumor infiltrating lymphocytes | Metastatic melanoma | I/II | Active, not recruiting | NCT01740557 | |
| GSK1325756 | Chronic obstructive pulmonary disease | I | Completed | NCT01209052 | |
| GSK1325756 | Nutritional status | I | Completed | NCT01209104 | |
| Danirixin | Chronic obstructive pulmonary disease | I | Completed | NCT03136380 | |
| Danirixin | Chronic obstructive pulmonary disease | II | Terminated | NCT03250689 | |
| Danirixin | Chronic obstructive pulmonary disease | II | Completed | NCT03034967 | |
| Danirixin | Infections, respiratory syncytial virus | I | Completed | NCT02201303 | |
| Danirixin | Chronic obstructive pulmonary disease | II | Completed | NCT02130193 | |
| SB‐656933‐AAA | Chronic obstructive pulmonary disease | I | Completed | NCT00504439 | |
| CXCL9 | Emapalumab | Hemophagocytic lymphohistiocytoses | II/III | Active, not recruiting | NCT03985423 |
| Emapalumab | Graft failure | II | Recruiting | NCT04731298 | |
| CXCL10 | NI‐0801 | Primary biliary cirrhosis | II | Terminated | NCT01430429 |
| MDX‐1100 | Combination with methotrexate in rheumatoid arthritis | II | Completed | NCT01017367 | |
| MDX‐1100 | Ulcerative colitis | I | Completed | NCT00295282 | |
| MDX‐1100 | Ulcerative colitis | II | Completed | NCT00656890 | |
| CXCL10 protein | COVID‐19 | Not Applicable | Completed | NCT04389645 | |
| CXCL10/CXCR3 | Ozone | Environmental and genetic factors on lung function | Early Phase I | Recruiting | NCT03599206 |
| CXCL12 | JVS‐100 | Peripheral arterial disease | II | Unknown | NCT02544204 |
| JVS‐100 | Critical limb ischemia | II | Completed | NCT01410331 | |
| ACRX‐100 | Heart failure | I | Completed | NCT01082094 | |
| NOX‐A12 | Combination with irradiation in glioblastoma | I/II | Recruiting | NCT04121455 | |
| NOX‐A12 | Combination with pembrolizumab in colorectal and pancreatic cancer | I/II | Completed | NCT03168139 | |
| NOX‐A12 | Combination with bortezomib and dexamethasone in relapsed multiple myeloma | II | Completed | NCT01521533 | |
| NOX‐A12 | Combination with bendamustine and rituximab in relapsed chronic lymphocytic leukemia | II | Completed | NCT01486797 | |
| NOX‐A12 | Hematopoietic stem cell transplantation | I | Completed | NCT01194934 | |
| CXCR4 | AMD3100 | Healthy volunteers | I | Completed | NCT00322127 |
| AMD3100 | Neutropenia | I | Completed | NCT01058993 | |
| AMD3100 | Acute myeloid leukemia | I/II | Completed | NCT00512252 | |
| AMD3100 | Lymphoma | I/II | Completed | NCT00733824 | |
| AMD070 | HIV Infections | I | Completed | NCT00063804 | |
| POL6326 | Healthy volunteers | I | Completed | NCT01841476 | |
| CXCR4 modified anti‐BCMA (B‐cell maturation antigen) CAR T cells | Multiple myeloma | Early Phase I | Not yet recruiting | NCT04727008 | |
| POL6326 | Large reperfused ST‐elevation myocardial infarction | II | Completed | NCT01905475 | |
| BL‐8040 | Chronic myeloid leukemia | I/II | Withdrawn | NCT02115672 | |
| BKT140 | Multiple myeloma | I/II | Completed | NCT01010880 | |
| Autologous CD4 T‐cells | HIV infections | Early Phase I | Completed | NCT03020524 | |
| AMD11070 | HIV infections | I/II | Completed | NCT00089466 | |
| AC220 | Acute myeloid leukaemia, high risk myelodysplastic syndrome | I/II | Completed | NCT01236144 | |
| BL‐8040 | Combination with pembrolizumab in metastatic pancreatic adenocarcinoma | II | Active, not recruiting | NCT02907099 | |
| ALX‐0651 | Healthy volunteers | I | Terminated | NCT01374503 | |
| BMS‐936564 | Multiple myeloma | I | Completed | NCT01359657 | |
| BMS‐936564 | Acute myelogenous leukemia and selected B‐cell cancers | I | Completed | NCT01120457 | |
| LY2510924 | Solid tumor | I | Terminated | NCT02737072 | |
| USL311 | Solid tumors, relapsed/recurrent glioblastoma multiforme | I/II | Terminated | NCT02765165 | |
| CXCL12/CXCR4 | Tipifarnib | Relapsed or refractory peripheral T‐cell lymphoma | II | Completed | NCT02464228 |
| CXCR5 | CXCR5 modified EGFR CAR‐T cells | Nonsmall cell lung cancer | Early phase I | Recruiting | NCT05060796 |
| SP01A | HIV infections | I/II | Completed | NCT00299338 |
Data from https://clinicaltrials.gov/.
Inhibitors against CCR2 are designed to disrupt the binding of CCR2 to its ligand CCL2, acting by blocking the activation of a series of signaling cascades downstream of the CCL2/CCR2 axis. A phase I study evaluated the CCR2 inhibitor PF‐04136309 in combination with FOLFIRINOX for the treatment of patients with advanced pancreatic cancer (NCT01413022). Among patients who completed a course of combination therapy, 97% achieved local tumor control and 49% achieved an objective tumor response. More importantly, the mean percentage of CCR2+ monocytes in the blood of the combination‐treated patients was significantly lower compared with the chemotherapy group, suggesting that chemotherapy plus PF04136309 prevents the drainage of CCR2+ monocytes into the peripheral circulation from the bone marrow and affects antitumor immunity. 153 Both CCX140‐B and CCX872‐B can selectively inhibit CCR2 and are mainly used to evaluate the effect on type 2 diabetes. 154 , 155 Several other CCR2 inhibitors (e.g., MK‐0812, JNJ‐41443532, JNJ‐17166864, AZD2423, etc.) have also been studied in clinical trials for CCL2/CCR2 axis‐related diseases, showing a favorable safety profile. 113 , 154 , 156 , 157 , 158 , 159
3.2. The CCL5/CCR5 axis
3.2.1. Characteristics of the CCL5/CCR5 signaling axis
When CCL5 (also known as RANTES: regulated upon activation normal T cell expressed and secreted) was first identified, it appeared to be a classic chemokine because of its ability to direct leukocytes to sites of inflammation. It has been well accepted that CCL5 can be secreted by most inflammatory cells, with monocytes and T cells being the most common sources of CCL5. 160 Although CCL5 can bind to several receptors, such as CCR1, CCR3–5, CD44, and GPR75, it has the highest affinity to CCR5. 161 The transcription of CCR5 (also known as CD195) is regulated by CREB‐1 and mRNA and protein expression are commonly observed in T‐lymphocytes, monocytes, macrophages, immature DCs, eosinophils, and microglia. 162 , 163 CCL5 is not the only ligand for CCR5 since CCR5 also binds to proteins with an N‐terminal extracellular tail, such as CCL3 (macrophage inflammatory protein [MIP]‐1α) and CCL4 (MIP‐1β). 163 Furthermore, CCR5 on the surface of CD4+ T cells is the most prominent coreceptor that assists HIV‐1 to infect cells through binding to GP120, therefore it is considered a prospective candidate for anti‐HIV therapies. 164 The CCL5/CCR5 axis has also been reported to be engaged in the activation of several signaling pathways, including JAK/STAT, PI3K/AKT/mTOR, HIF‐α, TGF‐β‐smad, and NF‐κB axes that are implicated in inflammation, angiogenesis, tumor cell proliferation, apoptosis, and metastasis. 165 , 166 , 167 In the TME, increased CCR5 levels may be the result of high CCR5 expression on the tumor cell surface or the aggregation of CCR5+ cells, such as monocytes, lymphocytes, adipocytes, and mesenchymal stem cells (MSCs). 161
3.2.2. The role of CCL5/CCR5 axis in cancer progression
The overexpression of CCL5 and/or its receptor CCR5 in various tumor cells (e.g. breast cancer, 168 acute lymphocytic leukemia, 169 multiple myeloma (MM), 170 Hodgkin lymphoma, 171 colorectal carcinoma 172 ) has long been elucidated. The expression of CCL5 is rare in normal ductal epithelium or benign breast tumor masses, but can be obtained during malignant transformation of cells. 173 Additionally, CCL5 is highly expressed in advanced triple‐negative breast cancer, 174 whereas CCL5 is not overexpressed in breast tissue from women with benign breast disease or those who have undergone breast reduction. 168 In vivo studies demonstrate that breast cancer cells stimulate MSCs to resecrete CCL5, which then acts in a paracrine manner on tumor cells. These researchers also found that lung metastasis and colonization of tumor cells increased after mice were given both breast cancer cells and MSCs, suggesting that CCL5 facilitates the metastatic ability of tumor cells. 175 The CCL5/CCR5 axis also has far‐reaching impact on the progression of hematologic malignancies. For example, higher levels of CCL5 are detected in the serum of patients with acute myeloid leukemia, who have a monocytic phenotype 176 or Fms‐like tyrosine kinase 3‐internal tandem duplication mutations. 177 Also, CCL5 secretion is increased when CD40 is cocultured with classical Hodgkin lymphoma cells or with MSCs derived from lymph nodes of Hodgkin lymphoma patients. 178 High levels of CCL5 in Hodgkin's lymphoma tissue are correlated with monocyte infiltration and poor prognosis. 171 In addition, in vivo studies have shown that inhibition of CCR5 by neutralizing antibodies or antagonists was able to retard the progression of MM, reduce osteolytic lesions, and inhibit osteoclastogenesis. 179 , 180 CCL5 and CCR5 are overexpressed in colorectal cancer (CRC) primary tumor cells as well as in metastasis cells of the liver and lung and are positively correlated with prognosis in CRC. 181 Furthermore, the CCR5/CCL5 axis also plays a critical role in the progression of multiple solid tumors, including gastric cancer (GC), 182 glioblastoma, 44 head and neck cancer, 183 lung cancer, 184 ovarian cancer, 185 and so on, often by promoting tumor cell proliferation, metastasis, and assisting in the establishment of immunosuppressive TME. 186
Briefly, the above results suggest that CCL5 and CCR5 are highly expressed in various tumor cells and promote tumor proliferation and metastasis by recruiting immune cells. Based on the finding that CCR5 antibodies retard tumor progression and inhibit angiogenesis, 179 , 180 we speculate that CCL5/CCR5 may be a potential target in cancer therapy.
3.2.3. The CCL5/CCR5 axis in inflammatory diseases
In addition to being involved in the progression of multiple tumors, aberrant CCL5/CCR5 interactions have been identified in multiple types of inflammation, 187 including AD, atherosclerosis, diabetes, hepatitis, and some viral infections.
With the intensive study by scientists of the pathology of atherosclerosis, it is now widely accepted that the disease is an inflammatory disease in which the continuous accumulation of macrophages in the intima and the rise in cytokine levels in peripheral blood and local tissues are important hallmarks. 188 It has been reported that the chemokine CCL5 assists in the recruitment of monocytes to the intima in the early stages of atherosclerosis and promotes the conversion of macrophages. 189 Recently, Jongstra‐Bilen et al. 190 showed that the expression of CCL5 mRNA as well as other ligands of the CCR5 receptor (CCL3 and CCL4) was induced in the aortic intima of Ldlr−/− mice 3 weeks after the onset of cholesterol‐rich diet‐induced hypercholesterolemia. Blockade of CCR5 significantly reduced the recruitment of monocytes at the lesion site, suggesting that CCL5 chemokine signaling through CCR5 is critical. Further, CCL5/CCR5 has been reported to attract T cells to the lesion site to release inflammatory factors (e.g. perforin‐1, IL‐6, selectin, tumor necrosis factor‐α [TNF‐α], etc.) and exacerbate inflammatory damage. 191 , 192 Besides, CCL5 levels were found to be elevated in damaged tissues of inflammatory bowel disease (IBD), which then induced an influx of inflammatory factors. Knockdown of the CCR5 gene reduced the recruitment and activation of CCR5(+) leukocytes in the mucosa, leading to greatly reduced symptoms of inflammation in a metastatic model of colitis. Similarly, the CCR5 inhibitor maraviroc attenuated the development of intestinal inflammation by selectively reducing the recruitment of CCR5(+) leukocytes. 193 Upon liver injury, resident Kupffer cells interact with hepatic cell populations and release chemokines to recruit circulating leukocytes, of which monocytes subsequently differentiate into macrophages in the liver, influencing the development of tissue inflammation. 194 It has been shown that the CCL5/CCR5 signaling pathway can accelerate the inflammatory process in the liver through the NF‐kB pathway. 195 A new study indicated that CCL5 could directly activate M1 polarization and impede M2 polarization through CCR1‐ and CCR5‐mediated activation of MAPK and NF‐κB pathways. Neutralizing antibodies or antagonists with CCL5 could greatly reduce liver injury and improve survival in drug‐injured mouse models. 196 Additionally, the CCL5/CCR5 axis is also involved in viral infections. COVID‐19, a pandemic currently plaguing people worldwide, is caused by SARS‐CoV‐2 infection. Recent studies have shown that inhibition of the CCL5/CCR5 axis by the monoclonal antibody leronlimab could alleviate the symptoms of patients with critical pneumonia. 197 Also, it was observed that the levels of inflammatory molecules such as CCL5, IL‐6, and TNF‐α in the serum of patients were reduced after anti‐CCR5 treatment. 198
3.2.4. Clinical trials of drugs targeting the CCL5/CCR5 axis
We have described above the seminal function of the CCL5/CCR5 axis on tumor progression in preclinical studies, which can be attenuated by knocking down CCL5, using antibody neutralization, and CCR5 antagonists to block ligand–receptor binding, setting the stage for drugs targeting the CCL5/CCR5 axis to enter clinical trials. Table 2 summarizes clinical trials of several CCR5 antagonists (maraviroc, vicriviroc, AZD5672, and leronlimab) for the treatment of metastatic CRC, advanced breast and pancreatic ductal carcinoma, and Kaposi's sarcoma associated with HIV infection (Table 2). In addition, maraviroc has been reported for the treatment of conditions other than tumors, including graft‐versus‐host disease, hypertriglyceridemia, stroke, and COVID‐19 (NCT00948753, NCT01133210, NCT03172026, and NCT04435522). Another dual chemokine receptor antagonist, cenicriviroc, which targets CCR2/CCR5 and inhibits monocyte migration, has also being evaluated for safety and efficacy in clinical trials. Cenicriviroc also inhibits HIV‐1 and HIV‐2 infection and has potent anti‐inflammatory and anti‐infective activity. 199 , 200 Clinical trials related to cenicriviroc have shown a role in the treatment of COVID‐19 (NCT04500418), nonalcoholic steatohepatitis (NCT03517540), primary sclerosing cholangitis (NCT02653625), type 2 diabetes mellitus (NCT02330549), and many other diseases.
3.3. The CCL19/CCL21/CCR7 axis
3.3.1. Brief introduction of the CCL19/CCL21/CCR7 axis
Chemokines CCL19 and CCL21 are highly expressed in lymphoid tissues and act upon binding to the same receptor CCR7. In primary lymphoid organs (e.g., thymus), CCR7+ thymocytes are subject to CCL19 and CCL21 chemotaxis and move between different tissue sections. In particular, the CCL19/CCL21/CCR7 axis plays an instrumental role in thymocyte differentiation, clonal selection, and negative selection. 201 Within secondary lymphoid organs (e.g., lymph nodes), CCL19 and CCL21 are continuously secreted by high endothelial veins and fibrous reticulocytes, which bind to CCR7 and then induce lymphocytes in the circulation to enter lymphatic vessels and lymph nodes. 201 CCR7 is mostly expressed on DCs and T cells for orchestrating immune responses. 202 , 203 The role of CCR7 in inducing targeted migration of lymphocytes in immune and inflammatory responses is well recognized. As the predominant class of antigen‐presenting cells, DCs can rapidly transform to a mature state upon antigen stimulation and upregulate major histocompatibility complex II and several costimulatory molecules, such as CD80, CD86, and CCR7. 204 The elevated levels of CCR7 allow DCs to sense the CCL19/CCL21 concentration gradient, move toward the highest concentration of this signaling protein, enter the lymph nodes for antigen‐presenting and then activate T cells. 205 , 206 CCR7 also controls the migration of thymocyte‐derived regulatory T cells and effector T cells from sites of inflammation to the second lymphoid organ (SLO) as well as their proper localization. 207 , 208 CCR7 expressed on the surface of cancer cells can direct malignant cells from the primary site into the lymphatic system by binding to appropriate signaling proteins, spreading in the body and eventually forming metastases in other tissues, which greatly increases the risk of death for patients. 209
3.3.2. Roles of the CCL19/CCL21/CCR7 axis in cancer progression
Because of the pivotal role of the CCL19/CCL21/CCR7 signaling axis in both antigen presentation and activation of T cell‐mediated responses, it has been postulated that increasing the levels of CCL19 and CCL21 within tumors could aid immunotherapy of cancer by enhancing the immune response to tumors. The first of these is to increase CCL19/21 concentration within the TME to sharpen the immune response to tumor. For instance, in mouse lung and colorectal models, intratumoral injection of CCL19 protein directly was shown to result in increased DCs as well as CD4+ and CD8+ T cells in the TME, promote enhanced secretion of proinflammatory factors, such as chemokines CXCL9 and CXCL10, as well as cytokines IL‐12, granulocyte‐macrophage colony stimulating factor (GM‐CSF), and IFN‐γ, and decrease levels of immunosuppressive molecules prostaglandin E2 and transforming growth factor‐β (TGF‐β); ultimately retarding tumor growth. 210 , 211 Similarly, in an orthotopic mouse model of breast cancer, intratumoral administration of CCL21 significantly increased the proportion of T cells, NK cells, and DCs within the tumor, reduced the size of the tumor and extended the survival time of tumor‐bearing mice. 212 Introduction of exogenous genes into tumor cells by recombinant plasmid techniques (e.g., lentiviral transfection) is another way to achieve elevated concentrations of CCL19/CCL21 protein. For example, mouse melanoma and ovarian cancer cells transfected respectively to express mouse CCL19 and then inoculated into C57BL/6 mice, showed significantly slower tumor growth compared with the control group without CCL19 expression. 213 In a mouse lung metastasis model, injection of endothelial progenitor cells overexpressing CCL19 by tail vein was able to reduce the number of lung metastatic nodules and prolong the survival of mice. 214 In addition, transfection of breast cancer MCF‐7 cells with CCL21 protein potentiated a range of functions of DCs, such as antigen uptake and presentation, migration, and antiapoptotic ability in vitro. 215
Therapeutic strategies that increase the intratumoral CCL19 or CCL21 levels may also be utilized in combination with other immunotherapies or nonimmunotherapies to improve antitumor efficacy. DNA vaccines have been a particularly attractive approach in recent years to enhance protective antitumor immunity by mobilizing leukocytes (e.g., cytotoxic T cells and NK cells) to the tumor. When combined with CCL21, the DNA vaccine showed higher efficacy than single treatment in a mouse model of orthotopic melanoma. 216 In addition, MSCs expressing CCL19 have been demonstrated to promote immune cell infiltration into TME and enhance the efficacy of anti‐PD‐L1 antibodies. 217 Therefore, targeting the CCL19/CCL21/CCR7 axis to inhibit lymphatic metastasis but maintaining a robust antitumor immune response has increasingly become a bright spot in tumor immunotherapy. 209
3.3.3. The CCL19/CCL21/CCR7 signaling axis in autoimmune diseases
RA is a chronic, systemic autoimmune disease of unknown etiology, characterized by intraarticular inflammatory cell infiltration and elevated proinflammatory cytokines, which can lead to multiple joint deformities and even loss of function. 218 Page et al. 219 detected DC subsets within the synovium of RA patients by immunohistochemical staining and showed that immature DCs were found only in the lining layer of the synovium, whereas mature DCs were found in the perivascular lymphatic aggregation zone. In addition, the expression of CCL19, CCL21, and CCR7 was only increased in the perivascular area, suggesting that the expression of these chemokines as well as CCR7 is associated with lymphocyte aggregation. In addition, upregulation of CCL19 and CCR7 gene expression was shown in psoriasis patients, whose key role is involved in establishing the typical inducible skin‐associated lymphoid tissue structures during disease progression, which can be clearly identified in the skin aggregates at the lesion site. 220 The majority of lymphocytes in healthy human cerebrospinal fluid (CSF) are CCR7+ central memory T cells, 221 whereas in patients with relapsed and progressive MS, increased expression of CCL19 is found in CSF, 222 implying that the CCL19/CCR7 axis may be involved in the normal immune surveillance of the brain. In experimental autoimmune encephalomyelitis (EAE) models, blocking CCR7 signaling has been proved to reduce the binding of T cells to inflammatory venues in EAE brain slices. 223 , 224 Collectively, these results suggest that the CCL19/CCR7 axis plays an important role in the progression of autoimmune diseases and is closely related to immune regulation at the site of the lesion. Blocking the CCL19/CCR7 axis is a potential therapeutic option for the treatment of autoimmune diseases.
3.3.4. Clinical trials of drugs targeting the CCL19/CCL21/CCR7 signaling axis
As mentioned above, modulating the function of the CCL19/CCL21/CCR7 axis may hold therapeutic potential for cancer as well as many inflammation‐related diseases, and there are several clinical trials currently underway.
One of the phase I trials is studying the side effects and optimal dose of autologous DC‐adenovirus CCL21 vaccine combined with intravenous pembrolizumab and seeing how well they work in treating patients with stage IV nonsmall cell lung cancer (NCT03546361). The researchers concluded that vaccines made from genetically modified viruses may help the body build an effective immune response to kill tumor cells, whereas monoclonal antibodies, such as pembrolizumab, may interfere with the ability of tumor cells to grow and spread, so giving a CCL21 genetically modified DC vaccine with pembrolizumab to treat patients with stage IV nonsmall cell lung cancer may work better. This clinical trial enrolled 24 patients, and up to 12 patients will participate in the dose escalation phase, during which 12 patients will be evaluated during the dose expansion. Two additional phase I clinical trials investigated the safety, toxicity and maximum tolerated dose of the autologous DC‐adenovirus CCL21 vaccine. The vaccine as an intratumoral injection was well tolerated by patients with advanced or recurrent nonsmall cell lung cancer and cutaneous melanoma (NCT00601094, NCT00798629). Besides, JBH492 is an antibody–drug conjugate that consists of an antibody against CCR7 on tumor cells combined with the steroid DM4, which leads to inhibition of tumor cell proliferation. A phase I/Ib Open‐label clinical trial (NCT04240704) is currently undertaken for investigating the preliminary effects of JBH492 monotherapy on non‐Hodgkin's lymphoma and chronic lymphocytic leukemia.
3.4. The CCL20/CCR6 axis
3.4.1. Major characteristics of the CCL20/CCR6 signaling axis
Since its discovery in the 1990s, CCL20 has gradually been attributed several names, such as MIP‐3α, liver and activation regulatory chemokine and exodus‐1, and it has gained increasing attention in molecular and cellular immunology. A very large number of cells in the body express CCL20, including CD8+ T cells, B lymphocytes, T helper 17 cells, macrophages, neutrophils, DCs, mast cells, and endothelial cells. 225 , 226 , 227 As an inflammatory chemokine, CCL20 strongly attracts lymphocytes and DCs to lymphoid tissues, thus participating in the formation and function of lymphoid tissues at various sites. CCL20 also has a predominant role in innate immunity, being upregulated by transcription factors, such as NFκB, 228 and induced by the TNF‐α and IL‐1β. 229 When the immune system is stimulated by inflammatory substances such as lipopolysaccharide, CCL20 is also quickly upregulated, leading to a prompt accumulation of immune cells in the spleen. 230 Currently, there is only one known receptor for CCL20, namely CCR6. 231 , 232 CCR6 is the hallmark chemokine receptor of immune cells. When CCL20 binds to its receptor CCR6, it not only participates in regulating the immune homeostasis of the body, but also serves to modulate the inflammatory response through the Th17 pathway, playing an essential role in the progression of autoimmune diseases and various malignant tumors. 59 , 233 , 234
3.4.2. The CCL20/CCR6 axis in cancer progression
With concerted, extensive efforts, the functional role played by the CCL20/CCR6 axis is gradually being unfolded, particularly in regulating cancer progression and metastasis within the TME. Ding et al. 235 revealed that tissue expression of CCL20 in clinical specimens of hepatocellular carcinoma (HCC) was related to tumor size, differentiation, recurrence, and vascular infiltration, and that high CCL20 expression was associated with worse PFS and OS in patients. In in vitro analysis, CCL20 greatly enhanced the invasive ability of triple‐negative breast cancer cell lines by increasing the secretion of matrix metalloproteinase (MMP)‐2 and MMP‐9; meanwhile, anti‐CCL20 antibody by intraperitoneal injection in a mouse breast cancer model could effectively inhibit the occurrence of bone metastases. 236 A study showed that serum CCL20 and IL‐17A levels were higher in CRC patients than those in healthy subjects, and the combination of CCL20 and IL‐17A signature curve analysis could differentiate CRC patients from healthy volunteers effectively. 237 In a case study of chemotherapy resistance to the FOLFOX regimen, CCL20 secreted by tumor cells was able to facilitate Tregs recruitment into the TME, which enhanced chemoresistance and closely correlated with poorer survival rates. 238 Additionally, IL‐4‐treated M2‐type macrophages highly express CCL20 and the CCL20/CCR6 axis promotes pancreatic cancer proliferation and distant metastasis via inducing epithelial–mesenchymal transition (EMT) in vivo. 239 Furthermore, CCL20 and/or CCR6 proteins were found to be highly expressed in lung, 240 cervical, 241 gastric, 242 ovarian cancer tissues, 243 and renal cell carcinoma, 244 facilitating tumor proliferation and directional migration through autocrine or paracrine modes.
3.4.3. The CCL20/CCR6 axis in autoimmune diseases
Similar to the CCL19/CCL21/CCR7 axis, the CCL20/CCR6 axis serves an essential role in the recruitment of inflammatory cells in the immune response and may contribute to a variety of autoimmune diseases, such as MS, IBD, psoriasis, and RA. 234 A study showed that stimulation of human epithelial cells with Th17 cytokines (IL‐17A, IL‐22, and TNF‐α) was able to induce a remarkable increase in CCL20 and CCR6 levels in their cultures in a dose‐ and time‐dependent manner. Similar results were obtained in in vivo tests, where subcutaneous injection of these Th17 cytokines also resulted in increased expression of CCL20 and CCR6, as well as infiltration of mature DCs and CD4+ T cells in the skin of mice. 245 , 246 In several animal models of psoriasis, the use of anti‐TNF‐α antibody infliximab, 247 anti‐CCR6 antibody, 248 and the anti‐CCL20 antibody, 249 respectively, significantly reduced regional infiltration of CCR6+CD4+ T cells and attenuated the inflammatory response in affected skin lesions. Significantly elevated levels of CCL20 were also found in the affected joints of RA patients, followed by a marked increase in CD4+CD45RO+CCR6+ memory T cells in the peripheral circulation of the patients. 250 Hirota et al. 251 found that in an animal model of RA, IL‐17‐producing Th17 cells predominantly expressed CCR6 and its ligand CCL20. Blockade of proinflammatory cytokines with infliximab or anti‐IL‐6R antibodies significantly decreased the CCL20 level and Th17 cells migration to the joints. 251 In the MS mouse model known as EAE, CCR6 and CCL20 expression was found to be upregulated in the spinal cord, 252 and CCR6‐deficient mice showed a milder development of EAE compared with wild‐type mice. 253 CCL20 and CCR6 also play an important contribution to the pathogenesis of IBD by regulating the delicate balance of Th17 and Tregs. 254 , 255 Data from these results indicate that the CCL20/CCR6 axis is implicated in the pathogenesis of autoimmune diseases and that antibodies or antagonists to CCR6 or CCL20 hold promise as an intriguing treatment tactic to ameliorate neuroinflammation and autoimmunity.
A human monoclonal antibody (MOR103) to GM‐CSF was well tolerated in randomized clinical trials and showed preliminary evidence of efficacy in RA (NCT01023256) but little improvement in the severity of MS (NCT01517282). Additional animal experiments and clinical trials are certainly needed to gain more insight into the pivotal roles of the CCL20/CCR6 axis in human disease.
3.5. The CXCL5/CXCR2 axis
3.5.1. Basics of the CXCL5/CXCR2 signaling axis
CXCL5, previously named epithelial neutrophil‐activating peptide‐78 (ENA‐78), is characterized by its ability to recruit neutrophils during the immune response, 27 , 256 contribute to angiogenesis and reshape the connective tissue. 257 CXCL5 is secreted by cells stimulated by the inflammatory cytokines IL‐1 or TNF‐α, whereas many immune cells (e.g., macrophages, 258 eosinophils 259 ) and nonimmune cells (e.g., mesothelial cells 260 and cancer‐associated fibroblasts 261 ) are also capable of expressing CXCL5. In addition, the secretion of CXCL5 and IL‐1β in TME interferes with the maturation of functional DCs, and CXCL5 expressed in eosinophils inhibits the secretion of IFN‐γ. 262 CXCL5 activates downstream signaling pathways by binding to the IL‐8B receptor, which later became known as CXCR2 and is highly expressed on neutrophils, although CXCR2 also binds to other ligands, including CXCL1–3 and CXCL6–8. 2
3.5.2. The CXCL5/CXCR2 signaling axis in cancer progression
The relationship between the CXCL5/CXCR2 signaling axis and carcinogenesis is increasingly being recognized. Abnormal elevations of CXCL5 and/or CXCR2 proteins have been noted in as many as 14 distinct malignant tumor types, including but not limited to CRC, 263 , 264 nonsmall cell lung cancer, 265 breast cancer, 266 bladder cancer, 267 nasopharyngeal carcinoma, 268 and so on. The expression intensity of CXCL5 was also detected in line with the malignancy degree, metastasis, and survival of cancer patients, which provides new potential for clinical application. 27 The CXCL5/CXCR2 signaling axis can also indirectly promote tumor progression via modulating the function of various immune cells within the TME. For instance, Zhou et al. 269 discovered that CXCL5 might contribute to the likelihood of tumor metastasis and recurrence in a mouse intrahepatic cholangiocarcinoma model via recruiting intratumoral neutrophils. Overexpression of CXCL5 on HCC stem cell‐like cells recruits immunosuppressive neutrophils and promotes lymphatic metastasis of tumor cells through binding CXCR2. 270 MDSCs in breast and renal cell carcinomas were shown to be related to tumor grade and positively correlated with CXCL5 expression. 271 , 272 In addition, a positive correlation was noted between CXCL5 expression and the number of CD8+ T cells, CD11b+MMP9+Ly6G+ granulocytes and macrophages in colorectal and pancreatic cancers. 263 , 273
Because of CXCL5's chemotactic effect on vascular endothelium, it is also considered to be a potent angiogenic factor. In animal studies of bladder, renal cell, and CRCs, CXCL5 secreted by tumor cells binds its receptor CXCR2 and activates the downstream AKT/NF‐κB signaling pathway, thereby stimulating the proliferation and aggregation of endothelial cells. 274 , 275 , 276 Data from several sources have identified the involvement of CXCL5 in the proliferation of other types of tumor cells, such as prostate cancer, 277 lung cancer, 265 cervical cancer, 278 hepatoblastoma, 279 osteosarcoma, 280 and papillary thyroid carcinoma. 281 In addition, the levels of CXCL5 were significantly higher in the lymph node metastatic tissue of head and neck squamous cell carcinomas than those in the primary tumor area. 282 , 283 CXCL5 expression was also stronger in HCC cell lines with high metastatic potential than that in their less aggressive counterparts, due to its ability to strongly activate the ERK1/2 and PI3K/AKT signaling pathways in tumor cells. 284 , 285
3.5.3. The CXCL5/CXCR2 axis in inflammatory diseases
Due to the strong chemotactic effect of CXCL5 on neutrophils, its role in regulating the inflammatory response has been widely noted. In a study of mechanical pain sensitization caused by ultraviolet B (UVB), Dawes et al. 286 explored changes in all chemokines and inflammatory factors in inflamed skin sites in humans and rats after UVB exposure. It was found that CXCL5 expression was most significantly elevated in the inflamed skin. Moreover, CXCL5 injection via the plantar area triggered a dose‐dependent decrease in mechanical pain threshold in rats, a result that reveals for the first time the mechanism of CXCL5 involvement in chronic inflammatory pain. 286 In addition, Xu et al. 287 found that the expression of CXCL5 and its receptor CXCR2 was elevated notably in spinal cord neurons of rats with chronic constriction injury of the sciatic nerve, and that the CXCL5/CXCR2 pathway regulated the phosphorylation of glycogen synthase kinase‐3 (GSK‐3β), which induced neuropathic pain in rats. Mild inflammation promotes retinal ganglion cell (RGC) survival and axonal regeneration after optic nerve (ON) injury with the involvement of infiltrating macrophages and neutrophils. Interestingly, the expression of Cxcl5 and Cxcr2 is increased when the ON and lens are injured. In retinal graft cultures, the addition of recombinant CXCL5 promoted RGC survival and neurite growth with an increase in the number of activated microglia, a phenomenon that was inhibited by the CXCR2 antagonist SB225002. 288
In pulmonary inflammation, the polymorphonuclear neutrophils (PMNs) recruited from the blood are essential for alveolar space defense and pathogen clearance. However, when PMNs are extensively translocated into the interstitial and alveolar spaces of the lung, they can lead to uncontrolled immune responses. 289 CXCR2 is obviously upregulated in airway epithelial cells during acute exacerbations of chronic obstructive pulmonary disease (COPD), and there is a significant positive correlation between CXCR2 expression and the number of neutrophils. Blocking CXCR2 reduced the proportion of neutrophils in bronchoalveolar lavage fluid in a mouse model. 290 Asthma is also a chronic inflammatory disease of the lungs, and although the role of neutrophils in stable asthma is unclear, a significant increase is observed in late responses to stimulation or asthma exacerbation, accompanied by increased levels of CXCL5 and CXCR2. 291 CXCL5 and its receptor CXCR2 were overexpressed in lung tissue of acute respiratory distress syndrome (ARDS) through the upregulation of MMP‐2 and MMP‐9. 292 In addition, CXCL5‐neutralizing antibodies effectively attenuated the inflammatory response, diffused alveolar injury and pulmonary edema, and reduced the expression levels of MMP‐2 and MMP‐9 in ARDS mouse models. 292 Further studies confirmed that CXCR2 is essential for the development of autoantibody‐mediated arthritis and that it upregulates the expression of the corresponding ligands CXCL1, CXCL2, and CXCL5. 293 , 294 , 295
The results of these studies suggest that blocking CXCL5/CXCR2 signaling appears to be a promising strategy for a wide range of inflammatory diseases and that in‐depth studies of this pathway are warranted.
3.5.4. Therapeutic strategies targeting the CXCL5/CXCR2 axis
In recent years, the CXCL5/CXCR2 axis has received increasing attention for its potential in cancer screening, tumor prognosis and personalized anticancer therapy. First, in vivo tests have shown that blocking CXCL5 or applying CXCR2 antagonists can slow disease progression by blocking the AKT/NF‐κB signaling pathway, thereby effectively reducing the blood supply to the tumor. 296 In addition, CXCL5‐neutralizing antibody‐treated mice showed reduced metastasis of breast cancer cells, mainly through inhibition of ERK/Snail signaling. 297 Meanwhile, therapeutic strategies targeting CXCL5/CXCR2 in combination with chemotherapy or immunotherapy have being explored. For example, in a mouse lung cancer model, CXCL5 antibodies synergistically enhanced the therapeutic effect of the tyrosine kinase inhibitor gefitinib through activating the AKT/NF‐κB and ERK/RSK1/2 signaling pathways. 298 Additionally, the CXCR2 antagonist SCH‐527123 not only inhibited tumor proliferation, invasion, and angiogenesis, but also enhanced the sensitivity of CRC to oxaliplatin treatment. 296 In a phase I clinical trial of patients with human epidermal growth factor receptor‐2 negative metastatic breast cancer, a 30% responsiveness was observed for orally administered noncompetitive CXCR1/2 antagonist reparixin adjuvant to paclitaxel, and there was no pharmacokinetic effect between the two drugs (NCT02001974). Navarixin (MK‐7123), a CXCR2/CXCR1 antagonist with oral bioavailability, is currently evaluated in clinical trials for its efficacy and safety in advanced/metastatic solid tumors, psoriasis, and COPD (NCT03473925, NCT00684593, NCT00688467, and NCT01006616). Danirixin, another selective CXCR2 antagonist with high affinity, is able to effectively inhibit the binding of CXCL8 (IL‐8) to CXCR2, and several clinical trials have focused on its improvement of lung function in patients with mild to severe COPD (NCT03136380, NCT03250689, NCT03034967, and NCT02130193).
3.6. The CXCL9, ‐10, ‐11/CXCR3 axis
3.6.1. Introduction to the CXCL9, ‐10, ‐11/CXCR3 axis
CXCL9, ‐10, and ‐11 are selective ligands of CXCR3. These ligands are primarily produced by cancer cells, endothelial cells, fibroblasts, and monocytes, and are commonly expressed at low levels in the homeostatic state, but are upregulated when stimulated by cytokines, such as TNF‐α and IFN‐γ. 299 , 300 CXCL9, which is referred to as monokine induced by gamma IFN (MIG), primarily mediates lymphocyte infiltration into the lesion site and inhibits tumor growth. 301 It has been reported that CXCL10, or IFN gamma‐inducible protein 10, is intensely induced by IFN‐α/β as well as IFN‐γ, but weakly induced by TNF‐α. 302 CXCL11 is considered the predominant CXCR3 agonist due to its stronger potency than CXCL9 or CXCL10, is a major chemoattractant for effector T cells, and stimulates calcium flux and receptor desensitization. 303 CXCR3 is predominantly expressed on CD4+ and CD8+ T lymphocytes. In the CD4+ subpopulation, CXCR3 is abundant on proinflammatory Th1 cells, but was also present on FOXp3+ Tregs. 304 It is widely believed that the CXCL9, ‐10, ‐11/CXCR3 signaling axis modulates immune cell polarization and activation and directs immune cells toward their focal sites, which include macrophages, cytotoxic lymphocytes (CTL), and NK cells, among others. 300 , 305
3.6.2. Targeting CXCL9, ‐10, ‐11/CXCR3 axis for cancer therapy
Recent studies have reported that CXCR3 expression levels in clinical tumor specimens correlate with metastatic potential and patient prognosis, making it feasible to use the CXCL9, ‐10, ‐11/CXCR3 axis as a predictor of treatment outcomes, although the relationship between expression of these three ligands and tumor recurrence or metastasis remains controversial. 31 , 306 , 307 Using shRNA‐mediated silencing, Wightman et al. 308 determined that CXCL10/CXCR3 coexpression increased tumor cell metastasis and recurrence in both in vitro and in vivo analyses of B16F1 melanoma. Simultaneous reduction of CXCR3, CXCL9, and/or CXCL10 expression suppresses cancer metastasis rates in melanoma, 309 CRC, 310 and breast cancer models. 311 Data from a study on radiotherapy for head and neck cancer showed that circulating lymphocyte populations correlated with serum CXCL10 concentrations, but not with CXCL9. 312 Mitsuhashi et al. 313 noted that the pretreatment serum concentrations of CXCL10 and CXCL11 in lung cancer patients receiving anti‐PD‐1 antibodies were significantly correlated with clinical outcomes, and identified tumor‐derived CXCL10/11 as a potential circulating biomarker for monitoring drug treatment sensitivity. However, some studies have found a contrary relationship between the expression levels of CXCL9/CXCL10 and poor prognosis, drawing negative conclusion for clinical development. 314 , 315
Drugs that enhance the expression of paracrine CXCL9, ‐10, and ‐11 and inactivate CXCR3 expression on cancer cells have exhibited antitumor activity in several tumor models. It has been suggested that blocking PD‐1 checkpoint in mouse colon cancer cells (MC38) resulted in increased levels of IFN‐γ‐induced chemokines CXCL9 and CXCL10. In contrast, reducing CXCL9 and CXCL10 expression in animal models significantly decreased the efficacy of anti‐PD‐1 drugs and limited the aggregation of CD8+ T cells within the TME. 316 In lung and renal cell carcinoma models, intratumoral injection of CXCL9 or CXCL10 proteins, respectively, reduced neovascularization and delayed tumor growth by inducing tumor‐infiltrating CXCR3+ monocytes. 317 , 318 Additionally, a novel CXCL10 fusion protein (IP10‐scFv) coadministered with CTLs successfully induced tumor‐infiltrating lymphocytes and prolonged survival in mice. 319 CXCL11 is a controversial target for cancer treatment because it helps induce Tregs migration. In a mouse model of mesothelioma, selective lysing virus transfected with CXCL11 was reported to enhance CTL and NK cell infiltration into TME, but not CD4+ T cells. 320 On the contrary, CXCL11 expression was remarkably upregulated in rectal adenocarcinoma, but was not correlated with a better prognosis in cancer patients. 321 Pharmacological antagonism of AMG487 against CXCR3 was effective in inhibiting the proliferation of osteosarcoma and colon cancer cells in vitro and attenuated lung metastasis in mouse tumor models. 322 , 323
3.6.3. Roles of the CXCL9, ‐10, ‐11/CXCR3 axis in neurological diseases
It has been shown that CXCR3 and its ligands are expressed at high levels in CSF and peripheral blood of patients with neurological disorders and are potentially useful regulators of neuroinflammatory response. 324 , 325 MS is a common chronic inflammatory disease of the CNS characterized by the damage of myelin and oligodendrocytes by inflammatory cells. Early in 1999, Balashov et al. 326 reported that CXCL10 was expressed by astrocytes in MS brain lesions and that CXCR3+ T cells were found to be increased in the blood of relapsed/advanced MS patients. However, treatment of rats with EAE with monoclonal antibodies against CXCL10 resulted in an increase in infiltrating CD4+ Th1 cells in the CNS and an exacerbation of disease grading in animal models, suggesting that CXCL10 may play a specific inhibitory role in Th1‐mediated disease development. 327 In another study, Chung and Liao 328 revealed no significant differences in time to disease onset and severity between CXCR3‐deficient (CXCR3–/–) mice and wild‐type mice. However, pathological sections revealed more severe and extensive demyelination phenomena and axonal damage in CXCR3–/– mice. Furthermore, in vitro studies indicated that astrocytes respond to infection by upregulating CXCL10 mRNA expression and releasing CXCL10 into the supernatant, which is completely abolished by CXCR3 antagonists. 329 Gliomas account for the majority of CNS tumors, with glioblastoma progression involving glioma stem cells (GSCs) that are refractory to diverse therapeutic options. Shono et al. 330 reported that CXCL10 and CXCR3 were increased in GSCs and a malignant glioma model, celecoxib inhibited the expression of CCL2 and CXCR3 in an NF‐κB‐dependent manner, and in addition, silencing of CCL2 led to a decrease in GSC viability. Sharma et al. 331 used high‐throughput tissue microarrays to detect CXCR3 and CXCL10 immuno‐expression in glioblastoma multiforme (GBM) and diffuse astrocytoma (DA) tissues. Their results showed that among 129 analyzable samples, strong CXCR3 and CXCL10 expression was observed in 72.7 and 50.7% of GBM cases, respectively, whereas CXCR3 and CXCL10 expression in DA cases was 31.8 and 24.5%, respectively. Moreover, CXCR3 antagonist NBI‐74330 inhibited the growth of GL261 gliomas and increased the median survival time of CXCR3–/– mice, whereas NBI‐74330 did not affect the infiltration of CXCR3+ NK and NKT cells within gliomas, suggesting that CXCR3 may not be a major pathway for NK and NKT cells to enter gliomas. 332 Xia et al. 333 used tissues from AD patients for immunohistochemistry to demonstrate extensive expression of CXCR3 in brain structures. In addition, they found that CXCL10‐positive astrocytes were also greatly increased in AD patients compared with controls. Recently, an experiment investigating CXCR3 antagonists in the amyloid precursor protein (APP)/presenilin 1 (PS1) transgenic mouse model of AD showed that CXCR3 antagonists increased Aβ phagocytosis in microglia and ameliorated behavioral deficits in diseased mice, suggesting that CXCR3 axis mediates AD‐like pathology in APP/PS1 mice and could be a therapeutic candidate for AD. 334
Taken together, the aforementioned studies indicate that targeting the CXCL9, ‐10, ‐11/CXCR3 axis may have potential for treatment of both cancers and neurodegeneration.
3.7. The CXCL12–CXCR4/CXCR7 signaling axis
3.7.1. Physiological role of the CXCL12–CXCR4/CXCR7 axis
When chemokines bind to their cognate receptors on target cells, they will perform a range of important functions within the tissues. CXCL12, also known as stromal cell‐derived factor‐1, is the only ligand for CXCR4, which is predominantly secreted by stromal fibroblasts, osteoblasts, and vascular endothelial cells. CXCL12 has been highly conserved during evolution and is one of the most primitive chemokines. 335 By interacting with CXCR4 and ACKR3, CXCL12 is essential for the development of the brain, cardiovascular system, hematopoietic organs, and reproductive cells, and thus the chemokine network of CXCL12/CXCR4/ACKR3 signaling is necessary for life. 336 Studies have shown that CXCL12 secretion is also involved in a range of pathological processes, such as cell damage, heart failure, and inflammation during chemotherapy or after organ irradiation. 336 Increased CXCL12 expression has also been observed in the hypoxic and proangiogenic environment within tumors or during autoimmune diseases. 337 Functioning as a GPCRs, CXCR4 is primarily expressed on the surface of endothelial mature cells, precursor cells, and pericytes. 338 Many factors are implicated in the regulation of CXCR4 expression, including hypoxia, stress, and injury, with HIF‐1α being the most important regulator. 338 Also, CXCR4 expression is influenced by IL‐17A released from T cells 339 as well as IL‐5, IFN‐γ, and TGF‐β secreted by stromal cells. 340 Although CXCR7 is a member of the GPCR family, it does not induce cellular signaling mediated by G protein subunits (α, β, and γ), its main role is to establish and maintain the gradient of its ligands CXCL11 and CXCL12 on both sides of the cell membrane. 341 The process for activating CXCR7 is as follows: CXCR7 binds to the ligand and the CXCR7–ligand complex is subsequently internalized by the cell membrane, a process that leads to the degradation of the ligand while the receptor moves back to the cell membrane.
The CXCL12/CXCR4 signaling axis exerts a regulatory effect on the secretion of cytokines and chemokines, as it is shown to induce the expression of TNF‐α, IL‐1α/β, CXCL5, and some other chemokines, but it does not affect the expression of IL‐2 or IFN‐γ. 336 The CXCL12/CXCR4 axis also modulates vascular endothelial adhesion and actin polymerization responses, as well as accommodates the migration of bone MSCs (BMSCs) underneath and beneath leukemic cells. 342 , 343 The two receptors CXCR4 and CXCR7 can interact to form a heterodimer, which results in enhanced CXCL12‐induced signaling via G proteins. Compared with cells transfected with CXCR4 only, coexpression of CXCR4 and CXCR7 on HEK293 cells resulted in higher calcium flux and more β‐arrestin recruitment through activation of downstream ERK signal cascade. 344 , 345 The interactions between CXCL12, CXCR4, and CXCR7 display considerable complexity under physiological conditions. When this interaction is disrupted, regulation of this axis can influence the progression of diseases including cancer, CNS, cardiac, and autoimmune diseases. 336 , 346
3.7.2. The CXCL12–CXCR4/CXCR7 contributes to cancer progression
Numerous studies have explored the role of the CXCL12–CXCR4/CXCR7 axis in various cancer types in recent years. 346 , 347 , 348 Under the regulation of the CXCL12–CXCR4/CXCR7 signaling axis, cancer tissues can exhibit enhanced cell migration and proliferation by activating signaling cascades within tumor cells, as well as regulate angiogenesis and induce metastasis through the vascular endothelial growth factor (VEGF). Thus, drugs targeting CXCR4 and/or CXCR7 can influence cancer progression pathways by regulating the CXCR4/CXCR7–CXCL12 axis. Several studies have reported that CXCL12 can stimulate the proliferation of various tumor cell lines, including melanoma, 349 glioma, 350 small cell lung cancer, 351 gastric cancer, 352 pancreatic cancer, 353 and CRC. 354 Moreover, CXCL12/CXCR4 regulates EMT in sacral chondrosarcoma, 355 oral squamous cell carcinoma, 356 and glioblastoma. 357 Furthermore, the CXCL12/CXCR4 axis is also critical for tumor cell metastasis and drug resistance in cancer therapy. 358 , 359 , 360 Inhibition of the PI3K/AKT/NF‐κB signaling pathway by downregulating CXCR4 significantly reduced cell proliferation and increased apoptosis in osteosarcoma cells. 361 Notch positively controls CXCL12/CXCR4 function in myeloma cell lines, and in vivo blockade of Notch markedly limits myeloma cell infiltration into bone marrow of mouse xenografts. 362 Interference with CXCR4 expression using CXCR4 antagonist or lentivirus shRNA can effectively inhibit tumor cell proliferation and invasion in breast cancer, 363 human hilar cholangiocarcinoma, 364 laryngeal squamous carcinoma, 365 and esophageal carcinoma. 366 Long et al. 367 investigated the ability to reduce the proliferation of HEC‐1‐A cells after inhibiting higher mRNA and protein expression levels of CXCR4 and CXCR7 in endometrial adenocarcinoma by RNA interference. Apart from these findings, immunohistochemistry showed that elevated expression of CXCR7 was related to increased tumor grade of prostate cancer, and its overexpression also increased the release of VEGF and the proinflammatory cytokine IL‐8, which might promote the invasiveness of tumor cells. 368 , 369 Importantly, enhanced CXCR7 expression was associated with poor prognosis in patients with prostate cancer and glioblastoma, suggesting that CXCR7 may serve as a prognostic biomarker for these two tumors. 370 , 371 In an animal model of prostate cancer, coadministration of CCX771 (a CXCR7 inhibitor) and enzalutamide remarkably inhibited tumor growth and macrovascular formation, thus suppressing the drug resistance of enzalutamide. 372
3.7.3. Other diseases involved in CXCL12–CXCR4/CXCR7 regulation
CXCL12 is involved in the release of inflammatory factors during the immune process and may influence the pathogenesis of atherosclerosis and osteoarthritis. 373 , 374 CXCL12 from the subchondral layer binds to CXCR4 in chondrocytes and induces articular cartilage degeneration by promoting a shift of TGF‐β receptor type I (TβRI) from activin receptor‐like kinase 5 (ALK5) to ALK1 in chondrocytes. 373 Gao et al. 375 found that CXCL12 interacted with CXCR4 to activate the GSK‐3β/β‐catenin/TCF21 pathway, thereby reducing plasma HDL‐C levels and the efficacy of reverse cholesterol transport, inhibiting ABCA1‐dependent cholesterol efflux from macrophages, and exacerbating atherosclerosis. During embryonic development, CXCR7 expression is critical for cardiovascular system function. In vitro studies have shown that CXCL12 induced migration of oligodendrocyte precursor cells and angiogenesis of HUVECs through CXCR4‐activated MEK/ERK and PI3K/AKT pathways. Knockdown of CXCR4 could also reverse these phenomena and downregulated the MEK/ERK and PI3K/AKT pathways. 376 , 377 Furthermore, CXCR7 also facilitates cardiac remodeling by activating endothelial cell proliferation and angiogenesis. 378 The gradient of CXCL12 directs CXCR4‐positive neural stem cells to differentiate into neuronal cells, including oligodendrocytes, astrocytes, and so on, toward the damaged tissue. 379 Similarly, the redistribution of CXCL12 due to the increased expression of CXCR7 at the marginal sites of endothelial cells explains the pathogenesis of MS. 380 Several modulators that have entered clinical trials targeting the CXCL12–CXCR4/CXCR7 signaling axis are summarized in Table 2.
3.8. The CXCL13/CXCR5 axis
3.8.1. A brief introduction of the CXCL13/CXCR5 axis
Originally known as B cell‐inducible chemokine 1 or B lymphocyte chelator, CXCL13 forms a unisexual ligand–receptor pair with CXCR5 that is essential for the homeostatic organization of the B cell compartment of secondary lymphoid tissue. CXCL13 is secreted constitutively by stromal cells (e.g., follicular high endothelial vein cells) in the B‐cell region of secondary lymphoid tissues (follicles), thus correctly orchestrating CXCR5+ T/B lymphocytes and macrophages from the blood into the follicles. 381 , 382 , 383 CXCR5 is also named Burkitt's lymphoma receptor 1 because it was initially isolated from Burkitt's lymphoma and its expression is detected on tonsillar B cells as well as in all peripheral blood. Similar to other chemokine receptors, CXCR5 is kinetically modulated on T cells and is upregulated on memory/effector T cells following T cell receptor stimulation, whereas IL‐2 causes its downregulation. 384 , 385 CXCR5 shares 40% amino acid homology with CXCR1, so when CXCL13 activates CXCR5, this signaling axis can lead to intracellular calcium ion influx and induce the activation of several intracellular signaling cascades, such as PI3K/AKT, MAPK/ERK, and Rac pathways, playing a role in immune disorders as well as tumor progression. 384 , 386
3.8.2. Roles of the CXCL13/CXCR5 axis in cancer progression
Abnormal activation of CXCL13/CXCR5 signaling has been implicated in the development of several advanced solid cancers as well as hematological malignancies. For instance, expression of CXCL13 and/or CXCR5 correlates significantly with tumorigenesis, and CXCL13 is considered a predictive factor for lung cancer progression and early diagnosis. 387 , 388 A recent interesting study focused on the correlation between CXCL13 and patient prognosis in patients with squamous lung cancer receiving corticosteroids and chemotherapy. 389 This study determined that perivascular CXCL13‐positive niches induced the forming of tertiary lymphoid structures, which was correlated with better patient survival. However, studies also have shown that treatment with steroids compromised the formation of these tertiary lymphoid structures, compared with those who were untreated. 389 There is also evidence that CXCL13 secreted by PD‐1 high expressing tumor infiltrating CD8+ lymphocytes helps to induce other immune cell subsets into TME, including B lymphocytes and T follicular helper cells (TFH cells). Moreover, CXCL13 and CXCL13+ immune cells in the TME of nonsmall cell lung cancer strongly predicted patients’ response to anti‐PD‐1 therapy, correlating with improved durable response and prolonged OS. 390 It seems that there is a strong correlation between the CXCL13/CXCR5 axis and breast cancer progression, which have been verified in several studies. Using microarray analysis, CXCL13 was found to be the most overexpressed chemokine in breast cancer tissues when normal breast tissues were used as controls, while a positive correlation was identified between the expression of CXCL13 and CXCR5. 391 In addition, expression levels of CXCL13 and CXCR5 could be potential biomarkers for diagnosis and prognosis for breast cancer. 392 , 393 , 394 Of interest, the results of a recent in vitro study have suggested that an anti‐CXCL13 antibody reduced the levels of activated ERK and cyclin D1 and potentiated the cleavage of caspase‐9, thereby reducing the viability of MDA‐MB‐231 breast cancer cells. 395 Furthermore, treatment with the anti‐CXCL13 antibody inhibited ERK activation and slowed tumor growth in a 4T1 mouse model of breast cancer, thus providing a rationale for clinical trials targeting CXCL13. 396 Immunohistochemical analysis of specimens from various tumors (including ovarian cancer, 397 CRC, 398 prostate cancer, 399 melanoma, 400 clear renal cell carcinoma, 401 and nasopharyngeal carcinoma 402 ) showed that CXCL13 and CXCR5 were markedly elevated in tumors, as compared with normal tissue, and contributed to the ability of tumor cells to proliferate, migrate, and invade, ultimately affecting tumor progression, metastasis, and OS. Elucidating CXCL13/CXCR5 signaling effects and downstream signaling pathways will help investigate the molecular mechanisms that control tumor progression and responses to targeted therapies, accelerating the translation of drug research into clinical precision medicine.
3.8.3. The CXCL13/CXCR5 axis in autoimmune and infectious diseases
The formation of CXCL13/CXCR5‐derived tertiary lymphoid structures has been associated with the evolution of a divergent range of diseases, including MS, myasthenia gravis, Sjögren's disease, RA, bullous pemphigoid, Graves thyroiditis, and infectious diseases. We already know that abnormal lymphocyte aggregates develop within the affected synovial membrane in patients with RA, and in fact, intense expression of CXCL13 mRNA and protein was detected in areas of B‐lymphocyte aggregation, thus facilitating endothelial progenitor cell homing and angiogenesis during RA progression. 403 A similar role of CXCL13 and/or CXCR5 in regulating the formation of ectopic lymphoid structures was subsequently found in myasthenia gravis, 404 Sjögren's syndrome, 405 and SLE. 406 Further, the expression levels of CXCL13 are associated with the progression and unfavorable prognosis of the above‐mentioned diseases and has been suggested as a biomarker to predict the progression of these diseases. Indeed, the CXCL13/CXCR5 axis not only affects the abnormal activity and differentiation of B cells, but also involves the drive of TFH cells. For example, CXCR5+/CD4+ T cells in circulation were similar to TFH cells and have been noted in SLE patients, and they are also engaged in promoting the differentiation of pathological B cells and are associated with disease progression. 407
Before the cloning of CXCL13, CXCR5 was determined to be as a coreceptor required during HIV‐2 infection of host cells, rendering TFH cells vulnerable to viral infection. During HIV infection, the expression of CXCL13 and CXCR5 was demonstrated to be dysregulated, for example, as HIV infection progressed, the number of CXCR5+ B lymphocytes decreased, whereas plasma levels of CXCL13 increased. 408 Subsequent studies confirmed the elevation of serum CXCL13 levels during chronic HIV infection and demonstrated an association of CXCL13 secretion with both viral load and disease progression. 408 , 409 , 410 Li et al. 411 revealed that in patients with chronic hepatitis B (CHB), CXCR5+CD8+ T cells were partially depleted but possessed greater antiviral capacity than the CXCR5− subpopulation; furthermore, the CXCL13 from CHB patients promoted the infiltration of intrahepatic CXCR5+CD8+ T cells, a subpopulation that produces anti‐HBV‐specific IFN‐γ and IL‐21 and improves treatment response in CHB patients. Notably, administration of CXCR5+CD8+ T cells to CHB mice resulted in a significant reduction in HBsAg expression in the same study. Intriguingly, overexpression of CXCL13 was detected in the muscles of monkeys chronically infected with the Lyme disease pathogen Borrelia burgdorferi, but the bacterium did not appear to have an effect on plasma levels of CXCL13 chemokines. In contrast, once B. burgdorferi infected the CNS, constitutively elevated levels of CXCL13 were observed in the CSF, contributing to the formation of ectopic lymphoid tissue within the CNS. 412 Neurosyphilis is often an advanced manifestation of a long‐term infection, usually presenting as stroke‐like symptoms or chronic meningitis. CXCL13 is also thought to be implicated in infection with Treponema pallidum (the causative agent of syphilis), with CXCL13 levels within the CSF of syphilis patients being 100‐fold higher than those in uninfected individuals. Pathologically, activation and enrichment of B cells and ectopic germinal centers were observed in the CNS of neurosyphilis patients, suggesting that T. pallidum infection leads to overexpression of CXCL13 in the CFS, causing a strong humoral response that promotes destruction of neural tissue. 413
4. CONCLUSIONS
The chemokine system is an extraordinarily complex defense entity in the body, consisting of a huge array of interplaying ligands, receptors, and regulatory molecules that are involved in various cellular processes. Among them, chemotaxis of immune cells (especially lymphocytes) is its core biological function, but its impact goes far beyond that. The contribution of the chemokine network in physiopathological processes is enormous, involving organ development, immune surveillance, inflammation, infection, as well as innate and adaptive immune responses. It has conclusively been shown that the chemokine/chemokine receptor axis has a tumorigenic role in many different cancer models and clinics (Figure 4) and is also involved in immunosuppression and protective TME formation and can serve as prognostic bioindicators for many hematologic tumors as well as solid tumors. Modulation of the expression of chemokines or their homologous receptors on tumor cells or immune cells in TME provides a basis for the exploitation of new drugs for clinical evaluation in cancer immunotherapy. In fact, in addition to its vital role in tumors, almost all inflammatory diseases involve chemokines and their receptors in one way or another. Nevertheless, many unknown aspects of the role of chemokines and chemokine receptors in human disease remain to be unfolded, which necessitates strong efforts in much more basic animal studies as well as clinical researches.
FIGURE 4.
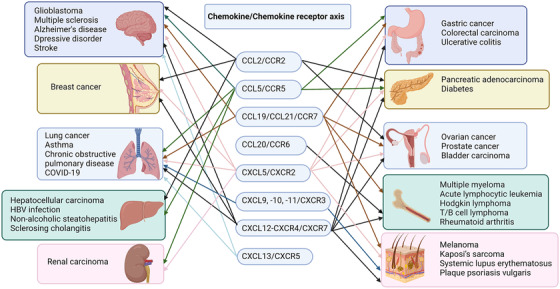
Chemokines/chemokine receptor axis in cancers and inflammatory diseases. Multiple chemokines/chemokine receptor axes play important roles in different tumor types and inflammation‐related diseases. Almost all organs of the body are regulated by the chemokines/chemokine receptor axes that predominantly affect the progression of tumors and the immune response during inflammation. Development of drugs targeting chemokines or their receptors is a potential strategy for the treatment of these diseases (the figure was created using biorender.com)
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors read and approved the final manuscript. H. L. wrote the initial manuscript and created the tables and figures. M. W. and X. Z. revised the manuscript and approved the final version.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
This work is supported by National Major Scientific and Technological Special Project for the “Significant New Drugs Development” (No. 2018ZX09201018‐013) and the National Science and Technology Major Projects for Major New Drugs Innovation and Development (No. 2018ZX09733001‐004).
Li H, Wu M, Zhao X. Role of chemokine systems in cancer and inflammatory diseases. MedComm. 2022;3:e147. 10.1002/mco2.147
Contributor Information
Min Wu, Email: min.wu@und.edu.
Xia Zhao, Email: xiazhaoscu@126.com.
DATA AVAILABILITY STATEMENT
The data included in this article are available upon request from the corresponding authors.
REFERENCES
- 1. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659‐702. [DOI] [PubMed] [Google Scholar]
- 2. Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonecchi R, Graham GJ. Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front Immunol. 2016;7:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCully ML, Kouzeli A, Moser B. Peripheral tissue chemokines: homeostatic control of immune surveillance T cells. Trends Immunol. 2018;39(9):734‐747. [DOI] [PubMed] [Google Scholar]
- 5. Anders HJ, Romagnani P, Mantovani A. Pathomechanisms: homeostatic chemokines in health, tissue regeneration, and progressive diseases. Trends Mol Med. 2014;20(3):154‐165. [DOI] [PubMed] [Google Scholar]
- 6. Mollica Poeta V, Massara M, Capucetti A, Bonecchi R. Chemokines and chemokine receptors: new targets for cancer immunotherapy. Front Immunol. 2019;10:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Legler DF, Thelen M. Chemokines: chemistry, biochemistry and biological function. Chimia (Aarau). 2016;70(12):856‐859. [DOI] [PubMed] [Google Scholar]
- 8. Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944‐2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540‐550. [DOI] [PubMed] [Google Scholar]
- 10. Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res. 2014;2(12):1125‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mukaida N, Sasaki S, Baba T. Chemokines in cancer development and progression and their potential as targeting molecules for cancer treatment. Mediators Inflamm. 2014;2014:170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity. 2021;54(5):859‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu H, Yang Z, Lu W, et al. Chemokines and chemokine receptors: a new strategy for breast cancer therapy. Cancer Med. 2020;9(11):3786‐3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18(3):121‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation‐associated diseases in organs. Oncotarget. 2018;9(6):7204‐7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol. 2015;7(5):a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burg JS, Ingram JR, Venkatakrishnan AJ, et al. Structural biology. Structural basis for chemokine recognition and activation of a viral G protein‐coupled receptor. Science. 2015;347(6226):1113‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fox JC, Thomas MA, Dishman AF, et al. Structure‐function guided modeling of chemokine‐GPCR specificity for the chemokine XCL1 and its receptor XCR1. Sci Signal. 2019;12(597):eaat4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao S, Wu B, Stevens RC. Advancing chemokine GPCR structure based drug discovery. Structure. 2019;27(3):405‐408. [DOI] [PubMed] [Google Scholar]
- 21. Soulika AM, Pleasure DE. Chemokines. Encyclopedia of the Neurological Sciences. 2014:764‐769. [Google Scholar]
- 22. Yung SC, Farber JM. Chemokines. Handbook of Biologically Active Peptides. 2013:656‐663. [Google Scholar]
- 23. Wang L, Zhang YL, Lin QY, et al. CXCL1‐CXCR2 axis mediates angiotensin II‐induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J. 2018;39(20):1818‐1831. [DOI] [PubMed] [Google Scholar]
- 24. Lepsenyi M, Algethami N, Al‐Haidari AA, et al. CXCL2‐CXCR2 axis mediates αV integrin‐dependent peritoneal metastasis of colon cancer cells. Clin Exp Metastasis. 2021;38(4):401‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun X, He X, Zhang Y, et al. Inflammatory cell‐derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast‐hijacked cancer escape mechanism. Gut. 2022;71(1):129‐147. [DOI] [PubMed] [Google Scholar]
- 26. Domschke G, Gleissner CA. CXCL4‐induced macrophages in human atherosclerosis. Cytokine. 2019;122:154141. [DOI] [PubMed] [Google Scholar]
- 27. Zhang W, Wang H, Sun M, et al. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target. Cancer Commun (Lond). 2020;40(2‐3):69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X, Dai Y, Zhang X, et al. CXCL6 regulates cell permeability, proliferation, and apoptosis after ischemia‐reperfusion injury by modulating Sirt3 expression via AKT/FOXO3a activation. Cancer Biol Ther. 2021;22(1):30‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang YH, Shen CY, Lin SC, et al. Monocytes secrete CXCL7 to promote breast cancer progression. Cell Death Dis. 2021;12(12):1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han ZJ, Li YB, Yang LX, Cheng HJ, Liu X, Chen H. Roles of the CXCL8‐CXCR1/2 axis in the tumor microenvironment and immunotherapy. Molecules. 2021;27(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neo SY, Lundqvist A. The multifaceted roles of CXCL9 within the tumor microenvironment. Adv Exp Med Biol. 2020;1231:45‐51. [DOI] [PubMed] [Google Scholar]
- 32. Karin N, Razon H. Chemokines beyond chemo‐attraction: cXCL10 and its significant role in cancer and autoimmunity. Cytokine. 2018;109:24‐28. [DOI] [PubMed] [Google Scholar]
- 33. Gao Q, Zhang Y. CXCL11 signaling in the tumor microenvironment. Adv Exp Med Biol. 2021;1302:41‐50. [DOI] [PubMed] [Google Scholar]
- 34. Janssens R, Struyf S, Proost P. The unique structural and functional features of CXCL12. Cell Mol Immunol. 2018;15(4):299‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao SH, Liu SZ, Wang GZ, Zhou GB. CXCL13 in cancer and other diseases: biological functions, clinical significance, and therapeutic opportunities. Life (Basel). 2021;11(12):1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cereijo R, Gavaldà‐Navarro A, Cairó M, et al. CXCL14, a brown adipokine that mediates brown‐fat‐to‐macrophage communication in thermogenic adaptation. Cell Metab. 2018;28(5):750‐763.e6. [DOI] [PubMed] [Google Scholar]
- 37. Broxmeyer HE, Cooper SH, Ropa J. CXCL15/Lungkine has suppressive activity on proliferation and expansion of multi‐potential, erythroid, granulocyte and macrophage progenitors in S‐phase specific manner. Blood Cells Mol Dis. 2021;91:102594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Korbecki J, Bajdak‐Rusinek K, Kupnicka P, et al. The role of CXCL16 in the pathogenesis of cancer and other diseases. Int J Mol Sci. 2021;22(7):3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiao S, Xie W, Zhou L. Mucosal chemokine CXCL17: what is known and not known. Scand J Immunol. 2021;93(2):e12965. [DOI] [PubMed] [Google Scholar]
- 40. Liu SS, Liu C, Lv XX, et al. The chemokine CCL1 triggers an AMFR‐SPRY1 pathway that promotes differentiation of lung fibroblasts into myofibroblasts and drives pulmonary fibrosis. Immunity. 2021;54(9):2042‐2056.e8. [DOI] [PubMed] [Google Scholar]
- 41. Lv LL, Feng Y, Wen Y, et al. Exosomal CCL2 from tubular epithelial cells is critical for albumin‐induced tubulointerstitial inflammation. J Am Soc Nephrol. 2018;29(3):919‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao X, Gu M, Xu X, et al. CCL3/CCR1 mediates CD14(+)CD16(−) circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthr Cartil. 2020;28(5):613‐625. [DOI] [PubMed] [Google Scholar]
- 43. Mukaida N, Sasaki SI, Baba T. CCL4 signaling in the tumor microenvironment. Adv Exp Med Biol. 2020;1231:23‐32. [DOI] [PubMed] [Google Scholar]
- 44. Kranjc MK, Novak M, Pestell RG, Lah TT. Cytokine CCL5 and receptor CCR5 axis in glioblastoma multiforme. Radiol Oncol. 2019;53(4):397‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li F, Du X, Lan F, et al. Eosinophilic inflammation promotes CCL6‐dependent metastatic tumor growth. Sci Adv. 2021;7(22):eabb5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xie C, Ye F, Zhang N, Huang Y, Pan Y, Xie X. CCL7 contributes to angiotensin II‐induced abdominal aortic aneurysm by promoting macrophage infiltration and pro‐inflammatory phenotype. J Cell Mol Med. 2021;25(15):7280‐7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Farmaki E, Kaza V, Chatzistamou I, Kiaris H. CCL8 promotes postpartum breast cancer by recruiting M2 macrophages. iScience. 2020;23(6):101217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li B, Zhang S, Huang N, et al. CCL9/CCR1 induces myeloid‑derived suppressor cell recruitment to the spleen in a murine H22 orthotopic hepatoma model. Oncol Rep. 2019;41(1):608‐618. [DOI] [PubMed] [Google Scholar]
- 49. Rump L, Mattey DL, Kehoe O, Middleton J. An initial investigation into endothelial CC chemokine expression in the human rheumatoid synovium. Cytokine. 2017;97:133‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Polosukhina D, Singh K, Asim M, et al. CCL11 exacerbates colitis and inflammation‐associated colon tumorigenesis. Oncogene. 2021;40(47):6540‐6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marazioti A, Kairi CA, Spella M, et al. Beneficial impact of CCL2 and CCL12 neutralization on experimental malignant pleural effusion. PLoS One. 2013;8(8):e71207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abu El‐Asrar AM, Berghmans N, Al‐Obeidan SA, et al. The CC chemokines CCL8, CCL13 and CCL20 are local inflammatory biomarkers of HLA‐B27‐associated uveitis. Acta Ophthalmol. 2019;97(1):e122‐e128. [DOI] [PubMed] [Google Scholar]
- 53. Gu Y, Li X, Bi Y, et al. CCL14 is a prognostic biomarker and correlates with immune infiltrates in hepatocellular carcinoma. Aging (Albany NY). 2020;12(1):784‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu LZ, Zhang Z, Zheng BH, et al. CCL15 recruits suppressive monocytes to facilitate immune escape and disease progression in hepatocellular carcinoma. Hepatology. 2019;69(1):143‐159. [DOI] [PubMed] [Google Scholar]
- 55. Shen W, Zhang X, Tang J, et al. CCL16 maintains stem cell‐like properties in breast cancer by activating CCR2/GSK3β/β‐catenin/OCT4 axis. Theranostics. 2021;11(5):2297‐2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen YT, Hsu H, Lin CC, et al. Inflammatory macrophages switch to CCL17‐expressing phenotype and promote peritoneal fibrosis. J Pathol. 2020;250(1):55‐66. [DOI] [PubMed] [Google Scholar]
- 57. Cardoso AP, Pinto ML, Castro F, et al. The immunosuppressive and pro‐tumor functions of CCL18 at the tumor microenvironment. Cytokine Growth Factor Rev. 2021;60:107‐119. [DOI] [PubMed] [Google Scholar]
- 58. Yan Y, Chen R, Wang X, et al. CCL19 and CCR7 expression, signaling pathways, and adjuvant functions in viral infection and prevention. Front Cell Dev Biol. 2019;7:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kadomoto S, Izumi K, Mizokami A. The CCL20‐CCR6 axis in cancer progression. Int J Mol Sci. 2020;21(15):5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sharma S, Kadam P, Dubinett S. CCL21 programs immune activity in tumor microenvironment. Adv Exp Med Biol. 2020;1231:67‐78. [DOI] [PubMed] [Google Scholar]
- 61. Ren G, Al‐Jezani N, Railton P, Powell JN, Krawetz RJ. CCL22 induces pro‐inflammatory changes in fibroblast‐like synoviocytes. iScience. 2021;24(1):101943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Faura J, Bustamante A, Penalba A, et al. CCL23: a chemokine associated with progression from mild cognitive impairment to Alzheimer's disease. J Alzheimers Dis. 2020;73(4):1585‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang Y, Wu X, Geng M, et al. CCL24 protects renal function by controlling inflammation in podocytes. Dis Markers. 2021;2021:8837825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu X, Sun M, Yang Z, et al. The roles of CCR9/CCL25 in inflammation and inflammation‐associated diseases. Front Cell Dev Biol. 2021;9:686548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen X, An Y, Zhang Y, et al. CCL26 is upregulated by nab‐paclitaxel in pancreatic cancer‐associated fibroblasts and promotes PDAC invasiveness through activation of the PI3K/AKT/mTOR pathway. Acta Biochim Biophys Sin (Shanghai). 2021;53(5):612‐619. [DOI] [PubMed] [Google Scholar]
- 66. Martínez‐Rodríguez M, Monteagudo C. CCL27 signaling in the tumor microenvironment. Adv Exp Med Biol. 2021;1302:113‐132. [DOI] [PubMed] [Google Scholar]
- 67. Mohan T, Deng L, Wang BZ. CCL28 chemokine: an anchoring point bridging innate and adaptive immunity. Int Immunopharmacol. 2017;51:165‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bergamaschi C, Pandit H, Nagy BA, et al. Heterodimeric IL‐15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN‐γ, CXCL9 and CXCL10. J Immunother Cancer. 2020;8(1):e000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fox JC, Nakayama T, Tyler RC, Sander TL, Yoshie O, Volkman BF. Structural and agonist properties of XCL2, the other member of the C‐chemokine subfamily. Cytokine. 2015;71(2):302‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pawelec P, Ziemka‐Nalecz M, Sypecka J, Zalewska T. The impact of the CX3CL1/CX3CR1 axis in neurological disorders. Cells. 2020;9(10):2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stewart TJ, Smyth MJ. Chemokine‐chemokine receptors in cancer immunotherapy. Immunotherapy. 2009;1(1):109‐127. [DOI] [PubMed] [Google Scholar]
- 72. Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469‐499. [DOI] [PubMed] [Google Scholar]
- 73. Gerber PA, Hippe A, Buhren BA, Müller A, Homey B. Chemokines in tumor‐associated angiogenesis. Biol Chem. 2009;390(12):1213‐1223. [DOI] [PubMed] [Google Scholar]
- 74. Fillion I, Ouellet N, Simard M, Bergeron Y, Sato S, Bergeron MG. Role of chemokines and formyl peptides in pneumococcal pneumonia‐induced monocyte/macrophage recruitment. J Immunol. 2001;166(12):7353‐7361. [DOI] [PubMed] [Google Scholar]
- 75. Fantuzzi L, Belardelli F, Gessani S. Monocyte/macrophage‐derived CC chemokines and their modulation by HIV‐1 and cytokines: a complex network of interactions influencing viral replication and AIDS pathogenesis. J Leukoc Biol. 2003;74(5):719‐725. [DOI] [PubMed] [Google Scholar]
- 76. Montero J, Coll J, Sevilla N, Cuesta A, Bols NC, Tafalla C. Interleukin 8 and CK‐6 chemokines specifically attract rainbow trout (Oncorhynchus mykiss) RTS11 monocyte‐macrophage cells and have variable effects on their immune functions. Dev Comp Immunol. 2008;32(11):1374‐1384. [DOI] [PubMed] [Google Scholar]
- 77. Li M, Chen L, Gao Y, et al. Recent advances targeting C‐C chemokine receptor type 2 for liver diseases in monocyte/macrophage. Liver Int. 2020;40(12):2928‐2936. [DOI] [PubMed] [Google Scholar]
- 78. Bier A, Khashab R, Sharabi Y, Grossman E, Leibowitz A. Melatonin prevents T lymphocyte infiltration to the kidneys of hypertensive rats, induced by a high‐salt diet, by preventing the expression of CXCR3 ligand chemokines. Nutrients. 2021;13(10):3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. De Filippo K, Dudeck A, Hasenberg M, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121(24):4930‐4937. [DOI] [PubMed] [Google Scholar]
- 80. Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282(1):121‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Menzies‐Gow A, Robinson DS. Eosinophil chemokines and chemokine receptors: their role in eosinophil accumulation and activation in asthma and potential as therapeutic targets. J Asthma. 2001;38(8):605‐613. [DOI] [PubMed] [Google Scholar]
- 82. Oliveira SH, Lukacs NW. The role of chemokines and chemokine receptors in eosinophil activation during inflammatory allergic reactions. Braz J Med Biol Res. 2003;36(11):1455‐1463. [DOI] [PubMed] [Google Scholar]
- 83. Shinagawa K, Trifilieff A, Anderson GP. Involvement of CCR3‐reactive chemokines in eosinophil survival. Int Arch Allergy Immunol. 2003;130(2):150‐157. [DOI] [PubMed] [Google Scholar]
- 84. Tecchio C, Cassatella MA. Neutrophil‐derived chemokines on the road to immunity. Semin Immunol. 2016;28(2):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rajarathnam K, Schnoor M, Richardson RM, Rajagopal S. How do chemokines navigate neutrophils to the target site: dissecting the structural mechanisms and signaling pathways. Cell Signal. 2019;54:69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Capucetti A, Albano F, Bonecchi R. Multiple roles for chemokines in neutrophil biology. Front Immunol. 2020;11:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu K, Wu L, Yuan S, et al. Structural basis of CXC chemokine receptor 2 activation and signalling. Nature. 2020;585(7823):135‐140. [DOI] [PubMed] [Google Scholar]
- 88. Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169(4):1485‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yoshimura T, Robinson EA, Tanaka S, Appella E, Leonard EJ. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin‐stimulated human blood mononuclear leukocytes. J Immunol. 1989;142(6):1956‐1962. [PubMed] [Google Scholar]
- 90. Hao Q, Vadgama JV, Wang P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun Signal. 2020;18(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kadomoto S, Izumi K, Mizokami A. Roles of CCL2‐CCR2 axis in the tumor microenvironment. Int J Mol Sci. 2021;22(16):8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. O'Connor T, Heikenwalder M. CCL2 in the tumor microenvironment. Adv Exp Med Biol. 2021;1302:1‐14. [DOI] [PubMed] [Google Scholar]
- 93. Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl‐terminal tails. Proc Natl Acad Sci USA. 1994;91(7):2752‐2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lou Q, Liu YL, Zhang SM, Li YY, Huang XF. CCL2 promotes angiogenesis of primary rat cardiac microvascular endothelial cells. Sheng Li Xue Bao. 2020;72(4):441‐448. [PubMed] [Google Scholar]
- 95. Bosteels C, Fierens K, De Prijck S, et al. CCR2‐ and flt3‐dependent inflammatory conventional type 2 dendritic cells are necessary for the induction of adaptive immunity by the human vaccine adjuvant system AS01. Front Immunol. 2020;11:606805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lim SY, Yuzhalin AE, Gordon‐Weeks AN, Muschel RJ. Targeting the CCL2‐CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7(19):28697‐28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tian DS, Peng J, Murugan M, et al. Chemokine CCL2‐CCR2 signaling induces neuronal cell death via STAT3 activation and IL‐1β production after status epilepticus. J Neurosci. 2017;37(33):7878‐7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Han R, Gu S, Zhang Y, et al. Estrogen promotes progression of hormone‐dependent breast cancer through CCL2‐CCR2 axis by upregulation of Twist via PI3K/AKT/NF‐κB signaling. Sci Rep. 2018;8(1):9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Guo F, Xu D, Lin Y, et al. Chemokine CCL2 contributes to BBB disruption via the p38 MAPK signaling pathway following acute intracerebral hemorrhage. FASEB J. 2020;34(1):1872‐1884. [DOI] [PubMed] [Google Scholar]
- 100. Kulbe H, Levinson NR, Balkwill F, Wilson JL. The chemokine network in cancer–much more than directing cell movement. Int J Dev Biol. 2004;48(5‐6):489‐496. [DOI] [PubMed] [Google Scholar]
- 101. Yoshimura T. The chemokine MCP‐1 (CCL2) in the host interaction with cancer: a foe or ally?. Cell Mol Immunol. 2018;15(4):335‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. O'Connor T, Borsig L, Heikenwalder M. CCL2‐CCR2 signaling in disease pathogenesis. Endocr Metab Immune Disord Drug Targets. 2015;15(2):105‐118. [DOI] [PubMed] [Google Scholar]
- 103. Bao X, Chen C, Yuan L. Triptolide attenuates neuropathic pain by regulating microglia polarization through the CCL2/CCR2 axis. Evid Based Complement Alternat Med. 2021;2021:8985721. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104. Vakilian A, Khorramdelazad H, Heidari P, Sheikh Rezaei Z, Hassanshahi G. CCL2/CCR2 signaling pathway in glioblastoma multiforme. Neurochem Int. 2017;103:1‐7. [DOI] [PubMed] [Google Scholar]
- 105. Liu H, Sun Y, O'Brien JA, et al. Necroptotic astrocytes contribute to maintaining stemness of disseminated medulloblastoma through CCL2 secretion. Neuro Oncol. 2020;22(5):625‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hedayati‐Moghadam M, Hosseinian S, Paseban M, et al. The role of chemokines in cardiovascular diseases and the therapeutic effect of curcumin on CXCL8 and CCL2 as pathological chemokines in atherosclerosis. Adv Exp Med Biol. 2021;1328:155‐170. [DOI] [PubMed] [Google Scholar]
- 107. Moadab F, Khorramdelazad H, Abbasifard M. Role of CCL2/CCR2 axis in the immunopathogenesis of rheumatoid arthritis: latest evidence and therapeutic approaches. Life Sci. 2021;269:119034. [DOI] [PubMed] [Google Scholar]
- 108. Ding X, Yang DR, Lee SO, et al. TR4 nuclear receptor promotes prostate cancer metastasis via upregulation of CCL2/CCR2 signaling. Int J Cancer. 2015;136(4):955‐964. [DOI] [PubMed] [Google Scholar]
- 109. Fang WB, Smart C, et al. Expression of CCL2/CCR2 signaling proteins in breast carcinoma cells is associated with invasive progression. Sci Rep. 2021;11(1):8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Avila MA, Berasain C. Targeting CCL2/CCR2 in tumor‐infiltrating macrophages: a tool emerging out of the box against hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol. 2019;7(2):293‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. An J, Xue Y, Long M, Zhang G, Zhang J, Su H. Targeting CCR2 with its antagonist suppresses viability, motility and invasion by downregulating MMP‐9 expression in non‐small cell lung cancer cells. Oncotarget. 2017;8(24):39230‐39240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang Z, Xie H, Zhou L, et al. CCL2/CCR2 axis is associated with postoperative survival and recurrence of patients with non‐metastatic clear‐cell renal cell carcinoma. Oncotarget. 2016;7(32):51525‐51534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19(13):3404‐3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen CH, Su LJ, Tsai HT, Hwang CF. ELF‐1 expression in nasopharyngeal carcinoma facilitates proliferation and metastasis of cancer cells via modulation of CCL2/CCR2 signaling. Cancer Manag Res. 2019;11:5243‐5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lu Y, Cai Z, Galson DL, et al. Monocyte chemotactic protein‐1 (MCP‐1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66(12):1311‐1318. [DOI] [PubMed] [Google Scholar]
- 116. Xu W, Wei Q, Han M, et al. CCL2‐SQSTM1 positive feedback loop suppresses autophagy to promote chemoresistance in gastric cancer. Int J Biol Sci. 2018;14(9):1054‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wang T, Zhan Q, Peng X, Qiu Z, Zhao T. CCL2 influences the sensitivity of lung cancer A549 cells to docetaxel. Oncol Lett. 2018;16(1):1267‐1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chang AL, Miska J, Wainwright DA, et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid‐derived suppressor cells. Cancer Res. 2016;76(19):5671‐5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yang H, Zhang Q, Xu M, et al. CCL2‐CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD‐1 signaling in esophageal carcinogenesis. Mol Cancer. 2020;19(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cho HR, Kumari N, Thi Vu H, Kim H, Park CK, Choi SH. Increased antiangiogenic effect by blocking CCL2‐dependent macrophages in a rodent glioblastoma model: correlation study with dynamic susceptibility contrast perfusion MRI. Sci Rep. 2019;9(1):11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kalbasi A, Komar C, Tooker GM, et al. Tumor‐derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2017;23(1):137‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fader AN, Rasool N, Vaziri SA, et al. CCL2 expression in primary ovarian carcinoma is correlated with chemotherapy response and survival outcomes. Anticancer Res. 2010;30(12):4791‐4798. [PubMed] [Google Scholar]
- 123. Li L, Liu YD, Zhan YT, et al. High levels of CCL2 or CCL4 in the tumor microenvironment predict unfavorable survival in lung adenocarcinoma. Thorac Cancer. 2018;9(7):775‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lavender N, Yang J, Chen SC, et al. The Yin/Yan of CCL2: a minor role in neutrophil anti‐tumor activity in vitro but a major role on the outgrowth of metastatic breast cancer lesions in the lung in vivo. BMC Cancer. 2017;17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gutierrez‐Arcelus M, Rich SS, Raychaudhuri S. Autoimmune diseases ‐ connecting risk alleles with molecular traits of the immune system. Nat Rev Genet. 2016;17(3):160‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kraus AU, Penna‐Martinez M, Meyer G, Badenhoop K. Vitamin D effects on monocytes' CCL‐2, IL6 and CD14 transcription in Addison's disease and HLA susceptibility. J Steroid Biochem Mol Biol. 2018;177:53‐58. [DOI] [PubMed] [Google Scholar]
- 127. Rana AK, Li Y, Dang Q, Yang F. Monocytes in rheumatoid arthritis: circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int Immunopharmacol. 2018;65:348‐359. [DOI] [PubMed] [Google Scholar]
- 128. Behfar S, Hassanshahi G, Nazari A, Khorramdelazad H. A brief look at the role of monocyte chemoattractant protein‐1 (CCL2) in the pathophysiology of psoriasis. Cytokine. 2018;110:226‐231. [DOI] [PubMed] [Google Scholar]
- 129. Kulkarni O, Anders HJ. CCL2/MCP1: a novel target in systemic lupus erythematosus and lupus nephritis. Z Rheumatol. 2008;67(3):220‐224. [DOI] [PubMed] [Google Scholar]
- 130. Lee YH, Song GG. Urinary MCP‐1 as a biomarker for lupus nephritis: a meta‐analysis. Z Rheumatol. 2017;76(4):357‐363. [DOI] [PubMed] [Google Scholar]
- 131. Devarapu SK, Kumar Vr S, Rupanagudi KV, et al. Dual blockade of the pro‐inflammatory chemokine CCL2 and the homeostatic chemokine CXCL12 is as effective as high dose cyclophosphamide in murine proliferative lupus nephritis. Clin Immunol. 2016;169:139‐147. [DOI] [PubMed] [Google Scholar]
- 132. Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci Biobehav Rev. 2014;42:93‐115. [DOI] [PubMed] [Google Scholar]
- 133. Duan L, Zhang XD, Miao WY, et al. PDGFRβ cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2. Neuron. 2018;100(1):183‐200.e8. [DOI] [PubMed] [Google Scholar]
- 134. Georgakis MK, Malik R, Björkbacka H, et al. Circulating monocyte chemoattractant protein‐1 and risk of stroke: meta‐analysis of population‐based studies involving 17 180 Individuals. Circ Res. 2019;125(8):773‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Dhaiban S, Al‐Ani M, Elemam NM, Maghazachi AA. Targeting chemokines and chemokine receptors in multiple sclerosis and experimental autoimmune encephalomyelitis. J Inflamm Res. 2020;13:619‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gutiérrez IL, González‐Prieto M, Caso JR, García‐Bueno B, Leza JC, Madrigal JLM. Reboxetine treatment reduces neuroinflammation and neurodegeneration in the 5xFAD mouse model of Alzheimer's disease: role of CCL2. Mol Neurobiol. 2019;56(12):8628‐8642. [DOI] [PubMed] [Google Scholar]
- 137. Olson A, Coote C, Snyder‐Cappione JE, Lin N, Sagar M. HIV‐1 transcription but not intact provirus levels are associated with systemic inflammation. J Infect Dis. 2021;223(11):1934‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sauce D, Pourcher V, Ferry T, Boddaert J, Slama L, Allavena C. Immune activation and chronic inflammation: is there an additional effect of HIV in a geriatric population?. Medicine (Baltimore). 2021;100(17):e25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Henderson LJ, Reoma LB, Kovacs JA, Nath A. Advances toward curing HIV‐1 Infection in tissue reservoirs. J Virol. 2020;94(3):e00375‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Covino DA, Sabbatucci M, Fantuzzi L. The CCL2/CCR2 axis in the pathogenesis of HIV‐1 infection: a new cellular target for therapy?. Curr Drug Targets. 2016;17(1):76‐110. [DOI] [PubMed] [Google Scholar]
- 141. Fantuzzi L, Spadaro F, Vallanti G, et al. Endogenous CCL2 (monocyte chemotactic protein‐1) modulates human immunodeficiency virus type‐1 replication and affects cytoskeleton organization in human monocyte‐derived macrophages. Blood. 2003;102(7):2334‐2337. [DOI] [PubMed] [Google Scholar]
- 142. Ansari AW, Kamarulzaman A, Schmidt RE. Multifaceted impact of host C‐C chemokine CCL2 in the immuno‐pathogenesis of HIV‐1/M. tuberculosis co‐infection. Front Immunol. 2013;4:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Vicenzi E, Alfano M, Ghezzi S, et al. Divergent regulation of HIV‐1 replication in PBMC of infected individuals by CC chemokines: suppression by RANTES, MIP‐1alpha, and MCP‐3, and enhancement by MCP‐1. J Leukoc Biol. 2000;68(3):405‐412. [PubMed] [Google Scholar]
- 144. Campbell GR, Spector SA. CCL2 increases X4‐tropic HIV‐1 entry into resting CD4+ T cells. J Biol Chem. 2008;283(45):30745‐30753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Sabbatucci M, Covino DA, Purificato C, et al. Endogenous CCL2 neutralization restricts HIV‐1 replication in primary human macrophages by inhibiting viral DNA accumulation. Retrovirology. 2015;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Chen S, Yang L, Nilsson‐Payant B, et al. SARS‐CoV‐2 infected cardiomyocytes recruit monocytes by secreting CCL2. Res Sq. 2020. 10.21203/rs.3.rs-94634/v1 [DOI] [Google Scholar]
- 147. Yang L, Nilsson‐Payant BE, Han Y, et al. Cardiomyocytes recruit monocytes upon SARS‐CoV‐2 infection by secreting CCL2. Stem Cell Rep. 2021;16(9):2274‐2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sandhu SK, Papadopoulos K, Fong PC, et al. A first‐in‐human, first‐in‐class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC‐chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71(4):1041‐1050. [DOI] [PubMed] [Google Scholar]
- 149. Pienta KJ, Machiels JP, Schrijvers D, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC‐chemokine ligand 2 (CCL2), in metastatic castration‐resistant prostate cancer. Invest New Drugs. 2013;31(3):760‐768. [DOI] [PubMed] [Google Scholar]
- 150. Brana I, Calles A, LoRusso PM, et al. an anti‐C‐C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open‐label, multicenter phase 1b study. Target Oncol. 2015;10(1):111‐123. [DOI] [PubMed] [Google Scholar]
- 151. Gilbert J, Lekstrom‐Himes J, Donaldson D, et al. Effect of CC chemokine receptor 2 CCR2 blockade on serum C‐reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein‐1 promoter region. Am J Cardiol. 2011;107(6):906‐911. [DOI] [PubMed] [Google Scholar]
- 152. Vergunst CE, Gerlag DM, Lopatinskaya L, et al. Modulation of CCR2 in rheumatoid arthritis: a double‐blind, randomized, placebo‐controlled clinical trial. Arthritis Rheum. 2008;58(7):1931‐1939. [DOI] [PubMed] [Google Scholar]
- 153. Nywening TM, Wang‐Gillam A, Sanford DE, et al. Targeting tumour‐associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single‐centre, open‐label, dose‐finding, non‐randomised, phase 1b trial. Lancet Oncol. 2016;17(5):651‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Di Prospero NA, Artis E, Andrade‐Gordon P, et al. CCR2 antagonism in patients with type 2 diabetes mellitus: a randomized, placebo‐controlled study. Diabetes Obes Metab. 2014;16(11):1055‐1064.</bib [DOI] [PubMed] [Google Scholar]
- 155. Bekker P, Charvat T, Miao S, et al. Clinical development of CCR2 antagonists CCX140‐B and CCX872‐B. Nephrology Dialysis Transplantation. 50th European‐Renal‐Association, Volume 28 2013;17‐17.
- 156. Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov. 2009;8(1):23‐33. [DOI] [PubMed] [Google Scholar]
- 157. Yang MG, Xiao Z, Cherney RJ, et al. Use of a conformational‐switching mechanism to modulate exposed polarity: discovery of CCR2 antagonist BMS‐741672. ACS Med Chem Lett. 2019;10(3):300‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kalliomäki J, Attal N, Jonzon B, et al. A randomized, double‐blind, placebo‐controlled trial of a chemokine receptor 2 (CCR2) antagonist in posttraumatic neuralgia. Pain. 2013;154(5):761‐767. [DOI] [PubMed] [Google Scholar]
- 159. Kalliomäki J, Huizar K, Kågedal M, Hägglöf B, Schmelz M. Evaluation of the effects of a metabotropic glutamate receptor 5‐antagonist on electrically induced pain and central sensitization in healthy human volunteers. Eur J Pain. 2013;17(10):1465‐1471. [DOI] [PubMed] [Google Scholar]
- 160. Appay V, Rowland‐Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22(2):83‐87. [DOI] [PubMed] [Google Scholar]
- 161. Aldinucci D, Borghese C, Casagrande N. The CCL5/CCR5 axis in cancer progression. Cancers (Basel). 2020;12(7):1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hemmatazad H, Berger MD. CCR5 is a potential therapeutic target for cancer. Expert Opin Ther Targets. 2021;25(4):311‐327. [DOI] [PubMed] [Google Scholar]
- 163. Oppermann M. Chemokine receptor CCR5: insights into structure, function, and regulation. Cell Signal. 2004;16(11):1201‐1210. [DOI] [PubMed] [Google Scholar]
- 164. Martínez‐Muñoz L, Barroso R, Dyrhaug SY, et al. CCR5/CD4/CXCR4 oligomerization prevents HIV‐1 gp120IIIB binding to the cell surface. Proc Natl Acad Sci USA. 2014;111(19):E1960‐E1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Huang CY, Fong YC, Lee CY, et al. CCL5 increases lung cancer migration via PI3K, Akt and NF‐kappaB pathways. Biochem Pharmacol. 2009;77(5):794‐803. [DOI] [PubMed] [Google Scholar]
- 166. Hermida MA, Dinesh Kumar J, Leslie NR. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv Biol Regul. 2017;65:5‐15. [DOI] [PubMed] [Google Scholar]
- 167. Johansen C, Rittig AH, Mose M, et al. STAT2 is involved in the pathogenesis of psoriasis by promoting CXCL11 and CCL5 production by keratinocytes. PLoS One. 2017;12(5):e0176994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Velasco‐Velázquez M, Jiao X, De La Fuente M, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012;72(15):3839‐3850. [DOI] [PubMed] [Google Scholar]
- 169. Zi J, Yuan S, Qiao J, et al. Treatment with the C‐C chemokine receptor type 5 (CCR5)‐inhibitor maraviroc suppresses growth and induces apoptosis of acute lymphoblastic leukemia cells. Am J Cancer Res. 2017;7(4):869‐880. [PMC free article] [PubMed] [Google Scholar]
- 170. Menu E, De Leenheer E, De Raeve H, et al. Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: a study in the 5TMM model. Clin Exp Metastasis. 2006;23(5‐6):291‐300. [DOI] [PubMed] [Google Scholar]
- 171. Casagrande N, Borghese C, Visser L, Mongiat M, Colombatti A, Aldinucci D. CCR5 antagonism by maraviroc inhibits Hodgkin lymphoma microenvironment interactions and xenograft growth. Haematologica. 2019;104(3):564‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Halama N, Zoernig I, Berthel A, et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti‐CCR5 therapy in cancer patients. Cancer Cell. 2016;29(4):587‐601. [DOI] [PubMed] [Google Scholar]
- 173. Velasco‐Velázquez M, Xolalpa W, Pestell RG. The potential to target CCL5/CCR5 in breast cancer. Expert Opin Ther Targets. 2014;18(11):1265‐1275. [DOI] [PubMed] [Google Scholar]
- 174. Khalid A, Wolfram J, Ferrari I, et al. Recent advances in discovering the role of CCL5 in metastatic breast cancer. Mini Rev Med Chem. 2015;15(13):1063‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557‐563. [DOI] [PubMed] [Google Scholar]
- 176. Yazdani Z, Mousavi Z, Ghasemimehr N, et al. Differential regulatory effects of chemotherapeutic protocol on CCL3_CCL4_CCL5/CCR5 axes in acute myeloid leukemia patients with monocytic lineage. Life Sci. 2020;240:117071. [DOI] [PubMed] [Google Scholar]
- 177. Waldeck S, Rassner M, Keye P, et al. CCL5 mediates target‐kinase independent resistance to FLT3 inhibitors in FLT3‐ITD‐positive AML. Mol Oncol. 2020;14(4):779‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Aldinucci D, Lorenzon D, Cattaruzza L, et al. Expression of CCR5 receptors on Reed‐Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. Int J Cancer. 2008;122(4):769‐776. [DOI] [PubMed] [Google Scholar]
- 179. Abe M, Hiura K, Wilde J, et al. Role for macrophage inflammatory protein (MIP)‐1alpha and MIP‐1beta in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100(6):2195‐2202. [PubMed] [Google Scholar]
- 180. Oba Y, Lee JW, Ehrlich LA, et al. MIP‐1alpha utilizes both CCR1 and CCR5 to induce osteoclast formation and increase adhesion of myeloma cells to marrow stromal cells. Exp Hematol. 2005;33(3):272‐278. [DOI] [PubMed] [Google Scholar]
- 181. Cambien B, Richard‐Fiardo P, Karimdjee BF, et al. CCL5 neutralization restricts cancer growth and potentiates the targeting of PDGFRβ in colorectal carcinoma. PLoS One. 2011;6(12):e28842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Aldinucci D, Casagrande N. Inhibition of the CCL5/CCR5 axis against the progression of gastric cancer. Int J Mol Sci. 2018;19(5):1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. González‐Arriagada WA, Lozano‐Burgos C, Zúñiga‐Moreta R, González‐Díaz P, Coletta RD. Clinicopathological significance of chemokine receptor (CCR1, CCR3, CCR4, CCR5, CCR7 and CXCR4) expression in head and neck squamous cell carcinomas. J Oral Pathol Med. 2018;47(8):755‐763. [DOI] [PubMed] [Google Scholar]
- 184. Lu Y, Luan XR. miR‐147a suppresses the metastasis of non‐small‐cell lung cancer by targeting CCL5. J Int Med Res. 2020;48(4):300060519883098. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 185. Long H, Xie R, Xiang T, et al. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem‐like cells via NF‐κB‐mediated MMP‐9 upregulation. Stem Cells. 2012;30(10):2309‐2319. [DOI] [PubMed] [Google Scholar]
- 186. Chang LY, Lin YC, Mahalingam J, et al. Tumor‐derived chemokine CCL5 enhances TGF‐β‐mediated killing of CD8(+) T cells in colon cancer by T‐regulatory cells. Cancer Res. 2012;72(5):1092‐1102. [DOI] [PubMed] [Google Scholar]
- 187. Zeng Z, Lan T, Wei Y, Wei X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. 2022;9(1):12‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Poupel L, Combadière C. Atherosclerosis : on the trail of chemokines. Biol Aujourdhui. 2010;204(4):285‐293. [DOI] [PubMed] [Google Scholar]
- 189. Kang H, Li X, Xiong K, et al. The entry and egress of monocytes in atherosclerosis: a biochemical and biomechanical driven process. Cardiovasc Ther. 2021;2021:6642927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Jongstra‐Bilen J, Tai K, Althagafi MG, et al. Role of myeloid‐derived chemokine CCL5/RANTES at an early stage of atherosclerosis. J Mol Cell Cardiol. 2021;156:69‐78. [DOI] [PubMed] [Google Scholar]
- 191. Li Y, Liu X, Duan W, et al. Batf3‐dependent CD8α(+) dendritic cells aggravates atherosclerosis via Th1 cell induction and enhanced CCL5 expression in plaque macrophages. EBioMedicine. 2017;18:188‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Chistiakov DA, Melnichenko AA, Grechko AV, Myasoedova VA, Orekhov AN. Potential of anti‐inflammatory agents for treatment of atherosclerosis. Exp Mol Pathol. 2018;104(2):114‐124. [DOI] [PubMed] [Google Scholar]
- 193. Mencarelli A, Cipriani S, Francisci D, et al. Highly specific blockade of CCR5 inhibits leukocyte trafficking and reduces mucosal inflammation in murine colitis. Sci Rep. 2016;6:30802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases‐diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18(1):45‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Luedde T, Schwabe RF. NF‐κB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8(2):108‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Li M, Sun X, Zhao J, et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell Mol Immunol. 2020;17(7):753‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Patterson BK, Seethamraju H, Dhody K, et al. Disruption of the CCL5/RANTES‐CCR5 pathway restores immune homeostasis and reduces plasma viral load in critical COVID‐19. medRxiv. 2020. 10.1101/2020.05.02.20084673 [DOI] [Google Scholar]
- 198. Agresti N, Lalezari JP, Amodeo PP, et al. Disruption of CCR5 signaling to treat COVID‐19‐associated cytokine storm: case series of four critically ill patients treated with leronlimab. J Transl Autoimmun. 2021;4:100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Kuwata T, Enomoto I, Baba M, Matsushita S. Incompatible natures of the HIV‐1 envelope in resistance to the CCR5 antagonist cenicriviroc and to neutralizing antibodies. Antimicrob Agents Chemother. 2016;60(1):437‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Visseaux B, Charpentier C, Collin G, et al. Cenicriviroc, a novel CCR5 (R5) and CCR2 antagonist, shows in vitro activity against R5 tropic HIV‐2 clinical isolates. PLoS One. 2015;10(8):e0134904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Nitta T, Nitta S, Lei Y, Lipp M, Takahama Y. CCR7‐mediated migration of developing thymocytes to the medulla is essential for negative selection to tissue‐restricted antigens. Proc Natl Acad Sci USA. 2009;106(40):17129‐17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Van Raemdonck K, Umar S, Palasiewicz K, et al. CCL21/CCR7 signaling in macrophages promotes joint inflammation and Th17‐mediated osteoclast formation in rheumatoid arthritis. Cell Mol Life Sci. 2020;77(7):1387‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Jaeger K, Bruenle S, Weinert T, et al. Structural basis for allosteric ligand recognition in the human CC chemokine receptor 7. Cell. 2019;178(5):1222‐1230.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J Immunol. 2004;172(5):2778‐2784. [DOI] [PubMed] [Google Scholar]
- 205. Haessler U, Pisano M, Wu M, Swartz MA. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc Natl Acad Sci USA. 2011;108(14):5614‐5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Ricart BG, John B, Lee D, Hunter CA, Hammer DA. Dendritic cells distinguish individual chemokine signals through CCR7 and CXCR4. J Immunol. 2011;186(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 207. Correale P, Rotundo MS, Botta C, et al. Tumor infiltration by T lymphocytes expressing chemokine receptor 7 (CCR7) is predictive of favorable outcome in patients with advanced colorectal carcinoma. Clin Cancer Res. 2012;18(3):850‐857. [DOI] [PubMed] [Google Scholar]
- 208. Aggarwal S, Sharma SC, S ND. Dynamics of regulatory T cells (T(regs)) in patients with oral squamous cell carcinoma. J Surg Oncol. 2017;116(8):1103‐1113. [DOI] [PubMed] [Google Scholar]
- 209. Zhang L, Wang F, Yao X, Ma S, Zhang L, Qin Z. Progress in targeting therapy of cancer metastasis by CCL21/CCR7 axis. Sheng Wu Gong Cheng Xue Bao. 2020;36(12):2741‐2754. [DOI] [PubMed] [Google Scholar]
- 210. Hillinger S, Yang SC, Batra RK, et al. CCL19 reduces tumour burden in a model of advanced lung cancer. Br J Cancer. 2006;94(7):1029‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Lu J, Ma J, Cai W, et al. CC motif chemokine ligand 19 suppressed colorectal cancer in vivo accompanied by an increase in IL‐12 and IFN‐γ. Biomed Pharmacother. 2015;69:374‐379. [DOI] [PubMed] [Google Scholar]
- 212. Phan‐Lai V, Kievit FM, Florczyk SJ, Wang K, Disis ML, Zhang M. CCL21 and IFNγ recruit and activate tumor specific T cells in 3D scaffold model of breast cancer. Anticancer Agents Med Chem. 2014;14(2):204‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Gao JQ, Sugita T, Kanagawa N, et al. Anti‐tumor responses induced by chemokine CCL19 transfected into an ovarian carcinoma model via fiber‐mutant adenovirus vector. Biol Pharm Bull. 2005;28(6):1066‐1070. [DOI] [PubMed] [Google Scholar]
- 214. Hamanishi J, Mandai M, Matsumura N, et al. Activated local immunity by CC chemokine ligand 19‐transduced embryonic endothelial progenitor cells suppresses metastasis of murine ovarian cancer. Stem Cells. 2010;28(1):164‐173. [DOI] [PubMed] [Google Scholar]
- 215. Wu S, Xing W, Peng J, et al. Tumor transfected with CCL21 enhanced reactivity and apoptosis resistance of human monocyte‐derived dendritic cells. Immunobiology. 2008;213(5):417‐426. [DOI] [PubMed] [Google Scholar]
- 216. Igoucheva O, Grazzini M, Pidich A, et al. Immunotargeting and eradication of orthotopic melanoma using a chemokine‐enhanced DNA vaccine. Gene Ther. 2013;20(9):939‐948. [DOI] [PubMed] [Google Scholar]
- 217. Iida Y, Yoshikawa R, Murata A, et al. Local injection of CCL19‐expressing mesenchymal stem cells augments the therapeutic efficacy of anti‐PD‐L1 antibody by promoting infiltration of immune cells. J Immunother Cancer. 2020;8(2):e000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Komatsu N, Takayanagi H. Autoimmune arthritis: the interface between the immune system and joints. Adv Immunol. 2012;115:45‐71. [DOI] [PubMed] [Google Scholar]
- 219. Page G, Lebecque S, Miossec P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002;168(10):5333‐5341. [DOI] [PubMed] [Google Scholar]
- 220. Mitsui H, Suárez‐Fariñas M, Belkin DA, et al. Combined use of laser capture microdissection and cDNA microarray analysis identifies locally expressed disease‐related genes in focal regions of psoriasis vulgaris skin lesions. J Invest Dermatol. 2012;132(6):1615‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. de Graaf MT, Smitt PA, Luitwieler RL, et al. Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom. 2011;80(1):43‐50. [DOI] [PubMed] [Google Scholar]
- 222. Gold R, Jawad A, Miller DH, et al. Expert opinion: guidelines for the use of natalizumab in multiple sclerosis patients previously treated with immunomodulating therapies. J Neuroimmunol. 2007;187(1‐2):156‐158. [DOI] [PubMed] [Google Scholar]
- 223. Alt C, Laschinger M, Engelhardt B. Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood‐brain barrier suggests their involvement in G‐protein‐dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur J Immunol. 2002;32(8):2133‐2144. [DOI] [PubMed] [Google Scholar]
- 224. Columba‐Cabezas S, Serafini B, Ambrosini E, Aloisi F. Lymphoid chemokines CCL19 and CCL21 are expressed in the central nervous system during experimental autoimmune encephalomyelitis: implications for the maintenance of chronic neuroinflammation. Brain Pathol. 2003;13(1):38‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Lee AY, Eri R, Lyons AB, Grimm MC, Korner H. CC chemokine ligand 20 and its cognate receptor CCR6 in mucosal T cell immunology and inflammatory bowel disease: odd couple or axis of evil?. Front Immunol. 2013;4:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Mita S, Nakakuki M, Ichioka M, et al. Dienogest inhibits C‐C motif chemokine ligand 20 expression in human endometriotic epithelial cells. Eur J Obstet Gynecol Reprod Biol. 2017;214:65‐70. [DOI] [PubMed] [Google Scholar]
- 227. Chen W, Qin Y, Liu S. CCL20 signaling in the tumor microenvironment. Adv Exp Med Biol. 2020;1231:53‐65. [DOI] [PubMed] [Google Scholar]
- 228. Fujiie S, Hieshima K, Izawa D, et al. Proinflammatory cytokines induce liver and activation‐regulated chemokine/macrophage inflammatory protein‐3alpha/CCL20 in mucosal epithelial cells through NF‐kappaB [correction of NK‐kappaB]. Int Immunol. 2001;13(10):1255‐1263. [DOI] [PubMed] [Google Scholar]
- 229. Skovdahl HK, Damås JK, Granlund AVB, et al. C‐C motif ligand 20 (CCL20) and C‐C motif chemokine receptor 6 (CCR6) in human peripheral blood mononuclear cells: dysregulated in ulcerative colitis and a potential role for CCL20 in IL‐1β release. Int J Mol Sci. 2018;19(10):3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Paradis M, Mindt BC, Duerr CU, et al. A TNF‐α‐CCL20‐CCR6 axis regulates Nod1‐induced B cell responses. J Immunol. 2014;192(6):2787‐2799. [DOI] [PubMed] [Google Scholar]
- 231. Baba M, Imai T, Nishimura M, et al. Identification of CCR6, the specific receptor for a novel lymphocyte‐directed CC chemokine LARC. J Biol Chem. 1997;272(23):14893‐14898. [DOI] [PubMed] [Google Scholar]
- 232. Yang D, Chertov O, Bykovskaia SN, et al. Beta‐defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286(5439):525‐528. [DOI] [PubMed] [Google Scholar]
- 233. Lee AYS, Körner H. The CCR6‐CCL20 axis in humoral immunity and T‐B cell immunobiology. Immunobiology. 2019;224(3):449‐454. [DOI] [PubMed] [Google Scholar]
- 234. Meitei HT, Jadhav N, Lal G. CCR6‐CCL20 axis as a therapeutic target for autoimmune diseases. Autoimmun Rev. 2021;20(7):102846. [DOI] [PubMed] [Google Scholar]
- 235. Ding X, Wang K, Wang H, et al. High expression of CCL20 is associated with poor prognosis in patients with hepatocellular carcinoma after curative resection. J Gastrointest Surg. 2012;16(4):828‐836. [DOI] [PubMed] [Google Scholar]
- 236. Lee SK, Park KK, Kim HJ, et al. Human antigen R‐regulated CCL20 contributes to osteolytic breast cancer bone metastasis. Sci Rep. 2017;7(1):9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Wang D, Yuan W, Wang Y, et al. Serum CCL20 combined with IL‐17A as early diagnostic and prognostic biomarkers for human colorectal cancer. J Transl Med. 2019;17(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Wang D, Yang L, Yu W, et al. Colorectal cancer cell‐derived CCL20 recruits regulatory T cells to promote chemoresistance via FOXO1/CEBPB/NF‐κB signaling. J Immunother Cancer. 2019;7(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Liu B, Jia Y, Ma J, et al. Tumor‐associated macrophage‐derived CCL20 enhances the growth and metastasis of pancreatic cancer. Acta Biochim Biophys Sin (Shanghai). 2016;48(12):1067‐1074. [DOI] [PubMed] [Google Scholar]
- 240. Zhang XP, Hu ZJ, Meng AH, Duan GC, Zhao QT, Yang J. Role of CCL20/CCR6 and the ERK signaling pathway in lung adenocarcinoma. Oncol Lett. 2017;14(6):8183‐8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Walch‐Rückheim B, Mavrova R, Henning M, et al. Stromal fibroblasts induce CCL20 through IL6/C/EBPβ to support the recruitment of Th17 cells during cervical cancer progression. Cancer Res. 2015;75(24):5248‐5259. [DOI] [PubMed] [Google Scholar]
- 242. Han G, Wu D, Yang Y, Li Z, Zhang J, Li C. CrkL meditates CCL20/CCR6‐induced EMT in gastric cancer. Cytokine. 2015;76(2):163‐169. [DOI] [PubMed] [Google Scholar]
- 243. Liu W, Wang W, Wang X, Xu C, Zhang N, Di W. Cisplatin‐stimulated macrophages promote ovarian cancer migration via the CCL20‐CCR6 axis. Cancer Lett. 2020;472:59‐69. [DOI] [PubMed] [Google Scholar]
- 244. Kadomoto S, Izumi K, Hiratsuka K, et al. Tumor‐associated macrophages induce migration of renal cell carcinoma cells via activation of the CCL20‐CCR6 axis. Cancers (Basel). 2019;12(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129(9):2175‐2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kim TG, Jee H, Fuentes‐Duculan J, et al. Dermal clusters of mature dendritic cells and T cells are associated with the CCL20/CCR6 chemokine system in chronic psoriasis. J Invest Dermatol. 2014;134(5):1462‐1465. [DOI] [PubMed] [Google Scholar]
- 247. Brunner PM, Koszik F, Reininger B, Kalb ML, Bauer W, Stingl G. Infliximab induces downregulation of the IL‐12/IL‐23 axis in 6‐sulfo‐LacNac (slan)+ dendritic cells and macrophages. J Allergy Clin Immunol. 2013;132(5):1184‐1193.e8. [DOI] [PubMed] [Google Scholar]
- 248. Robert R, Ang C, Sun G, et al. Essential role for CCR6 in certain inflammatory diseases demonstrated using specific antagonist and knockin mice. JCI Insight. 2017;2(15):e94821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Mabuchi T, Singh TP, Takekoshi T, et al. CCR6 is required for epidermal trafficking of γδ‐T cells in an IL‐23‐induced model of psoriasiform dermatitis. J Invest Dermatol. 2013;133(1):164‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Ruth JH, Shahrara S, Park CC, et al. Role of macrophage inflammatory protein‐3alpha and its ligand CCR6 in rheumatoid arthritis. Lab Invest. 2003;83(4):579‐588. [DOI] [PubMed] [Google Scholar]
- 251. Hirota K, Yoshitomi H, Hashimoto M, et al. Preferential recruitment of CCR6‐expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803‐2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Hou L, Yuki K. CCR6 and CXCR6 identify the Th17 cells with cytotoxicity in experimental autoimmune encephalomyelitis. Front Immunol. 2022;13:819224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Elhofy A, Depaolo RW, Lira SA, Lukacs NW, Karpus WJ. Mice deficient for CCR6 fail to control chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213(1‐2):91‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Skovdahl HK, Granlund A, Østvik AE, et al. Expression of CCL20 and its corresponding receptor CCR6 is enhanced in active inflammatory bowel disease, and TLR3 mediates CCL20 expression in colonic epithelial cells. PLoS One. 2015;10(11):e0141710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Godefroy E, Alameddine J, Montassier E, et al. Expression of CCR6 and CXCR6 by gut‐derived CD4(+)/CD8α(+) T‐regulatory cells, which are decreased in blood samples from patients with inflammatory bowel diseases. Gastroenterology. 2018;155(4):1205‐1217. [DOI] [PubMed] [Google Scholar]
- 256. Mathur AN, Zirak B, Boothby IC, et al. Treg‐cell control of a CXCL5‐IL‐17 inflammatory axis promotes hair‐follicle‐stem‐cell differentiation during skin‐barrier repair. Immunity. 2019;50(3):655‐667.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Duchene J, Lecomte F, Ahmed S, et al. A novel inflammatory pathway involved in leukocyte recruitment: role for the kinin B1 receptor and the chemokine CXCL5. J Immunol. 2007;179(7):4849‐4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Rousselle A, Qadri F, Leukel L, et al. CXCL5 limits macrophage foam cell formation in atherosclerosis. J Clin Invest. 2013;123(3):1343‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Persson T, Monsef N, Andersson P, et al. Expression of the neutrophil‐activating CXC chemokine ENA‐78/CXCL5 by human eosinophils. Clin Exp Allergy. 2003;33(4):531‐537. [DOI] [PubMed] [Google Scholar]
- 260. Peng Y, Kajiyama H, Yuan H, et al. PAI‐1 secreted from metastatic ovarian cancer cells triggers the tumor‐promoting role of the mesothelium in a feedback loop to accelerate peritoneal dissemination. Cancer Lett. 2019;442:181‐192. [DOI] [PubMed] [Google Scholar]
- 261. Xu WW, Li B, Guan XY, et al. Cancer cell‐secreted IGF2 instigates fibroblasts and bone marrow‐derived vascular progenitor cells to promote cancer progression. Nat Commun. 2017;8:14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Yu PF, Huang Y, Han YY, et al. TNFα‐activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2(+) neutrophils. Oncogene. 2017;36(4):482‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Yildirim K, Colak E, Aktimur R, et al. Clinical value of CXCL5 for determining of colorectal cancer. Asian Pac J Cancer Prev. 2018;19(9):2481‐2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Zhao J, Ou B, Han D, et al. Tumor‐derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk‐1/Snail and AKT/GSK3β/β‐catenin pathways. Mol Cancer. 2017;16(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Wang L, Shi L, Gu J, et al. CXCL5 regulation of proliferation and migration in human non‐small cell lung cancer cells. J Physiol Biochem. 2018;74(2):313‐324. [DOI] [PubMed] [Google Scholar]
- 266. Romero‐Moreno R, Curtis KJ, Coughlin TR, et al. The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nat Commun. 2019;10(1):4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Wang W, Zhang M, Huang Z, et al. Knockdown of CXCL5 inhibits the invasion, metastasis and stemness of bladder cancer lung metastatic cells by downregulating CD44. Anticancer Drugs. 2022;33(1):e103‐e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Qiu WZ, Zhang HB, Xia WX, et al. The CXCL5/CXCR2 axis contributes to the epithelial‐mesenchymal transition of nasopharyngeal carcinoma cells by activating ERK/GSK‐3β/snail signalling. J Exp Clin Cancer Res. 2018;37(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Zhou SL, Dai Z, Zhou ZJ, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. 2014;35(3):597‐605. [DOI] [PubMed] [Google Scholar]
- 270. Soler‐Cardona A, Forsthuber A, Lipp K, et al. CXCL5 facilitates melanoma cell‐neutrophil interaction and lymph node metastasis. J Invest Dermatol. 2018;138(7):1627‐1635. [DOI] [PubMed] [Google Scholar]
- 271. Li X, Wang M, Gong T, et al. A S100A14‐CCL2/CXCL5 signaling axis drives breast cancer metastasis. Theranostics. 2020;10(13):5687‐5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Najjar YG, Rayman P, Jia X, et al. Myeloid‐derived suppressor cell subset accumulation in renal cell carcinoma parenchyma is associated with intratumoral expression of IL1β, IL8, CXCL5, and Mip‐1α. Clin Cancer Res. 2017;23(9):2346‐2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Jiang SH, Zhu LL, Zhang M, et al. GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4‐mediated Ca(2+) signalling in a GABA‐independent manner. Gut. 2019;68(11):1994‐2006. [DOI] [PubMed] [Google Scholar]
- 274. Huang Z, Zhang M, Chen G, et al. Bladder cancer cells interact with vascular endothelial cells triggering EGFR signals to promote tumor progression. Int J Oncol. 2019;54(5):1555‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Guan Z, Li C, Fan J, He D, Li L. Androgen receptor (AR) signaling promotes RCC progression via increased endothelial cell proliferation and recruitment by modulating AKT → NF‐κB → CXCL5 signaling. Sci Rep. 2016;6:37085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Chen C, Xu ZQ, Zong YP, et al. CXCL5 induces tumor angiogenesis via enhancing the expression of FOXD1 mediated by the AKT/NF‐κB pathway in colorectal cancer. Cell Death Dis. 2019;10(3):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Qi Y, Zhao W, Li M, et al. High C‐X‐C motif chemokine 5 expression is associated with malignant phenotypes of prostate cancer cells via autocrine and paracrine pathways. Int J Oncol. 2018;53(1):358‐370. [DOI] [PubMed] [Google Scholar]
- 278. Bai L, Yao N, Qiao G, Wu L, Ma X. CXCL5 contributes to the tumorigenicity of cervical cancer and is post‐transcriptionally regulated by miR‐577. Int J Clin Exp Pathol. 2020;13(12):2984‐2993. [PMC free article] [PubMed] [Google Scholar]
- 279. Yang Y, Hou J, Shao M, et al. CXCL5 as an autocrine or paracrine cytokine is associated with proliferation and migration of hepatoblastoma HepG2 cells. Oncol Lett. 2017;14(6):7977‐7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Dang H, Wu W, Wang B, et al. CXCL5 plays a promotingrRole in osteosarcoma cell migration and invasion in autocrine‐ and paracrine‐dependent manners. Oncol Res. 2017;25(2):177‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Cui D, Zhao Y, Xu J. Activation of CXCL5‐CXCR2 axis promotes proliferation and accelerates G1 to S phase transition of papillary thyroid carcinoma cells and activates JNK and p38 pathways. Cancer Biol Ther. 2019;20(5):608‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Lee BS, Jang JY, Seo C, Kim CH. Crosstalk between head and neck cancer cells and lymphatic endothelial cells promotes tumor metastasis via CXCL5‐CXCR2 signaling. FASEB J. 2021;35(1):e21181. [DOI] [PubMed] [Google Scholar]
- 283. Li Y, Wu T, Gong S, et al. Analysis of the prognosis and therapeutic value of the CXC chemokine family in head and neck squamous cell carcinoma. Front Oncol. 2020;10:570736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Jia X, Wei S, Xiong W. CXCL5/NF‐κB pathway as a therapeutic target in hepatocellular carcinoma treatment. J Oncol. 2021;2021:9919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Nie Y, Jiang MC, Liu C, Liu Q, Zhu X. CXCL5 has potential to be a marker for hepatocellular carcinoma prognosis and was correlating with immune infiltrates. Front Oncol. 2021;11:637023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Dawes JM, Calvo M, Perkins JR, et al. CXCL5 mediates UVB irradiation‐induced pain. Sci Transl Med. 2011;3(90):90ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Xu W, Zhu M, Yuan S, Yu W. Spinal CXCL5 contributes to nerve injury‐induced neuropathic pain via modulating GSK‐3β phosphorylation and activity in rats. Neurosci Lett. 2016;634:52‐59. [DOI] [PubMed] [Google Scholar]
- 288. Liu YF, Liang JJ, Ng TK, et al. CXCL5/CXCR2 modulates inflammation‐mediated neural repair after optic nerve injury. Exp Neurol. 2021;341:113711. [DOI] [PubMed] [Google Scholar]
- 289. Quinton LJ, Nelson S, Zhang P, Happel KI, Gamble L, Bagby GJ. Effects of systemic and local CXC chemokine administration on the ethanol‐induced suppression of pulmonary neutrophil recruitment. Alcohol Clin Exp Res. 2005;29(7):1198‐1205. [DOI] [PubMed] [Google Scholar]
- 290. Pedersen F, Waschki B, Marwitz S, et al. Neutrophil extracellular trap formation is regulated by CXCR2 in COPD neutrophils. Eur Respir J. 2018;51(4):1700970. [DOI] [PubMed] [Google Scholar]
- 291. Sokulsky LA, Garcia‐Netto K, Nguyen TH, et al. A critical role for the CXCL3/CXCL5/CXCR2 neutrophilic chemotactic axis in the regulation of type 2 responses in a model of rhinoviral‐induced asthma exacerbation. J Immunol. 2020;205(9):2468‐2478. [DOI] [PubMed] [Google Scholar]
- 292. Wang CY, Shang M, Zhou CL, Feng LZ, Zhou QS, Hu K. Mechanism of Cxc chemokine ligand 5 (CXCL5)/Cxc chemokine receptor 2 (CXCR2) bio‐axis in mice with acute respiratory distress syndrome. Med Sci Monit. 2019;25:5299‐5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Jacobs JP, Ortiz‐Lopez A, Campbell JJ, Gerard CJ, Mathis D, Benoist C. Deficiency of CXCR2, but not other chemokine receptors, attenuates autoantibody‐mediated arthritis in a murine model. Arthritis Rheum. 2010;62(7):1921‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Barsante MM, Cunha TM, Allegretti M, et al. Blockade of the chemokine receptor CXCR2 ameliorates adjuvant‐induced arthritis in rats. Br J Pharmacol. 2008;153(5):992‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Podolin PL, Bolognese BJ, Foley JJ, et al. A potent and selective nonpeptide antagonist of CXCR2 inhibits acute and chronic models of arthritis in the rabbit. J Immunol. 2002;169(11):6435‐6444. [DOI] [PubMed] [Google Scholar]
- 296. Ning Y, Labonte MJ, Zhang W, et al. The CXCR2 antagonist, SCH‐527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther. 2012;11(6):1353‐1364. [DOI] [PubMed] [Google Scholar]
- 297. Hsu YL, Hou MF, Kuo PL, Huang YF, Tsai EM. Breast tumor‐associated osteoblast‐derived CXCL5 increases cancer progression by ERK/MSK1/Elk‐1/snail signaling pathway. Oncogene. 2013;32(37):4436‐4447. [DOI] [PubMed] [Google Scholar]
- 298. Kuo PL, Huang MS, Hung JY, et al. Synergistic effect of lung tumor‐associated dendritic cell‐derived HB‐EGF and CXCL5 on cancer progression. Int J Cancer. 2014;135(1):96‐108. [DOI] [PubMed] [Google Scholar]
- 299. Li Q, Sun J, Cao Y, et al. Bu‐Shen‐Fang‐Chuan formula attenuates T‐lymphocytes recruitment in the lung of rats with COPD through suppressing CXCL9/CXCL10/CXCL11‐CXCR3 axis. Biomed Pharmacother. 2020;123:109735. [DOI] [PubMed] [Google Scholar]
- 300. Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation ‐ A target for novel cancer therapy. Cancer Treat Rev. 2018;63:40‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Gorbachev AV, Kobayashi H, Kudo D, et al. CXC chemokine ligand 9/monokine induced by IFN‐gamma production by tumor cells is critical for T cell‐mediated suppression of cutaneous tumors. J Immunol. 2007;178(4):2278‐2286. [DOI] [PubMed] [Google Scholar]
- 302. Qian C, An H, Yu Y, Liu S, Cao X. TLR agonists induce regulatory dendritic cells to recruit Th1 cells via preferential IP‐10 secretion and inhibit Th1 proliferation. Blood. 2007;109(8):3308‐3315. [DOI] [PubMed] [Google Scholar]
- 303. Karin N. CXCR3 ligands in cancer and autoimmunity, chemoattraction of effector T cells, and beyond. Front Immunol. 2020;11:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Paust HJ, Riedel JH, Krebs CF, et al. CXCR3+ regulatory T cells control TH1 responses in crescentic GN. J Am Soc Nephrol. 2016;27(7):1933‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Schoenborn JR, Wilson CB. Regulation of interferon‐gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41‐101. [DOI] [PubMed] [Google Scholar]
- 306. Humblin E, Kamphorst AO. CXCR3‐CXCL9: it's all in the tumor. Immunity. 2019;50(6):1347‐1349. [DOI] [PubMed] [Google Scholar]
- 307. Reschke R, Yu J, Flood B, Higgs EF, Hatogai K, Gajewski TF. Immune cell and tumor cell‐derived CXCL10 is indicative of immunotherapy response in metastatic melanoma. J Immunother Cancer. 2021;9(9):e003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Wightman SC, Uppal A, Pitroda SP, et al. Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome. Br J Cancer. 2015;113(2):327‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Bagheri H, Pourhanifeh MH, Derakhshan M, et al. CXCL‐10: a new candidate for melanoma therapy?. Cell Oncol (Dordr). 2020;43(3):353‐365. [DOI] [PubMed] [Google Scholar]
- 310. Bai M, Chen X, Ba YI. CXCL10/CXCR3 overexpression as a biomarker of poor prognosis in patients with stage II colorectal cancer. Mol Clin Oncol. 2016;4(1):23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Gomes‐Santos IL, Amoozgar Z, Kumar AS, et al. Exercise training improves tumor control by increasing CD8(+) T‐cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol Res. 2021;9(7):765‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Sridharan V, Margalit DN, Lynch SA, et al. Definitive chemoradiation alters the immunologic landscape and immune checkpoints in head and neck cancer. Br J Cancer. 2016;115(2):252‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Mitsuhashi A, Kondoh K, Horikawa K, et al. Programmed death (PD)‐1/PD‐ligand 1 blockade mediates antiangiogenic effects by tumor‐derived CXCL10/11 as a potential predictive biomarker. Cancer Sci. 2021;112(12):4853‐4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Bolomsky A, Schreder M, Hübl W, Zojer N, Hilbe W, Ludwig H. Monokine induced by interferon gamma (MIG/CXCL9) is an independent prognostic factor in newly diagnosed myeloma. Leuk Lymphoma. 2016;57(11):2516‐2525. [DOI] [PubMed] [Google Scholar]
- 315. Sato Y, Motoyama S, Nanjo H, et al. CXCL10 expression status is prognostic in patients with advanced thoracic esophageal squamous cell carcinoma. Ann Surg Oncol. 2016;23(3):936‐942. [DOI] [PubMed] [Google Scholar]
- 316. Chow MT, Ozga AJ, Servis RL, et al. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti‐PD‐1 therapy. Immunity. 2019;50(6):1498‐1512.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Gudowska‐Sawczuk M, Kudelski J, Mroczko B. The role of chemokine receptor CXCR3 and its ligands in renal cell carcinoma. Int J Mol Sci. 2020;21(22):8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Jie X, Chen Y, Zhao Y, et al. Targeting KDM4C enhances CD8(+) T cell mediated antitumor immunity by activating chemokine CXCL10 transcription in lung cancer. J Immunother Cancer. 2022;10(2):e003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Wang X, Lu XL, Zhao HY, Zhang FC, Jiang XB. A novel recombinant protein of IP10‐EGFRvIIIscFv and CD8(+) cytotoxic T lymphocytes synergistically inhibits the growth of implanted glioma in mice. Cancer Immunol Immunother. 2013;62(7):1261‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Liu Z, Ravindranathan R, Li J, Kalinski P, Guo ZS, Bartlett DL. CXCL11‐Armed oncolytic poxvirus elicits potent antitumor immunity and shows enhanced therapeutic efficacy. Oncoimmunology. 2016;5(3):e1091554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Cao Y, Jiao N, Sun T, et al. CXCL11 correlates with antitumor immunity and an improved prognosis in colon cancer. Front Cell Dev Biol. 2021;9:646252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Pradelli E, Karimdjee‐Soilihi B, Michiels JF, et al. Antagonism of chemokine receptor CXCR3 inhibits osteosarcoma metastasis to lungs. Int J Cancer. 2009;125(11):2586‐2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Cambien B, Karimdjee BF, Richard‐Fiardo P, et al. Organ‐specific inhibition of metastatic colon carcinoma by CXCR3 antagonism. Br J Cancer. 2009;100(11):1755‐1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Koper OM, Kamińska J, Sawicki K, Kemona H. CXCL9, CXCL10, CXCL11, and their receptor (CXCR3) in neuroinflammation and neurodegeneration. Adv Clin Exp Med. 2018;27(6):849‐856. [DOI] [PubMed] [Google Scholar]
- 325. Zhou YQ, Liu DQ, Chen SP, et al. The role of CXCR3 in neurological diseases. Curr Neuropharmacol. 2019;17(2):142‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP‐1alpha and IP‐10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96(12):6873‐6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Narumi S, Kaburaki T, Yoneyama H, Iwamura H, Kobayashi Y, Matsushima K. Neutralization of IFN‐inducible protein 10/CXCL10 exacerbates experimental autoimmune encephalomyelitis. Eur J Immunol. 2002;32(6):1784‐1791. [DOI] [PubMed] [Google Scholar]
- 328. Chung CY, Liao F. CXCR3 signaling in glial cells ameliorates experimental autoimmune encephalomyelitis by restraining the generation of a pro‐Th17 cytokine milieu and reducing CNS‐infiltrating Th17 cells. J Neuroinflammation. 2016;13(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Piotrowska A, Ciapała K, Pawlik K, Kwiatkowski K, Rojewska E, Mika J. Comparison of the effects of chemokine receptors CXCR2 and CXCR3 pharmacological modulation in neuropathic pain model‐in vivo and in vitro study. Int J Mol Sci. 2021;22(20):11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Shono K, Yamaguchi I, Mizobuchi Y, et al. Downregulation of the CCL2/CCR2 and CXCL10/CXCR3 axes contributes to antitumor effects in a mouse model of malignant glioma. Sci Rep. 2020;10(1):15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Sharma I, Siraj F, Sharma KC, Singh A. Immunohistochemical expression of chemokine receptor CXCR3 and its ligand CXCL10 in low‐grade astrocytomas and glioblastoma multiforme: a tissue microarray‐based comparison. J Cancer Res Ther. 2016;12(2):793‐797. [DOI] [PubMed] [Google Scholar]
- 332. Liu C, Luo D, Reynolds BA, et al. Chemokine receptor CXCR3 promotes growth of glioma. Carcinogenesis. 2011;32(2):129‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Xia MQ, Bacskai BJ, Knowles RB, Qin SX, Hyman BT. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP‐10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer's disease. J Neuroimmunol. 2000;108(1‐2):227‐235. [DOI] [PubMed] [Google Scholar]
- 334. Krauthausen M, Kummer MP, Zimmermann J, et al. CXCR3 promotes plaque formation and behavioral deficits in an Alzheimer's disease model. J Clin Invest. 2015;125(1):365‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Zhou Y, Cao H‐B, Li W‐J, Zhao L. The CXCL12 (SDF‐1)/CXCR4 chemokine axis: oncogenic properties, molecular targeting, and synthetic and natural product CXCR4 inhibitors for cancer therapy. Chin J Natural Med. 2018;16(11):801‐810. [DOI] [PubMed] [Google Scholar]
- 336. Mousavi A. CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted‐therapy. Immunol Lett. 2020;217:91‐115. [DOI] [PubMed] [Google Scholar]
- 337. Huynh C, Dingemanse J, Sidharta PN. Relevance of the CXCR4/CXCR7‐CXCL12 axis and its effect in pathophysiological conditions. Pharmacol Res. 2020;161:105092. [DOI] [PubMed] [Google Scholar]
- 338. Bianchi ME, Mezzapelle R. The chemokine receptor CXCR4 in cell proliferation and tissue regeneration. Front Immunol. 2020;11:2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Wang M, Wang L, Ren T, Xu L, Wen Z. IL‐17A/IL‐17RA interaction promoted metastasis of osteosarcoma cells. Cancer Biol Ther. 2013;14(2):155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross‐talk between paracrine‐acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67(9):4244‐4253. [DOI] [PubMed] [Google Scholar]
- 341. Lounsbury N. Advances in CXCR7 modulators. Pharmaceuticals (Basel). 2020;13(2):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Cho BS, Kim HJ, Konopleva M. Targeting the CXCL12/CXCR4 axis in acute myeloid leukemia: from bench to bedside. Korean J Intern Med. 2017;32(2):248‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Liu C, Weng Y, Yuan T, et al. CXCL12/CXCR4 signal axis plays an important role in mediating bone morphogenetic protein 9‐induced osteogenic differentiation of mesenchymal stem cells. Int J Med Sci. 2013;10(9):1181‐1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Décaillot FM, Kazmi MA, Lin Y, Ray‐Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta‐arrestin to enhance cell migration. J Biol Chem. 2011;286(37):32188‐32197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Ceholski DK, Turnbull IC, Pothula V, et al. CXCR4 and CXCR7 play distinct roles in cardiac lineage specification and pharmacologic β‐adrenergic response. Stem Cell Res. 2017;23:77‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Shi Y, Riese DJ, Shen J. The role of the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Front Pharmacol. 2020;11:574667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Santagata S, Ieranò C, Trotta AM, et al. CXCR4 and CXCR7 signaling pathways: a focus on the cross‐talk between cancer cells and tumor microenvironment. Front Oncol. 2021;11:591386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Sun X, Cheng G, Hao M, et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29(4):709‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Mitchell B, Mahalingam M. The CXCR4/CXCL12 axis in cutaneous malignancies with an emphasis on melanoma. Histol Histopathol. 2014;29(12):1539‐1546. [DOI] [PubMed] [Google Scholar]
- 350. Chen L, Zhu M, Yu S, et al. Arg kinase mediates CXCL12/CXCR4‐induced invadopodia formation and invasion of glioma cells. Exp Cell Res. 2020;389(1):111893. [DOI] [PubMed] [Google Scholar]
- 351. Wang M, Lin T, Wang Y, et al. CXCL12 suppresses cisplatin‐induced apoptosis through activation of JAK2/STAT3 signaling in human non‐small‐cell lung cancer cells. Onco Targets Ther. 2017;10:3215‐3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Daniel SK, Seo YD, Pillarisetty VG. The CXCL12‐CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin Cancer Biol. 2020;65:176‐188. [DOI] [PubMed] [Google Scholar]
- 353. Malik S, Westcott JM, Brekken RA, Burrows FJ. CXCL12 in pancreatic cancer: its function and potential as a therapeutic drug target. Cancers (Basel). 2021;14(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Khare T, Bissonnette M, Khare S. CXCL12‐CXCR4/CXCR7 axis in colorectal cancer: therapeutic target in preclinical and clinical studies. Int J Mol Sci. 2021;22(14):7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Yang P, Wang G, Huo H, Li Q, Zhao Y, Liu Y. SDF‐1/CXCR4 signaling up‐regulates survivin to regulate human sacral chondrosarcoma cell cycle and epithelial‐mesenchymal transition via ERK and PI3K/AKT pathway. Med Oncol. 2015;32(1):377. [DOI] [PubMed] [Google Scholar]
- 356. Duan Y, Zhang S, Wang L, et al. Targeted silencing of CXCR4 inhibits epithelial‐mesenchymal transition in oral squamous cell carcinoma. Oncol Lett. 2016;12(3):2055‐2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Yao C, Li P, Song H, et al. CXCL12/CXCR4 axis upregulates twist to induce EMT in human glioblastoma. Mol Neurobiol. 2016;53(6):3948‐3953. [DOI] [PubMed] [Google Scholar]
- 358. Mortezaee K. CXCL12/CXCR4 axis in the microenvironment of solid tumors: a critical mediator of metastasis. Life Sci. 2020;249:117534. [DOI] [PubMed] [Google Scholar]
- 359. Zhou W, Guo S, Liu M, Burow ME, Wang G. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr Med Chem. 2019;26(17):3026‐3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Hirbe AC, Morgan EA, Weilbaecher KN. The CXCR4/SDF‐1 chemokine axis: a potential therapeutic target for bone metastases?. Curr Pharm Des. 2010;16(11):1284‐1290. [DOI] [PubMed] [Google Scholar]
- 361. Jiang C, Ma S, Hu R, et al. Effect of CXCR4 on apoptosis in osteosarcoma cells via the PI3K/Akt/NF‐κβ signaling pathway. Cell Physiol Biochem. 2018;46(6):2250‐2260. [DOI] [PubMed] [Google Scholar]
- 362. Mirandola L, Apicella L, Colombo M, et al. Anti‐Notch treatment prevents multiple myeloma cells localization to the bone marrow via the chemokine system CXCR4/SDF‐1. Leukemia. 2013;27(7):1558‐1566. [DOI] [PubMed] [Google Scholar]
- 363. Guo S, Xiao D, Liu H, Zheng X, Liu L, Liu S. Interfering with CXCR4 expression inhibits proliferation, adhesion and migration of breast cancer MDA‐MB‐231 cells. Oncol Lett. 2014;8(4):1557‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Tan XY, Chang S, Liu W, Tang HH. Silencing of CXCR4 inhibits tumor cell proliferation and neural invasion in human hilar cholangiocarcinoma. Gut Liver. 2014;8(2):196‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Luo HN, Wang ZH, Sheng Y, et al. MiR‐139 targets CXCR4 and inhibits the proliferation and metastasis of laryngeal squamous carcinoma cells. Med Oncol. 2014;31(1):789. [DOI] [PubMed] [Google Scholar]
- 366. Wang DF, Lou N, Qiu MZ, Lin YB, Liang Y. Effects of CXCR4 gene silencing by lentivirus shRNA on proliferation of the EC9706 human esophageal carcinoma cell line. Tumour Biol. 2013;34(5):2951‐2959. [DOI] [PubMed] [Google Scholar]
- 367. Long P, Sun F, Ma Y, Huang Y. Inhibition of CXCR4 and CXCR7 for reduction of cell proliferation and invasion in human endometrial cancer. Tumour Biol. 2016;37(6):7473‐7480. [DOI] [PubMed] [Google Scholar]
- 368. Saha A, Ahn S, Blando J, Su F, Kolonin MG, DiGiovanni J. Proinflammatory CXCL12‐CXCR4/CXCR7 signaling axis drives Myc‐induced prostate cancer in obese mice. Cancer Res. 2017;77(18):5158‐5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Luo Y, Li Q, Yang X, et al. Overexpression of CXCR7 is a novel indicator for enzalutamide resistance in castration‐resistant prostate cancer patients. Dis Markers. 2021;2021:6649579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Yang J, Tang H, Huang J, An H. Upregulation of CXCR7 is associated with poor prognosis of prostate cancer. Med Sci Monit. 2018;24:5185‐5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Deng L, Zheng W, Dong X, et al. Chemokine receptor CXCR7 is an independent prognostic biomarker in glioblastoma. Cancer Biomark. 2017;20(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 372. Li S, Fong KW, Gritsina G, et al. Activation of MAPK signaling by CXCR7 leads to enzalutamide resistance in prostate cancer. Cancer Res. 2019;79(10):2580‐2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Qin HJ, Xu T, Wu HT, et al. SDF‐1/CXCR4 axis coordinates crosstalk between subchondral bone and articular cartilage in osteoarthritis pathogenesis. Bone. 2019;125:140‐150. [DOI] [PubMed] [Google Scholar]
- 374. Murad HAS, Rafeeq MM, Alqurashi TMA. Role and implications of the CXCL12/CXCR4/CXCR7 axis in atherosclerosis: still a debate. Ann Med. 2021;53(1):1598‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Gao JH, He LH, Yu XH, et al. CXCL12 promotes atherosclerosis by downregulating ABCA1 expression via the CXCR4/GSK3β/β‐catenin(T120)/TCF21 pathway. J Lipid Res. 2019;60(12):2020‐2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Tian Y, Yin H, Deng X, Tang B, Ren X, Jiang T. CXCL12 induces migration of oligodendrocyte precursor cells through the CXCR4‑activated MEK/ERK and PI3K/AKT pathways. Mol Med Rep. 2018;18(5):4374‐4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Song ZY, Wang F, Cui SX, Qu XJ. Knockdown of CXCR4 inhibits CXCL12‐induced angiogenesis in HUVECs through downregulation of the MAPK/ERK and PI3K/AKT and the Wnt/β‐catenin pathways. Cancer Invest. 2018;36(1):10‐18. [DOI] [PubMed] [Google Scholar]
- 378. Hao H, Hu S, Chen H, et al. Loss of endothelial CXCR7 impairs vascular homeostasis and cardiac remodeling after myocardial infarction: implications for cardiovascular drug discovery. Circulation. 2017;135(13):1253‐1264. [DOI] [PubMed] [Google Scholar]
- 379. Cheng X, Wang H, Zhang X, et al. The role of SDF‐1/CXCR4/CXCR7 in neuronal regeneration after cerebral ischemia. Front Neurosci. 2017;11:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Cruz‐Orengo L, Holman DW, Dorsey D, et al. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J Exp Med. 2011;208(2):327‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B‐cell‐homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor‐1. Nature. 1998;391(6669):799‐803. [DOI] [PubMed] [Google Scholar]
- 382. Shi K, Hayashida K, Kaneko M, et al. Lymphoid chemokine B cell‐attracting chemokine‐1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol. 2001;166(1):650‐655. [DOI] [PubMed] [Google Scholar]
- 383. Rubio AJ, Porter T, Zhong X. Duality of B cell‐CXCL13 axis in tumor immunology. Front Immunol. 2020;11:521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Kazanietz MG, Durando M, Cooke M. CXCL13 and its receptor CXCR5 in cancer: inflammation, immune response, and beyond. Front Endocrinol (Lausanne). 2019;10:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Wan S, Lin M, Mao Y, Chen X, Liang D. Altered expression of CXCL13 and its chemokine receptor CXCR5 on B lymphocytes during active graves' orbitopathy. Curr Eye Res. 2021;46(2):210‐216. [DOI] [PubMed] [Google Scholar]
- 386. Hussain M, Liu J, Wang GZ, Zhou GB. CXCL13 signaling in the tumor microenvironment. Adv Exp Med Biol. 2021;1302:71‐90. [DOI] [PubMed] [Google Scholar]
- 387. Tian C, Li C, Zeng Y, et al. Identification of CXCL13/CXCR5 axis's crucial and complex effect in human lung adenocarcinoma. Int Immunopharmacol. 2021;94:107416. [DOI] [PubMed] [Google Scholar]
- 388. Chao CC, Lee WF, Wang SW, et al. CXC chemokine ligand‐13 promotes metastasis via CXCR5‐dependent signaling pathway in non‐small cell lung cancer. J Cell Mol Med. 2021;25(19):9128‐9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Siliņa K, Soltermann A, Attar FM, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 2018;78(5):1308‐1320. [DOI] [PubMed] [Google Scholar]
- 390. Thommen DS, Koelzer VH, Herzig P, et al. A transcriptionally and functionally distinct PD‐1(+) CD8(+) T cell pool with predictive potential in non‐small‐cell lung cancer treated with PD‐1 blockade. Nat Med. 2018;24(7):994‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Panse J, Friedrichs K, Marx A, et al. Chemokine CXCL13 is overexpressed in the tumour tissue and in the peripheral blood of breast cancer patients. Br J Cancer. 2008;99(6):930‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Jiang L, Wang D, Sheng M, et al. CXCL13/CXCR5 are potential biomarkers for diagnosis and prognosis for breast cancer. J BUON. 2020;25(6):2552‐2561. [PubMed] [Google Scholar]
- 393. Heimes AS, Madjar K, Edlund K, et al. Subtype‐specific prognostic impact of different immune signatures in node‐negative breast cancer. Breast Cancer Res Treat. 2017;165(2):293‐300. [DOI] [PubMed] [Google Scholar]
- 394. Schmidt M, Weyer‐Elberich V, Hengstler JG, et al. Prognostic impact of CD4‐positive T cell subsets in early breast cancer: a study based on the FinHer trial patient population. Breast Cancer Res. 2018;20(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Ma JJ, Jiang L, Tong DY, Ren YN, Sheng MF, Liu HC. CXCL13 inhibition induce the apoptosis of MDA‐MB‐231 breast cancer cells through blocking CXCR5/ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(24):8755‐8762. [DOI] [PubMed] [Google Scholar]
- 396. Xu L, Liang Z, Li S, Ma J. Signaling via the CXCR5/ERK pathway is mediated by CXCL13 in mice with breast cancer. Oncol Lett. 2018;15(6):9293‐9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Yang M, Lu J, Zhang G, et al. CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD‐1 checkpoint blockade in high‐grade serous ovarian cancer. J Immunother Cancer. 2021;9(1):e001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Zhu Z, Zhang X, Guo H, Fu L, Pan G, Sun Y. CXCL13‐CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway. Mol Cell Biochem. 2015;400(1‐2):287‐295. [DOI] [PubMed] [Google Scholar]
- 399. Ohandjo AQ, Liu Z, Dammer EB, et al. Transcriptome network analysis identifies CXCL13‐CXCR5 signaling modules in the prostate tumor immune microenvironment. Sci Rep. 2019;9(1):14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Si Z, Hu H. Identification of CXCL13 as an immune‐related biomarker associated with tumorigenesis and prognosis in cutaneous melanoma patients. Med Sci Monit. 2021;27:e932052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Zheng Z, Cai Y, Chen H, et al. CXCL13/CXCR5 axis predicts poor prognosis and promotes progression through PI3K/AKT/mTOR pathway in clear cell renal cell carcinoma. Front Oncol. 2018;8:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Li JP, Wu CY, Chen MY, et al. PD‐1(+)CXCR5(−)CD4(+) Th‐CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J Immunother Cancer. 2021;9(7):e002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Tsai CH, Chen CJ, Gong CL, et al. CXCL13/CXCR5 axis facilitates endothelial progenitor cell homing and angiogenesis during rheumatoid arthritis progression. Cell Death Dis. 2021;12(9):846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Weiss JM, Robinet M, Aricha R, et al. Novel CXCL13 transgenic mouse: inflammation drives pathogenic effect of CXCL13 in experimental myasthenia gravis. Oncotarget. 2016;7(7):7550‐7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Aqrawi LA, Ivanchenko M, Björk A, et al. Diminished CXCR5 expression in peripheral blood of patients with Sjögren's syndrome may relate to both genotype and salivary gland homing. Clin Exp Immunol. 2018;192(3):259‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Bao YQ, Wang JP, Dai ZW, et al. Increased circulating CXCL13 levels in systemic lupus erythematosus and rheumatoid arthritis: a meta‐analysis. Clin Rheumatol. 2020;39(1):281‐290. [DOI] [PubMed] [Google Scholar]
- 407. Moser B. CXCR5, the defining marker for follicular B helper T (TFH) cells. Front Immunol. 2015;6:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Mehraj V, Ramendra R, Isnard S, et al. CXCL13 as a biomarker of immune activation during early and chronic HIV infection. Front Immunol. 2019;10:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Bekele Feyissa Y, Chiodi F, Sui Y, Berzofsky JA. The role of CXCL13 in antibody responses to HIV‐1 infection and vaccination. Front Immunol. 2021;12:638872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Widney DP, Breen EC, Boscardin WJ, et al. Serum levels of the homeostatic B cell chemokine, CXCL13, are elevated during HIV infection. J Interferon Cytokine Res. 2005;25(11):702‐706. [DOI] [PubMed] [Google Scholar]
- 411. Li Y, Tang L, Guo L, et al. CXCL13‐mediated recruitment of intrahepatic CXCR5(+)CD8(+) T cells favors viral control in chronic HBV infection. J Hepatol. 2020;72(3):420‐430. [DOI] [PubMed] [Google Scholar]
- 412. Schmidt C, Plate A, Angele B, et al. A prospective study on the role of CXCL13 in Lyme neuroborreliosis. Neurology. 2011;76(12):1051‐1058. [DOI] [PubMed] [Google Scholar]
- 413. Yu Q, Cheng Y, Wang Y, et al. Aberrant humoral immune responses in neurosyphilis: cXCL13/CXCR5 play a pivotal role for B‐cell recruitment to the cerebrospinal fluid. J Infect Dis. 2017;216(5):534‐544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data included in this article are available upon request from the corresponding authors.


