Abstract
Fatty acids (FA) are one of the substrates that can be oxidized for energy production. The blood concentration of all types of FA varies according to different nutrition conditions, and follicular fluid levels are generally in line with serum levels. Elevated levels of FA, especially non-esterified fatty acids (NEFA), are commonly found in females with metabolic issues, which are often related to subfertility in many species including humans, pigs, cattle, and mice. Long-time exposure to an excessive quantity of fatty acids impairs the cell structure and functions causing injuries in tissues and organs, resulting in lipotoxicity and eventually hampering health and fertility. High levels of saturated NEFA can have detrimental effects on granulosa cells, oocyte quality, and embryo development. Although the harmful effects of FA are established in reproductive tissues, how granulosa cells and cumulus cells respond and cooperate with oocytes when exposed to NEFA requires further understanding. This review provides a summary of the adverse impacts of exposure to NEFA during in vitro maturation on oocytes, follicular cells, and embryos. A comprehensive understanding of the effects of NEFA on oocytes in vitro would improve our understanding of the impacts of natural exposure in vivo.
Lay summary
Exposure to excess FAs affects the health of eggs, early embryos, and children born from these. The way different cell types react to excess FAs has not been studied very extensively, especially in pigs which provide a good model to investigate the impact of nutrition on the ovaries in humans. This review also looks at the way cells surrounding the egg react to FAs to help our understanding of the impact of excess fatty acids on female fertility.
Keywords: fatty acids, oocyte, granulosa cells, cumulus cells, blastocyst
Introduction
Female fertility relies heavily on ovarian function and the resulting oocyte quality. The oocyte, which oversees subsequent development, is a relatively dependent entity. Although it can be matured and fertilized in vitro, its development in vivo is dependent on the status of its microenvironment before fertilization, which includes the follicular cells and the follicular fluid. In the follicle, the developing oocyte is surrounded by cumulus cells attached to the mural granulosa cells, and the roles of these two types of granulosa cells in maintaining the quality of the oocyte are determinant. Cumulus cells are regarded as nutritive cells because they provide the oocyte with paracrine factors and metabolic substrates including pyruvate. Mural granulosa cells communicate with cumulus cells via cell junctions to exchange metabolites, growth factors, and signaling messengers. Besides, the follicular fluid (FF) serves as another substrate supplier and the amounts of nutrients in FF are dependent on those in the bloodstream. Therefore, the composition of the FF and the oocyte reflects the mother’s nutrition. The facts that mitochondria are the units responsible for energy production and that most mitochondria in an individual originate from the oocyte, and are therefore derived from the mother, highlight the influence of maternal nutrition on the oocyte and potentially on the energy metabolism of the offspring.
Fatty acid (FA) concentrations are affected by the diet and the lipolysis of stored lipids. The FA concentrations, especially non-esterified fatty acids (NEFA), which are also known as free fatty acids (FFA), are increased during both over- and under-nutrition conditions, and the elevated levels are associated with low embryo development in humans, cattle, and pigs (Leroy et al. 2005, Hoving et al. 2012, Valckx et al. 2014b ). Some of the excess FAs are stored in lipid droplets, some exist as FFA, some are transferred into mitochondria and β-oxidized for energy production, and some remain in the cytosol and either undergo peroxidation and generate ROS or affect the endoplasmic reticulum (ER) calcium store and cause ER stress. The level of toxicity for cells strongly depends on the type of FA, saturated or unsaturated, to which cells are exposed.
In recent years, more studies focused on the effects of elevated levels of NEFA on granulosa cells, cumulus cells, oocytes, and early embryo development in various species (Mu et al. 2001, Leroy et al. 2005, Aardema et al. 2011, Van Hoeck et al. 2011, Aardema et al. 2013, Desmet et al. 2016, Sutton-McDowall et al. 2016, Aardema et al. 2017, Itami et al. 2018, Ogawa et al. 2018, Shi & Sirard 2021a ). Additionally, ovarian dysfunctions induced by high fat diets were also investigated in humans (Chavarro et al. 2007), mice (Yi et al. 2012) and cattle (Zachut et al. 2010). Previous reviews paid more attention to species such as human, cattle, and mice. Because of some similarities including a very comparable digestive physiology and metabolism (monogastric) and an approximate length of IVM in humans and pigs, the pig is an excellent model to study the impacts of nutrition on ovarian function in humans without all the ethical limitations. Considering that the relationships between the different follicular cell types under the influence of FA have not been studied very extensively in the past, this review presents our revised understanding of the effects of elevated NEFA on female reproductive success and also of the protective functions of granulosa and cumulus cells on the oocytes of pigs and other mammals.
Energy metabolism in cumulus–oocyte complexes
The oocyte is the female germ cell involved in reproduction, and it is the source of life in mammals. Its development and the initial days of the subsequent embryo development are energy-consuming processes. As opposed to somatic cells, oocytes and early embryos need to metabolize not only the principal energy sources from exogenous nutrients but also energy sources from intracellular stores (Sturmey & Leese 2003). Substrates stored in oocytes can support the energy requirements of the early days of embryo development since sufficient and appropriate energy metabolism plays a critical role in maintaining developmental competence. In vitro matured oocytes and in vitro produced embryos have reduced developmental competence and they have reduced capacity to utilize glucose, suggesting that energy deficiency is an indicator of lower oocyte and embryo qualities (Krisher et al. 2007). When considering the metabolism of the oocyte, it is important to consider the interactions between the oocyte, cumulus cells, and granulosa cells because these cells produce and exchange metabolites during development.
Carbohydrate metabolism
Glucose is metabolized via glycolysis, the pentose phosphate pathway (PPP), and tricarboxylic acid (TCA) in oocytes. Metabolism of pyruvate, glutamine, and glycine by the Krebs cycle increases during in vitro maturation, indicating that oxidative metabolism plays an essential role in providing energy for oocyte maturation. Compared to immature cumulus–oocyte complexes (COCs), mature COCs consume higher amounts of substrates and store more energy for fertilization and subsequent early embryo development. Moreover, during all the stages of development from immature oocyte to expanded blastocyst, most of the ATP (about 95%) is generated by oxidative metabolism (Sturmey & Leese 2003). Different from mouse and cow oocytes, which prefer to use pyruvate produced by and transferred from cumulus cells, porcine oocytes prefer to utilize glucose as their primary substrate for energy production, and the pathways of glucose metabolism are also different between oocytes matured in vivo and in vitro (Krisher et al. 2007).
Lipid metabolism
In addition to playing important roles in many biological functions including biological membrane construction, cell–cell interactions, steroid synthesis, and signaling, lipids also act as a source of nutrients. Decreased triglyceride, phospholipids, cholesterol, and total lipids during oocyte maturation suggest the important role of lipid metabolism in oocyte maturation (Ferguson & Leese 1999, Sturmey & Leese 2003). FAs are utilized via β-oxidation in mitochondria, and the lipid–mitochondrial microstructures highlight the importance of lipids in generating ATP production. Considering one single molecule of FAs (such as palmitate) and glucose, the use of lipids yields substantially higher amounts of ATP compared to carbohydrates. Studies of genes involved in lipid metabolism were conducted in porcine and bovine ovaries (Uzbekova et al. 2015, Bertevello et al. 2018), and the different transcription patterns of genes related to FA metabolism in oocytes, theca cells, granulosa cells, and cumulus cells indicated that the different follicular compartments play different roles in lipid homeostasis.
The β-oxidation of FA was reduced in both cumulus cells and oocytes matured in vitro compared to cumulus cells and oocytes matured in vivo in pig (Yuan et al. 2011) and mouse (Dunning et al. 2014). In vitro maturated oocytes display a lower developmental competence, and lipid oxidation seems to be involved in creating this difference.
Lipids are required for energy production during oocyte maturation
FAs, stored in lipid droplets as triglycerides, are metabolized by β-oxidation and the TCA cycle in mitochondria. Closed location of mitochondria and lipid droplets in oocytes makes them a ‘metabolic unit’, which ensures a steady and readily available supply for lipid oxidation processes (Kruip et al. 1983, Sturmey et al. 2006). The co-localization of mitochondria and surrounding lipid droplets (with a distance of 6–10 nm) reinforces the important role of lipid metabolism during oocyte maturation (Sturmey et al. 2006). The essential role of lipid β-oxidation can be also demonstrated by its inhibition and activation since the alteration of β-oxidation can lead to impairment in oocyte maturation and development (Ferguson & Leese 2006, Dunning et al. 2011). A study investigating the effects of FA on granulosa cells showed that the inhibition of FA oxidation inhibits proliferation and progesterone secretion in bovine granulosa cells (Elis et al. 2015). Therefore, moderate β-oxidation that provides enough ATP but without excess production of ROS during maturation is very important for oocyte developmental competence. However, the degree of dependence on lipid metabolism in oocytes varies between species. Compared to bovine and mouse oocytes, pig oocytes contain more lipids and a higher number of lipid droplets, and partial inhibition of FA β-oxidation can inhibit porcine oocyte maturation while the inhibition of FA β-oxidation do not impair mouse and bovine oocyte maturation since they are capable to use carbohydrate metabolism to compensate for a deficiency in energy produced by FA β-oxidation (Paczkowski et al. 2013). This study suggests that the lipid metabolism during oocyte maturation is more important in the species of which oocytes contain more lipids (McEvoy et al. 2000).
Balance between lipid oxidation and glucose consumption
We know that oocytes metabolize carbohydrates (pyruvate and lactate) and lipids (free fatty acids) to generate energy to maintain and support their biological development; however, these metabolic processes produce side products such as reactive oxygen species (ROS). Basal ROS production is an unavoidable natural mechanism in cells, but prolonged and high ROS production can cause oxidative stress which induces apoptosis. The ‘quiet embryo hypothesis’ described by Leese (2002) stipulates that having enough energy and maintaining a minimum level of ROS is optimal for oocyte and early embryo development. In such conditions, the activity of some oocyte and embryo mitochondria is ‘shut down’ to maintain the quiet status. Oocytes and cleavage-stage embryos have lower oxygen consumption than blastocyst, suggestion ‘shut down’ metabolic activity (Sturmey & Leese 2003). Prolonged exposure to high levels of extracellular lipid in cells resulted in excess peroxidation of free FA, leading to the production of excess ROS and toxic lipid peroxides, which together are known as lipotoxicity (Igosheva et al. 2010). Therefore, the balanced metabolism of pyruvate and FA, which keeps a low level of ROS, is important for oocyte quality and developmental competence.
Lipid profiles in follicular cells
The lipid content of oocytes varies from species to species. Before maturation, pig oocytes contain 74 ng of triglycerides out of a total lipid content of 161 ng, and bovine oocytes contain 23 ng of triglycerides out of the total lipid content of 63 ng (McEvoy et al. 2000). Oocytes that contain a large amount of intracellular lipids are more difficult to cryopreserve because they are prone to cryoinjury and are more sensitive to temperature changes. The reasons why the lipid content varies between species are unknown, but there is one hypothesis that the species with a high lipid content in oocytes have a longer duration from ovulation to implantation because the stored lipids can act as endogenous energy reserves to maintain their basic needs until implantation (Sturmey et al. 2009, Bradley & Swann 2019). More studies are needed to explore the mechanisms responsible for the differences in lipid contents between species.
Dynamics of lipid profiles in oocytes
The analysis of FA profiles in pigs showed that the proportions of free saturated FA are 46, 40, and 37% in oocytes, FF, and serum, respectively; and the proportions of free mono-unsaturated FA are 27, 36, and 46% in oocytes, FF, and serum, respectively; and the proportions of free polyunsaturated FA are 27, 24, and 15% in oocytes, FF, and serum, respectively (Yao et al. 1980, Homa et al. 1986). The ratios of total free FA in oocytes are closer to those in FF, suggesting that the free FA content is derived directly from the FF rather than from the serum. It is important to notice that lipid metabolism is a dynamic process, and the composition of fatty acids is easily influenced by the maternal energetic state. Besides, palmitic acid (PA), oleic acid (OA), and stearic acid (SA) are the major FA of oocytes, FF, oviductal fluid, uterine fluid, and serum in pigs and cows, and they comprise approximately 60–80% of the total FA (Yao et al. 1980, Homa et al. 1986).
It is well known that organisms need nutrients to produce energy to maintain their biological functions besides surviving. There are differences in nutrient needs and energy storage in domestic animals between winter and summer. The FA composition of phospholipids in FF, granulosa cells, and oocytes in cows changes from summer to winter. In summer, there is a relatively higher level of saturated FA than that in winter (Zeron et al. 2001). Seasonal changes were associated with cow fertility and oocyte quality both in vivo and in vitro studies (Ryan et al. 1993), and the varying FA compositions between seasons may explain the decreased fertility in summer since the saturated FAs have more detrimental effects than unsaturated FAs on oocyte quality and development. Similarly, lower fertility, with decreased follicle and oocyte development, was also observed in pigs according to season (Bertoldo et al. 2010). Therefore, it is interesting to investigate whether there is a seasonal difference in the FA composition of porcine oocytes and verify the relationship between the FA composition and oocyte quality.
In addition to seasonal changes, lipid profiles also vary during follicle and oocyte development. Yao et al. found that the composition of free and esterified FA in porcine FF was different at the different follicle development stages (1980). The NEFA content decreased during follicular development, and the large follicles had the lowest level of NEFA which was similar to the serum level. In porcine oocytes, the amount of triglycerides, which is the major constituent of total lipid, decreased during maturation but remained stable until blastocyst development (Sturmey & Leese 2003). A similar pattern was observed during cow oocyte maturation and early embryo development (Ferguson & Leese 1999). Besides, another study using Nile Red fluorescent probe reported that the levels of phospholipids and cholesterol in lipid droplets also significantly decreased from immature to mature porcine oocytes (Romek et al. 2011). Although there was no decrease in triglycerides and phospholipids from the zygote to the morula stage, decreases were observed from morula to blastocyst and from blastocyst to hatched blastocyst (Romek et al. 2011). The divergences between these results and the results mentioned above can be explained by the different methods used to measure the triglyceride content. The decreased lipid content during porcine oocyte and early embryo development highlights the metabolic role of lipids, but it is necessary to consider the roles of other supplements, the serum supplemented in the IVM medium increases the lipid content in oocytes for example, that can mask these changes in endogenous reserves.
Varied levels of FA in abnormal metabolic conditions
It was mentioned before that the FA content of the follicular fluid may influence the relative FA content of the oocyte, and the concentrations of NEFA in the follicular fluid are dependent on their concentrations in the serum, which are easily influenced by the nutrition status (Leroy et al. 2005, Jungheim et al. 2010, Aardema et al. 2013). Therefore, dietary FA can disrupt the original lipid profile of the FF and thus of the oocyte, and these maternal changes are associated with oocyte quality, subsequent development, and metabolic diseases in offspring which may cross generations (Valckx et al. 2014a , Chambers et al. 2016). Besides, abnormal nutrition conditions such as diabetes in women and negative energy balance in cows and pigs were associated with elevated levels of NEFA (Leroy et al. 2005, Hoving et al. 2012, Valckx et al. 2014b ).
Negative energy balance (NEB) is a metabolic status achieved in the period after calving when the food intake does not satisfy the high energy demand of milk production in cattle. During this period, cows suffer from metabolic and endocrine issues, resulting in ovarian problems, poor oocyte quality, and reduced reproductive performance. Another obvious change is the two- to three-fold increase in the serum and follicular fluid NEFA levels in postpartum cows compared to the levels of prepartum cows (Leroy et al. 2005). There is a similar problem in sows, where weight loss during lactation has negative effects on ovulation rates, oocyte quality, embryo survival, and litter development (Vinsky et al. 2006). Elevated levels of follicular NEFA were also observed in sows experiencing lactation weight loss (Hoving et al. 2012).
Meanwhile, elevated levels of NEFA were also observed in women with high BMI values and obesity (Valckx et al. 2014b ); and FA concentrations in FF and infertility rates were positively correlated to increased BMI. A high-fat diet can also modify NEFA levels, and mice fed a high-fat diet had a higher concentration of NEFA in the serum (Tian et al. 2016). Besides, the high-fat diet increased the number of abnormal mitochondria and induced spindle and chromosome alignment defects in mouse oocytes (Luzzo et al. 2012). Besides, studies in mice demonstrated that diets rich in specific lipids could alter the lipid profile of ovarian cells, and along with lipotoxicity responses, impaired fertilization (Wakefield et al. 2008, Wu et al. 2010). In mouse and rat studies, metabolism dysfunctions induced by high-fat diets could be transmitted to next generations (Dunn & Bale 2009, Chambers et al. 2016).
The NEFA concentrations and composition profiles in the serum and FF of cows, humans, and pigs either with normal or abnormal nutritional status are listed in Table 1. There are differences and similarities between the NEFA concentrations of serum and FF of different species. The physiological concentrations of NEFA in bovine serum and FF are similar, but the concentration of NEFA in serum was increased with a higher level than that in FF under NEB conditions (Leroy et al. 2005). The NEFA concentration in human FF is lower than in the serum, and the altered levels under obesity are almost the same in both FF and serum (Igosheva et al. 2010, Valckx et al. 2012, 2014b ). However, the concentration of NEFA in pig FF is slightly higher in the serum (Yao et al. 1980). No study measured the concentration of NEFA in the FF of sows experiencing NEB. Most differences can be explained as species differences, but the large variation in pig serum may be due to breed differences. Besides, the reflection of an increase in the total NEFA levels from serum in the FF demonstrates their close relationship.
Table 1.
NEFA concentrations and composition profiles of physiological and pathological status of bovine, human, and porcine.
| Bovine | Human | Porcine | ||||
|---|---|---|---|---|---|---|
| Serum | FF | Serum | FF | Serum | FF | |
| Physiological NEFA | ||||||
| Concentrations (μM) | 100–300 | 100–300 | 600–700 | 200 | 200–400; 600–800 | 600–900 |
| Composition profile | ||||||
| PA | 17% | 22% | – | 23% | 20% | 27% |
| SA | 28% | 15% | – | 9% | 17% | 12% |
| OA | 22% | 30% | – | 33% | 44% | 33% |
| Pathological NEFA | ||||||
| Concentrations (μM) | 400–1200 | 200–600 | 700–900 | 300 | 600–1200 | – |
| Composition profile | ||||||
| PA | 21% | 22% | - | 22% | – | – |
| SA | 24% | 13% | - | 13% | – | – |
| OA | 35% | 37% | - | 37% | – | – |
| References | Leroy et al. (2005) | Leroy et al. (2005) | Igosheva et al. (2010), Valckx et al. (2012, 2014b) | Valckx et al. (2014b) | Yao et al. (1980), Hoving et al. (2012) | Yao et al. (1980) |
The conditions mentioned above reveal that NEFA levels may play important roles in regulating oocyte quality and development, which could have impacts on progeny since the maternal nutrition status has been highly correlated with metabolic disorders in offspring (Valckx et al. 2014a , Chambers et al. 2016).
Oocyte maturation and its subsequent development are sensitive to the lipid environment
Lipid uptake and storage
As mentioned above, lipid storage undergoes dramatic changes during oocyte maturation and early embryo development, varies seasonally, or differs according to the nutritional status. But whether other supplementations to in vitro culture media could increase lipid storage needs to be addressed separately. Oocytes matured in the presence of 10% fetal calf serum had more lipid stored, including triglycerides and cholesterol, compared to oocytes cultured in serum-free medium (Yang et al. 2010). It should be noted that the serum FA composition changes under specific metabolic stress, and these changes are reflected in the FF, oocyte, and ovarian cells but to different extents. The differentially expressed lipid metabolism-related genes are corresponding to the different functions and the differential lipid profile of follicular cells and oocytes (Uzbekova et al. 2015).
Besides, the supplementation of SA or PA decreased lipid accumulation in bovine oocytes, but a high level of OA or linoleic acid could increase neutral lipid accumulation (Aardema et al. 2011, Carro et al. 2013). When mouse oocytes were exposed to human FF enriched in triglycerides and FFA during maturation, the neutral lipid content increased (Yang et al. 2012). Also, when the culture medium of pig COCs was supplemented with a mix of NEFAs (PA, SA, and OA) or SA alone, the lipid content of the cumulus cells reflected the altered composition of the medium, but the lipid content of oocytes was not affected (Ogawa et al. 2018, Pawlak et al. 2020). It is similar to bovine where exposure to a high level of the mix of NEFAs (PA, SA, and OA) during IVM resulted in a massive lipid accumulation in cumulus cells but not in the oocyte (Aardema et al. 2013). However, supplementation with PA was able to increase the lipid content in porcine oocytes (Shibahara et al. 2020). Although differences exist between species and variations also depend on concentrations used, it can be speculated that cumulus cells play the role of accumulating extra FA when exposed to the high level of the mix of NEFAs and the presence of unsaturated FFA promotes this accumulation process.
Saturated FFA have detrimental effects
The addition of extra NEFAs to the culture medium not only changed the lipid content but long-term excessive lipid exposure could also result in detrimental effects, which are collectively referred to as lipotoxicity (Igosheva et al. 2010). Studies in bovine, the most studied model, showed that an elevated level of NEFA reduced cell viability and steroidogenesis and increased apoptosis of granulosa, cumulus, and theca cells (Leroy et al. 2005, Vanholder et al. 2006, Wu et al. 2012, Sutton-McDowall et al. 2016, Aardema et al. 2017, Sharma et al. 2019, Wang et al. 2020). Besides, high levels of NEFA in the IVM medium resulted in delayed maturation, low fertilization, and decreased blastocyst formation. The blastocysts displayed lower cell numbers, higher apoptotic ratios, and aberrant metabolism (Van Hoeck et al. 2011, Desmet et al. 2016).
Supplementation of NEFA also had negative effects on porcine granulosa cell proliferation and viability and increased the lipid content in granulosa and cumulus cells (Ogawa et al. 2018, Pawlak et al. 2020, Shibahara et al. 2020). Besides, the high level of NEFA altered transcriptomic pattern which is enriched in pathways related to metabolism, inflammation, and impaired mitochondrial function in granulosa cells (Shi & Sirard 2021a). After ovulation, the epithelial–mesenchymal transition (EMT) of granulosa cells to luteal cells occurs, which is associated with changes in estradiol and progesterone production. Inhibition of EMT and altered steroidogenesis in response to high levels of NEFA partly explain the anovulation and impaired follicle development observed in pathological nutritional status (Foong et al. 2006, Chaput & Sirard 2020, Shi & Sirard 2021a). Moreover, supplementation with NEFA during IVM also decreased the blastocyst formation (Shibahara et al. 2020, Shi & Sirard 2021c). However, another study in porcine found that supplementation with NEFA-rich FF or NEFA was not harmful to the oocyte developmental competence (Ogawa et al. 2018). These controversial results can be explained by the different concentrations used. The concentration of that increased blastocyst formation was less than half of the actual level in FF and much lower than the pathological level, and the other factors in the supplemented FF that do not exist in control (only with BSA) may be responsible for this beneficial effect.
Similarly, the survival of human granulosa cells was reduced under elevated SA and PA concentrations (Mu et al. 2001). Human embryos originating from oocytes from follicles with a higher percentage of saturated FA, especially PA and SA, failed to cleave (O’Gorman et al. 2013). Moreover, the IVF outcome of obese patients could be improved if the oocytes were from a donor with normal BMI; however, oocytes from a mother on a high-fat diet suffered the same defects and failed to develop into blastocysts even if they were transferred to a surrogate with normal BMI (Jungheim et al. 2013). It could be concluded that the high-fat environment has stronger effects at the oocyte level. In mice, the supplementation of NEFA reduced follicular antrum formation, increased progesterone synthesis, induced apoptosis in granulosa cells, and impaired oocyte maturation and development (Valckx et al. 2014a, Chen et al. 2019).
Further studies aimed at understanding the mechanisms involved in the effects of elevated NEFA via analyses of transcriptome and DNA methylation. The methylation pattern of the maternal imprinted genes and several metabolism-related genes were altered in oocytes from both obese mice and their offspring (Ge et al. 2014). In bovine, blastocysts originating from oocytes exposed to elevated NEFA had different transcriptomic patterns in pathways related to lipid and carbohydrate metabolism and cell death (Van Hoeck et al. 2015). Similarly to gene expression, the DNA methylation pattern of blastocysts originating from oocytes exposed to NEFA was altered in pathways involved in lipid and carbohydrate metabolism, cell death, immune response, and metabolic disorders (Desmet et al. 2016). In pigs, transcriptome and DNA methylation patterns of blastocysts from oocytes exposed to NEFA were also altered, and affected pathways were mainly related to metabolism and inflammation (Shi & Sirard 2021c). Besides, transcriptomic analysis of GCs cocultured with COCs showed that metabolism, inflammatory responses, mitochondrial dysfunctions, and the EMT were all affected by the elevated level of NEFA (Shi & Sirard 2021a). The differences and similarities in the effects of high levels of NEFA in different species are listed in Table 2.
Table 2.
NEFA effects on biological functions in different cell types of different species.
| Species/exposure | Cell type | Affected biological functions | Reference |
|---|---|---|---|
| Humans | |||
| In vitro | GCs | Cell viability, cell proliferation, apoptosis, steroidogenesis, fatty acid metabolism | Mu et al. (2001) |
| Bovine | |||
| In vitro | GCs, CCs, TCs | Cell proliferation, apoptosis, steroidogenesis, ROS production, ER stress, EMT | Leroy et al. (2005), Vanholder et al. (2006), Wu et al. (2012), Aardema et al. (2013), Sutton-McDowall et al. (2016), Sharma et al. (2019), Wang et al. (2020), Yenuganti et al. (2021) |
| Oocyte | Maturation, fertilization, development competence, apoptosis | Leroy et al. (2005), Aardema et al. (2011), Carro et al. (2013) | |
| Blastocyst | Blastocyst formation, blastocyst formation, energy metabolism, ER stress, inflammation, transcriptome, DNA methylation pattern | Leroy et al. (2005), Van Hoeck et al. (2011, 2013, 2015), Desmet et al. (2016), Sutton-McDowall et al. (2016) | |
| In vivo | GCs, CCs | Lipid storage, lipid profile | Zachut et al. (2010), Aardema et al. (2013) |
| Porcine | |||
| In vitro | GCs, CCs | Lipid storage, cell proliferation, cell viability, metabolism, EMT | Ogawa et al. (2018), Pawlak et al. (2020), Shibahara et al. (2020) |
| Oocyte | Maturation, mitochondrial functions, lipid storage, development competence | Itami et al. (2018), Ogawa et al. (2018), Shibahara et al. (2020) | |
| Blastocyst | Lipid storage, development competence, metabolism, inflammation, transcriptome, DNA methylation pattern | Pawlak et al. (2020), Shibahara et al. (2020), Shi and Sirard (2021b) | |
| In vivo | Ovary | Follicle development, apoptosis, antioxidants production | Xu et al. (2016) |
| Mouse | |||
| In vitro | GCs | Steroidogenesis, cell viability, apoptosis | Valckx et al. (2014a), Chen et al. (2019) |
| Oocyte | Lipid storage, ER stress, maturation, development ability | Valckx et al. (2014a), Yang et al. (2012) | |
| Blastocyst | Development ability, ER stress, lipid storage, mitochondrial superoxide level | Valckx et al. (2014a), Yousif (2019) | |
| In vivo | GCs, CCs, oocyte, blastocyst | Mitochondrial activity, ROS production, apoptosis, ER stress, ovulation, fertilization, blastocyst formation | Igosheva et al. (2010), Wu et al. (2010) |
| Sheep | |||
| In vivo | GCs, oocyte, blastocyst | Fatty acid composition, steroidogenesis, cell proliferation, embryo development | Wonnacott et al. (2010) |
CCs, cumulus cells; EMT, epithelial–mesenchymal transition; ER, endoplasmic reticulum; GCs, granulosa cells; ROS, reactive oxygen species; TCs, theca cells.
It seems that different types of FA impair cell functions via different mechanisms. A high-fat diet or direct exposure to excessive FA induced mitochondrial abnormalities in oocytes that could be countered by antioxidants (Boots et al. 2016); however, antioxidants seemed useless to counter the injuries caused by PA in early embryo development (Nonogaki et al. 1994). In the case of PA, endoplasmic reticulum calcium ATPase pumps were negatively affected, and the ER stress inhibitor salubrinal® could reverse the impaired oocyte development induced by PA exposure (Wu et al. 2012). Elevated SA and NEFA levels upregulated redox genes in oocytes but decreased their relative expression in cumulus cells, indicating their different metabolism in oocytes and cumulus cells (Van Hoeck et al. 2013).
Mono-unsaturated FA counteracts the adverse effects of saturated FA
Among the saturated FA, different types have different mechanisms to affect oocyte quality and development. Excess saturated FA undergo peroxidation, leading to increased apoptosis and reduced success of fertilization and development (Nonogaki et al. 1994, Itami et al. 2018). However, unsaturated FA have beneficial effects on fertility, and they can counteract the adverse effects of saturated FA. Linoleic acid and OA improved bovine and mouse embryo development in a dose-dependent manner, but a high dose impaired maturation (Marei et al. 2010, Aardema et al. 2011). Similarly, studies in cows found that supplementation with either linolenic or alpha-linolenic acid increased the corresponding FA in FF, granulosa cells, COCs, as well as improved the cleavage rate (Zachut et al. 2010). Besides, a diet supplemented with omega 3 and 6 affected the FA composition and development of granulosa cells, oocytes, and blastocysts in sheep (Wonnacott et al. 2010). Higher concentrations of total unsaturated FA are related to better follicle growth, oocyte quality, fertilization, and early embryo development. Unlike saturated FA, unsaturated FA is preferentially and more easily stored as triglycerides, and activation of the peroxisome proliferator-activated receptors (PPARs) by unsaturated FA can reduce lipotoxicity of saturated FA (Nolan & Larter 2009). Reduced lipotoxicity was demonstrated when supplementation of OA improved embryo morphology and blastocyst formation under exposure to PA and SA (Aardema et al. 2011, Valckx et al. 2014a ). However, the ultimate effect of unsaturated FA, especially OA, was dependent on their concentration and the ratio of OA to other FA: a low concentration of OA and its low ratio to saturated FA may not have any beneficial effect.
Short-term changes in NEFA levels by fasting do change the composition of NEFA in serum, FF and cumulus cells, but does not change that of oocytes nor the subsequent developmental competence (Aardema et al. 2013). However, elevated NEFA levels in vitro changed the FA composition profiles of all cell types and hampered oocyte quality and its developmental competence (Vanholder et al. 2006, Valckx et al. 2014a , Van Hoeck et al. 2015, Desmet et al. 2016, Sutton-McDowall et al. 2016, Pawlak et al. 2020, Shibahara et al. 2020, Wang et al. 2020, Shi & Sirard 2021b ). Distinctions between in vivo and in vitro studies could be explained by the different environments since the in vivo environment is more homeostatically precise and dynamic under the regulation of all the follicular cells and the FF. Additionally, storage and utilization by other tissues and cells, their interactions in vivo, and other factors might be responsible for these differences. Therefore, when exploring the effects of NEFA on oocytes, we need to consider the role of the other ovarian cell types.
Protective functions of cumulus and granulosa cells exposed to NEFA
The follicular environment and the orchestrated crosstalk between somatic cells and the oocyte are essential conditions for the oocyte to achieve competence for fertilization. In antral follicles, specialized granulosa cells which surround the oocyte are named cumulus cells. Cumulus cells and the connected mural granulosa cells supply nutrients to the oocyte. Gap junctions between cumulus cells and the oocyte or mural granulosa cells allow bidirectional paracrine signaling, including signals for the regulation of chromatin remodeling and RNA synthesis in the oocyte. Besides, the utilization of glucose by cumulus cells is the way to provide pyruvate to the oocyte, which has a low capacity to utilize glucose and support maturation. Cumulus cells are involved in providing FA to oocytes and affect their lipid metabolism. The removal of cumulus cells before IVM decreased bovine oocyte developmental competence, and this suboptimal cytoplasmic maturation was associated with affected lipid metabolism probably via increased lipolysis and FA oxidation in the oocyte (Aardema et al. 2013, Auclair et al. 2013, Lolicato et al. 2015, Aardema et al. 2017). Therefore, it is believed that cumulus and granulosa cells have protective functions to ensure proper oocyte development, especially during exposure to excess NEFA.
Lipid storage
FAs in circulation are transported into cells via either FA transporter proteins or by direct diffusion through the lipid bilayer. The transportation of follicular fluid FA into cumulus cells and oocytes is with the help of albumin or lipoproteins. However, cumulus cells and oocytes have different abilities to uptake and utilize FAs and lipids, as demonstrated by the different expression patterns of genes related to lipid metabolism in cumulus cells and oocytes (Uzbekova et al. 2015). Whether these cells prefer to use their lipid stores or the lipids from the extracellular milieu and how they interact with each other under lipid stress are not fully clear.
Bovine oocytes matured in vitro in the absence of cumulus cells have fewer lipid content, which implies that denuded oocytes may use more stored lipid (Auclair et al. 2013). With the exposure to NEFAs, there was a higher level of FA incorporation when the oocytes were maturated without cumulus cells (Lolicato et al. 2015). Besides, the oocytes matured without cumulus cells were more vulnerable when they were exposed to saturated FA (Aardema et al. 2017). These studies stress the important roles of cumulus cells in regulating the lipid content in oocyte and in protecting oocytes from the damages of excess saturated FA. In the presence of excess NEFA, granulosa cells, cumulus cells, and oocytes accumulated lipids (Yang et al. 2012, Carro et al. 2013, Ogawa et al. 2018, Pawlak et al. 2020, Shibahara et al. 2020). Besides, lipid storage in response to exposure to mono-unsaturated FA is one possible mechanism to explain the counteracting effects of OA on the negative effects of other saturated FA. The more lipid stored, the fewer free FA can undergo peroxidation and less ROS are generated. Therefore, lipid storage in granulosa cells and cumulus cells can decrease the amount of NEFA that oocytes are exposed to.
A cellular enzyme, stearoyl-CoA desaturase (SCD), can desaturate saturated FA into mono-unsaturated FA. The inhibition of SCD decreased lipid storage in bovine cumulus cells and impaired blastocyst formation, suggesting a protective role of cumulus cells that decrease the negative effects of NEFA on oocytes (Aardema et al. 2017). Unlike in bovine, SCD is expressed not only in porcine cumulus cells but also in oocytes and embryos at different stages (Lee et al. 2019, Pawlak et al. 2020). This might help porcine oocytes store FA as lipid droplets since mono-saturated FAs have more potential to promote lipid storage than other NEFA. Besides, the expression of SCD in porcine oocytes suggests that they may be more tolerant of high levels of NEFA because they can desaturate the saturated FA, and the high level of saturated FAs is more potentially toxic than the same level of unsaturated FA.
Increased β-oxidation
In addition to being stored, FAs are oxidized for energy production in mitochondria or undergo peroxidation in the cytoplasm. Increased FA uptake via supplementation with L-carnitine or 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) can rescue cardiac mitochondria from lipotoxicity (Oyanagi et al. 2011), suggesting that an increased metabolism of FA is a kind of protection to decrease the excess FA available for peroxidation in the cytoplasm. Therefore, the oxidation of FA in cumulus cells and granulosa cells is another way to decrease the total amount of free NEFA in cells and decrease the lipotoxicity of NEFA to the oocyte.
Interactions between oocytes and granulosa cells
The transfer of factors through gap junctions between granulosa and cumulus cells and between cumulus cells and the oocyte plays important roles in maintaining proper growth and development. Cumulus cells can protect the oocyte from ROS by regulating redox homeostasis and providing glutathione to the oocyte. Under exposure to elevated levels of saturated NEFAs, more ROS are produced and the redox regulation of cumulus cells is unable to supply enough protection to the oocyte from ROS, which was shown as the ROS accumulation in COCs and the deterioration of cumulus cells (Lolicato et al. 2015). Compared to blastocysts from COCs cultured without a monolayer of granulosa cells, blastocysts originated from cocultured COCs had anti-inflammatory responses to the exposure to high levels of NEFA, as demonstrated by downregulation of inflammatory factors including SP1 transcription factor (SP1), tumor necrosis factor (TNF), and nuclear factor kappa B (NFKB), and increase of anti-apoptosis factors such as BCL2 like 1 (BCL2L1) (Shi & Sirard 2021b ). It can be speculated that some factors originating from cocultured granulosa cells can regulate inflammation. However, these factors have not been identified, and more studies are needed to find and verify them.
Mural granulosa cells and cumulus cells are barriers that protect oocytes from direct exposure to the hazardous environment. In the case of excess FAs, granulosa cells decrease the amount the oocyte is exposed to, either by storage and utilization like cumulus cells, or by activating response mechanisms including anti-inflammatory actions to make the oocyte healthier.
Conclusions
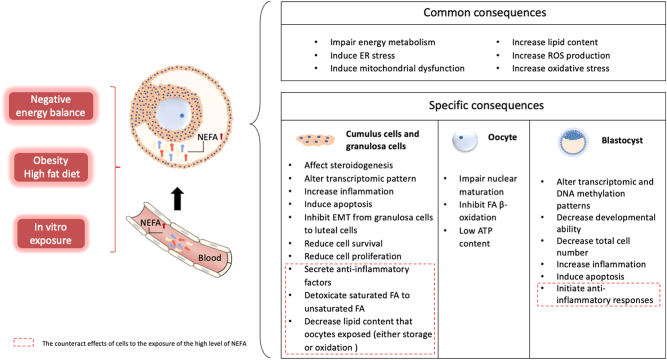
An elevated level of NEFA is one of the metabolic issues that impair oocyte quality and development, and the resulting defects may be retained in the following generations. In this study, we summarized the common and specific effects of NEFA on ovarian cells in humans, cows, mice, and pigs (Fig. 1). Fatty acids can be used as substrate to generate energy to maintain biological functions, and their storage and oxidation are important for oocyte and embryo development. Humans and domestic animals are both affected by abnormal FA levels which are regulated by the nutrition status. The bovine model is the most studied, and studies of humans are limited because of ethical problems. Nutrition-related fertility problems in pigs are less studied. More studies should focus on pigs, not only because they have physiological, nutritional, genomic homologies, and preimplantation development similarities compared to humans (Madeja et al. 2019) but also because the same problems also exist in this species, and the related mechanisms should be identified.
Figure 1.
The effects of the high levels of NEFA in ovarian cells. Some pathological nutrition statuses including negative energy balance or obesity or high-fat diet and in vitro exposure cause elevated NEFA levels in serum and follicular fluid. Impaired energy metabolism, ER stress, mitochondrial dysfunction, increased lipid content, increased ROS production, and increased oxidative stress are the common consequences of high levels of NEFA on ovarian cells (including granulosa cells, cumulus cells, and theca cells), oocytes, and blastocysts. Besides, there are some specific consequences in different cell types. The cell survival, proliferation, and steroidogenesis of granulosa cells and cumulus cells are affected by exposure to the high levels of NEFA. Besides, the epithelial-mesenchymal transition of granulosa cells to luteal cells is also negatively affected, and the transcriptomic pattern was altered. In oocytes, the high level of NEFA impairs the nuclear maturation, inhibits FA β-oxidation, and decreases ATP content. Blastocysts originating from oocytes exposed to the high level of NEFA showed less total cell number and altered transcriptomic and DNA methylation patterns. Moreover, granulosa cells and cumulus cells protect the oocyte when they are exposed to a high fatty acid environment via reducing the quantity of NEFAs that oocyte-exposed either through lipid droplets storage or oxidization. Besides, the granulosa cells can produce and deliver some anti-inflammatory factors to cumulus cells and oocytes through the bidirectional communication. Therefore, the anti-inflammatory response exists in blastocyst originated from the high level of NEFAs-exposed oocyte.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This study was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC grant number 445230-12). Fonds de recherche du Québec – Nature et technologies (FRQNT) provided a fellowship to Meihong Shi.
Author contribution statement
Meihong Shi designed and wrote the paper, and Marc-André Sirard designed and revised the paper.
Acknowledgement
The authors thank Sylvie Bilodeau for language editing.
References
- Aardema H, Vos PL, Lolicato F, Roelen BA, Knijn HM, Vaandrager AB, Helms JB, Gadella BM.2011Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biology of Reproduction 8562–69. ( 10.1095/biolreprod.110.088815) [DOI] [PubMed] [Google Scholar]
- Aardema H, Lolicato F, Van De Lest CH, Brouwers JF, Vaandrager AB, Van Tol HT, Roelen BA, Vos PL, Helms JB, Gadella BM.2013Bovine cumulus cells protect maturing oocytes from increased fatty acid levels by massive intracellular lipid storage. Biology of Reproduction 88164. ( 10.1095/biolreprod.112.106062) [DOI] [PubMed] [Google Scholar]
- Aardema H, Van Tol HTA, Wubbolts RW, Brouwers JFHM, Gadella BM, Roelen BAJ.2017Stearoyl-CoA desaturase activity in bovine cumulus cells protects the oocyte against saturated fatty acid stress. Biology of Reproduction 96982–992. ( 10.1095/biolreprod.116.146159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair S, Uzbekov R, Elis S, Sanchez L, Kireev I, Lardic L, Dalbies-Tran R, Uzbekova S.2013Absence of cumulus cells during in vitro maturation affects lipid metabolism in bovine oocytes. American Journal of Physiology: Endocrinology and Metabolism 304E599–E613. ( 10.1152/ajpendo.00469.2012) [DOI] [PubMed] [Google Scholar]
- Bertevello PS, Teixeira-Gomes AP, Seyer A, Vitorino Carvalho A, Labas V, Blache MC, Banliat C, Cordeiro LAV, Duranthon V, Papillier P, et al. 2018Lipid identification and transcriptional analysis of controlling enzymes in bovine ovarian follicle. International Journal of Molecular Sciences 19 3261. ( 10.3390/ijms19103261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoldo M, Holyoake PK, Evans G, Grupen CG.2010Oocyte developmental competence is reduced in sows during the seasonal infertility period. Reproduction, Fertility, and Development 221222–1229. ( 10.1071/RD10093) [DOI] [PubMed] [Google Scholar]
- Boots CE, Boudoures A, Zhang W, Drury A, Moley KH.2016Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Human Reproduction 312090–2097. ( 10.1093/humrep/dew181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Swann K.2019Mitochondria and lipid metabolism in mammalian oocytes and early embryos. International Journal of Developmental Biology 6393–103. ( 10.1387/ijdb.180355ks) [DOI] [PubMed] [Google Scholar]
- Carro M, Buschiazzo J, Rios GL, Oresti GM, Alberio RH.2013Linoleic acid stimulates neutral lipid accumulation in lipid droplets of maturing bovine oocytes. Theriogenology 79687–694. ( 10.1016/j.theriogenology.2012.11.025) [DOI] [PubMed] [Google Scholar]
- Chambers TJG, Morgan MD, Heger AH, Sharpe RM, Drake AJ.2016High-fat diet disrupts metabolism in two generations of rats in a parent-of-origin specific manner. Scientific Reports 631857. ( 10.1038/srep31857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput C, Sirard MA.2020Embryonic response to high beta-hydroxybutyrate (BHB) levels in postpartum dairy cows. Domestic Animal Endocrinology 72106431. ( 10.1016/j.domaniend.2019.106431) [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC.2007Dietary fatty acid intakes and the risk of ovulatory infertility. American Journal of Clinical Nutrition 85231–237. ( 10.1093/ajcn/85.1.231) [DOI] [PubMed] [Google Scholar]
- Chen Z, Lei L, Wen D, Yang L.2019Melatonin attenuates palmitic acid-induced mouse granulosa cells apoptosis via endoplasmic reticulum stress. Journal of Ovarian Research 12 43. ( 10.1186/s13048-019-0519-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet KL, Van Hoeck V, Gagne D, Fournier E, Thakur A, O'Doherty AM, Walsh CP, Sirard MA, Bols PE, Leroy JL.2016Exposure of bovine oocytes and embryos to elevated non-esterified fatty acid concentrations: integration of epigenetic and transcriptomic signatures in resultant blastocysts. BMC Genomics 17 1004. ( 10.1186/s12864-016-3366-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GA, Bale TL.2009Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 1504999–5009. ( 10.1210/en.2009-0500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning KR, Akison LK, Russell DL, Norman RJ, Robker RL.2011Increased beta-oxidation and improved oocyte developmental competence in response to l-carnitine during ovarian in vitro follicle development in mice. Biology of Reproduction 85548–555. ( 10.1095/biolreprod.110.090415) [DOI] [PubMed] [Google Scholar]
- Dunning KR, Anastasi MR, Zhang VJ, Russell DL, Robker RL.2014Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS ONE 9 e87327. ( 10.1371/journal.pone.0087327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elis S, Desmarchais A, Maillard V, Uzbekova S, Monget P, Dupont J.2015Cell proliferation and progesterone synthesis depend on lipid metabolism in bovine granulosa cells. Theriogenology 83840–853. ( 10.1016/j.theriogenology.2014.11.019) [DOI] [PubMed] [Google Scholar]
- Ferguson EM, Leese HJ.1999Triglyceride content of bovine oocytes and early embryos. Journal of Reproduction and Fertility 116373–378. ( 10.1530/jrf.0.1160373) [DOI] [PubMed] [Google Scholar]
- Ferguson EM, Leese HJ.2006A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Molecular Reproduction and Development 731195–1201. ( 10.1002/mrd.20494) [DOI] [PubMed] [Google Scholar]
- Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA.2006Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome patients undergoing gonadotropin therapy for in vitro fertilization. Journal of Clinical Endocrinology and Metabolism 912327–2333. ( 10.1210/jc.2005-2142) [DOI] [PubMed] [Google Scholar]
- Ge ZJ, Luo SM, Lin F, Liang QX, Huang L, Wei YC, Hou Y, Han ZM, Schatten H, Sun QY.2014DNA methylation in oocytes and liver of female mice and their offspring: effects of high-fat-diet-induced obesity. Environmental Health Perspectives 122159–164. ( 10.1289/ehp.1307047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa ST, Racowsky C, Mcgaughey RW.1986Lipid analysis of immature pig oocytes. Journal of Reproduction and Fertility 77425–434. ( 10.1530/jrf.0.0770425) [DOI] [PubMed] [Google Scholar]
- Hoving LL, Soede NM, Feitsma H, Kemp B.2012Lactation weight loss in primiparous sows: consequences for embryo survival and progesterone and relations with metabolic profiles. Reproduction in Domestic Animals 471009–1016. ( 10.1111/j.1439-0531.2012.02007.x) [DOI] [PubMed] [Google Scholar]
- Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, Mcconnell J.2010Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE 5 e10074. ( 10.1371/journal.pone.0010074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itami N, Shirasuna K, Kuwayama T, Iwata H.2018Palmitic acid induces ceramide accumulation, mitochondrial protein hyperacetylation, and mitochondrial dysfunction in porcine oocytes. Biology of Reproduction 98644–653. ( 10.1093/biolre/ioy023) [DOI] [PubMed] [Google Scholar]
- Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH.2010Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology 1514039–4046. ( 10.1210/en.2010-0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Schon SB, Schulte MB, Deugarte DA, Fowler SA, Tuuli MG.2013IVF outcomes in obese donor oocyte recipients: a systematic review and meta-analysis. Human Reproduction 282720–2727. ( 10.1093/humrep/det292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisher RL, Brad AM, Herrick JR, Sparman ML, Swain JE.2007A comparative analysis of metabolism and viability in porcine oocytes during in vitro maturation. Animal Reproduction Science 9872–96. ( 10.1016/j.anireprosci.2006.10.006) [DOI] [PubMed] [Google Scholar]
- Kruip TAM, Cran DG, Van Beneden TH, Dieleman SJ.1983Structural changes in bovine oocytes during final maturation in vivo. Gamete Research 829–47. ( 10.1002/mrd.1120080105) [DOI] [Google Scholar]
- Lee DK, Choi KH, Hwang JY, Oh JN, Kim SH, Lee CK.2019Stearoyl-coenzyme A desaturase 1 is required for lipid droplet formation in pig embryo. Reproduction 157235–243. ( 10.1530/REP-18-0556) [DOI] [PubMed] [Google Scholar]
- Leese HJ.2002Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. BioEssays 24845–849. ( 10.1002/bies.10137) [DOI] [PubMed] [Google Scholar]
- Leroy JL, Vanholder T, Mateusen B, Christophe A, Opsomer G, De Kruif A, Genicot G, Van Soom A.2005Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 130485–495. ( 10.1530/rep.1.00735) [DOI] [PubMed] [Google Scholar]
- Lolicato F, Brouwers J, Van De Lest C, Wubbolts R, Adedema H, Riore P, Roelen B, Helms J, Gadella B.2015The cumulus cell layer protects the bovine maturing oocyte against fatty acid-induced lipotoxicity. Biology of Reproduction 921–16. [DOI] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH.2012High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS ONE 7 e49217. ( 10.1371/journal.pone.0049217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeja Z E Pawlak P & Piliszek A.. 2019. Beyond the mouse: non-rodent animal models for study of early mammalian development and biomedical research. International Journal of Developmental Biology 63187–201. ( 10.1387/ijdb.180414ap) [DOI] [PubMed] [Google Scholar]
- Marei WF, Wathes DC, Fouladi-Nashta AA.2010Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction 139 979–988. ( 10.1530/REP-09-0503) [DOI] [PubMed] [Google Scholar]
- McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JS, Speake BK.2000Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. Journal of Reproduction and Fertility 118163–170. ( 10.1530/jrf.0.1180163) [DOI] [PubMed] [Google Scholar]
- Mu YM, Yanase T, Nishi Y, Tanaka A, Saito M, Jin CH, Mukasa C, Okabe T, Nomura M, Goto K, et al. 2001Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology 1423590–3597. ( 10.1210/endo.142.8.8293) [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Larter CZ.2009Lipotoxicity: Why Do Saturated Fatty Acids Cause and Monounsaturates Protect against It? W; iley Online Library. [DOI] [PubMed] [Google Scholar]
- Nonogaki T, Noda Y, Goto Y, Kishi J, Mori T.1994Developmental blockage of mouse embryos caused by fatty acids. Journal of Assisted Reproduction and Genetics 11482–488. ( 10.1007/BF02215713) [DOI] [PubMed] [Google Scholar]
- O’Gorman A, Wallace M, Cottell E, Gibney MJ, Mcauliffe FM, Wingfield M, Brennan L.2013Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction 146389–395. ( 10.1530/REP-13-0184) [DOI] [PubMed] [Google Scholar]
- Ogawa K, Itami N, Ueda M, Kansaku K, Shirasuna K, Kuwayama T, Iwata H.2018Non-esterified fatty acid-associated ability of follicular fluid to support porcine oocyte maturation and development. Reproductive Medicine and Biology 17155–163. ( 10.1002/rmb2.12084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyanagi E, Yano H, Uchida M, Utsumi K, Sasaki J.2011Protective action of L-carnitine on cardiac mitochondrial function and structure against fatty acid stress. Biochemical and Biophysical Research Communications 41261–67. ( 10.1016/j.bbrc.2011.07.039) [DOI] [PubMed] [Google Scholar]
- Paczkowski M, Silva E, Schoolcraft WB, Krisher RL.2013Comparative importance of fatty acid beta-oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biology of Reproduction 88111. ( 10.1095/biolreprod.113.108548) [DOI] [PubMed] [Google Scholar]
- Pawlak P, Malyszka N, Szczerbal I, Kolodziejski P.2020Fatty acid induced lipolysis influences embryo development, gene expression and lipid droplet formation in the porcine cumulus cells. Biology of Reproduction 10336–48. ( 10.1093/biolre/ioaa045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romek M, Gajda B, Krzysztofowicz E, Kepczynski M, Smorag Z.2011New technique to quantify the lipid composition of lipid droplets in porcine oocytes and pre-implantation embryos using Nile red fluorescent probe. Theriogenology 7542–54. ( 10.1016/j.theriogenology.2010.06.040) [DOI] [PubMed] [Google Scholar]
- Ryan DP, Prichard JF, Kopel E, Godke RA.1993Comparing early embryo mortality in dairy cows during hot and cool seasons of the year. Theriogenology 39719–737. ( 10.1016/0093-691x(9390257-6) [DOI] [PubMed] [Google Scholar]
- Sharma A, Baddela VS, Becker F, Dannenberger D, Viergutz T, Vanselow J.2019Elevated free fatty acids affect bovine granulosa cell function: a molecular cue for compromised reproduction during negative energy balance. Endocrine Connections 8493–505. ( 10.1530/EC-19-0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Sirard MA.2021aCocultured porcine granulosa cells respond to excess non-esterified fatty acids during in vitro maturation. Journal of Ovarian Research 14 142. ( 10.1186/s13048-021-00904-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Sirard MA.2021bEffects of NEFAs during IVM on pig embryos from granulosa cell-cocultured oocytes. Molecular Reproduction and Development 88805–816. ( 10.1002/mrd.23548) [DOI] [PubMed] [Google Scholar]
- Shi M, Sirard MA.2021cTranscriptome and epigenome analysis of porcine embryos from non-esterified fatty acid-exposed oocytes. Domestic Animal Endocrinology 76106605. ( 10.1016/j.domaniend.2021.106605) [DOI] [PubMed] [Google Scholar]
- Shibahara H, Ishiguro A, Inoue Y, Koumei S, Kuwayama T, Iwata H.2020Mechanism of palmitic acid-induced deterioration of in vitro development of porcine oocytes and granulosa cells. Theriogenology 14154–61. ( 10.1016/j.theriogenology.2019.09.006) [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Leese HJ.2003Energy metabolism in pig oocytes and early embryos. Reproduction 126197–204. ( 10.1530/rep.0.1260197) [DOI] [PubMed] [Google Scholar]
- Sturmey RG, O’Toole PJ, Leese HJ.2006Fluorescence resonance energy transfer analysis of mitochondrial:lipid association in the porcine oocyte. Reproduction 132829–837. ( 10.1530/REP-06-0073) [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Reis A, Leese HJ, Mcevoy TG.2009Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reproduction in Domestic Animals 44 (Supplement 3) 50–58. ( 10.1111/j.1439-0531.2009.01402.x) [DOI] [PubMed] [Google Scholar]
- Sutton-McDowall ML, Wu LL, Purdey M, Abell AD, Goldys EM, Macmillan KL, Thompson JG, Robker RL.2016Nonesterified fatty acid-induced endoplasmic reticulum stress in cattle cumulus oocyte complexes alters cell metabolism and developmental competence. Biology of Reproduction 9423. ( 10.1095/biolreprod.115.131862) [DOI] [PubMed] [Google Scholar]
- Tian Y, Ma J, Wang W, Zhang L, Xu J, Wang K, Li D.2016Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Molecular and Cellular Biochemistry 42275–84. ( 10.1007/s11010-016-2807-x) [DOI] [PubMed] [Google Scholar]
- Uzbekova S, Elis S, Teixeira-Gomes AP, Desmarchais A, Maillard V, Labas V.2015MALDI mass spectrometry imaging of lipids and gene expression reveals differences in fatty acid metabolism between follicular compartments in porcine ovaries. Biology 4216–236. ( 10.3390/biology4010216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valckx SD, De Pauw I, De Neubourg D, Inion I, Berth M, Fransen E, Bols PE, Leroy JL.2012BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Human Reproduction 273531–3539. ( 10.1093/humrep/des350) [DOI] [PubMed] [Google Scholar]
- Valckx SD, Hoeck VV, Arias-Alvarez M, Maillo V, Lopez-Cardona AP, Gutierrez-Adan A, Berth M, Cortvrindt R, Bols PE, Leroy JL.2014aElevated non-esterified fatty acid concentrations during in vitro murine follicle growth alter follicular physiology and reduce oocyte developmental competence. Fertility and Sterility 102 1769.e1–177. ( 10.1016/j.fertnstert.2014.08.018) [DOI] [PubMed] [Google Scholar]
- Valckx SD, Arias-Alvarez M, De Pauw I, Fievez V, Vlaeminck B, Fransen E, Bols PE, Leroy JL.2014bFatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: a descriptive cross-sectional study. Reproductive Biology and Endocrinology 12 13. ( 10.1186/1477-7827-12-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoeck V, Sturmey RG, Bermejo-Alvarez P, Rizos D, Gutierrez-Adan A, Leese HJ, Bols PE, Leroy JL.2011Elevated non-esterified fatty acid concentrations during bovine oocyte maturation compromise early embryo physiology. PLoS ONE 6e23183. ( 10.1371/journal.pone.0023183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoeck V Leroy J Alvarez MA Rizos D Gutierrez-Adan A Schnorbusch K Bols P Leese HJ & Sturmey RG. 2013. Oocyte developmental failure in response to elevated nonesterified fatty acid concentrations: mechanistic insights. Reproduction 14533–44. ( 10.1530/REP-12-0174) [DOI] [PubMed] [Google Scholar]
- Van Hoeck V, Rizos D, Gutierrez-Adan A, Pintelon I, Jorssen E, Dufort I, Sirard MA, Verlaet A, Hermans N, Bols PE, et al. 2015Interaction between differential gene expression profile and phenotype in bovine blastocysts originating from oocytes exposed to elevated non-esterified fatty acid concentrations. Reproduction, Fertility, and Development 27372–384. ( 10.1071/RD13263) [DOI] [PubMed] [Google Scholar]
- Vanholder T, Leroy JL, Van Soom A, Maes D, Coryn M, Fiers T, De Kruif A, Opsomer G.2006Effect of non-esterified fatty acids on bovine theca cell steroidogenesis and proliferation in vitro. Animal Reproduction Science 9251–63. ( 10.1016/j.anireprosci.2005.05.014) [DOI] [PubMed] [Google Scholar]
- Vinsky MD, Novak S, Dixon WT, Dyck MK, Foxcroft GR.2006Nutritional restriction in lactating primiparous sows selectively affects female embryo survival and overall litter development. Reproduction, Fertility, and Development 18347–355. ( 10.1071/rd05142) [DOI] [PubMed] [Google Scholar]
- Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, Mitchell M.2008Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. American Journal of Physiology: Endocrinology and Metabolism 294E425–E434. ( 10.1152/ajpendo.00409.2007) [DOI] [PubMed] [Google Scholar]
- Wang Y, Li C, Li J, Wang G, Li L.2020Non-esterified fatty acid-induced reactive oxygen species mediated granulosa cells apoptosis is regulated by Nrf2/p53 signaling pathway. Antioxidants 9 523. ( 10.3390/antiox9060523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott KE, Kwong WY, Hughes J, Salter AM, Lea RG, Garnsworthy PC, Sinclair KD.2010Dietary omega-3 and-6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Reproduction 139 57–69. ( 10.1530/REP-09-0219) [DOI] [PubMed] [Google Scholar]
- Wu LL-Y, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL.2010High-fat diet causes lipotoxicity responses in cumulus–oocyte complexes and decreased fertilization rates. Endocrinology 1515438–5445. ( 10.1210/en.2010-0551) [DOI] [PubMed] [Google Scholar]
- Wu LL, Russell DL, Norman RJ, Robker RL.2012Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (ΔΨm), and embryo development. Molecular Endocrinology 26562–573. ( 10.1210/me.2011-1362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Che L, Yang Z, Zhang P, Shi J, Li J, Lin Y, Fang Z, Che L, Feng B, et al. 2016Effect of high fat dietary intake during maternal gestation on offspring ovarian health in a pig model. Nutrients 8 498. ( 10.3390/nu8080498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Dunning KR, Wu LL-Y, Hickey TE, Norman RJ, Russell DL, Liang X, Robker RL.2010Identification of perilipin-2 as a lipid droplet protein regulated in oocytes during maturation. Reproduction, Fertility, and Development 221262–1271. ( 10.1071/RD10091) [DOI] [PubMed] [Google Scholar]
- Yang X, Wu LL, Chura LR, Liang X, Lane M, Norman RJ, Robker RL.2012Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertility and Sterility 971438–1443. ( 10.1016/j.fertnstert.2012.02.034) [DOI] [PubMed] [Google Scholar]
- Yao JK, Ryan RJ, Dyck PJ.1980The porcine ovarian follicle. VI. Comparison of fatty acid composition of serum and follicular fluid at different developmental stages. Biology of Reproduction 22141–147. ( 10.1095/biolreprod22.2.141) [DOI] [PubMed] [Google Scholar]
- Yenuganti VR, Koczan D, Vanselow J.2021Genome wide effects of oleic acid on cultured bovine granulosa cells: evidence for the activation of pathways favoring folliculo-luteal transition. BMC Genomics 22486. ( 10.1186/s12864-021-07817-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D, Zeng S, Guo Y.2012A diet rich in n-3 polyunsaturated fatty acids reduced prostaglandin biosynthesis, ovulation rate, and litter size in mice. Theriogenology 7828–38. ( 10.1016/j.theriogenology.2012.01.013) [DOI] [PubMed] [Google Scholar]
- Yousif MD.2019Oleic acid attenuates palmitic acid-induced impairments in mouse blastocyst development. [Google Scholar]
- Yuan Y, Ida JM, Paczkowski M, Krisher RL.2011Identification of developmental competence-related genes in mature porcine oocytes. Molecular Reproduction and Development 78565–575. ( 10.1002/mrd.21351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachut M, Dekel I, Lehrer H, Arieli A, Arav A, Livshitz L, Yakoby S, Moallem U.2010Effects of dietary fats differing in n-6: n-3 ratio fed to high-yielding dairy cows on fatty acid composition of ovarian compartments, follicular status, and oocyte quality. Journal of Dairy Science 93529–545. ( 10.3168/jds.2009-2167) [DOI] [PubMed] [Google Scholar]
- Zeron Y, Ocheretny A, Kedar O, Borochov A, Sklan D, Arav A.2001Seasonal changes in bovine fertility: relation to developmental competence of oocytes, membrane properties and fatty acid composition of follicles. Reproduction 121447–454. ( 10.1530/rep.0.1210447) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a