Abstract
Summary
Treatment of insulinoma can be challenging, while surgical resection is considered the first line. When surgery is contraindicated or is refused, minimally invasive procedures such as selective arterial embolization, local ablative techniques including alcohol ablation, radiofrequency ablation and microwave ablation are being used of late. The world’s first microwave ablation of insulinoma was performed in 2015, after which there have been only a handful of reported cases. A 78-year-old female presented with painful swelling of the left lower limb. She was drowsy and was previously misdiagnosed as epilepsy when she had similar episodes since 2 years ago. She had hypoglycaemia with high serum insulin and C-peptide, and mildly high adjusted calcium, serum prolactin. MRI did not show pituitary adenoma. Lower limb venous duplex scan showed left lower limb deep vein thrombosis for which she was treated with anticoagulation. CT of the abdomen showed a tumour measuring 1.8 cm, located in the antero-superior aspect of the body of the pancreas, with the superior surface being abutted by the splenic artery and the inferior surface being 3 mm above the pancreatic duct, suggestive of an insulinoma. Selective transcatheter arterial embolization of the pancreatic tumour was attempted but was abandoned due to multiple small feeding arteries. Microwave ablation of the tumour was performed successfully. Since there was a possibility of the ablation being compromised due to the heat sink at the splenic artery, 2 mL of 99% alcohol was injected into the rim of the tumour near the artery. She was subsequently normoglycaemic. She defaulted follow up for repeat imaging of pancreas and screening for MEN1 syndrome due to the impact of the COVID-19 pandemic. Minimally invasive procedures are preferred over surgery in selected patients with insulinoma, out of which microwave ablation could be preferentially recommended due to its efficacy and minimal complications. We report the first case of MWA performed in combination with AA in successfully treating insulinoma to our knowledge. This is also the first reported case of DVT associated with isolated insulinoma prior to intervention, though it is rarely reported in MEN1 syndrome.
Learning points
Novel therapeutic minimally invasive procedures are successful in treating selected cases of insulinoma.
Microwave ablation could be recommended preferentially over selective trans-arterial embolization, and radiofrequency ablation in treating insulinoma due to its efficacy and minimal complications.
We report the first case of microwave ablation performed in combination with alcohol ablation in successfully treating insulinoma to our knowledge.
Patient Demographics: Geriatric, Female, White, Sri Lanka
Clinical Overview: Pancreas, Tumours and neoplasia
Related Disciplines: Gastroenterology, Radiology/Rheumatology
Publication Details: Novel treatment, May, 2022
Background
Insulinomas are rare neuroendocrine tumours (NET) with an incidence of 1–4 per million population (1). They account for 1–2% of all pancreatic neoplasms and are the commonest functioning endocrine neoplasm of the pancreas (1). Insulinomas follow the 90% rule, where 90% are solitary, 90% benign, 90% <2 cm in size, >90% occur at intrapancreatic sites, and about 10% are associated with multiple endocrine neoplasia syndrome type 1 (MEN1) (1). Surgical resection is the first line of treatment of benign and malignant insulinomas (2, 3). Diazoxide and somatostatin analogues are drug options which can be used until definitive therapy (2). When surgery is contraindicated or is refused, minimally invasive procedures (MIP) such as selective transcatheter arterial embolization (STAE), local ablative techniques including alcohol ablation (AA), radiofrequency ablation (RFA), and microwave ablation (MWA) are being increasingly used since of late (2, 3). We present a case of successfully treated insulinoma, with challenges in management including contraindications for surgery, complex tumour vasculature, and concomitant treatment of insulinoma associated deep vein thrombosis (DVT) with risk of bleeding.
Ablative techniques in treating insulinoma were initiated only in the recent past, evidenced by the world’s first AA, RFA, laparoscopic RFA, and MWA being performed in the years 2006, 2009, 2012, and 2015, respectively (4, 5, 6, 7). Reported cases of insulinoma treated with MWA worldwide are a handful, including a series of seven cases in Russia, following the world’s first case performed in the United States (3, 7). Koshyet al. presented a case of metastatic insulinoma treated by repeated episodes of combination therapy with STAE and MWA following pancreatic surgery with failed tumour clearance (8). We report the first case of MWA performed in combination with AA in successfully treating insulinoma to our knowledge.
Case presentation
A 78-year-old female presented to the Sri Jayewardenepura General Hospital, Sri Lanka, in July 2020, with 1 month history of painful swelling of the left lower limb. She had no preceding history of immobility, limb fractures, or limb or pelvic surgeries. During admission, she was noted to be drowsy and ill, when she was found to be hypoglycaemic. She denied the use of any antidiabetic medication or illicit drugs. She had been given a diagnosis of epilepsy when she had similar episodes of reduced consciousness and occasionally generalized tonic–clonic seizures since 2 years ago. She was evaluated by a general practitioner and found to have normal MRI of the brain and EEG. She had no family history of tumours or hormonal dysfunction. She was haemodynamically stable. The left lower limb was swollen with calf tenderness. She had no rashes or skin nodules.
Investigations
On admission she was found to have a random blood sugar of 35 mg/dL. Concomitant serum insulin was 306.33 pmol/L (<174 pmol/L), and serum C-peptide 7.11 nmol/L (0.26–1.03 nmol/L). Adjusted calcium was 10.6 mg/dL (8.6–10 mg/dL), and serum prolactin was 737.1 mIU/L (102–496 mIU/L). MRI of the brain showed no pituitary adenoma, and she was not on any drugs which could account for hyperprolactinemia. Serum amylase was 73 IU/L (0–80 IU/L) with normal renal and liver function tests. She had a high D-dimer of 5.71 mg/L (<0.55 mg/L), with an otherwise normal platelet count and clotting profile. Lower limb venous duplex scan showed left lower limb deep vein thrombosis (DVT) extending from the noncompressible calf veins and popliteal vein into the superficial, caudal part of common femoral and profunda femoris veins with superficial thrombophlebitis.
She was manged in liaison with the consultant haematologist and endocrinologist. Hypoglycaemia was managed with intravenous (IV) dextrose with difficulty in maintaining persistent normoglycemia, after which she was started on diazoxide. Subcutaneous (SC) enoxaparin and warfarin were commenced for DVT. Since the DVT was below the level of the inguinal ligament she did not require an IVC filter to prevent pulmonary thromboembolism.
Ultrasound scan (USS) of the abdomen showed a hypoechoic round lesion measuring 1.8 cm, with a focus on calcification in the body of the pancreas. Contrast enhanced CT (CECT) of the abdomen showed a well defined homogeneous highly enhanced exophytic lesion bulging out antero-superiorly, measuring 1.8 cm with a less enhanced calcified centre, with the superior surface being abutted by the splenic artery and the inferior surface being 3mm above the pancreatic duct (Figs 1 and 2).
Figure 1.
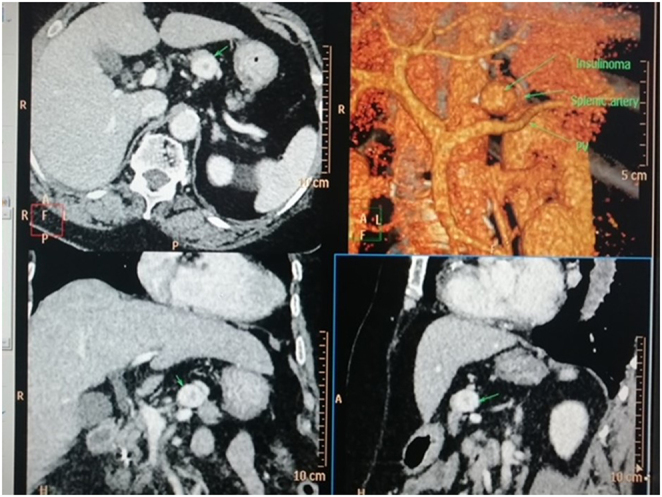
Arterial phase-contrast CT with multiplanar reconstruction and volume rendering image showing avidly enhancing lesion in the body of the pancreas (arrow).
Figure 2.
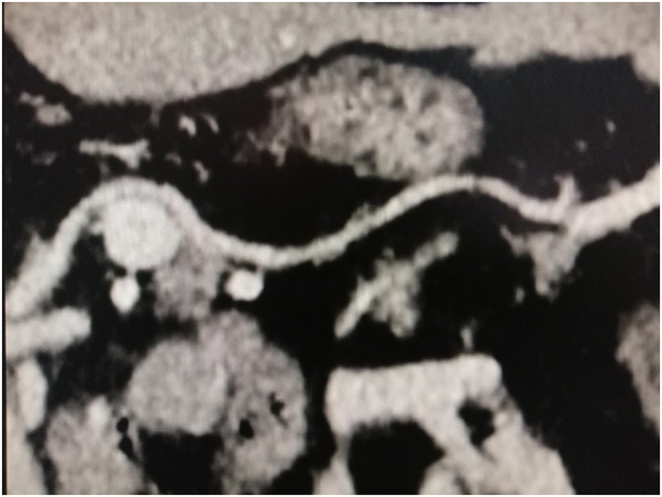
Arterial phase CT scan demonstrating insulinoma (arrow) with the splenic artery (arrowhead) halfway around the lesion.
Treatment
She was highly symptomatic needing immediate intervention. However, the multidisciplinary team involved in her management including the surgeon, endocrinologist, interventional radiologist, and haematologist decided she was not a suitable candidate for surgery, which prompted minimally invasive modalities. The consultant interventional radiologist opted to perform selective transcatheter arterial embolization (STAE) of the pancreatic tumour. Anticoagulants were with-held timely prior to the procedure. The international normalized ratio (INR) prior to the procedure was 2.7, and platelet count was 250 × 103.
Digital subtraction angiogram (DSA) was performed in view of STAE of the pancreatic tumour by the interventional radiologist under local anaesthesia. The right common femoral artery was punctured and a 5F sheath was inserted. A 5F catheter was advanced to the coeliac trunk, and DSA was performed. Multiple small feeding arteries were identified from the splenic artery, which was not amenable for STAE. Therefore, the procedure was abandoned. MWA of the pancreatic tumour was subsequently planned.
One week after the attempted STAE, USS, and CT guided MWA of the pancreatic insulinoma was performed under sedation and local anaesthesia. The pancreatic lesion was identified, and a 15 cm, 17 Gauge microwave needle was advanced into the lesion at the midline. The lesion was anterior to the spine, slightly to the left from the midline. Therefore, the lesion was accessed abdominally, via a 1-cm window between the liver and the stomach, and the needle directly approached the lesion (Fig. 3). The ablation time was 2 min and 30 s for an ablative dose of 60 W of magnitude. Her need for anticoagulation for DVT and risk of bleeding were carefully balanced during the MWA procedure which was a challenge. The glycaemic state was closely maintained with a continuous IV dextrose infusion. CECT of the abdomen was repeated immediately while the microwave catheter was in situ. A very thin rim of enhancement was noted in the superolateral aspect of the ablated lesion close to the splenic artery (Fig. 4). Since there may be a heat sink near the splenic artery, with dissipation of heat due to fast splenic blood flow close to the lesion, there was a possibility of the effectiveness of ablation being compromised. Therefore, 2 mL of 99% alcohol was injected into the lesion again under CT guidance via a 20G Chiba needle into the area of the tumour adjacent to the splenic artery. This could possibly be an artefact due to the microwave antenna in situ, or the immediate effect that is expected at the tumour margin following vascular compromise. CECT was repeated for 3 days, which showed more than 90% of the tumour is avascular. The pancreas and the splenic artery appeared otherwise normal. The blood sugars (BS) were closely monitored and there was no recurrence of hypoglycaemia, while maintaining sugar around 90 mg/dL. She did not require further functional imaging. She was discharged with plans to follow up at the endocrinology and haematology clinics. An interval CECT abdomen was planned for 6 weeks to see the residual enhancement of the tumour.
Figure 3.
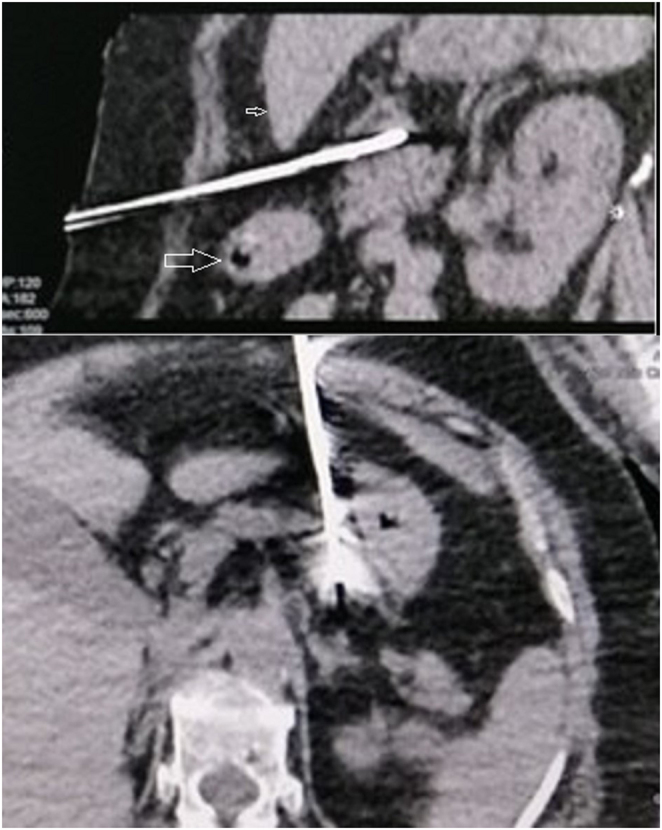
CT guided percutaneous microwave ablation using 17G antenna positioned within the insulinoma. Needle path between left lobe of the liver (small arrow) and stomach (large arrow).
Figure 4.
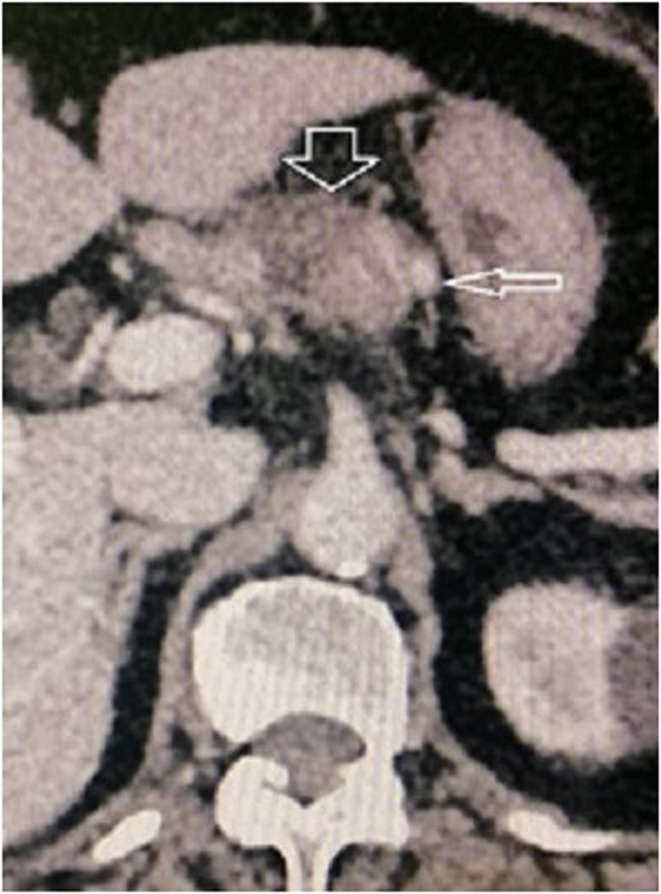
Post-ablation day 3 contrast CT arterial phase showing near total ablation of the insulinoma (thick arrow) except a thin crescent adjacent to the splenic artery (thin arrow).
Outcome and follow-up
Three weeks after microwave ablation she presented with epigastric pain, abdominal distension and reduced bowel opening. She was haemodynamically stable, with gaseous distension of the abdomen with reduced bowel sounds. She had serum amylase of 389 IU/L and BS was 97 mg/dL. Liver enzymes and renal functions were normal. X-ray of the abdomen showed dilated large intestinal loops. USS of abdomen showed an irregular area with heterogeneous echotexture measuring 3.5 cm × 2.6 cm in the body of the pancreas, with no internal vascularity noted. She was treated for focal pancreatitis, after which she improved clinically and biochemically.
Warfarin was withheld after 6 months with resolution of DVT on lower limb venous duplex scan. She defaulted follow up and interval CECT abdomen could not be done as planned due to safety concerns and financial difficulties during the COVID-19 pandemic. However, by following her up over the telephone, 8 months after microwave ablation of insulinoma, she was found to be well without any recurrence of hypoglycaemic symptoms with good glycaemic control.
Discussion
We present a patient who was previously misdiagnosed as epilepsy and was found to have likely hypoglycaemia induced seizures in the past, due to an insulinoma of the body of the pancreas, which was successfully treated with MWA. The management was challenging due to the concomitant DVT and anticoagulation.
Venous thrombosis (VT), mostly DVT is a recognised complication of pancreatic adenocarcinomas with an incidence of 6–16% (9). The risk of thrombosis in NET is less compared to pancreatic adenocarcinoma but is higher than the risk of the normal population (9). There are reported cases of thrombosis following pancreatectomy in insulinoma, but no such reported cases pre-operatively (10). VT was found to develop on average after 2 years of diagnosis of the NET, as in this patient who had symptoms of the NET since 2 years ago (5). Though the rare possibility of DVT being associated with insulinoma in this case may be spiculated, it can not be confirmed as the causative factor, as DVT maybe common in that age group even in the absence of significant risk factors.
The incidence of VT associated with NET including insulinoma was found to be higher when associated with MEN1 according to Leeet al. (11). However, this patient had no convincing evidence of MEN1. The marginally high prolactin level with no pituitary lesions on MRI could be secondary to a possible hypoglycaemic seizure on admission, whereas a MEN1-related prolactinoma would expect to secrete much higher prolactin levels.
A malignant insulinoma could be suspected biochemically if the fasting time for the occurrence of hypoglycaemia is less than 8 h, serum insulin > 28 µU/mL, and serum C-peptide > 4 ng/mL (12). The imaging features suggestive of a malignant insulinoma are; tumour size > 2.5 cm, extra-pancreatic regional lymphadenopathy, and remote extension, which this patient did not have (12).
Surgical resection is the first-line treatment for insulinoma, and the choice of procedure depends on the association with MEN1, number of insulinomas, tumour size, location in the pancreas, anatomical proximity to the pancreatic duct and major blood vessels, and the likelihood of malignancy (13). Parenchyma sparing procedures such as enucleation, and partial or central pancreatectomy are preferred in isolated sporadic insulinoma, by which the risk of subsequent exocrine and endocrine insufficiency can be reduced (1). To avoid the risk of post-procedure pancreatic duct leak, enucleation is considered safe only in tumours more than 2–3 mm away from the pancreatic duct (13). This patient’s tumour was only 3 mm from the pancreatic duct, making it a high-risk procedure. Radical resection is considered for lesions near the main pancreatic duct, >4 cm in diameter, poorly capsulated, and multiple lesions (1). The risk of peri- or post-surgical complications range from 33 to 52%, including pancreatic fistulas in 8–19% of cases (3). The recurrence rate of insulinoma following open surgery is 7.2%, and the mortality rate is 3.7% (14). Furthermore, this patient was also at potential risk of bleeding during surgery due to being on anticoagulants, and at increased risk of propagation of DVT and further thromboembolic complications postoperatively (10). The multidisciplinary team opted for MIP instead of surgery due to her poor general condition, advanced age, having frequent episodes of symptomatic hypoglycaemia, presence of concomitant DVT, and increased risk of post-operative thrombotic complications (1).
MIPs including STAE, and ablative procedures are recommended when surgery is contraindicated or refused. These MIPs are now increasingly preferred over surgery due to their minimal invasiveness, repeatability, limited hospital stay, and lower risk of postprocedural complications, including abdominal pain, pancreatitis and transient diabetes (15). However, MIPs require expensive equipment, operator experience and expertise. The inability to obtain histology for grading and prognostication is a limitation in embolization and ablative therapies. Islet cells are surrounded by a rich blood supply, receiving 10–15% of the pancreatic blood flow. Insulinomas are highly vascular tumours making STAE a good therapeutic option (1, 15). This technique directly approaches the tumour via the vasculature and embolizes substances like microfibrillar collagen, polyvinyl alcohol particles or trisacryl gelatin microspheres (15). There are no reported cases of STAE of insulinoma in Sri Lanka up to now. STAE carries the risks of conventional angiography and inadvertent embolization of nontargeted structures, which can be minimized by obtaining a super-selective arterial approach to the tumour area (15). This could not be performed in this patient because microcatheters could not be advanced into the multiple small feeding arteries shown on DSA.
CT or endoscopic USS-guided (EUS) AA, RFA, and MWA are successful ablative therapies for insulinoma. Only tumours <2 cm were eligible for AA and need caution in tumours adjacent to blood vessels, due to the risk of bleeding (14). The superior surface of this insulinoma was abutted by the splenic artery. Therefore performing alcohol ablation as the primary ablative procedure would have carried a risk of bleeding, especially in this patient who had a risk of bleeding while being on anticoagulants. Yaoet al.showed that 4 out of 26 patients (15.4%) who underwent AA had a recurrence of insulinoma, which was higher compared to the following surgery but had the advantage that AA could be repeated (14). There are no reported cases of RFA or MWA of insulinoma in Sri Lanka to date. RFA and MWA are safe only for insulinomas originating in the anterosuperior and inferior parts of the pancreas, to avoid puncture or thermal damage to the pancreatic duct, which commonly originates from the posterosuperior part of the pancreas (14). Since the head, neck, and body of the pancreas, except for the tail is located retroperitoneally, percutaneous RFA or MWA of tumours in those parts have a risk of puncture and thermal damage to organs situated in front of the pancreas (14). This patient’s tumour was situated in the antero-superior aspect of the body of the pancreas, with a narrow window between the stomach and the liver, enabling safe percutaneous access with no collateral damage. EUS guided ablation of insulinoma enables an easier target of the tumour than the percutaneous approach, with minimal damage to other organs, and the added advantage of acquiring tissue biopsy specimens (14). Laparoscopic RFA has the benefit of direct visualization of the tumour, and the ability to gain tissue samples for histology and immunohistochemistry (14). RFA and MWA cause electromagnetic waves to produce agitation of water molecules and induce frictional heating of the tumour tissue, resulting in coagulative necrosis and cell death (3). The wavelengths of radiofrequency waves and microwaves are 30 cm to 1000 m and 1 mm to 1 m, respectively (16). MWA has added advantages compared to RFA, including its effect being independent of tissue impedance, absence of electrical circuit, not requiring cooling, less susceptibility to heat-sinks due to the shorter wavelength compared to RFA (3). The ablation area is not limited by water vaporization or charring, as in RFA due to electrical insulation. Therefore, the microwave field penetrates the tissue deeper, causing more effective transfer of the microwave energy to tissues, thus significantly reducing the ablation time (3).
MIPs have a risk of localized abdominal pain, mild pancreatitis, pseudocysts and duodenal ulceration, but are less frequent than following surgery (14). AA has a higher incidence of pancreatitis compared to RFA and MWA, and rarely necrotising pancreatitis (17). Pancreatitis occurs with an injection of >2 mL ethanol, and direct injection of ethanol into the parenchyma has a risk of severe pancreatitis due to direct cytotoxic effect and pancreatic ductal hypertension (17). Pancreatitis due to RFA and MWA is rare and self-resolving and is thought to be due to thermal damage, which is less in MWA compared to RFA (3, 17). This patient developed mild focal pancreatitis, which could also be attributed to the injection of 2 mL of 99% alcohol to the lesion than due to MWA itself. This is completely resolved with treatment.
Conclusion
With advancing technology, novel MIPs in treating insulinoma are increasingly preferred over surgery worldwide since of late. MWA could be recommended preferentially over STAE, AA, and RFA due to its efficacy and minimal complications. We report the first case of MWA performed in combination with AA in successfully treating insulinoma to our knowledge. This is also the first reported case of DVT associated with isolated insulinoma prior to intervention, though it is rarely reported in MEN1 syndrome. However, such patients need to be monitored for the possibility of underlying MEN1 syndrome, which was a limitation in this patient. This is an example of the impact of the COVID-19 pandemic on non-COVID-19 related disease.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Author contribution statement
K P J, D T M, C K, L P, B A A were involved in investigating and managing the patient. K P J did the literature review and wrote the article. D T M and B A A supervised. All authors read and approved the final manuscript.
Acknowledgement
The authors all express our gratitude to the patient who kindly gave consent for this case to be presented in this paper.
References
- 1.Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K. Diagnosis and management of insulinoma. World Journal of Gastroenterology 201319829–837. ( 10.3748/wjg.v19.i6.829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozkirli E, Bakiner O, Abali H, Andic C, Yapar AF, Kayaselcuk F, Ertorer E. A case of inoperable malignant insulinoma with resistant hypoglycemia who experienced the most significant clinical improvement with everolimus. Case Reports in Endocrinology 20132013 636175. ( 10.1155/2013/636175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egorov AV, Vasilyev IA, Musayev GH, Mironova AV. The role of microwave ablation in management of functioning pancreatic neuroendocrine tumors. Gland Surgery 20198766–772. ( 10.21037/gs.2019.12.07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jürgensen C, Schuppan D, Neser F, Ernstberger J, Junghans U, Stölzel U. EUS-guided alcohol ablation of an insulinoma. Gastrointestinal Endoscopy 200663 1059–1062. ( 10.1016/j.gie.2005.10.034) [DOI] [PubMed] [Google Scholar]
- 5.Limmer S, Huppert PE, Juette V, Lenhart A, Welte M, Wietholtz H. Radiofrequency ablation of solitary pancreatic insulinoma in a patient with episodes of severe hypoglycemia. European Journal of Gastroenterology and Hepatology 2009211097–1101. ( 10.1097/meg.0b013e328323d70e) [DOI] [PubMed] [Google Scholar]
- 6.Prochazka V, Hlavsa J, Andrasina T, Stary K, Muckova K, Kala Z, Valek V. Laparoscopic radiofrequency ablation of functioning pancreatic insulinoma: video case report. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques 201222e312–e315. ( 10.1097/SLE.0b013e318264b607) [DOI] [PubMed] [Google Scholar]
- 7.Chen OT, Dojki FK, Weber SM, Hinshaw JL. Percutaneous microwave ablation of an insulinoma in a patient with refractory symptomatic hypoglycemia. Journal of Gastrointestinal Surgery 2015191378–1381. ( 10.1007/s11605-015-2831-2) [DOI] [PubMed] [Google Scholar]
- 8.Koshy AA, Gordon IO, Van Ha TG, Kaplan EL, Philipson LH. Metastatic insulinoma following resection of nonsecreting pancreatic islet cell tumor: a case report and review of the literature. Journal of Investigative Medicine High Impact Case Reports 201312324709612473274. ( 10.1177/2324709612473274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massironi S, Cavalcoli F, Artoni A, Sciola V, Zilli A, Ciafardini C, Rossi RE. Thrombotic risk in gastroenteropancreatic neuroendocrine tumor patients: a single-center experience. Annals of Gastroenterology 202134588–593. ( 10.20524/aog.2021.0613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber-Alvarez P, Weber-Sánchez LA, Sánchez-Brito LG. Thrombophilia and multiple thrombosis post pancreatectomy due to insulinoma: case report. Journal of Clinical Investigation and Studies 201811–3. ( 10.15761/JCIS.1000116) [DOI] [Google Scholar]
- 11.Lee ME, Ortega-Sustache YM, Agarwal SK, Tepede A, Welch J, Mandl A, Bansal R, Tirosh A, Piaggi P, Cochran Cet al. Patients with MEN1 are at an increased risk for venous thromboembolism. Journal of Clinical Endocrinology and Metabolism 2021106e460–e468. ( 10.1210/clinem/dgaa501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuo F, Anastasopoulou C. Insulinoma. [Updated 2020 Jun 27]. In StatPearls [Internet]. Treasure Island (FL): StatPearls; Publishing, 2021. (available at: https://www.ncbi.nlm.nih.gov/books/NBK544299/) [Google Scholar]
- 13.Chinthakindi DM, Prashanth DK, Thogari DR, Pratap Reddy DR. A study of diagnosis and surgical management of pancreatic insulinoma. Surgical Update 2018459–67. ( 10.17511/ijoso.2018.i01.10) [DOI] [Google Scholar]
- 14.Yao C, Wang X, Zhang Y, Kong J, Gao J, Ke S, Ding X, Xin Z, Xu W, Wang Set al. Treatment of insulinomas by laparoscopic radiofrequency ablation: case reports and literature review. Open Medicine 20201584–91. ( 10.1515/med-2020-0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mele C, Brunani A, Damascelli B, Tichà V, Aimaretti G, Scacchi M, Marzullo P. Embolization for insulinoma in complicated severe obesity: description of an inoperable case and review of the literature. Journal of the Pancreas 201718287–293. [Google Scholar]
- 16.Britannica, The Editors of Encyclopaedia. ‘Radio Wave’. Encyclopedia Britannica, 2020. (available at: https://www.britannica.com/science/radio-wave). Accessed on 14 May 2021. [Google Scholar]
- 17.So H, Oh D, Seo DW. Recent developments in endoscopic ultrasound-guided ablation treatment. International Journal of Gastrointestinal Intervention 20209135–141. ( 10.18528/ijgii200036) [DOI] [Google Scholar]



 This work is licensed under a
This work is licensed under a