Abstract
Summary
We report a 26-year-old Japanese man who visited our outpatient clinic presenting fever immediately after i.m. injection of the second dose of a coronavirus disease 2019 (COVID-19) vaccine (Moderna®). At the first visit, the patient had a fever of 37.7°C and a swollen thyroid gland with mild tenderness. He was diagnosed with subacute thyroiditis (SAT) based on the presence of thyrotoxicosis (free tri-iodothyronine, 32.3 pg/mL; free thyroxine, >7.77 ng/dL; and thyroid-stimulating hormone (TSH) < 0.01 μIU/mL), high C-reactive protein level (7.40 mg/dL), negative TSH receptor antibody, and characteristic ultrasound findings. His HLA types were A*02:01/24:02, B*15:11/35:01, Cw*03:03, DRB1*09:01/12:01, DQB1*03:03, and DPB1*05: 01/41:01. He was initially administered prednisolone 15 mg/day, following which the fever subsided. After 10 days, he developed limb weakness and could not walk. The serum potassium level decreased to 1.8 mEq/L, which confirmed the diagnosis of thyrotoxic periodic paralysis (TPP). Potassium supplementation was initiated. The muscle weakness gradually decreased. Prednisolone therapy was terminated 6 weeks after the first visit. His thyroid function returned to normal 5 months after the first visit, through a hypothyroid state. To our knowledge, this is the first reported case of TPP-associated SAT following COVID-19 vaccination. Persistent fever following vaccination should be suspected of SAT. Additionally, TPP may be associated with SAT in Asian male patients.
Learning points
Following coronavirus disease 2019 (COVID-19) vaccination, subacute thyroiditis may develop regardless of the vaccine type.
If persistent fever, anterior neck pain, swelling and tenderness of thyroid gland, and symptoms of thyrotoxicosis are observed immediately after the COVID-19 vaccination, examination in consideration of the onset of subacute thyroiditis is recommended.
HLA-B35 may be associated with the onset of subacute thyroiditis after the COVID-19 vaccination.
Although rare, subacute thyroiditis can be associated with thyrotoxic periodic paralysis, especially in Asian men.
Glucocorticoid therapy for subacute thyroiditis may induce thyrotoxic periodic paralysis through hypokalemia.
Patient Demographics: Adult, Male, Asian - Japanese, Japan
Clinical Overview: Thyroid, Thyroid
Related Disciplines: General practice, Genetics
Publication Details: Unusual effects of medical treatment, May, 2022
Background
Subacute thyroiditis (SAT) is a self-limiting inflammatory disease characterized by fever, painful swelling of the thyroid gland, and thyrotoxicosis (1). Viral infection has often been considered the cause of SAT (1). Recently, there has been a surge in the number of cases of SAT following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (2). Then, three cases of SAT after vaccination for COVID-19 have been reported for the first time (3). In accordance with the recent systematic review, 51 cases of SAT after vaccination for COVID-19 had been reported (4). The cases of SAT after vaccination for COVID-19 have been found in Europe, North America, Asia, and Australia. SAT was observed regardless of the vaccine type and occurred in the first, second, and third doses (4).
Thyrotoxic periodic paralysis (TPP) is a rare muscle disease that presents with acute painless muscle weakness. Patients usually have both hypokalemia and hyperthyroidism. This muscle weakness is more severe in the proximal muscles and lower extremities (5).
TPP is mainly caused by Graves’ disease, among all thyroid diseases. TPP cases associated with SAT are very rare (5).
Herein, we report a Japanese male with SAT associated with TPP following COVID-19 vaccination.
Case presentation
The patient was a 26-year-old Japanese man with no familial or medical history of thyroid disease, hypokalemia, and periodic paralysis. On day minus 28, he received his first dose of COVID-19 vaccine (Moderna®, Takeda Pharmaceutical Company Ltd, Japan/Moderna Biotech Ltd., Spain), which was administered intramuscularly, with no adverse effects. Subsequently, on day 0, he received the second dose; then, he experienced persistent fever and headache, which prompted him to visit our outpatient clinic on day 12. Before the onset of fever, no symptoms of upper respiratory tract infections were noted. His height was 171 cm and weighed 76 kg, which decreased by 9 kg after onset. His heart rate, blood pressure, and body temperature were 112 b.p.m, 134/92 mmHg, and 37.7°C. He also complained of mild neck pain, and palpation revealed a mild enlargement and tenderness of the thyroid gland. He was diagnosed with SAT, and on the same day, prednisolone was administered at a dose of 15 mg/day.
On the morning of day 22, he developed limb weakness and reported difficulty in walking. Therefore, he was transported to our hospital in an ambulance. His serum potassium level dropped to 1.8 mEq/L. He did not have a history of heavy alcohol consumption or high carbohydrate intake.
Investigation
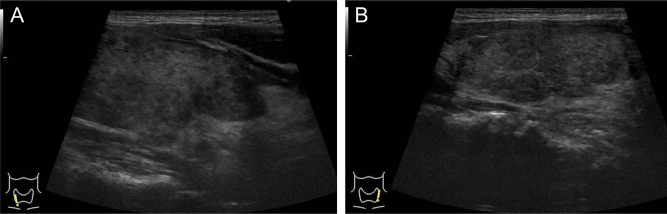
The results of the laboratory tests at the first visit to our hospital on day 12were as follows: free tri-iodothyronine (FT3), 32.3 (reference range, 2.3–4.0) pg/mL; free thyroxine (FT4), >7.77 (0.9–1.7) ng/dL; thyroid-stimulating hormone (TSH), <0.01 (0.5–5.0) μIU/mL; thyroglobulin, 667 (2–31) ng/mL; and C-reactive protein, 7.40 (<0.3) mg/dL. Anti-thyroglobulin antibody (TgAb) was positive at 40 (<12) IU/mL, while anti-thyroid peroxidase antibody (TPOAb) and TSH receptor antibody (TRAb, third generation) were negative at 4.2 IU/mL (<5.1) and 1.0 IU/L (<2.0), respectively. A PCR of a nasal swab was negative for SARS-CoV-2, but the SARS-CoV-2 antibody was positive at 40 600 (<0.8) U/mL. The serum potassium level was normal at 4.3 mEq/L. Ultrasonography revealed predominant swelling of the right lobe of the thyroid, and both lobes were mostly occupied by heterogeneous hypoechoic lesions with decreased vascularity, as observed using color Doppler ultrasonography (Fig. 1). These hypoechoic regions coincided with the tender sites.
Figure 1.
Ultrasonography of the thyroid gland. Longitudinal view of the right lobe (A) and left lobe (B). The sizes of the isthmus, right lobe, and left lobe in the thyroid gland were 27.9 × 23.0 × 8.0 mm, 66.4 × 19.7 × 26.0 mm, and 60.3 × 19.3 × 18.8 mm (length, width, and thickness), respectively. In both lobes, most of the areas of the thyroid gland are replaced by irregular hypoechoic lesions with decreased vascularity (color Doppler ultrasound image is not shown).
On day 22, his serum potassium level dropped to 1.8 mEq/L. Furthermore, FT3, FT4, and TSH levels were 12.3 pg/mL, 5.22 μg/dL, and <0.01 μIU/mL, respectively.
The HLA types were identified to be A * 02:01/24:02, B * 15:11/35:01, Cw * 03:03, DRB1 * 09:01/12:01, DQB1 * 03:03, and DPB1 * 05:01/41:01.
Treatment
On day 12, the patient was administered prednisolone at a dose of 15 mg/day. The prednisolone dose was reduced by 5 mg every 2 weeks and discontinued after 6 weeks. On day 22, i.v. administration of potassium chloride, with a maximum dose of 80 mEq/day, was started for hypokalemia. After the normalization of the serum potassium level, it was switched to oral administration and continued with dose reduction from 14.4 to 7.2 mEq/day until prednisolone was discontinued.
Outcome and follow-up
Table 1 summarizes the clinical course of the patient. On day 12, he was diagnosed with SAT and administered prednisolone at a dose of 15 mg/day on the same day. The fever subsided the following day.
Table 1.
Clinical course of the present case.
| Day 12 | Day 19 | Day 22 | Day 23 | Day 25 | Day 33 | Day 47 | Day 61 | Day 160 | |
|---|---|---|---|---|---|---|---|---|---|
| TSH (μIU/mL) | <0.01 | <0.01 | <0.01 | N.D. | <0.01 | <0.01 | 12.80 | 6.05 | 3.92 |
| Free T3 (pg/mL) | 32.30 | 16.30 | 12.30 | N.D. | 6.32 | 3.12 | 2.22 | 3.03 | 3.63 |
| Free T4 (ng/dL) | >7.77 | >7.77 | 5.22 | N.D. | 3.02 | 1.42 | 0.86 | 1.04 | 1.19 |
| C-reactive protein (mg/dL) | 7.40 | 0.10 | 0.37 | 1.50 | 0.45 | 0.19 | 0.10 | 0.21 | N.D. |
| Serum potassium (mEq/L) | 4.3 | N.D. | 1.8 | 2.2 | 3.4 | 4.1 | 4.9 | 4.1 | 4.6 |
| Body temperature (°C) | 37.7 | N.D. | 37.4 | 37.5 | 36.3 | N.D. | N.D. | N.D. | N.D. |
| Muscle weakness of the limbs | − | − | ++ | ++ | + | ± | ± | − | − |
| Prednisolone (mg/day) | 15* | 15 | 15 | 15 | 15 | 10 | 5 | − | − |
| Potassium supplementation (mEq/day) | − | − | 80 | 27.2 | 20 | 14.4 | 7.2 | − | − |
*Prednisolone was administered following blood examination on the same day; Severity of muscle weakness: ++, severe; +, intermediate; ±, mild; −, none. Reference range: TSH, 0.5–5.0 μIU/mL; FT3, 2.3–4.0 pg/mL; FT4, 0.9–1.7 ng/dL; C-reactive protein, <0.30 mg/dL; serum potassium, 3.6–5.0 mEq/L.
N.D., not done.
On day 22, the patient was taken to our hospital because of lower limb weakness and walking difficulty. Potassium supplementation was started for hypokalemia. The serum potassium level subsequently increased to 4.6 mEq/L, limb weakness subsided, and an i.v. potassium supplementation was switched to oral medication; however, severe weakness of the lower limbs recurred the following night. The patient continued to have mild paroxysmal weakness of the lower limbs, which subsided by day 31. Since the lower limb weakness was alleviated, he was discharged 7 days later and continued to visit the outpatient clinic.
On day 33, FT3 and FT4 levels were approximately within the normal limit. On day 47, the patient exhibited low FT3 and FT4 levels and high TSH levels. The prednisolone dose was reduced by 5 mg every 2 weeks and discontinued after 6 weeks. On day 61, FT3 and FT4 levels returned to normal values, and TgAb became negative at 11.0 IU/mL. On day 160, the TSH level was normalized.
Discussion
In the present case, there was no preceding cold-like symptom, and fever persisted from the day of the second inoculation of the Moderna vaccine for COVID-19. At the first visit to our hospital, fever, anterior neck pain, thyroid gland tenderness, remarkable thyrotoxicosis, and increased CRP were observed. Negative TRAb and TPOAb and positive TgAb, which became negative 2 months later, were observed. On ultrasonography, most of the areas of both lobes of the thyroid gland were replaced with amorphous hypoechoic lesions. The glucocorticoid therapy markedly improved the fever and inflammatory findings, and thyroid function was normalized after transient hypothyroidism. Based on the above course, the present case was diagnosed with SAT triggered by COVID-19 vaccination.
The mechanisms underlying SAT development following COVID-19 vaccination remain unclear. SAT developed in patients following the administration of an inactivated vaccine, with aluminum hydroxide as an adjuvant. The vaccine might have induced SAT as a phenomenon of post-vaccination autoimmune/inflammatory syndrome induced by adjuvants (ASIA) syndrome (3). In addition, SAT developed after viral vector vaccines and mRNA vaccines (4). In the present case, the patient was administrated with an mRNA vaccine. Lipid nanoparticles in mRNA vaccines and polysorbate 80 in viral vector vaccines used as an excipient may act as adjuvants and cause SAT.
Vojdani et al. reported that SARS-CoV-2 spike protein, nucleoprotein, and membrane protein were shown to cross-react with thyroid peroxidase, and numerous thyroid peroxidase peptide sequences shared homology or similarity with sequences in various SARS-CoV-2 proteins (6). Based on these findings, they suggested that the cross-reactivity between thyroid cell antigens and the spike protein of the coronavirus produced by vaccines might promote autoimmune thyroiditis and that SAT after COVID-19 vaccination might occur by a similar mechanism (6). However, TPOAb was negative in the present case. Another mechanism is related to the binding of the SARS-CoV-2 spike protein produced by the vaccines to the endothelial cells, which can lead to mitochondrial damage and thyroid dysfunction (7).
Genetic factors are considered involved in SAT development, and up to 70% of patients with SAT carry HLA-B35 (8). The present patient also had HLA-B35, although the HLA type was not investigated in other cases with SAT after COVID-19 vaccination.
The present patient developed periodic paralysis during the clinical course of SAT. Because he had no family history of hypokalemia and periodic paralysis, he was thought to have developed acquired periodic paralysis due to thyrotoxicosis (TPP), not familial PP. TPP is mainly caused by Graves’ disease, among all thyroid diseases (5). SAT-associated TPP very rarely occurs. There is a report that 1 among 135 cases with TPP was associated with SAT (9). In addition, the frequency of TPP in patients with SAT is unknown.
The onset of TTP may be associated with a genetic predisposition. TTP is more common in men (5), and it is ten times more common among patients with hyperthyroidism in Asia than in similar patients in North America (5). It has been reported that the development of TPP was associated with HLA-A2 and HLA-BW22, HLA-AW19 and HLA-B17, HLA-B5, HLA-BW46, and HLA-DRw8 (10). Cavan et al. have reported that HLA-B46, HLA-DR9, and HLA-DQB1*0303 haplotype were only weakly associated in male patients with TPP associated with Graves’ disease in Hong Kong Chinese subjects (11). The present patient had HLA-A2, HLA-DR9, and HLA-DQB1*0303.
The principal biochemical abnormality during a TPP attack is hypokalemia. It is thought to be related to the increased Na/K-ATPase pump activity in the skeletal muscles because of the direct stimulation by thyroid hormones, β-adrenergic hormones, and insulin (5). Glucocorticoids are also thought to cause hypokalemia and TPP (12). Glucocorticoids may induce hypokalemia from a transcellular potassium shift caused by several mechanisms, such as an increased Na/K-ATPase pool in the skeletal muscle, steroid-induced hyperinsulinemia, and hyperglycemia (12). There are more than ten reports of TPP in patients with thyrotoxicosis who received glucocorticoids. There were various types of glucocorticoid drugs. Many of the patients received a pulse therapy of methylprednisolone or high-dose dexamethasone (12, 13, 14).
In the present patient, TTP was thought to be mainly caused by thyrotoxicosis. Since the present patient was an Asian man and had some other genetic factors, he may have been more likely to develop periodic paralysis when he had thyrotoxicosis. Furthermore, the effect of glucocorticoids on the reduction of serum potassium may have contributed to the onset of TPP.
The COVID-19 pandemic is still ongoing, and opportunities for COVID-19 vaccination will continue to increase. The prevalence of SAT after the COVID-19 inoculation is unknown, but it occurs regardless of the vaccine type. Therefore, SAT may occur after COVID-19 vaccination. Thus, SAT should be suspected when persistent fever, anterior neck pain, thyroid swelling, and symptoms of thyrotoxicosis are observed. Although it is a rare case, TPP may be associated with SAT, especially in Asian men.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Patient consent
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
Author contribution statement
All authors participated in the treatment of the patient, collected data, interpreted the data, and wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.Nishihara E, Ohye H, Amino N, Takata K, Arishima T, Kubo T, Ito M, Kubota S, Fukata S, Miyauchi A. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Internal Medicine 200847725–729. ( 10.2169/internalmedicine.47.0740) [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri RM, Campennì A, Deandreis D, Siracusa M, Tozzoli R, Ovčariček PP, Giovanella L. SARS-COV-2-related immune-inflammatory thyroid disorders: facts and perspectives. Expert Review of Clinical Immunology 202117737–759. ( 10.1080/1744666X.2021.1932467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.İremli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination ASIA syndrome. Journal of Clinical Endocrinology and Metabolism 20211062600–2605. ( 10.1210/clinem/dgab373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ippolito S, Gallo D, Rossini A, Patera B, Lanzo N, Fazzino GFM, Piantanida E, Tanda ML. SARS-CoV-2 vaccine-associated subacute thyroiditis: insights from a systematic review. Journal of Endocrinological Investigation 20221–12. Online ahead of print( 10.1007/s40618-022-01747-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal QZ, Niazi M, Zia Z, Sattar SBA. A literature review on thyrotoxic periodic paralysis. Cureus 202012 e10108. ( 10.7759/cureus.10108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Frontiers in Immunology 202011617089. ( 10.3389/fimmu.2020.617089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, Zhang Y, Yin Q, Cho Y, Andrade Let al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circulation Research 20211281323–1326. ( 10.1161/CIRCRESAHA.121.318902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stasiak M, Lewiński A. New aspects in the pathogenesis and management of subacute thyroiditis. Reviews in Endocrine and Metabolic Disorders 2021221027–1039. ( 10.1007/s11154-021-09648-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang CC, Cheng CJ, Sung CC, Chiueh TS, Lee CH, Chau T, Lin SH. A 10-year analysis of thyrotoxic periodic paralysis in 135 patients: focus on symptomatology and precipitants. European Journal of Endocrinology 2013169529–536. ( 10.1530/EJE-13-0381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel M, Ladak K. Thyrotoxic periodic paralysis: a case report and literature review. Clinical Medicine and Research 202119148–151. ( 10.3121/cmr.2021.1610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavan DA, Penny MA, Jacobs KH, Kelly MA, Jenkins D, Mijovic C, Chowt C, Cockramt CS, Hawkins BR, Barnett AH. The HLA association with Graves’ disease is sex-specific in Hong Kong Chinese subjects. Clinical Endocrinology 19944063–66. ( 10.1111/j.1365-2265.1994.tb02444.x) [DOI] [PubMed] [Google Scholar]
- 12.Polamaung W, Kongkit J, Yimnoi P, Boonchaya-Anant P, Snabboon T. Thyrotoxic hypokalemic periodic paralysis triggered by dexamethasone administration. Acta Medica 20206391–93. ( 10.14712/18059694.2020.24) [DOI] [PubMed] [Google Scholar]
- 13.Ahamed R, McCalley S, Sule AA. Steroids and thyrotoxicosis precipitate periodic paralysis. Cureus 201810 e2106. ( 10.7759/cureus.2106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Affram KO, Reddy TL, Osei KM. A rare case of thyrotoxic periodic paralysis after epidural steroid injection: a case report and literature review. American Journal of Case Reports 2018191453–1458. ( 10.12659/AJCR.911270) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a