Abstract
We cloned the genomic DNA and cDNA of bglA, which encodes β-glucosidase in Aspergillus kawachii, based on a partial amino acid sequence of purified cell wall-bound β-glucosidase CB-1. The nucleotide sequence of the cloned bglA gene revealed a 2,933-bp open reading frame with six introns that encodes an 860-amino-acid protein. Based on the deduced amino acid sequence, we concluded that the bglA gene encodes cell wall-bound β-glucosidase CB-1. The amino acid sequence exhibited high levels of homology with the amino acid sequences of fungal β-glucosidases classified in subfamily B. We expressed the bglA cDNA in Saccharomyces cerevisiae and detected the recombinant β-glucosidase in the periplasm fraction of the recombinant yeast. A. kawachii can produce two extracellular β-glucosidases (EX-1 and EX-2) in addition to the cell wall-bound β-glucosidase. A. kawachii in which the bglA gene was disrupted produced none of the three β-glucosidases, as determined by enzyme assays and a Western blot analysis. Thus, we concluded that the bglA gene encodes both extracellular and cell wall-bound β-glucosidases in A. kawachii.
Aspergillus kawachii, an industrial fungus, has been used to prepare shochu koji, which is used to make shochu, a traditional Japanese alcoholic beverage. This species, like other Aspergillus species, secretes large amounts of various enzymes into the medium, and it can be a useful host for heterologous protein production. A. kawachii also releases large amounts of citric acid into the environment, causing it to become acidic. To adapt to this acidic environment, some of the enzymes of A. kawachii such as amylase, protease, cellulase, and hemicellulase, are more acid stable than the same enzymes secreted by other species (17, 18, 27, 28, 42).
One of the acid-stable enzymes is β-glucosidase (1,4-β-d-glucosidase; EC 3.2.1.21). β-Glucosidase catalyzes the hydrolysis of compounds containing β-glucosidic links, such as p-nitrophenyl-β-d-glucopyranoside and cellobiose, and acts by splitting off the terminal nonreducing β-d-glucose residue and releasing β-d-glucose as the reaction product (3, 4). Because of this activity, β-glucosidase plays an important role in the biological saccharification of cellulosic materials. Hydrolysis of cellulose is accomplished by the synergistic reactions of cellulase family enzymes. Exo-1,4-β-d-glucan cellobiohydrolase (exocellulase or cellobiohydrolase; EC 3.2.1.91) and endo-1,4-β-d-glucan glucanohydrolase (endoglucanase; EC 3.2.1.4) can directly solubilize crystalline cellulose. This results mainly in the generation of cellobiose, which is a product inhibitor for these enzymes. β-Glucosidase is involved in the final step of cellulose saccharification; in this step it degrades cellobiose to glucose and releases exocellulase and endoglucanase from cellobiose inhibition (11, 40).
In addition to its role in cellulose saccharification, β-glucosidase plays other important biological roles. Some β-glucosidases catalyze not only hydrolysis reactions but also transglycosylation reactions. This transglycosylation activity is believed to be important in the production of cellulase inducers in some fungi, but the details of this process are not clear (5, 37, 44, 46). In plants, β-glucosidases are important in flavor formation in fruits. They are also important in the production of wine and sweet potato shochu. Some monoterpene alcohols (linalool, α-terpineol, citronellol, nerol, and geranol) contribute to the flavor of sweet potato shochu and wine. These monoterpene alcohols are present in sweet potatoes and grape berries as nonvolatile glycosides and are released by the action of β-glucosidase (13, 23, 29, 30, 39, 45).
During production of sweet potato shochu, the β-glucosidases are supplied by A. kawachii (30). In a previous study we purified two extracellular β-glucosidases (EX-1 and EX-2) and one cell wall-bound β-glucosidase (CB-1) from A. kawachii (19). These three enzymes were very unstable after purification, but they became stable when cell wall material from A. kawachii was added. The cell wall material adsorbed all of the purified β-glucosidases, but it did not inhibit the activities of the enzymes. Although the N-terminal amino acid sequences of the enzymes were identical, the molecular masses were different, as follows: EX-1, 145 kDa; EX-2, 130 kDa; and CB-1, 120 kDa. Our data suggested that these three β-glucosidases are products of the same gene and are modified by different degrees of glycosylation (19).
In this study, we cloned the gene encoding β-glucosidase in A. kawachii and analyzed the sequence. We found that both of the extracellular β-glucosidases (EX-1 and EX-2) and the cell wall-bound β-glucosidase (CB-1) are encoded by a single gene, which we designated bglA.
MATERIALS AND METHODS
Strains, plasmids, and media.
A. kawachii IFO4308 was used as a donor of genomic DNA and mRNA. Saccharomyces cerevisiae YPH499 (MATa ura3 lys2 ade2 trp1 his3 leu2) was used as the host for expression of bglA cDNA. Escherichia coli JM109 and LE392 were used for DNA manipulation. Plasmid pUSC, which was a gift from O. Yamada, was used for transformation of A. kawachii and isolation of the Aspergillus nidulans sC gene (47). Basic medium (0.1% Bacto-Tryptone [Difco], 0.5% yeast extract, 0.1% NaNO3, 0.1% K2HPO4, 0.05% MgSO4 · 7H2O, 0.001% FeSO4 · 7H2O; pH 5.0) containing various carbon sources was used for cultivation of A. kawachii IFO4308. Solid cultivation was carried out as described previously by using rice grain (19). Minimal medium was used to select A. kawachii transformants (6). For yeast cultures, we used YNBD medium supplemented with the appropriate amino acids (2). E. coli was grown in Luria-Bertani medium supplemented with 100 μg of ampicillin per ml (38).
Purification of cell wall-bound β-glucosidase CB-1.
Cell wall-bound β-glucosidase CB-1 was purified from a lysate of 4-day-old mycelia as previously described (19).
Partial amino acid sequence of CB-1.
Purified CB-1 (200 μg) was digested with lysyl endopeptidase (Achromobacter protease I; Wako Pure Chemicals) by using the method of Kamei et al. (20). The resulting peptide fragments were separated by reverse-phase high-performance liquid chromatography by using a μBondasphere C-8 100-Å column (Waters Corp.) and a linear 0 to 100% acetonitrile gradient in which the concentration increased at a rate of 1.5% per min. The peptide fragments in peaks were sequenced with a gas phase protein sequencer (model 491 Procise; Applied Biosystems).
General DNA manipulation technique.
All of the DNA manipulation procedures (subcloning, purification of plasmids, etc.) were carried out by using standard methods, as described by Sambrook et al. (38).
Cloning of genomic DNA.
The genomic DNA of A. kawachii was amplified with a primer set (primer 1 [5′-GGTATTCAAGACGGAGGTGTTGTCGCGACTGCAAA-3′] and primer 2 [5′-GGCAGCCCAGTCCGACATAACAAAGCC-3′]) by performing PCR, and then two DNA fragments (probes A and B) were isolated. The λEMBL3 A. kawachii genomic library was screened independently with these probes as previously described (16).
Cloning of cDNA.
A. kawachii was grown in basic medium containing 1% glucose and 2% xylan as carbon sources for 3 days, and the total RNA was isolated from mycelia as described by Hata et al. (14). The poly(A)+ mRNA was separated from the total RNA by chromatography on an oligo(dT)-cellulose column (Amersham Pharmacia Biotech). The full-length cDNA was isolated from the mRNA by using a Marathon cDNA amplification kit (Clontech). On the basis of the genomic sequence of bglA, the following deoxyoligonucleotide primers were synthesized: Boligo5 (primer for rapid amplification of cDNA 5′ ends [5′-RACE]; 5′-CATGAGGTTCACTTTGATTGA-3′) and Boligo3 (primer for 3′-RACE; 5′-CGTAGCTAGCATCCCCAGAAG-3′). Using these primers and adapter primer 1 (5′-GTCAATGTCCCAAACGTCACCAGA-3′), we performed 5′- and 3′-RACE PCR. The amplified DNA fragments were cloned into pCR2 by using a TA cloning kit (Invitrogen), and 15 clones were isolated. We used one of longest of these clones to obtain full-length cDNA. The 5′- and 3′-RACE products were ligated at the EcoRV site in order to isolate full-length bglA cDNA (pYbglA21).
DNA sequencing.
Restriction enzyme fragments were subcloned into pUC118 by using E. coli JM109 as the host. The nucleotide sequence was determined by the dideoxy chain termination method of Messing with an automated DNA sequencer (model 371A; Applied Biosystems) (26). The complete nucleotide sequences of both strands were determined by overlapping at every junction.
The deduced amino acid sequence was compared with the sequences of enzymes related to β-glucosidases in the SwissProt database by using the FASTA program (11a).
Construction of bglA expression vector in S. cerevisiae.
The bglA expression vector pGBGA1 was synthesized as follows. Full-length β-glucosidase cDNA was isolated from pYbglA21 by EcoRI digestion and inserted into the SacI site of pG-1, which yielded pGBGA1.
The pYKBA1 vector, which contained the bglA gene with the exchanged signal sequence, was synthesized as follows. Full-length mature β-glucosidase cDNA was amplified by PCR by using the upper primer (5′-CCGGAGCTCGGATGAATTGGCTTACTCCCCA-3′) and the lower primer (5′-CCGGAGTCTAATTCATATACCACGGCCATCA-3′). The amplified DNA fragment was partially digested by SacI and then inserted into the SacI site of pYEX-S1 (Nippon Gene), which yielded pYKBA1. This plasmid was sequenced to confirm that the region encoding the mature protein was ligated in frame following the secretion signal from Kluyveromyces lactis.
Transformation of S. cerevisiae and A. kawachii.
Transformation of S. cerevisiae was carried out as described by Becker and Guarente (2). Transformation of A. kawachii was carried out as described by Punt and van den Hondel (33).
β-Glucosidase assay.
β-Glucosidase activity was measured by using p-nitrophenyl glycoside (PNPG) as a substrate, as previously described (19). A plate assay was performed by using 4-methylumbelliferyl-β-d-glucoside as a substrate. Transformants of S. cerevisiae were grown on minimal medium plates at 30°C for 72 h. The plates were overlaid with 0.75% top agarose containing 10 mM 4-methylumbelliferyl-β-d-glucoside and incubated for 5 min at room temperature. The fluorescence of the methylumbelliferol that was released by the enzyme reaction was observed under UV light (wavelength, 350 nm).
Subcellular fractionation.
Fractionation of yeast cells was carried out as described by Pines and London (32), with the following modifications. A growth medium fraction and a periplasm fraction were obtained from 100 ml of a yeast culture (100 ml containing 6 × 107 cells/ml), and protoplasts were recovered as described by Pines and London. The recovered protoplasts were resuspended in 15 ml of buffer B and were vortexed for 5 min with 0.33 volume of glass beads (diameter, 0.4 to 0.45 mm). The homogenate was assayed as the cytoplasm and membrane fraction.
Immunoblot analysis and DEAE chromatography.
Yeast strains were cultivated in YNBD medium supplemented with the appropriate amino acids for 1 day. The yeast cells were harvested by centrifugation and washed twice with H2O. The washed cells were extracted by boiling them for 5 min in extraction buffer (125 mM Tris-HCl [pH 6.0], 1 mM phenylmethylsulfonyl fluoride, 1× protease inhibitor cocktail [Boehringer Mannheim], 5% 2-mercaptoethanol, 2% sodium dodecyl sulfate [SDS], 5% sucrose) and then centrifuging them at 12,000 × g for 5 min. The culture medium was concentrated by ultrafiltration (type UFC3 TGC 00 filter; Millipore), and appropriate amounts were used for an immunoblot analysis.
Aspergillus strains were cultivated in basal medium containing 1% glucose and 2% xylan at 30°C for 3 days. The mycelia were harvested, washed, and then lysed in an isoosmotic solution (0.8 N NaCl, 50 mM sodium acetate buffer [pH 5.0]) containing 3 mg of Yatalase (Takara) per ml. The lysate was centrifuged at 5,000 rpm for 10 min, and the supernatant was used for the immunoblot analysis. The immunoblot analysis was performed with β-glucosidase antiserum as previously described (19).
DEAE chromatography was performed as described below. The cell wall lysate was placed on an anion-exchange column containing TSK gel DEAE-5PW (Tosoh Co., Ltd.) and equilibrated with 20 mM sodium acetate buffer (pH 5.0), and the column was eluted with a linear gradient consisting of 20 mM sodium acetate buffer (pH 5.0) containing 0 to 0.4 N NaCl. The β-glucosidase activity was measured by using PNPG as the substrate.
Mutagenesis and selection of sC− strains of A. kawachii.
Mutagenesis and isolation of sC− strains were carried out as described by Buxton et al. (6), and 11 sC− strains were isolated. The growth rates, morphological features, and enzyme production characteristics of selected sC− strains were investigated in order to eliminate unnecessary mutations. Three of the strains selected were transformed by pUSC, which contained the A. nidulans sC gene. This transformation restored the abilities of all three strains to grow on sulfate as the sole sulfur source. One of these three sC− strains was used for subsequent experiments and was designated strain SC60.
Disruption of bglA gene.
The SacI-SacI region of the bglA gene was replaced with the A. nidulans sC gene as follows. The genomic DNA of the bglA gene was subcloned into pYUM201 (Nippon Gene) by using the BamHI and KpnI sites (pYBA201). This plasmid was digested with SacI to remove a 2.8-kbp SacI-SacI fragment. The sC gene, which was isolated from pUSC by BamHI-SphI digestion, was inserted into the SacI site. The resulting plasmid (pDbglA) was used as the disruption vector.
The disruption vector was digested with BamHI and KpnI to recover the DNA fragment, which was bglA genomic DNA in which the SacI-SacI fragment of bglA was replaced by the sC gene. The A. kawachii sC− strain was transformed with this DNA fragment, and transformants were isolated by using minimal medium. The transformants were incubated in the basic medium containing 3% glucose, and the genomic DNAs were purified from the transformants as described by Iimura et al. and then were used for a Southern blot analysis to isolate the bglA disruptant (15).
Nucleotide sequence accession number.
The nucleotide sequence of the bglA gene and the deduced amino acid sequence have been deposited in the DDBJ database under accession no. AB003470 (8a).
RESULTS
Partial amino acid sequence of A. kawachii β-glucosidase CB-1.
After β-glucosidase CB-1 was purified from the cell wall lysate of A. kawachii, it produced a single band on SDS-polyacrylamide gel electrophoresis gels corresponding to a molecular weight of 120,000. This protein was digested with lysyl endopeptidase and was applied to a reverse-phase chromatography gel. Three fragments (P1, P2, and P3) were sequenced. The N-terminal amino acid sequences of these peptides were as follows: P1, GIQDAGVVATAK; P2, NDGALPLTGK; and P3, TREAYQDYLVLEPNNG.
A search of the SwissProt database revealed no sequences homologous to P2 or P3, while part of one protein, the β-glucosidase precursor in Candida pelliculosa, was found to exhibit 75% identity with P1.
Cloning of the genomic DNA (bglA) encoding β-glucosidase.
Barnett et al. reported that the amino acid sequence around the known active site of the β-glucosidase gene of Aspergillus wentii is highly conserved in other fungal β-glucosidase genes, such as Tricoderma reesei bgl1, Saccharomycopsis fibuligera bgl1 and bgl2, and the β-glucosidase gene of C. pelliculosa (1). Thus, we expected that this region around the active site would also be conserved in the β-glucosidase of A. kawachii. On the basis of the amino acid sequence of the PI region of β-glucosidase CB-1 and the conserved region around the active center in the C. pelliculosa enzyme (GFVMTDWGA), we attempted to clone the genomic DNA which encoded β-glucosidase CB-1. In this study, we isolated two phage clones, one obtained with probe 1 (designated bglA) and the other obtained with probe 2 (bglB), from the λEMBL3 A. kawachii genomic library.
After A. kawachii was grown for 3 days in basic medium containing 1% glucose and 2% xylan, total RNA was isolated from the mycelia and subjected to a Northern analysis in which bglA and bglB were used as probes. A signal was detected with bglA DNA but not with bglB (data not shown). Moreover, both strands of bglA and bglB genomic DNAs were sequenced. On the basis of the Northern blot analysis and DNA sequencing results, we concluded that bglA is the gene that encodes β-glucosidase CB-1.
Cloning of bglA cDNA.
Following the cloning of genomic bglA, we performed a Northern blot analysis to examine expression of the bglA gene (Fig. 1). Expression of bglA was not observed in a 1-day-old culture but was observed in 2- to 4-day-old cultures (Fig. 1, lanes 2 through 4). Since the greatest intensity was observed in the 3-day-old culture, we purified mRNA from mycelia in this culture and cloned bglA cDNA by the 5′- and 3′-RACE method. Fifteen clones of 5′- and 3′-RACE products were isolated. All of the 5′- and 3′-RACE products isolated were sequenced in order to verify the cDNA sequence, and no errors were detected in any of the 5′- and 3′-RACE products. The longest 5′-RACE product and the longest 3′-RACE product were ligated to isolate full-length bglA cDNA.
FIG. 1.
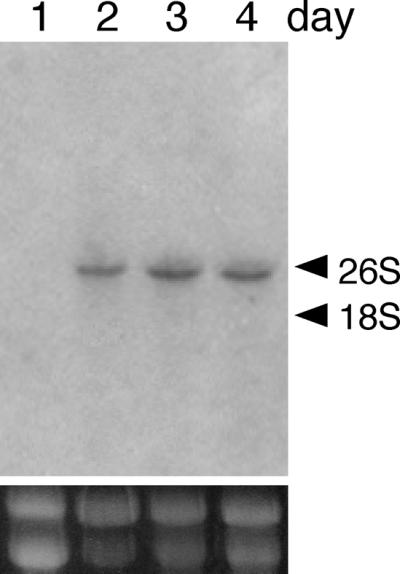
Northern blot analysis of bglA of A. kawachii IFO4308. Total RNA was isolated from mycelia after growth for 1 day (lane 1), 2 days (lane 2), 3 days (lane 3), and 4 days (lane 4) (5 μg per lane). Hybridization was performed by using a 32P-labeled SalI-SalI fragment of genomic bglA. The positions of rRNAs are indicated on the right.
Southern blot analysis.
Figure 2 shows the results of a Southern blot analysis of A. kawachii genomic DNA. In all of the samples digested with BamHI, EcoRI, or HindIII, one strong band was observed. This result indicated that the A. kawachii genomic DNA did not contain any genes that were homologous to the bglA gene of A. kawachii.
FIG. 2.
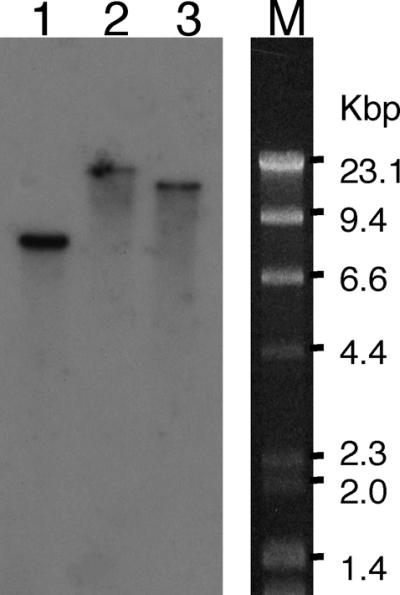
Southern blot analysis of A. kawachii genomic DNA. Genomic DNA was digested with BamHI (lane 1), EcoRI (lane 2), or HindIII (lane 3). Hybridization was performed overnight at 65°C by using a 32P-labeled SalI-SalI fragment of genomic bglA as a probe, and the membrane was washed three times for 30 min in 2× SSC–1% SDS at 65°C (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate).
Nucleotide sequence analysis.
The bglA nucleotide sequence contained a 2,933-bp open reading frame (ORF) which encoded 860 amino acids and was interrupted by six introns. The bglA product had a predicted molecular weight of 91,000 and 14 possible N-glycosylation sites.
Partial amino acid sequences P1, P2, and P3 were recognized from Gly-178 to Lys-189, from Asn-405 to Lys-414, and from Thr-603 to Gly-618, respectively. The N-terminal amino acid sequence was recognized from Asp-20 to Pro-32. Consequently, we hypothesized that the 19-amino-acid peptide from Met-1 to Ala-19 is a signal peptide.
The bglA gene was interrupted by six introns. All of the introns started with GT and ended with AG; these sequences are known to be general features of introns.
In the upstream region, a TATAA-like sequence was present at position −137; this sequence is known to be required for transcription initiation by RNA polymerase II in higher eukaryotes. Three CCAAT sequences were present at positions −341, −655, and −668. We isolated 15 5′-RACE products, 3 of which had longer 5′-end-flanking regions than the others. The 5′-end-flanking regions of these three clones started 85 bases upstream of the initiation codon. Five CREA binding sites (5′-[G/C][C/T]GG[G/A]G-3′) were present at positions −226, −526, −609, −687, and −752 (9, 10, 41, 43). This sequence is known to be required for CREA to mediate carbon catabolite repression.
In the 3′-end-flanking region of bglA, a putative polyadenylation signal, AATAAA, was present at position 2,978. The poly(A) tail started 11 bases downstream of the polyadenylation signal.
Comparison of amino acid sequences.
We searched the SwissProt database for sequences similar to the deduced amino acid sequence of bglA. Significant levels of similarity were found with β-glucosidases from Aspergillus aculeatus (21), S. fibuligera (bgl1 and bgl2) (25), C. pelliculosa (22), Kluyveromyces fragilis (34), Agrobacterium tumefaciens (8), Clostridum thermocellum (12), and T. reesei (1). A. aculeatus bgl1 exhibited the highest level of homology (81%) with A. kawachii bglA. All of these enzymes have been classified in the same family, β-glucosidase subfamily B, according to Rojas et al. (this subfamily includes yeast and fungal β-glucosidases, while subfamily A includes vegetal and prokaryotic β-glucosidases) (35).
The amino acid sequences of the β-glucosidases of A. kawachii, A. aculeatus, C. pelliculosa, S. fibuligera (bgl1) and T. reesei are aligned in Fig. 3. Rojas et al. identified the following eight conserved sequences in subfamily B β-glucosidases: DGP, GRNFE, DPYL, KHF, SDW, GLD, VLLKN, and FGYLSY (Fig. 3) (36). Each of these sequences was found in the β-glucosidase of A. kawachii. We designed the DNA sequence of primer 2 on the basis of the conserved sequence of C. pelliculosa β-glucosidases. This region was the region from Gly-275 to Ala-283, and it was conserved in other fungal β-glucosidases. The SDW motif was included in this region. Legler et al. reported that the Asp residue of the SDW motif in the β-glucosidase of A. wentii is the active site (24). This region, including the SDW motif, is used as a signature region for the active site of glycosyl hydrolase family 3 enzymes (accession no. PS00775) by the PROSITE data bank (42a). Based on these results, we concluded that the A. kawachii β-glucosidase is a subfamily B β-glucosidase.
FIG. 3.
Alignment of the amino acid sequence of A. kawachii β-glucosidase with the amino acid sequences of other fungal β-glucosidases. Amino acid residues are indicated by single-letter codes. Alignment was maximized by introducing gaps, which are indicated by dashes. The numbers indicate the multiple alignment positions from the N terminus. Conserved residues are shaded. The solid bars indicate the regions conserved in β-glucosidase subfamily B, and the open bar indicates an SDW motif. Stars indicate the putative active site (Asp) and proton donor (His). Abbreviations: AK BGKA, A. kawachii bglA; AA BGL1, A. aculeatus bgl1 (DDBJ/EMBL/GenBank accession no. D64088); SF BGL1, S. fibuligera bgl1 (SwissProt/GenBank/PIR accession no. P22506/M22475); TR BGL1, T. reesei bgl1 (DDBJ/EMBL/GenBank accession no. U09580).
Expression of bglA cDNA in S. cerevisiae.
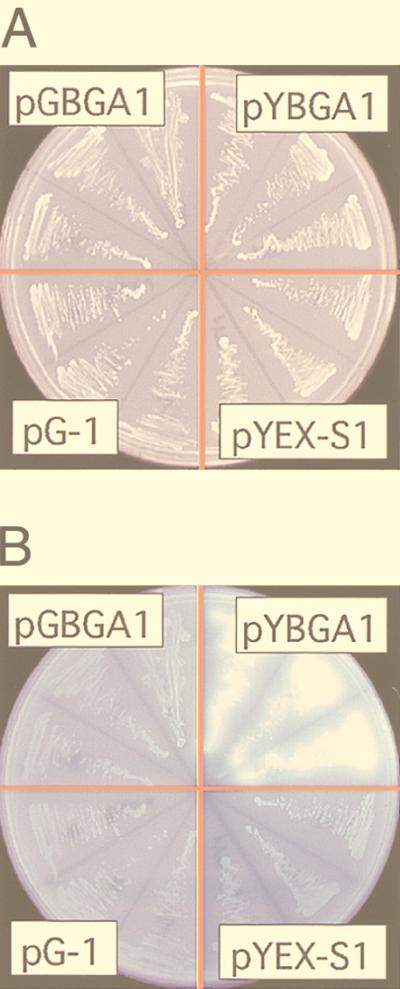
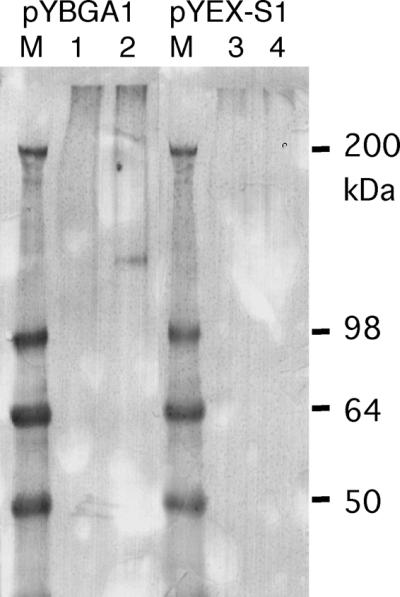
The bglA cDNA was expressed in S. cerevisiae YPH499 under the control of the glyceraldehyde-3-diphosphate dehydrogenase promoter and the phosphoglycerate kinase (PGK) terminator in multicopy expression plasmid pG-1. However, β-glucosidase production was not observed (Fig. 4). One possible reason for this result is that the signal sequence of A. kawachii β-glucosidase was not functional in the S. cerevisiae transformant. Therefore, we used the signal sequence from K. lactis (7) instead of the original bglA signal sequence. The bglA gene with the exchanged signal sequence was expressed under the control of the PGK promoter and the PGK terminator. As a result, bright fluorescence due to released methlyumbelliferyl was observed with the cells transformed with pYKBA1 (Fig. 4). On the basis of these results, we concluded that the bglA gene encodes the β-glucosidase of A. kawachii.
FIG. 4.
Expression of bglA in yeast. S. cerevisiae YPH499 was transformed with pG-1, pGBGA1, pYEX-S1, and pYBGA1. Three independent cultures transformed with each plasmid were streaked onto YNBD agar plates and incubated at 30°C for 2 days (A). These plates were overlaid with 0.75% agarose containing 10 mM 4-methylumbelliferyl-β-d-glucose and were incubated for 5 min at room temperature. β-Glucosidase activity was detected by UV-stimulated fluorescence of cleaved substrates (B).
Characterization of the yeast transformants.
The transformants containing pYEX-S1 or pYKBA1 were grown in YNBD medium for 24 h. The rate of increase in cell density of the recombinant strain containing pYKBA1, as measured by determining the optical density at 660 nm, was not significantly less than the rate of increase in cell density of the control recombinant strain containing pYEX-S1 (data not shown). In addition, microscopic examination revealed no morphological changes in the cells. These results indicated that production of recombinant β-glucosidase did not adversely affect the growth of the yeast.
To better understand the localization of recombinant β-glucosidase production, yeast cells were fractionated, and β-glucosidase activity was measured by using PNPG as a substrate. Almost all of the β-glucosidase activity was found in the periplasmic fraction of the recombinant yeast culture, and only a small amount was secreted into the medium (Table 1). As determined by Western blot analysis, the recombinant β-glucosidase was not present in the broth fraction (Fig. 5, lane 1), but it was present in the whole-cell fraction (Fig. 5, lane 2), and it had an apparent molecular weight of 120,000. We assumed that this molecular weight, which is greater than the calculated molecular weight (91,000), was due to N-linked glycosylation.
TABLE 1.
Localization of recombinant β-glucosidase in yeast
| Fraction | β-Glucosidase activity (U)a | % |
|---|---|---|
| Total | 920 ± 17.6b | 100 |
| Growth medium | 44 ± 2.5 | 4.8 |
| Periplasm | 701 ± 15.5 | 76.2 |
| Cytoplasm and membrane | 175 ± 6.7 | 19.0 |
The β-glucosidase activity of each fraction was measured by using PNPG as the substrate.
Mean ± standard error of the mean.
FIG. 5.
Western blot analysis of recombinant β-glucosidase produced by a yeast transformant. The transformant was incubated in YNBD medium at 30°C for 1 day. The protein in the culture medium (lanes 1 and 3) and cell lysate (lanes 2 and 4) was detected by performing a Western blot analysis with anti-β-glucosidase antiserum. Lanes M contained high-molecular-mass markers.
β-Glucosidase assays performed with the recombinant cells revealed that the enzyme was satiable at pH values ranging from 2.0 to 9.0 and at temperatures lower than 30°C (data not shown).
Disruption of bglA.
A. kawachii produces two extracellular β-glucosidases, EX-1 (145 kDa) and EX-2 (130 kDa), in addition to one cell wall-bound β-glucosidase (CB-1). Our previous results suggested that these three β-glucosidases are products of the same gene. To examine this possibility, we attempted to disrupt the bglA gene of A. kawachii by using sC− strain SC60 and the A. nidulans sC gene as a selective marker. A 2.8-kbp SacI-SacI fragment containing the promoter region and part of the ORF of the bglA gene was replaced by the sC gene from A. nidulans.
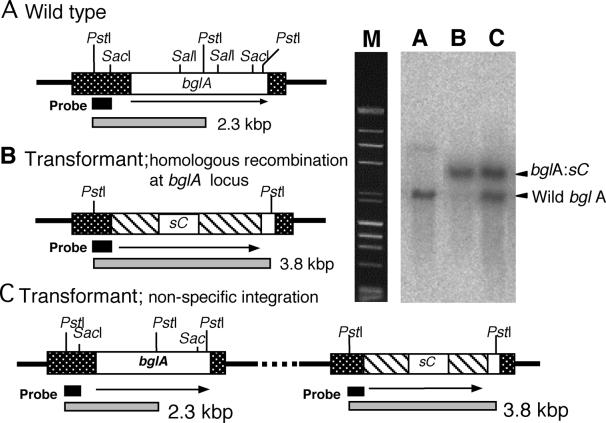
We investigated nine transformants by performing a Southern blot analysis to screen for the bglA-deleted strain. With the wild-type bglA strain (SC60), the signal should have been observed at a position corresponding to 2.3 kbp (Fig. 6, lane A). When the bglA gene was disrupted by homologous recombination with the introduced DNA fragment, the signal should have been shifted to 3.8 kbp (Fig. 6, lane B). When the introduced DNA fragment was integrated into a different locus of bglA, two signals should have appeared (Fig. 6, lane C). One of the nine transformants analyzed was found to have the hybridization pattern associated with a disrupted bglA gene (Fig. 6, lane B). We designated this transformant strain BAD574.
FIG. 6.
Gene structure and Southern blot analysis of A. kawachii IFO4308 (wild-type) bglA (A), a bglA disruptant (B), and a nonhomologous recombinant (C). Isolated genomic DNA was digested by PstI, and then a Southern blot analysis was performed by using a 368-bp PstI-SacI bglA fragment as the probe. Lane M contained a DNA molecular marker (λHindIII digest and φ174 HaeIII digest). The open box indicates the bglA ORF. The stippled boxes indicate 5′- and 3′-end-flanking regions. The hatched boxes indicate the sC gene of A. nidulans. The solid boxes indicate the regions where the probe bound.
Characterization of the bglA disruptant.
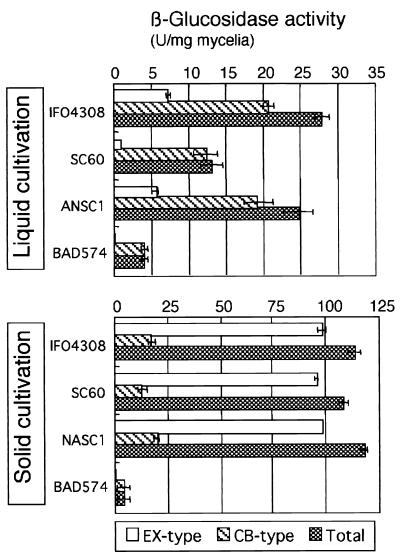
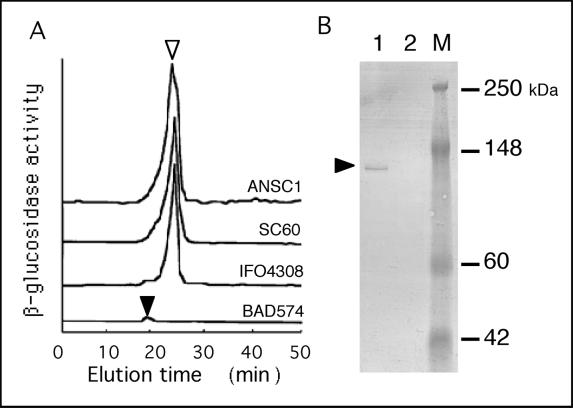
Both the extracellular and cell wall-bound β-glucosidase activities of the bglA disruptant were measured to determine whether both types of β-glucosidases are encoded by bglA. The cell wall-bound β-glucosidase activity of the bglA disruptant was at least 80% lower than the cell wall-bound β-glucosidase activity of the wild-type strain in both liquid and solid cultures (Fig. 7). However, what was the source of the remaining β-glucosidase activity in the bglA disruptant? DEAE ion-exchange chromatography of the solubilized cell-bound enzymes from the wild-type strain, the sC− mutant strain, and the pUSC-transformed sC− mutant strain resulted in a peak with a retention time of 25 min in each case (Fig. 8A). However, this peak was absent in the bglA-disrupted strain, and a minor peak was observed at 20 min. Moreover, a Western blot analysis of the bglA disruptant in which anti-β-glucosidase CB-1 antiserum was used revealed no β-glucosidase signal (Fig. 8B). These results show that β-glucosidase CB-1 was not produced by the bglA disruptant. Thus, the remaining β-glucosidase activity was produced by another gene that is not homologous to bglA.
FIG. 7.
Production of extracellular and cell wall-bound β-glucosidases by different strains of A. kawachii in liquid and solid media. IFO4308 was a wild-type strain; SC60 was an sC− strain; NASC was an SC60 strain transformed with the A. nidulans sC gene; and BAD574 was a bglA disruptant. Each strain was incubated in basic medium containing 1% glucose and 2% xylan (liquid culture) at 30°C for 3 days or on steamed rice grains for 2 days. β-Glucosidase activity was measured by using PNPG as the substrate.
FIG. 8.
Analysis of cell wall-bound β-glucosidase in the bglA disruptant. Each strain was incubated in basic medium containing 1% glucose and 2% xylan (liquid culture) at 30°C for 3 days. The mycelia were washed and lysed by using Yatalase (Takara) in isoosmotic buffer for 3 h and then centrifuged. The supernatant was isolated and used as a sample. (A) Elution profile of cell wall-bound β-glucosidase activity obtained with a DEAE-5PW column. The open triangle indicates the main peak at an elution time of 25 min, and the solid triangle indicates a minor peak at an elution time of 20 min. (B) Western blot analysis of cell wall-bound β-glucosidase. Cell wall lysates were detected with anti-β-glucosidase antiserum. Lane 1, A. kawachii IFO4308; lane 2, BAD574; lane M, molecular mass markers. The solid triangle indicates the band corresponding to β-glucosidase.
Production of extracellular β-glucosidase was not observed in liquid cultures, and the amount produced was less than 1% of the amount produced by the wild-type bglA strains in solid cultures (Fig. 7). Moreover, a Western blot analysis of the extracellular fraction of the solid culture in which anti-β-glucosidase antiserum was used revealed no signal with the bglA disruptant (data not shown). These results clearly indicate that the extracellular β-glucosidases are also encoded by the bglA gene.
DISCUSSION
The results obtained in this study indicate that the bglA gene of A. kawachii encodes both extracellular β-glucosidases and cell wall-bound β-glucosidase CB-1.
Although one β-glucosidase cDNA has been isolated from an Aspergillus species (A. aculeatus) and described, its genomic sequence has not been described. In the present study, we determined the positions of the introns and the sequence of the bglA promoter region. The first intron interrupts the signal peptide and N-terminal amino acid sequence of the mature protein, and the other introns are in the mature protein. In contrast, the β-glucosidase of the filamentous fungus T. reesei (bgl1) has only two introns, and their positions are different from the positions of the A. kawachii bglA introns.
The mature BglA protein consists of 842 amino acids and has an apparent molecular weight of 91,000. However, the molecular weight of β-glucosidase CB-1 was determined to be 120,000 by SDS-polyacrylamide gel electrophoresis. β-Glucosidase CB-1 is known to be a glycoprotein, so the difference in molecular weight is most likely due to the carbohydrate portion. Fourteen potential N-glycosylation sites were found in the predicted amino acid sequence of BglA. We previously determined the molecular weight of β-glucosidase CB-1 after we eliminated N-linked sugar chains by using N-endo-β-acetylglucosaminidase H. The molecular weight (98,000) agreed well with the predicted molecular mass of the BglA protein.
In a previous paper (19), we described purification and some of the characteristics of the two extracellular β-glucosidases of A. kawachii (EX-1 and EX-2). The molecular masses of these proteins were different from the molecular mass of β-glucosidase CB-1, but the three proteins have the same N-terminal amino acid sequence. When both the extracellular and cell wall-bound β-glucosidases were treated with N-endo-β-acetylglucosaminidase, the molecular masses were the same, 98 kDa. Moreover, all of the enzymatic properties of these three enzymes were identical. These results suggest that the two extracellular β-glucosidases are also encoded by bglA and are modified by different degrees of glycosylation. The results obtained with the bglA disruptant clearly showed that the bglA gene encodes both extracellular β-glucosidases (EX-1 and EX-2) and a cell wall-bound β-glucosidase (CB-1) and is the major β-glucosidase gene in A. kawachii.
The extracellular β-glucosidases and cell wall-bound β-glucosidase are encoded by the same gene, despite the different destinations of the enzymes. Culture conditions were found to affect the destination of BglA. In liquid cultures, about 80% of the β-glucosidase activity was attributed to cell wall-bound β-glucosidase (Fig. 7). In a previous study, we found that cell wall-bound β-glucosidase CB-1 adsorbed tightly, but not covalently, to the cell wall fraction of A. kawachii and consequently localized in the cell wall fraction. Furthermore, when we expressed the cDNA of bglA in yeast, most of the recombinant BglA proteins were localized in the yeast periplasmic fraction, and only small amounts of the β-glucosidases were secreted into the medium. Based on these results, we suspect that the recombinant BglA protein adsorbs to the cell wall and consequently is localized in the cell wall fraction of yeast cells. On the other hand, in solid cultures, about 80% of the β-glucosidase activity was attributed to extracellular β-glucosidase. Purified extracellular β-glucosidases also adsorbed tightly to the purified cell wall fraction of A. kawachii. Some soluble cell wall material seemed to be involved in the location of these enzymes. However, it is still not clear whether the three β-glucosidases are translated from the same mRNA transcribed from the bglA gene. Some types of posttranscriptional modification, such as processing, different ways of splicing, partial duplication of transcription, etc., may be involved. Additional experiments are needed to more fully understand the factors that regulate the activity and control the destination of β-glucosidases. Additional studies of bglA mRNA transcription in solid cultures are needed to understand these factors.
Ohta et al. reported that the β-glucosidase of A. kawachii has a role in flavor formation during production of sweet potato shochu (29, 30). The compounds that most strongly affect the flavor of sweet potato shochu are linalool, α-terpineol, citronellol, nerol, and geranol. However, these compounds are also present in the form of two nonvolatile precursors in sweet potato, neryl-β-glucoside and geranyl-β-glucoside. These terpenyl-β-glucosides are hydrolyzed by shochu koji β-glucosidases in the shochu mash. The liberated aglycons, which are nerol and geraniol, are transformed into citronellol by the shochu yeast in the shochu mash and into linalool and α-terpineol by acid and heat during distillation. As a result, there are several free monoterpene alcohols that contribute to the sweet potato shochu flavor. Thus, the β-glucosidase of A. kawachii is the key enzyme that affects flavor formation during production of sweet potato shochu. Almost all of the β-glucosidase activity (about 95% in a solid culture) is due to the enzyme encoded by bglA. Thus, it should be possible to regulate flavor formation in sweet potato shochu by controlling bglA gene expression.
When A. kawachii was grown in solid cultures in which rice or barley was used as the medium, the production of β-glucosidase was 5- to 10-fold higher than the production in liquid cultures when xylan and xylose were used. In addition, Ohta et al. reported that production of β-glucosidase rapidly increased in the late phase of cultures and was greater at low temperatures than at high temperatures (31). In view of these results, numerous factors must be involved in the regulation of bglA expression. We are currently attempting to determine how such factors control the production of β-glucosidase.
ACKNOWLEDGMENT
We are very grateful to Tadaaki Hashimoto for helpful discussions.
REFERENCES
- 1.Barnett C C, Berka R M, Fowler T. Cloning and amplification of the gene encoding an extracellular β-glucosidase from Trichoderma reesei: evidence for improved rates of saccharification of cellulosic substrates. Bio/Technology. 1991;9:562–567. doi: 10.1038/nbt0691-562. [DOI] [PubMed] [Google Scholar]
- 2.Becker D M, Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- 3.Béguin P. Molecular biology of cellulose degradation. Annu Rev Microbiol. 1990;44:219–248. doi: 10.1146/annurev.mi.44.100190.001251. [DOI] [PubMed] [Google Scholar]
- 4.Béguin P, Aubert J P. The biological degradation of cellulose. FEMS Microbiol Rev. 1994;13:25–58. doi: 10.1111/j.1574-6976.1994.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 5.Bhat K M, Gaikwad J S, Maheshwari R. Purification and characterization of an extracellular β-glucosidase from the thermophilic fungus S. thermophileand its influence on cellulase activity. J Gen Microbiol. 1993;139:2825–2832. [Google Scholar]
- 6.Buxton F P, Gwynne D I, Davies R W. Cloning of a new bidirectionally selectable marker for Aspergillusstrains. Gene. 1989;84:329–334. doi: 10.1016/0378-1119(89)90507-6. [DOI] [PubMed] [Google Scholar]
- 7.Castelli L A, Mardon C J, Strike P M, Azad A A, Macreadie I G. High-level secretion of correctly processed β-lactamase from Saccharomyces cerevisiaeusing a high-copy-number secretion vector. Gene. 1994;142:113–117. doi: 10.1016/0378-1119(94)90364-6. [DOI] [PubMed] [Google Scholar]
- 8.Castle L A, Smith K D, Morris R O. Cloning and sequencing of an Agrobacterium tumefaciensβ-glucosidase gene involved in modifying a vir-inducing plant signal molecule. J Bacteriol. 1992;174:1478–1486. doi: 10.1128/jb.174.5.1478-1486.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.DNA Data Bank of Japan. 21 September 1999, revision date. Database searches. [Online.] http://www.ddbj.nig.ac.jp/. [2 April 1999, last date accessed.]
- 9.Dowzer C E, Kelly J M. Cloning of the creA gene from Aspergillus nidulans: a gene involved in carbon catabolite repression. Curr Genet. 1989;15:457–459. doi: 10.1007/BF00376804. [DOI] [PubMed] [Google Scholar]
- 10.Drysdale M R, Kolze S E, Kelly J M. The Aspergillus niger carbon catabolite repressor encoding gene, creA. Gene. 1993;130:241–245. doi: 10.1016/0378-1119(93)90425-3. [DOI] [PubMed] [Google Scholar]
- 11.Enari T M, Niku-Paavola M L. Enzymatic hydrolysis of cellulose. Is the current theory of the mechanisms of hydrolysis valid? Crit Rev Biothechnol. 1987;5:67–87. doi: 10.3109/07388558709044153. [DOI] [PubMed] [Google Scholar]
- 11a.Genome Net Website. 25 September 1997, revision date. FASTA program. [Online.] http://www.genome.ad.jp/. [2 April 1999, last date accessed.]
- 12.Grabnitz F, Rucknagel K P, Seiss M, Staudenbauer W L. Nucleotide sequence of the Clostridium thermocellum bgIBgene encoding thermostable β-glucosidase B: homology to fungal β-glucosidases. Mol Gen Genet. 1989;217:70–76. doi: 10.1007/BF00330944. [DOI] [PubMed] [Google Scholar]
- 13.Gunata Y Z, Bayonove C L, Baumes R L, Cordonnier R E. The aroma of grapes. I. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J Chromatogr. 1985;331:83–90. [Google Scholar]
- 14.Hata Y, Kitamoto K, Gomi K, Kumagai C, Tamura G, Hara S. The glucoamylase cDNA from Aspergillus oryzae: its cloning, nucleotide sequence, and expression in Saccharomyces cerevisiae. Agric Biol Chem. 1991;55:941–949. [PubMed] [Google Scholar]
- 15.Iimura Y, Gomi K, Uzu H, Hara S. Transformation of Aspergillus oryzaethrough plasmid-mediated complementation of the methionine-auxotrophic mutation. Agric Biol Chem. 1987;51:323–328. [Google Scholar]
- 16.Ito K, Ikemasu T, Ishikawa T. Cloning and sequencing of the xynA gene encoding xylanase A of Aspergillus kawachii. Biosci Biotechnol Biochem. 1992;56:906–912. doi: 10.1271/bbb.56.906. [DOI] [PubMed] [Google Scholar]
- 17.Ito K, Iwashita K, Iwano K. Cloning and sequencing of the xynC gene encoding acid xylanase of Aspergillus kawachii. Biosci Biotechnol Biochem. 1992;56:1338–1340. doi: 10.1271/bbb.56.1338. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Ogasawara H, Sugimoto T, Ishikawa T. Purification and properties of acid stable xylanases from Aspergillus kawachii. Biosci Biotechnol Biochem. 1992;56:547–550. doi: 10.1271/bbb.56.547. [DOI] [PubMed] [Google Scholar]
- 19.Iwashita K, Todoroki K, Kimura H, Shimoi H, Ito K. Purification and characterization of extracellular and cell wall bound β-glucosidases from Aspergillus kawachii. Biosci Biotechnol Biochem. 1998;62:1938–1946. doi: 10.1271/bbb.62.1938. [DOI] [PubMed] [Google Scholar]
- 20.Kamei K, Yamamura Y, Hara S, Ikenaka T. Amino-acid sequence of chitinase from Streptomyces erythraeus. J Biochem. 1989;105:979–985. doi: 10.1093/oxfordjournals.jbchem.a122791. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi T, Enoki T, Tsurumaki S, Sumitani J, Ueda M, Ooi T, Arai M. Cloning and sequencing of the cDNA encoding β-glucosidase 1 from Aspergillus aculeatus. Gene. 1996;173:287–288. doi: 10.1016/0378-1119(96)00179-5. [DOI] [PubMed] [Google Scholar]
- 22.Kohchi C, Toh-e A. Nucleotide sequence of Candida pelliculosaβ-glucosidase gene. Nucleic Acids Res. 1985;13:6273–6282. doi: 10.1093/nar/13.17.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lecas M, Gunata Z Y, Sapis J, Bayonove C L. Purification and partial characterization of β-glucosidase from grape. Phytochemistry. 1991;30:451–454. [Google Scholar]
- 24.Legler G, Roeser K R, Illig H K. Reaction of β-d-glucosidase A3 from Aspergillus wentii with d-glucal. Eur J Biochem. 1979;101:85–92. doi: 10.1111/j.1432-1033.1979.tb04219.x. [DOI] [PubMed] [Google Scholar]
- 25.Machida M, Ohtsuki I, Fukui S, Yamashita I. Nucleotide sequences of Saccharomycopsis fibuligera genes for extracellular β-glucosidases as expressed in Saccharomyces cerevisiae. Appl Environ Microbiol. 1988;54:3147–3155. doi: 10.1128/aem.54.12.3147-3155.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messing J. New M13 vector for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- 27.Mikami S, Iwano K. Properties of enzymes produced by Aspergillus kawachii. J Brew Soc Jpn. 1988;83:791–796. . (In Japanese.) [Google Scholar]
- 28.Mikami S, Iwano K, Shiinoki S, Shimada T. Purification and some properties of acid-stable α-amylase from shochu-koji (Aspergillus kawachii) Agric Biol Chem. 1987;51:2495–2501. [Google Scholar]
- 29.Ohta T, Ikuya R, Nakashima M, Morimitsu Y, Samuta T, Saiki H. Characteristic flavor of Kansho-shochu(sweet potato spirit) Agric Biol Chem. 1990;54:1353–1357. [Google Scholar]
- 30.Ohta T, Omori T, Shimojo H, Hashimoto K, Samuta T, Ohba T. Identification of monoterpene alcohol β-glucoside in sweet potatoes and purification of a Shiro-kojiβ-glucosidase. Biosci Biotechnol Biochem. 1991;55:1811–1816. [Google Scholar]
- 31.Ohta T, Shimojo H, Hashimoto K, Kondo H, Samuta T, Ohba T. β-Glucosidase activity in Shiro-Koji and its contribution to sweet potato Shochuflavor. J Brew Soc Jpn. 1991;86:536–539. . (In Japanese.) [Google Scholar]
- 32.Pines O, London A. Expression and secretion of staphylococcal nuclease in yeast: effects of amino-terminal sequences. J Gen Microbiol. 1991;137:771–778. doi: 10.1099/00221287-137-4-771. [DOI] [PubMed] [Google Scholar]
- 33.Punt P J, van den Hondel C A. Transformation of filamentous fungi based on hygromycin B and phleomycin resistance markers. Methods Enzymol. 1992;216:447–457. doi: 10.1016/0076-6879(92)16041-h. [DOI] [PubMed] [Google Scholar]
- 34.Raynal A, Gerbaud C, Francingues M C, Guerineau M. Sequence and transcription of the β-glucosidase gene of Kluyveromyces fragilis cloned in Saccharomyces cerevisiae. Curr Genet. 1987;12:175–184. doi: 10.1007/BF00436876. [DOI] [PubMed] [Google Scholar]
- 35.Rojas A, Arola L, Romeu A. β-Glucosidase families revealed by computer analysis of protein sequences. Biochem Mol Biol Int. 1995;35:1223–1231. [PubMed] [Google Scholar]
- 36.Rojas A, Romeau A. A sequence analysis of the β-glucosidase subfamily B. FEBS Lett. 1996;378:93–97. doi: 10.1016/0014-5793(95)01412-8. [DOI] [PubMed] [Google Scholar]
- 37.Ryu D D, Mandels M. Cellulases: biosynthesis and applications. Enzyme Microb Technol. 1980;2:91–101. [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 39.Shimizu K. The role of monoterpenes in flavor of alcoholic beverages. J Brew Soc Jpn. 1994;89:594–600. . (In Japanese.) [Google Scholar]
- 40.Sternberg D. β-Glucosidase of Tricoderma: its biosynthesis and role in saccharification of cellulose. Appl Environ Microbiol. 1976;31:164–178. doi: 10.1128/aem.31.5.648-654.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strauss J, Mach R L, Zeilinger S, Hartler G, Stoffler G, Wolschek M, Kubicek C P. Cre1, the carbon catabolite repressor protein from Trichoderma reesei. FEBS Lett. 1995;376:103–107. doi: 10.1016/0014-5793(95)01255-5. [DOI] [PubMed] [Google Scholar]
- 42.Sudo S, Ishikawa T, Takayasu-Sakamoto Y, Sato K, Oba T. Characteristics of acid-stable α-amylase production by submerged culture of Aspergillus kawachii. J Ferment Bioeng. 1993;76:105–110. [Google Scholar]
- 42a.Swiss Institute of Bioinformatics Website. 5 October 1999, revision date. PROSITE. [Online.] http://www.expasy.ch/. [2 April 1999, last date accessed.]
- 43.Takashima S, Nakamura A, Iikura H, Masaki H, Uozumi T. Cloning of a gene encoding a putative carbon catabolite repressor from Trichoderma reesei. Biosci Biotechnol Biochem. 1996;60:173–176. doi: 10.1271/bbb.60.173. [DOI] [PubMed] [Google Scholar]
- 44.Umezurike G M. Kinetic analysis of the mechanism of action of β-glucosidase from B. theobromae. Biochim Biophys Acta. 1975;31:648–654. doi: 10.1016/0005-2744(75)90190-4. [DOI] [PubMed] [Google Scholar]
- 45.Williams P J, Strauss C R, Wilson B, Massy-Westropp R A. Novel monoterpene disaccharide glycosides of Vitis viniferagrapes and wines. Phytochemistry. 1982;21:2012–2020. [Google Scholar]
- 46.Wood T M, McCrae S I. Purification and some properties of the extracellular β-d-glucosidase of the cellulolytic fungus, T. koningii. J Gen Microbiol. 1982;128:2973–2982. [Google Scholar]
- 47.Yamada O, Lee B R, Gomi K. Transformation system for Aspergillus oryzae with double auxotrophic mutations, niaD and sC. Biosci Biotechnol Biochem. 1997;61:1367–1369. [Google Scholar]