Abstract
A cross-sectional online survey, including a discrete choice experiment (DCE), was used to investigate first-line treatment preferences in patients with classical Hodgkin lymphoma (cHL) in the United States; 141 patients (median age 35.0 years) participated. In the DCE, risk of progression at 2 years (progression free survival) had the highest relative importance to patients (31.3%) when considering first-line treatments, followed by 2-year overall survival (OS; 26.9%), on-treatment pulmonary toxicity (23.3%), and on-treatment peripheral neuropathy (18.5%). Marginal rate of substitution analyses demonstrated that a 0.44% and 0.09% increase in 2-year OS was required for patients to accept a 1% increase in the risk of disease progression at 2 years and peripheral neuropathy, respectively. A 2.6% increase in 2-year OS was needed to accept a 7% rather than a 2% risk of pulmonary toxicity. In summary, patients with cHL rated survival attributes as more important than drug-related toxicity when considering first-line treatments.
Keywords: Hodgkin, discrete choice experiment, patient preference, first-line treatment
Introduction
The prognosis for patients with classical Hodgkin lymphoma (cHL) has improved dramatically over the last 50 years [1,2], driven in part by the adoption of multi-agent combination chemotherapy [3]. Observations from clinical trials have demonstrated that approximately 80–90% of patients with early-stage disease and up to 70% of those with advanced-stage HL achieve complete remission with first-line therapy [4–6]. Furthermore, it now is estimated that over 86% of patients with HL in the United States (US) survive for 5 or more years following their diagnosis [7]. The treatment of cHL varies across regions and countries, but in the US, the combination of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) is the most frequently used first-line regimen for cHL [8–10].
Despite the significant improvements in prognosis since the introduction of ABVD in the mid-1970s, there remains a considerable unmet clinical need in patients with cHL. For example, at 4 to 5 years of follow-up, approximately 30% of patients with stage III or IV cHL are either refractory to or relapse following treatment with ABVD; these results are worse in high-risk patient groups [4,5]. The toxicities associated with ABVD and other multi-agent combination chemotherapy regimens are a major concern, especially for younger patients who may have many years of life remaining [6]. These toxicities include pulmonary toxicity, cardiotoxicity, infertility, and a 4.6% increased risk of secondary tumors when compared with the general population [11–15]. The pulmonary toxicity associated with bleomycin (BPT) is a significant concern and can be fatal or lead to permanent disability [14,16]. Withdrawal of bleomycin from the therapeutic program of patients following an interim negative PET scan may lower the rates of BPT with minimal risk to treatment failure [17].
In an attempt to further improve outcomes and reduce the risk of toxicity for patients with cHL, several novel agents and chemotherapy combinations are being explored [9]. For example, PET-adapted de-escalation techniques starting with a high dose multi-agent chemotherapy such as BEACOPP (bleomycin, etoposide, doxorubicin hydrochloride, cyclophosphamide, vincristine, procarbazine, prednisone) and de-escalating to ABVD as appropriate may represent a cost-effective method to reduce toxicities and potentially improve 5-year progression free survival (PFS) [18]. Additionally, brentuximab vedotin (BV), an anti-CD30 antibody-drug conjugate is indicated in the US for the treatment of adult patients with previously untreated stage III or IV cHL, in combination with doxorubicin, vinblastine, and dacarbazine (BV+AVD) [19]. In the most recent analysis of the phase 3 ECHELON-1 trial, treatment with BV+AVD was associated with a 30% reduction in the relative risk of a modified PFS event compared with ABVD (hazard ratio [HR] 0.70; p=.005), with 3-year modified PFS rates of 83.1% vs. 76.0%, respectively [20]. A higher rate of peripheral neuropathy was observed with BV+AVD (67% vs. 43% in the ABVD group), which was improved or resolved in the majority of patients at last follow-up [10]. Pulmonary toxicity was more frequent in the ABVD arm (3% vs. <1% in BV+AVD for grade 3 or higher), including 11 deaths due to pulmonary toxicity vs. none in the BV+AVD arm [10]; no difference in overall survival was observed between the two arms.
The availability of new treatment options with efficacy and tolerability profiles that differ from existing therapies necessitates reassessment of standard management of patients with cHL. Such a reassessment should give weight to patient preferences for one regimen over another and the factors driving these preferences. This information can help guide physicians as they involve patients in regimen decisions, an approach that is becoming increasingly recognized as important, as demonstrated by the US Food and Drug Administration (FDA) Patient Preference Initiative [21]. Investigators have begun to evaluate patient preferences in a number of different hematologic and solid malignancies [22–29]. However, the only study that included patients with cHL was conducted in Germany, France, and the United Kingdom [22], where cultural and clinical differences exist compared with the US. These factors may all impact on patient preferences in the US in comparison with Europe.
This study investigated first-line regimen preferences of patients with cHL in the US regardless of stage at diagnosis. The relative importance of various regimen attributes and patients’ willingness to accept trade-offs between these attributes was investigated.
Materials and methods
Study design
The study comprised a cross-sectional online survey, including a discrete choice experiment (DCE), adapted from the survey conducted in Europe. [22]. The survey and DCE analyses were modified for the US based on material collected during interviews with US clinical experts, targeted literature reviews, ECHELON-1 trial data, and a pilot test. The appropriateness and level of complexity of the final layout was confirmed using patient interviews.
Patients
Following approval from the Western Institutional Review Board, patients were identified from a cHL patient database held by Medefield Ltd (New York, NY, US), which recruits patients from websites, associations, and support forums. Included patients were residing in the US, had sufficient written fluency in English or Spanish to be able to complete the survey, had a self-reported diagnosis of cHL, and had initiated or were about to undergo a first-line chemotherapy regimen for cHL in 2016 or later. Patients were reimbursed by Medefield for participation in the study, in accordance with their agreement with Medefield.
Patient survey and DCE
The survey was designed to be completed in approximately 30 minutes and included multiple-choice questions on demographics, clinical characteristics, treatment history, and the patient’s experience with the treatment decision-making process. The survey included Likert scale questions on the importance of 18 different treatment attributes (both positive and negative), and patients were asked to rate the importance of each attribute in their decision-making using a scale ranging from ‘not important’ to ‘very important’.
Guidance from the International Society for Pharmacoeconomics and Outcomes Research Task Force notes that design algorithms can be used to optimize efficiency of DCEs [30]. Based on this guidance, a D-efficient design rather than a full-choice DCE was created using Ngene (Choice-Metrics, http://www.choice-metrics.com/features.html). A blocked design was used to reduce participant burden, with each participant responding to eight scenarios. In each scenario, participants were shown two hypothetical treatment regimens using a combination of infographics and plain-language text. Participants were asked to indicate their preference for one or the other hypothetical regimen, or neither. In addition, a dominant scenario logic test was included in the DCE; this presented a scenario with a dominant regimen option that was preferable in all attributes to the comparator regimen option (i.e. longer OS and PFS, lower incidence of adverse events [AEs]). Participants that chose the non-dominant option were excluded from the main analysis. The DCE also included a repeat scenario to check for answer consistency.
Regimen attributes and levels used in the DCE
Regimen attributes and levels included in the DCE were developed based on the major differences between BV+AVD and ABVD regimens observed in the primary analysis of ECHELON-1 [10]. These included 2-year OS, 2-year PFS (presented to patients as risk of relapse or progression to assist comprehension), peripheral neuropathy, and pulmonary toxicity. Pulmonary toxicity was coded as a categorical variable (2% vs. 7% increase in risk); the other variables were coded as continuous, based on linearity established in the European study.
The patient survey is provided in Appendix 2.
Data analysis
Non-DCE survey components (e.g. demographics, clinical characteristics, treatment history, self-reported importance of regimen attributes, and decision-making experience) were summarized with descriptive statistics.
A mixed logit (MXL) model was used to analyze DCE responses, with effect coding used for categorical variables. The MXL model incorporates random effects to adjust for individual differences in preference, hence providing more accurate estimates of the mean and standard deviation of preference weights under the assumption of normal distribution. Differences in regimen preferences between patient subgroups (male vs. female, age < median vs. ≥ median, and early- vs. advanced-stage disease) were examined by including these categories as interaction terms in the model.
For the categorical variable (pulmonary toxicity), a positive coefficient indicated an above-average preference weight across all levels (i.e. the level is preferred), while a negative coefficient indicated the level was not preferred. For the remaining three continuous variables, the mean coefficient represented the average increase or decrease in preference per 1% increase of the attribute value. Standard error (SE) and coefficients were reported for each attribute. The relative importance of each attribute was calculated by determining the differences between the maximum and minimum coefficients of each attribute, which were then normalized, presented as percentages, and ranked.
Trade-offs between attributes and OS were assessed using the marginal rate of substitution (MRS) to identify the minimum change in OS required for patients to be willing to accept a regimen with a 1% increased risk of progression or relapse, a 1% increase in the risk of peripheral neuropathy, or a pulmonary toxicity risk of 7% as opposed to 2%. These analyses were repeated for PFS, comparing the minimum decrease in risk of progression or relapse required.
A sensitivity analysis was conducted to include only patients who passed the dominant scenario logic test and who had consistent responses.
Results
Baseline patient characteristics
A total of 141 patients participated in the study (Table 1). Participants had a median age of 35.0 years (range 19.0–69.0) and the majority were males (60.3%) and generally well educated, with more than 85% reporting post-secondary education. Although patients were recruited from across the US, over 90% reported living in a city or suburban area. All patients reported having some form of health insurance. Most patients (58.9%) were never smokers and only 6.4% were current smokers. Ninety-four patients (66.7%) self-reported their disease stage as early stage (stage I or II) and 47 (33.3%) classified their disease stage as advanced stage (stage III or IV).
Table 1.
Patient demographic, clinical, and treatment characteristics.
| Total (N = 141) | ||
|---|---|---|
| Characteristica | n | % |
|
| ||
| Age, years | ||
| Mean (SD) | 35.6 (7.8) | |
| Median (range) | 35.0 (19.0–69.0) | |
| Sex | ||
| Men | 85 | 60.3% |
| Women | 56 | 39.7% |
| Region | ||
| Northeast | 52 | 36.9% |
| Midwest | 23 | 16.3% |
| South | 48 | 34.0% |
| West | 18 | 12.8% |
| Community | ||
| City | 64 | 45.4% |
| Suburb | 67 | 47.5% |
| Small town or village | 10 | 7.1% |
| Did not disclose | 0 | 0 |
| Education | ||
| Below high school or equivalent | 0 | 0 |
| High school or equivalent | 10 | 7.1% |
| Technical school/training | 10 | 7.1% |
| Some college, university, or other post-secondary education | 30 | 21.3% |
| College, university, or other post-secondary education | 64 | 45.4% |
| Graduate degree | 27 | 19.1% |
| Stage of HL at diagnosis | ||
| Early/Intermediate (stage I or II) | 94 | 66.7% |
| Advanced (stage III or IV) | 47 | 33.3% |
| Time since HL diagnosis, years | ||
| Mean (SD) | 4.1 (9.4) | |
| Median (range) | 1.1 (0.0–39.1) | |
| Source of information on treatment for HL b | ||
| Oncologist | 118 | 83.7% |
| Nurse/other HCP | 110 | 78.0% |
| Family/friends | 68 | 48.2% |
| Other patients/patient advocacy organization | 45 | 31.9% |
| Internet/television/magazine | 89 | 63.1% |
| None | 3 | 2.1% |
| Treatment status | ||
| No treatment decision made | 8 | 5.7% |
| Decision made, not yet started treatment | 32 | 22.7% |
| Currently on treatment | 59 | 41.8% |
| Completed treatment | 42 | 29.8% |
SD: standard deviations; HL: Hodgkin lymphoma; HCP: healthcare professional/provider.
n, % except where otherwise indicated.
Percentages may sum to more than 100%.
Patients reported receiving information about the treatment of cHL from multiple sources, including their oncologist (83.7%), another healthcare provider (78%), and media sources (63.1%, primarily the internet). Family, friends, other patients, and patient advocacy groups also provided information. At the time of survey, 59 patients (41.8%) were on chemotherapy and 42 (29.8%) had completed their treatment regimen. An additional 32 patients (22.7%) had made a decision but had not yet started the chemotherapy regimen and eight (5.7%) had not yet made a decision. The 59 patients who were on active treatment when they completed the survey self-reported their median time since the initiation of first-line therapy as 12 months; 66.1% received ABVD and 22% received BEACOPP or BEACOPPescalated in the first line. One in ten patients (10.2%) reported participating in a clinical trial and 1.7% reported their first-line regimen as unknown.
The 42 patients who had completed their cHL regimen reported a median time of 9 months since the initiation of first-line therapy and an even mix of first-line regimens (ABVD 50%; BEACOPP or BEACOPPescalated 42.9%; treatment in a clinical trial 4.8%; unknown 2.4%). Most (71.4%) of these patients had achieved a complete response or were in remission, consistent with complete response rates reported in the literature across early and advanced stage patients (70–90%) [4–6].
Patient regimen preferences
Among 18 specific attributes (and a nineteenth ‘other’) of cHL regimens rated by patients, survival attributes were ranked as most important and side effects ranked second. Notable side effects of concern to patients included risk of short- or long-term damage to the lungs, long-term damage to the heart, secondary neoplasia, or peripheral neuropathy (Supplementary Table S1). Out-of-pocket expense was rated as least important by patients; however, all attributes had an average rank of ‘fairly important’ or higher. The pattern of ranking was similar when attributes were ordered by mean and median values, respectively (Supplementary Table S2).
For the DCE portion of the study, 41 (29.1%) patients showed inconsistent preferences between the first and second occurrence of the repeat scenario and 17 (12.1%) patients failed the dominant scenario logic test; these 17 patients were excluded from the main DCE analysis.
All coefficients generated from the MXL model were statistically significant (p < .001) and indicated that patients preferred longer survival and a lower risk of progression/relapse over a reduction in toxicity (Supplementary Table S3). A 1% improvement in OS had the strongest impact on regimen preference (coefficient 0.31; SE 0.04), whereas a 1% increase in risk of progression or relapse had a lesser impact (coefficient –0.14; SE 0.02). The impact of a 1% increase in the risk of peripheral neuropathy was much smaller than either a 1% improvement in OS or a 1% increased risk of progression/relapse (coefficient –0.03; SE 0.0) (Supplementary Table S3).
MRS analysis of patient data
The MRS analyses demonstrated that for patients to accept a 1% increase in the risk of progression, a 0.4% increase in OS would be required (Table 2). A smaller increase in OS (0.1%) would be required to accept a 1% increase in the risk of peripheral neuropathy. A 2.6% minimum improvement in OS would be required to accept an increase in the risk of pulmonary toxicity from 2% to 7%. A minimum decrease of 2.3% in the risk of progression or relapse was required to accept a 1% decrease in OS. Decreases of 0.2% and 6.0%, respectively, in the risk of progression or relapse would be required to accept a 1% increase in the risk of peripheral neuropathy or an increase in the risk of pulmonary toxicity from 2% to 7%.
Table 2.
Marginal rates of substitutions for overall and progression-free survival.
| Attribute for patient to accept | Minimum gain in overall survival required (%) |
|---|---|
|
| |
| A 1% increase in the risk of progression or relapse | 0.44% |
| A 1% increase in the risk of peripheral neuropathy | 0.09% |
| A 7% vs. a 2% risk of pulmonary toxicity | 2.6% |
|
| |
| Minimum decrease in risk of progression or relapse required (%) | |
|
| |
| A 1% decrease in overall survival | 2.29% |
| A 1% increase in the risk of peripheral neuropathy | 0.20% |
| A 7% vs. a 2% risk of pulmonary toxicity | 5.97% |
Relative importance of attributes to patients
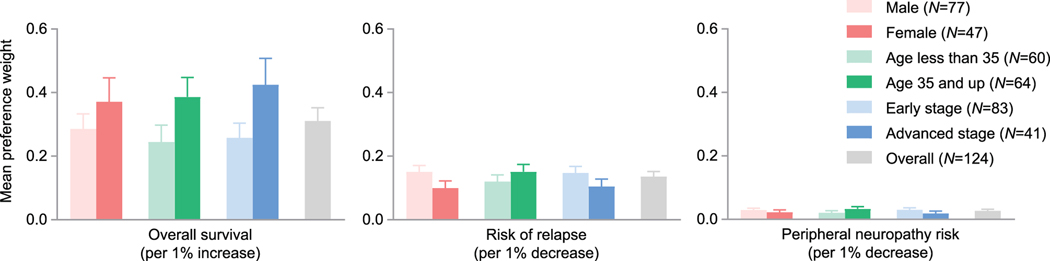
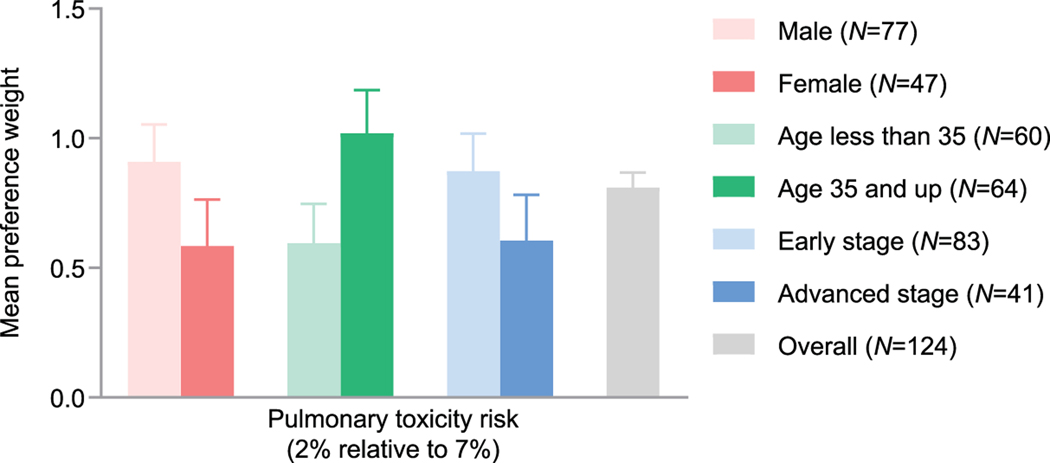
The risk of progression or relapse had the highest relative importance to patients (31.3%), followed by OS (26.9%), pulmonary toxicity (23.3%), and peripheral neuropathy (18.5%). Overall mean preference weights by patient subgroups are shown in Figure 1. Patients 35 years or older had a significantly higher mean preference weight for lower pulmonary toxicity risk than patients younger than 35 (p = .048; Figure 2).
Figure 1.
Mean patient preference weights for improved overall survival, decreased risk of relapse, or decreased risk of peripheral neuropathy by patient gender, age, and disease stage.
Figure 2.
Mean patient preference weights for the risk of pulmonary toxicity by patient gender, age, and disease stage.
The sensitivity analysis supported the main findings. Significant outcomes were retained across all attributes, with similar coefficients in the subset of patients (n = 95) who passed the dominant scenario logic test and gave consistent responses to repeat scenarios. When all 141 patients were included rather than only the 124 who passed the logic test, results were similar (Supplementary Table S4).
Discussion
Although many recent studies have investigated the treatment preferences of patients for a range of different cancer diagnoses and regimens [23,24,26–28,31–33], only one has evaluated patients with cHL [22]; our study addresses this knowledge gap by providing data for US patients. Participants reflected a wide range of demographics and included both early and advanced stage disease.
With the FDA’s increasing recognition of the importance of patient perspectives [21], this study is timely in providing direct insight from patients with cHL regarding their regimen preferences and willingness to trade-off between survival, risk of relapse/progression, and toxicity. Patients rated survival attributes as more important than drug-related toxicity when considering first-line regimens for cHL. According to the MRS, the level of trade-off acceptable to patients in terms of survival rates was a 1% decrease in OS to achieve a 2.3% reduction in risk of relapse/progression. There was a greater trade-off required to accept a higher risk (7% vs. 2%) of pulmonary toxicity. Patients were prepared to accept the higher levels with a 2.6% increase in OS or 6.0% decrease in risk of relapse/progression.
Approximately 14% of patients failed the dominant scenario logic test and were excluded from the analysis. However, excluding these patients did not impact the study outcomes, as the sensitivity analyses that included all patients showed consistent results. Likewise, including patients who showed inconsistent preferences between the first and second occurrence of a repeat scenario in the main analysis did not influence the results. The high percentage of patients who failed the sensitivity analyses highlights the complexity in designing a patient-focused DCE.
In the Likert scale ranking of the importance of different attributes in regimen decision-making, patients rated survival and long-term AEs most highly. Other aspects of treatment that were not captured in the DCE analysis and ranked highly with patients included risk of a secondary neoplasia or long-term cardiovascular disease. An option to enable patients to add additional drug-regimen attributes was not used by any of the patients, suggesting the list presented in the survey included the most important attributes.
In the DCE, patients placed a greater importance on 2-year survival attributes than on pulmonary toxicity and neuropathy, with a slightly higher preference weight for PFS than for OS. In interpreting the PFS and OS findings, it is important to note that preference weights are based on the coefficients per 1% increase, multiplied by the maximum range between attribute levels. The range for PFS (8%) was larger than that for OS (3%), which may be in part responsible for the differences in preference weights for PFS and OS.
In this study, regimen preferences of patients with cHL were broadly consistent across subgroups, although patients with more advanced disease tended to place greater importance on OS than patients with early-stage cHL. In addition, older patients placed a greater importance on pulmonary toxicity than younger patients, perhaps due to heightened awareness of, and elevated risk of, BPT [14]. In contrast, the importance of pulmonary toxicity in patients was not influenced by stage of disease and the interaction models showed no significant effect of sex, age, or stage of disease on preferences for OS, risk of progression, peripheral neuropathy, or pulmonary toxicity, with the exception of age on pulmonary toxicity.
Based on the available literature [10,11] and expert opinion [34], it was anticipated that most patients in the study would have received ABVD in the first line. Among patients who were receiving a drug regimen for cHL when they completed the survey, approximately two-thirds (66.1%) self-reported ABVD in the first line and just over one-fifth (22.0%) BEACOPP or BEACOPPescalated. This unexpected diversity in treatment regimen adds to the generalizability of the data.
The findings of this US study are broadly in line with the earlier European study [22]. Although results cannot be directly compared due to difference in attributes and levels of the DCE, patients in both studies rated survival attributes as more important than drug-related toxicity. Similarly, a survey conducted by the German Hodgkin Study Group found that primary cure was the most important aspect in the choice of treatment among 74% of relapse-free and 61% of relapsed cHL survivors [35]. Taken together, all three studies show that patients are willing to accept increased toxicity if it comes with improved disease control and survival. It would be interesting to explore how differences between European and US health care systems affect patient preferences in cHL treatment in future studies.
Limitations
As patients who completed the survey were either currently receiving or had previously received a drug regimen to treat cHL, insight into the views of patients who were in the process of making drug-regimen decisions was limited; this sample was chosen to ensure that a sufficient number of patients were included, although the sample size (n=141) remained relatively small. While recruitment was focused on patients with advanced cHL, heterogeneity of the patients accrued made it difficult to draw firm conclusions about specific disease stage, or decision-making around delivery of radiation therapy. The patients included in this study were relatively young (median age 35), well educated, insured, and living in a city or suburban area, perhaps due to the online format of the study; the findings may not be generalizable to older patients, uninsured patients, or those living in rural areas. Inconsistent (29.1%) or unlogic responses (12.1%) occurred frequently, reflecting the complexity of conducting a DCE with patients. Another limitation is that patient-reported information may have been affected by recall bias. The results of DCEs are only applicable to the attributes and levels selected in the study; while conclusions were made based on the included attributes and levels, we cannot say that the same preferences would be chosen if different attributes or levels are considered. While DCE levels were based on ECHELON-1 trial data for ABVD or BV-AVD given the regional (US) focus of the analysis, a relevant proportion of patients reported receiving BEACOPP as first-line treatment (22.1%) and these levels and attributes would not be accurately captured in the survey; modern PET-guided approaches to treatment are also not reflected by the DCE levels. Finally, although reported averages identified here are useful to guide patient–physician discussions about regimen options, such data cannot replace an informed discussion between an individual patient and physician.
Conclusions
In the US, patients with cHL prefer first-line treatments that improve survival and reduce the risk of progression, and are willing to accept some measure of increased toxicity to achieve such outcomes. Patient preferences and perspectives are increasingly being viewed as important across all aspects of healthcare and clinical development. This information is valuable to payers as they consider novel therapies that have different risk-benefit profiles compared with existing regimens, and can be used to help inform discussions between patients and treating oncologists with regard to decision-making in the treatment of HL. With the availability of multiple regimens used for first-line treatment of advanced HL, the need for informed decision-making that helps patients weigh risks and benefits is important; our findings can meaningfully contribute to such discussions.
Supplementary Material
Acknowledgments
This study was funded by Seattle Genetics. Editorial support for the development of this manuscript was provided by Karen Smoyer and Jon Edwards of Envision Pharma Group, which was funded by Seattle Genetics.
Footnotes
Disclosure of interest
Joseph Feliciano and Mayvis Rebeira were employees of Seattle Genetics at the time of the research. Kerstin Müller and Mary He are employees of ICON plc, which has a research and consultancy agreement with Seattle Genetics. Rei Tao and Ellen Korol were employees of ICON plc when this study was conducted. Mehul Dalal is an employee of Millennium Pharmaceuticals. Niloufer Khan has received research funding from Gilead Sciences. Matthew Matasar has received research support and honoraria from Seattle Genetics, Genentech, Roche, Johnson & Johnson, Bayer, and Rocket.
References
- 1.Baxi SS, Matasar MJ. State-of-the-art issues in Hodgkin’s lymphoma survivorship. Curr Oncol Rep. 2010;12(6):366–373. [DOI] [PubMed] [Google Scholar]
- 2.Engert A. ABVD or BEACOPP for advanced Hodgkin lymphoma. J Clin Oncol. 2016;34(11):1167–1169. [DOI] [PubMed] [Google Scholar]
- 3.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27(27):4548–4554. [DOI] [PubMed] [Google Scholar]
- 4.Carde P, Karrasch M, Fortpied C, et al. Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, International Prognostic Score ≥3, high-risk Hodgkin lymphoma: first results of the phase III EORTC 20012 Intergroup Trial. J Clin Oncol. 2016;34(17):2028–2036. [DOI] [PubMed] [Google Scholar]
- 5.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31(6):684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flerlage JE, Metzger ML, Bhakta N. The management of Hodgkin lymphoma in adolescents and young adults: burden of disease or burden of choice? Blood. 2018;132(4):376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCI. SEER Cancer Stat Facts: Hodgkin lymphoma [Internet]. Bethesda (MD): National Cancer Institute; 2018. [cited 2019 February 26]. Available from: https://seer.cancer.gov/statfacts/html/hodg.html [Google Scholar]
- 8.Nikolaenko L, Chen R, Herrera AF. Current strategies for salvage treatment for relapsed classical Hodgkin lymphoma. Ther Adv Hematol. 2017;8(10):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansell SM. Hodgkin lymphoma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol. 2018;93(5):704–715. [DOI] [PubMed] [Google Scholar]
- 10.Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378(4):331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2016;17(9):1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behringer K, Mueller H, Goergen H, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol. 2013;31(2):231–239. [DOI] [PubMed] [Google Scholar]
- 13.Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;373(26):2499–2511. [DOI] [PubMed] [Google Scholar]
- 14.Martin WG, Ristow KM, Habermann TM, et al. Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin’s lymphoma. J Clin Oncol. 2005;23(30):7614–7620. [DOI] [PubMed] [Google Scholar]
- 15.Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120(2):617–624. [DOI] [PubMed] [Google Scholar]
- 16.Fox KM, Feliciano J, Alzola C, et al. Incidence and predictors of pulmonary events among patients with Hodgkin lymphoma treated with bleomycin in the US Departments of Defense healthcare system. Chemotherapy. 2018;7:261. [Google Scholar]
- 17.Johnson P, Federico M, Kirkwood A, et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. N Engl J Med. 2016;374(25):2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vijenthira A, Chan K, Cheung MC, et al. Cost-effectiveness of first-line treatment options for patients with advanced-stage Hodgkin lymphoma: a modelling study. Lancet Haematol. 2020;7(2):e146–e156. [DOI] [PubMed] [Google Scholar]
- 19.ADCETRIS [prescribing information]. Bothell, WA: Seattle Genetics, Inc; 2019. [Google Scholar]
- 20.Straus DJ, Dlugosz-Danecka M, Alekseev S, et al. Brentuximab vedotin with chemotherapy for stage III/IV classical Hodgkin lymphoma: 3-year update of the ECHELON-1 study. Blood. 2020;135(10):735–742. [DOI] [PubMed] [Google Scholar]
- 21.FDA. Patient preference initiative [Internet]. Silver Spring (MD): US Food and Drug Administration; 2018. [cited 2019 February 26]. Available from: https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHPatientEngagement/ucm462830.htm [Google Scholar]
- 22.Bröckelmann PJ, McMullen S, Wilson JB, et al. Patient and physician preferences for first-line treatment of classical Hodgkin lymphoma in Germany, France and the United Kingdom. Br J Haematol. 2018;184(2):202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Bekker-Grob EW, Bliemer MC, Donkers B, et al. Patients’ and urologists’ preferences for prostate cancer treatment: a discrete choice experiment. Br J Cancer. 2013;109(3):633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson P, Bancroft T, Barron R, et al. Discrete choice experiment to estimate breast cancer patients’ preferences and willingness to pay for prophylactic granulocyte colony-stimulating factors. Value Health. 2014;17(4):380–389. [DOI] [PubMed] [Google Scholar]
- 25.Muhlbacher AC, Bethge S. Patients’ preferences: a discrete-choice experiment for treatment of non-small-cell lung cancer. Eur J Health Econ. 2015;16(6):657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muhlbacher AC, Nubling M. Analysis of physicians’ perspectives versus patients’ preferences: direct assessment and discrete choice experiments in the therapy of multiple myeloma. Eur J Health Econ. 2011;12(3):193–203. [DOI] [PubMed] [Google Scholar]
- 27.Qian Y, Arellano J, Hauber AB, et al. Patient, caregiver, and nurse preferences for treatments for bone metastases from solid tumors. Patient. 2016;9(4):323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shafey M, Lupichuk SM, Do T, et al. Preferences of patients and physicians concerning treatment options for relapsed follicular lymphoma: a discrete choice experiment. Bone Marrow Transplant. 2011;46(7):962–969. [DOI] [PubMed] [Google Scholar]
- 29.Stenehjem D, Korytowsky B, Oderda G, et al. Understanding the impact of patient and physician preferences in personalized treatment for melanoma using a discrete choice experiment. Value Health. 2015;18(7):A469–470. [Google Scholar]
- 30.Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health--a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. [DOI] [PubMed] [Google Scholar]
- 31.PEBC’s Ovarian Oncology Guidelines Group. A systematic review of patient values, preferences and expectations for the treatment of recurrent ovarian cancer. Gynecol Oncol. 2017;146(2):392–398. [DOI] [PubMed] [Google Scholar]
- 32.Havrilesky LJ, Alvarez Secord A, Ehrisman JA, et al. Patient preferences in advanced or recurrent ovarian cancer. Cancer. 2014;120(23):3651–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marshall DA, Deal K, Bombard Y, et al. How do women trade-off benefits and risks in chemotherapy treatment decisions based on gene expression profiling for early-stage breast cancer? A discrete choice experiment. BMJ Open. 2016;6(6):e010981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NCCN. Clinical practice guidelines in oncology (NCCN guidelines) Hodgkin lymphoma [Internet]. Plymouth Meeting (PA): National Comprehensive Cancer Network; 2016. [cited 2019 February 27]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf [Google Scholar]
- 35.Kreissl S, Goergen H, Muller H, et al. Survivors’ perspectives on risks and benefits of Hodgkin lymphoma treatment: results of a survey by the German Hodgkin Study Group. Leuk Lymphoma. 2019;60(6):1389–1398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.