Abstract
Background
Evidence on the efficacy and safety of anticoagulation in preventing stroke and thromboembolic events in people with thyrotoxic atrial fibrillation is scarce.
Objective
We evaluated the efficacy and safety of anticoagulation in people with thyrotoxic atrial fibrillation.
Methods
Our study protocol was published in the International Prospective Register of Systematic Reviews (registration no. CRD42020222782). Four databases and two systematic review registers were searched through 25 November 2020 for interventional and observational studies comparing anticoagulation therapy with active comparators, placebo, or no treatment in people with thyrotoxic atrial fibrillation. Random-effects meta-analysis and sensitivity analysis were performed. Quality of evidence was described using the GRADE framework.
Results
In the study, 23,145 records were retrieved. One randomized controlled trial and eight cohort studies were ultimately included. Effect estimates on the efficacy and safety of anticoagulation were extracted. Meta-analysis using the inverse variance and random-effects methods was conducted on four cohort studies with 3443 participants and 277 events. Anticoagulation in people with thyrotoxic atrial fibrillation reduced the risk of ischemic stroke and systemic thromboembolism by 3% (95% CI: 1–6%). Warfarin may prevent ischemic stroke in people with thyrotoxic atrial fibrillation if the CHA2DS2-VASc score exceeds 1 and when atrial fibrillation persists beyond 7 days. Direct oral anticoagulants may be associated with fewer bleeding events than warfarin.
Conclusions
Anticoagulation prevents ischemic stroke and systemic thromboembolism in people with thyrotoxic atrial fibrillation. Direct oral anticoagulants may be associated with fewer bleeding events.
Keywords: thyrotoxicosis, atrial fibrillation, stroke, thromboembolism, anticoagulation, direct oral anticoagulants
Introduction
Atrial fibrillation (AF) occurs in 6–28% of people with hyperthyroidism (1, 2, 3, 4, 5, 6). Thyrotoxicosis increases the activities of thrombin, fibrinogen, factor VIII, factor IX, von Willebrand factor, and tissue plasminogen activator inhibitor-1 (7, 8). Increased D-dimer and shortened activated partial thromboplastin time also occurs in thyrotoxicosis (9). The hypercoagulable and hypofibrinolytic states and atrial blood stasis predispose people with thyrotoxic AF (TAF) to ischemic strokes (IS) and systemic thromboembolism (SE).
Rationale for this systematic review and meta-analysis
Current guidelines make no specific recommendations on anticoagulation for people with TAF. Experts recommend anticoagulation in TAF until an euthyroid state and sinus rhythm are achieved (10, 11). Evidence is conflicting on the efficacy and safety of anticoagulation in people with TAF and most randomized controlled trials (RCTs) on anticoagulation in AF have excluded people with TAF. Warfarin was superior to aspirin or no treatment in reducing IS in people whose CHA2DS2-VASc score was 1 or more (7, 12, 13) and apixaban was superior to warfarin in reducing stroke and SE in the post-hoc analysis of a RCT (14). However, warfarin and direct oral anticoagulants (DOACs) did not prevent stroke in two retrospective cohort studies (6, 15). There are no systematic reviews or Cochrane reviews on this topic.
Materials and methods
The primary objective is the efficacy and safety of anticoagulation in people with TAF. The secondary objectives are frequency of intracranial hemorrhage (ICH), gastrointestinal bleeding (GIB), and quality of life in people with TAF with and without anticoagulation.
Methods
Interventional and observational studies reporting the efficacy and safety of anticoagulation, antiplatelet therapy, or no therapy in people with TAF were eligible for inclusion. Interventional studies include non-randomized studies and RCTs. Observational studies include cross-sectional studies, retrospective cohort studies, and prospective cohort studies. Studies unrelated to human beings, on people age thirteen and below, or unrelated to TAF were excluded.
The interventions were anticoagulation (warfarin, DOACs, or low molecular weight heparins) and the comparators are antiplatelet therapy or no treatment. The primary outcomes are IS, SE, bleeding, and all-cause mortality. The secondary outcomes are ICH, GIB, and quality of life.
We searched the Cochrane Register of Controlled Trials (CENTRAL), Embase, PubMed/MEDLINE, and Science Citation Index Expanded databases for relevant studies. The Cochrane Database of Systematic Review and the International Prospective Register of Systematic Reviews (PROSPERO) were searched for similar systematic reviews and meta-analyses. Search strategies are found in the Supplementary Material (see section on supplementary materials given at the end of this article) and online repository (https://doi.org/10.5281/zenodo.4855376). We searched the reference lists of review papers and shortlisted articles and unindexed studies. The number of articles retrieved from each database was recorded.
Two reviewers (YST and ATA) independently screened the titles and abstracts of articles. Articles were labeled as ‘include’, ‘uncertain’, or ‘exclude’ by the reviewers. Articles were included for full-text screening if both reviewers labeled the articles as ‘include’. Articles were excluded from full-text screening if both reviewers labeled the articles as ‘exclude’. The reviewers discussed articles that were labeled discordantly and those labeled as ‘uncertain’. A senior reviewer (ELT) arbitrated unresolved differences in opinions. Two reviewers (YST and ATA) repeated the same process for full-text screening.
Two reviewers (NCYY and ZW) extracted data on the authors, year of publication, location of study, study design, setting, details on interventions, number of participants, number of drop outs, age of participants, number of events for outcomes of interest, and risk of bias (ROB). The outcomes included any stroke, IS, SE, composite of IS and SE (IS/SE), any bleeding, major bleeding, hemorrhagic stroke, ICH, GIB, and all-cause mortality. Data extraction was done using a standardized spreadsheet on Microsoft Excel 2013. Discrepancies were discussed between the reviewers and a senior reviewer (ELT) arbitrated unresolved differences in opinions. Investigators were contacted via email for missing or ambiguous data.
Quality of evidence was assessed by two reviewers (NCYY and ZW) using the Revised Cochrane risk-of-bias tool for randomized trials (16) and the Risk of Bias in Non-randomized Studies of Interventions tool (17). Justifications were recorded. ROB plots were generated using the robvis tool (18).
No imputations of missing data or conversion of data were done for statistical analysis. Homogeneous data were meta-analyzed using Revman 5.4 (19). Statistical analysis using the random-effects model and inverse variance method was performed. The inverse variance method was chosen to give greater weight to studies with smaller variance. Sensitivity analysis using the fixed-effects model was not performed because there was significant heterogeneity between the cohorts in the included studies. Reporting bias was assessed using the funnel plot method.
Results
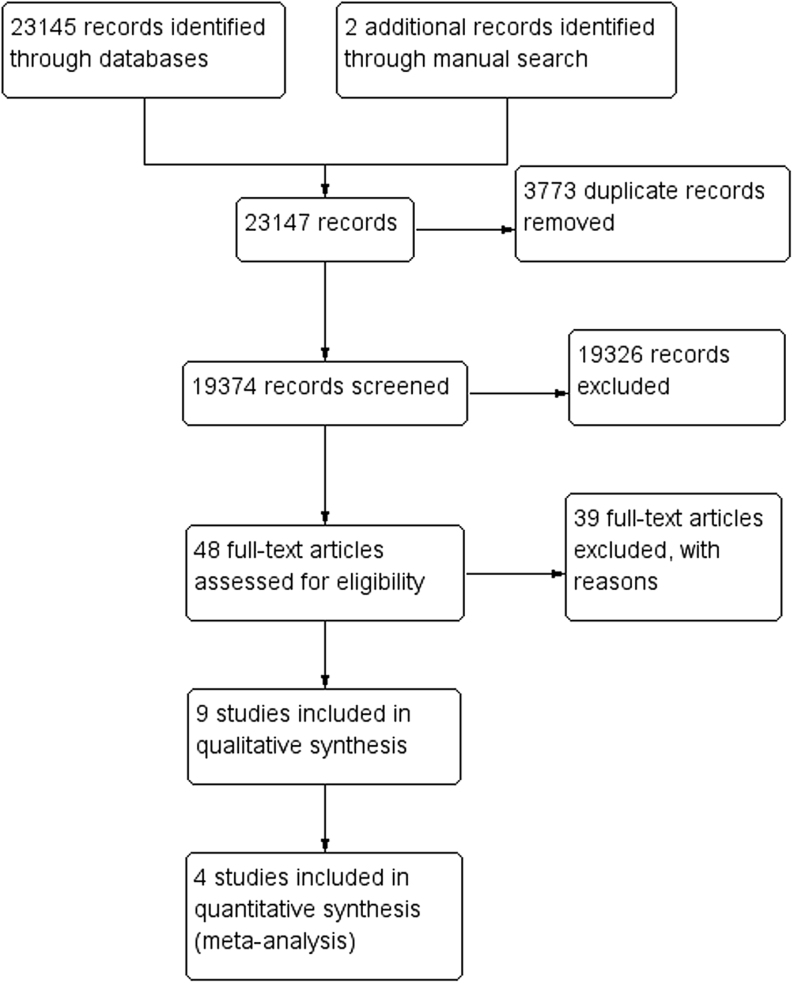
For the study, 23,145 records were retrieved from MEDLINE (1882), Embase (14358), CENTRAL (325), and Science Citation Index (6578). Two articles were retrieved from shortlisted articles. There were no similar systematic reviews or meta-analyses identified from the Cochrane Database of Systematic Reviews and PROSPERO. The study flow diagram is shown in Fig. 1. There were 3773 duplicate records. A total of 19,326 studies were deemed ineligible. YST selected 23 articles and ATA selected 32 articles from title and abstract screening. There was mutual agreement on inclusion of 10 articles. Forty-eight studies were retrieved for full-text screening and 39 studies were deemed ineligible. Nine studies (one RCT (14) and eight cohort studies (6, 7, 12, 13, 15, 20, 21, 22)) were included in the systematic review. Four cohort studies were included in the meta-analysis.
Figure 1.
Study flow diagram.
The characteristics of included studies are summarized in Table 1.
Table 1.
Characteristics of included studies.
| Study | Study characteristics | Patient characteristics | Outcomes |
|---|---|---|---|
| Chan 2015 (12) | Retrospective cohort study in Hong Kong. Follow-up duration: 24 months. Hyperthyroidism was defined as serum FT4 > 23 pmol/L with concomitant TSH < 0.03 mIU/L.Definition of AF was not stated. |
Six hundred forty-two patients with new-onset non-valvular TAF. Mean age of patients with TAF = 71.9 ± 14.6 years.One hundred thirty-six patients with TAF were on warfarin, 243 on aspirin, and 263 had no treatment. Mean CHA2DS2-VASc score was 3.3 ± 1.9 for all patients, 2.8 ± 1.7 for those on no treatment, 3.8 ± 1.9 for those on aspirin, and 3.2 ± 2.0 for those on warfarin. Mean HAS-BLED score was 1.8 ± 1.0 for all patients, 1.6 ± 0.9 for those on no treatment, 2.0 ± 1.0 for those on aspirin, and 1.7 ± 1.1 for those on warfarin.Baseline characteristics of patients on warfarin and those on antiplatelet therapy were compared. There were statistically significant differences in mean age, hypertension, CAD, prior stroke or TIA, mean CHA2DS2-VASc score, and mean HAS-BLED score between groups. | Stroke and ICH based on medical records and discharge summaries during the follow-up period. Stroke was defined as a sudden neurologic deficit persisting > 24 hours corresponding to a vascular territory in the absence of primary haemorrhage or other causes and confirmed by CT or MRI of the brain.ICH was determined based on new-onset neurological symptoms with radiological confirmation. |
| Chan 2020 (20) | Retrospective cohort study in Taiwan. Follow-up from date of first prescription of anticoagulant until occurrence of outcome or end of study. Hyperthyroidism and AF were determined by ICD-9-CM codes recorded in the Taiwan National Health Insurance Program. |
Four thousand ninety-four patients with non-valvular TAF. Onset of AF was not mentioned. Mean age of patients with TAF = 69.5 ± 13.3 years. 1181 patients with TAF were on warfarin and 3213 were on DOACs. Mean CHA2DS2-VASc score was 2.5 ± 2.0 for TAF patients on warfarin and 3.2 ± 1.8 for TAF patients on DOACs. HAS-BLED score was not stated.Baseline characteristics of TAF patients with and without DOACs were compared. P-values were not stated. | IS/SE, ICH, hospitalized GIB, and all major bleeding based on ICD-9-CM codes in the National Health Insurance Research Database. |
| Chen 2014 (7) | Prospective cohort study in China. Follow-up duration: 34.1 ± 0.6 months.Hyperthyroidism was defined as serum FT4 > 23 pmol/L with concomitant TSH < 0.03 mIU/L.AF was confirmed by ≥ two ECGs recorded ≥ four hours apart. |
Sixty-two patients with TAF. Patients with RHD were excluded. Onset of AF was not stated but eight patients had persistent AF. Mean age of patients with TAF = 55.1 ± 1.7 years. four patients with TAF were on warfarin, 12 were on aspirin, and 58 had no treatment. Mean CHA2DS2-VASc score was 1.6 ± 0.2 for TAF patients. HAS-BLED score was not stated.Baseline characteristics of TAF and non-thyroid AF patients were compared. There was statistically significant difference in the mean age between groups. | IS, SE, and TIA. IS was defined as a sudden neurologic deficit persisting > 24 hours corresponding to a vascular territory in the absence of primary haemorrhage or other causes and confirmed by CT or MRI of the brain.Definition of SE was not stated. |
| Chen 2019 (21) | Retrospective cohort study in Taiwan. Follow-up from index date to death, loss to follow-up, or end of study. Hyperthyroidism and AF were determined based on ICD-9-CM codes recorded in the Taiwan National Health Insurance Program. Only patients with ≥ two consensus-diagnosed episodes of thyrotoxicosis or who had been prescribed anti-thyroid drugs were included. |
One thousand eight hundred sixty-eight patients with new-onset TAF. Patients with RHD were excluded. Mean age of patients with TAF was not stated. Four hundred fifty patients with TAF were on warfarin, six on were DOACs, and 1163 were on aspirin. Number of patients with TAF on no treatment was not stated. Of the TAF patients, 19.7% had CHA2DS2-VASc score of 0, 33.9% had score of 1, 18.5% had score of 2, and 27.9% had score ≥ 3. HAS-BLED score was not stated.Baseline characteristics of TAF and non-thyroid AF patients were compared. There were statistically significant differences in ACEI/ARB, beta blocker, digoxin, and statin use between groups. | IS, mortality, major bleeding, hemorrhagic stroke, minor GIB, and bleeding during re-operation based on ICD-9-CM codes in the National Health Insurance Research Database. |
| Fauchier 2016 (22) | Retrospective cohort study in France. Patients were followed-up from first record of non-valvular AF to end of study, with mean follow-up duration of 944 days. Definition of hyperthyroidism was not stated. AF was diagnosed based on the replacement of consistent P waves by rapid oscillations or fibrillatory waves that vary in amplitude, shape, and timing, associated with an irregular, frequently rapid ventricular response when atrioventricular conduction was intact. |
Thirty-six patients with TAF. Onset of AF was not stated. Patients with valvular AF were excluded. Mean age of patients with TAF was not stated. Number of patients with TAF on warfarin, DOACs, aspirin, and no treatment were not stated. CHA2DS2-VASc and HAS-BLED scores of patients with TAF were not stated. Baseline characteristics of patients with AF from temporary and non-temporary causes were compared. There were statistically significant differences in age, heart failure, hypertension, diabetes, vascular disease, CAD, previous myocardial infarction, previous percutaneous intervention, renal failure, chronic pulmonary disease, CHA2DS2-VASc score, HAS-BLED score, ACEI/ARB, and diuretic use between groups. |
Stroke, SE, bleeding, and death based on ICD-10 codes in the discharge data from a computerized system, and mortality data from an online regional database. |
| Goldstein 2019 (14) | Double-blind randomized controlled trial in North America, South America, Europe, and Asian Pacific countries. Follow-up duration: 21.6 months (median). | Three hundred twenty-one patients with new-onset and existing TAF. Patients with moderate-to-severe mitral stenosis and prosthetic mechanical valves were excluded. Median age of patients with TAF = 69 years (IQR: 62–76). Number of patients with TAF on warfarin and DOACs were not stated. Eighty-six patients with TAF were on aspirin and seven were on clopidogrel. Mean CHA2DS2-VASc score of patients with TAF was 2.2 ± 1.2. HAS-BLED score was not stated. | Composite of ischemic or hemorrhagic stroke or SE, major bleeding, any stroke, IS, hemorrhagic stroke, all-cause mortality, myocardial infarction, hospitalization for heart failure, any bleeding. Outcomes were adjudicated based on pre-specified criteria established by a clinical events committee. |
| Hyperthyroidism was determined based on investigator responses on the ARISTOTLE data intake form. Patients who were noted to be taking antithyroid therapy were included as hyperthyroid. AF or atrial flutter were determined by ECG ≥ two weeks apart in the 12 months before enrolment. |
Baseline characteristics of patients with TAF and non-thyroid AF were compared. There were statistically significant differences in gender, ethnicity, weight, prior bleeding, and prior vitamin K antagonist use within 1 month between groups. | IS was defined as a focal neurological deficit, from a nontraumatic cause, lasting ≥ 24 hours and was categorized as ischemic, hemorrhagic, or of uncertain type.Bleeding was defined according to the ISTH criteria, as clinically overt bleeding accompanied by a decrease in the hemoglobin level of ≥2 g/dL or transfusion of ≥2 units of packed cells, occurring at a critical site, or resulting in death. Clinically relevant non-major bleeding was defined as clinically overt bleeding that did not satisfy the criteria for major bleeding and that led to hospital admission, physician-guided medical or surgical treatment, or a change in antithrombotic therapy.Definition of SE, all-cause mortality, myocardial infarction, and hospitalization for heart failure were not stated. | |
| Gundlund 2019 (15) | Retrospective cohort study in Denmark. Patients were followed-up from 4 weeks after discharge until outcome occurred, death, 5 years after discharge, emigration, or end of study. Hyperthyroidism and AF were determined by ICD-8 and ICD-10 codes obtained from the Danish Registries. |
Two thousand five hundred seven patients with new-onset TAF. Patients with valvular AF were excluded. Median age of patients with TAF = 73 years (IQR: 63–81). One thousand hundred patients with TAF were on anticoagulation and 1407 had no anticoagulation. Median CHA2DS2-VASc score of patients with TAF was 3 (IQR: 2–4) and median HAS-BLED score of patients with TAF was 2 (IQR: 1–3).Baseline characteristics of TAF and non-thyroid AF patients were compared. P-values were not reported. | Composite of IS, TIA, or SE, AF re-hospitalization and all-cause mortality based on ICD-8 and ICD-10 codes in the Danish Registries. |
| Siu 2009 (13) | Prospective cohort study in Hong Kong. Follow-up duration: 12 months. Hyperthyroidism was defined as serum FT4 > 23 pmol/L with concomitant TSH < 0.03 mIU/L.AF was confirmed by ≥ two ECGs recorded ≥ four hours apart. |
One hundred sixty patients with new-onset TAF. Patients with structural heart disease were excluded. Mean age of patients with TAF = 64.7 ± 1.3 years. 47 patients with TAF were on warfarin and 62 were on aspirin. Mean CHA2DS2-VASc score of patients with TAF was 0.7 ± 0.1. HAS-BLED score was not stated.Baseline characteristics of TAF and non-thyroid AF patients were compared. There were statistically significant differences in hypertension, CHADS2 score, beta blocker, calcium antagonist, digoxin, propafenone, sotalol, and amiodarone use between groups. | IS and sinus rhythm conversion based on data from discharge summaries and outpatient clinic records. IS was defined as a sudden neurologic deficit persisting > 24 hours corresponding to a vascular territory in the absence of primary hemorrhage or other causes and confirmed by CT or MRI of the brain. |
| Wong 2017 (6) | Retrospective cohort study in Hong Kong. Follow-up duration = 47 months (median) (IQR: 24 – 82).Hyperthyroidism was defined as elevated FT4 or FT3 and concomitant suppressed TSH. AF or atrial flutter was confirmed with standard 12-lead ECG. |
One hundred thirty-three patients with TAF. Onset of AF was not stated. Patients with significant mitral valve disease were excluded. 16 patients had moderate-to-severe mitral regurgitation. Mean age of patients with TAF was not stated. 12 patients with TAF were on warfarin, 1 was on DOACs, and 50 were on aspirin. Mean CHA2DS2-VASc score of patients with TAF and IS was 2.9 and that of patients with TAF and no IS was 2.3. HAS-BLED score was not stated. Baseline characteristics of patients with spontaneous AF resolution and those with persistent AF were compared. There were statistically significant differences in smoking status, FT4 level, history of thyrotoxicosis, ACEI/ARB, and digitalis use between groups. |
IS and sinus rhythm conversion based on data from a Clinical Data Analysis and Repository System. IS was defined as a sudden neurologic deficit persisting > 24 hours corresponding to a vascular territory in the absence of primary hemorrhage or other causes and confirmed by CT or MRI of the brain. |
ACEI: angiotensin-converting enzyme inhibitor, AF: atrial fibrillation, ARB: angiotensin-2 receptor blocker, CAD: coronary artery disease, CT: CT, DOACs: direct oral anticoagulants, ECG: ECG, FT3: free thyronine, FT4: free thyroxine, GIB: gastrointestinal bleeding, ICD: International Classification of Diseases, ICD-9-CM: International Classification of Diseases 9th Revision Clinical Modification, ICH: intracranial hemorrhage, IQR: inter-quartile range, IS/SE: ischemic stroke or systemic thromboembolism, ISTH: International Society on Thrombosis and Haemostasis, MRI: MRI, RHD: rheumatic heart disease, TAF: thyrotoxic atrial fibrillation, TIA: transient ischemic attack, TSH: thyroid stimulating hormone.
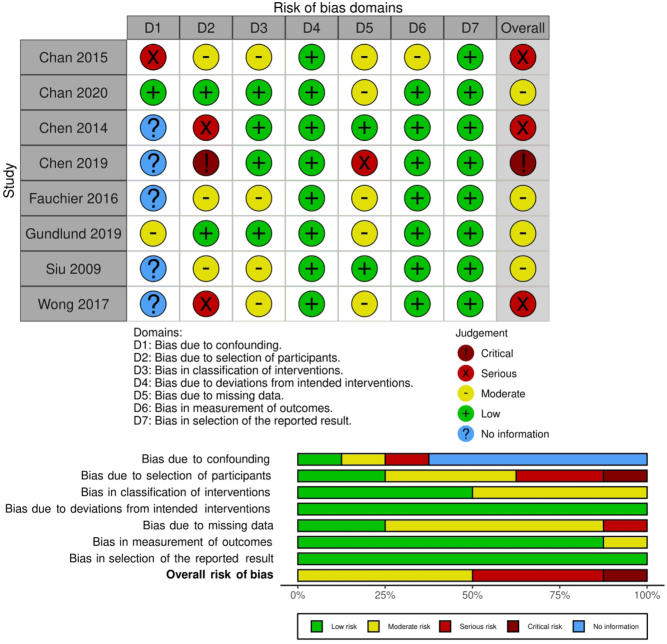
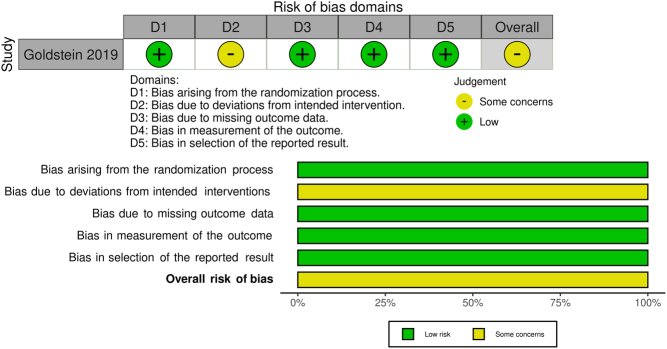
Methodological quality, ROB, and quality of evidence (based on the GRADE framework) are found in the online repository (https://doi.org/10.5281/zenodo.4855376). Briefly, the RCT (14) was judged to have some concerns for ROB. Four cohort studies (13, 15, 20, 22) were judged to be at moderate ROB. Three cohort studies (6, 7, 12) were judged to be at serious ROB. One cohort study (21) was judged to be at critical ROB. ROB of the observational studies is summarized in Fig. 2. ROB of the RCT is summarized in Fig. 3.
Figure 2.
ROB of observational studies.
Figure 3.
ROB of the RCT.
The characteristics of excluded studies are found in Table 1, Supplementary Material and online repository (https://doi.org/10.5281/zenodo.4855376).
Findings
The average age of participants was between 55 and 73 years. The youngest participants were 18 years old (15, 21). Important baseline characteristics were statistically significantly different in some studies. Patients in the DOACs arm were older compared to the warfarin arm (71.36 ± 12.48 vs 64.57 ± 14.22) in the study by Chan et al. (20). The CHA2DS2-VASc score was different between the warfarin and non-warfarin arms in people with TAF (3.20 ± 2.0 vs 3.28 ± 1.87) in the study by Chen et al. (7). The CHA2DS2-VASc score was different between the DOACs and warfarin arms (3.21 ± 1.78 vs 2.50 ± 1.95) in the study by Chan et al. (20). Fewer patients had hypertension, coronary artery disease, peripheral artery disease, and prior stroke in the warfarin arm compared to the non-warfarin arm in the study by Chen et al. (7). More patients had hypertension, prior stroke, hyperlipidemia, and chronic kidney disease in the DOACs arm compared to the warfarin arm in the study by Chan et al. (20). The HAS-BLED score was higher in the non-warfarin arm compared to the warfarin arm (1.79 ± 0.97 vs 1.70 ± 1.1) in the study by Chen et al. (7). Detailed baseline characteristics were not reported in the remaining studies (13, 22).
Meta-analysis
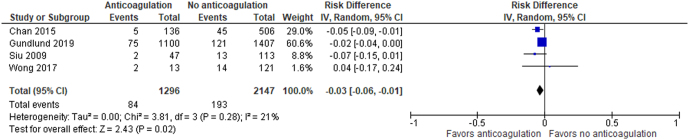
Meta-analysis was conducted on four cohort studies (6, 12, 13, 15). The study designs of observational and interventional studies are substantially different so the RCT (14) was not included in the meta-analysis, because the resultant heterogeneity in data will be high, which makes the summary estimate unreliable. There were 1296 people in the anticoagulation arm and 2147 people in the non-anticoagulation arm. There were 84 events in the anticoagulation arm and 193 events in the non-anticoagulation arm. Risk difference was reported as the effect estimate because risk difference is easy to interpret.
Comparing anticoagulation to no anticoagulation in people with TAF, the risk difference for IS/SE is −0.03, 95% CI (−0.06, −0.01) using random-effects analysis. Chi2 = 3.81 and I2 = 21%. Figure 4 shows the data table and forest plot.
Figure 4.
Data table and forest plot of the meta-analysis.
Ischemic stroke/systemic thromboembolism
Goldstein et al. (14) compared DOACs to warfarin in people with TAF. Hazard ratio (HR) for IS/SE was 1.47, 95% CI (0.25–8.77). Chan et al. (20) compared DOACs to warfarin in people with TAF. HR for IS/SE was 1.25, 95% CI (0.88–1.78).
Ischemic stroke
Chan et al. (12) reported an incidence for IS of 7.8%. Patients with CHA2DS2-VASc 0 did not develop IS. Warfarin prevented IS in patients with CHA2DS2-VASc one or more and AF lasting beyond 7 days. Chen et al. (7) reported an annual incidence rate of IS of 7.6%/year in people with TAF. Older age (60 ± 2.6 years vs 54 ± 2.0 years) and higher CHADS-VASc score (2.80 ± 0.3 vs 1.40 ± 0.2) were risk factors for stroke. Siu et al. (13) reported an incidence of IS of 9.4% in people with TAF. Majority (>70%) of strokes occurred when AF was present and within 30 days of presentation. The HR for IS in people on warfarin therapy was 0.17, 95% CI (0.04–0.79). Wong et al. (6) reported an incidence of 12.0% for IS in TAF, and the predictive factors were old age (>75 years) and renal failure. Goldstein et al. (14) compared DOACs to warfarin in people with TAF. The HR for IS in people on DOACs is 1.98, 95% CI (0.18–21.87).
Systemic thromboembolism
Gundlund et al. (15) reported a 5-year absolute risk of SE in people with TAF at 8.5%. Comparing people with TAF to those with primary AF, the HR for SE in the first year was 0.81, 95% CI (0.60–1.10). In comparison, the incidence of SE in various cohort studies was between 8 and 24% (23, 24, 25, 26). Gundlund et al. (15) also compared anticoagulation in those with TAF vs primary AF, and the HR for thromboembolism was 0.92, 95% CI (0.67–1.26). When they compared TAF vs primary AF patients not on anticoagulation, the HR for thromboembolism was 0.75, 95% CI (0.61–0.99).
Any bleeding events
Goldstein et al. (14) compared the risk of any bleeding with DOACs vs warfarin in people with TAF. The HR was 0.72, 95% CI (0.49–1.07).
Major bleeding events
Goldstein et al. (14) compared the risk of major bleeding with DOACs vs warfarin in people with TAF. HR for major bleeding was 0.47, 95% CI (0.14–1.57). Chan et al. (20) made a similar comparison and the HR was 0.65, 95% CI (0.44–0.96).
Intracranial hemorrhage
Chan et al. (20) compared DOACs to warfarin in people with TAF. HR for ICH was 0.84, 95% CI (0.46–1.53). None of the patients in the study by Chan et al. (12) had ICH.
Gastrointestinal bleeding
Chan et al. (20) compared DOACs to warfarin in people with TAF. HR for hospitalized GIB was 0.56, 95% CI (0.31–1.00).
All-cause mortality
Goldstein et al. (14) compared DOACs to warfarin in people with TAF. HR for all-cause mortality was 0.56, 95% CI (0.22–1.42). Gundlund et al. (15) compared TAF vs primary AF patients on anticoagulation and the HR for death was 0.94, 95% CI (0.78–1.14). When they compared TAF vs primary AF patients not on anticoagulation, the HR for death was 1.07, 95% CI (0.94–1.22). Chen et al. (21) found that anticoagulation reduced the risk of mortality within the first year, with HR of 0.47, 95% CI (0.27–0.83), but the benefit disappeared thereafter.
Possible sources of heterogeneity
Clinical and methodological diversity contributed to heterogeneity in this meta-analysis. Differences in patient characteristics of included studies caused clinical heterogeneity. However, only Chan 2015 (12) compared baseline characteristics of participants in the warfarin vs antiplatelet groups. Gundlund 2019 (15) and Siu 2009 (13) compared baseline characteristics of participants with TAF vs those with non-thyroid AF rather than in intervention groups. Furthermore, Gundlund 2019 (15) did not provide P -values on the comparison of patient characteristics. Wong 2017 (6) compared participants with AF that reverted spontaneously vs those with permanent AF. Thus, only one study made direct comparison between participants from intervention groups while the remaining studies did not provide such information. All studies had clear pre-established definitions of TAF and IS, and the diagnosis of these conditions are usually clear-cut, so there is unlikely to be clinical heterogeneity arising from the misclassification of exposure, intervention, and outcomes in these studies. Differences in study designs and ROB in the studies contributed to methodological heterogeneity in this meta-analysis.
Despite the clinical and methodological heterogeneity, our meta-analysis has no significant statistical heterogeneity, indicating low variability in the intervention effects in the studies. This is shown by the good overlap of CI of effect estimates in all studies in the meta-analysis. Furthermore, with the exception of Wong 2017 (6), all studies had the effect estimate in the same direction, in favor of anticoagulation. The Higgin’s I2 value of 21% indicated that heterogeneity is probably not important, while the chi2 P -value of 0.28 indicated the effect estimates are unlikely to be statistically different.
Measures taken to mitigate heterogeneity
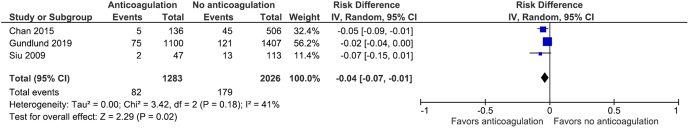
We performed meta-analysis using the random-effects model as this model assumes that different studies have different estimated intervention effects, and analysis using this model addresses heterogeneity that cannot be readily explained by other factors. The inverse variance method was used because outcomes were binary. This method gives greater weight to studies with lesser variance, and some studies with lesser variance in this meta-analysis had few events (12, 13). Sensitivity analysis was conducted to explore the effects of clinical heterogeneity on the meta-analysis. Data from Wong 2017 (6) were excluded from analysis as this data set had a small effect estimate that was in the opposite direction from the other studies and had a wide CI that crossed the line of no effect. The result of the sensitivity analysis was similar to the meta-analysis, with risk difference for IS/SE of −0.04, 95% CI (−0.07, −0.01) using random-effects analysis. Figure 5 shows the data table and forest plot.
Figure 5.
Data table and forest plot of the sensitivity analysis.
There was insufficient data for subgroup analysis. The exact number of subjects on warfarin, DOACs, antiplatelet agents, and no treatment were not reported in the studies included in the meta-analysis. There was inadequate data for subgroup analysis in subjects with renal impairment. Meta-regression was not performed as there are fewer than ten studies in the meta-analysis.
Assessment of reporting bias
Less than ten studies were included in the meta-analysis so funnel plot method and statistical methods for assessing the funnel plot asymmetry could not be performed. Statistical methods for assessing reporting bias were not performed as removal of studies with small effect estimates will further reduce the number of studies.
Reporting bias is very likely to be present because there are many studies with unpublished data on the relevant outcomes. Five studies identified during title and abstract screening (27, 28, 29, 30, 31) possibly had unpublished data related to the population and outcomes of interest. The authors of these studies did not respond to requests. The complete number of events was not available in four studies (7, 14, 21, 22) and only two authors replied with relevant information (6, 15).
Discussion
Summary of main results
Anticoagulation reduces the risk for IS/SE in people with TAF whose CHA2DS2-VASc score are above 1 when AF lasts beyond 7 days. DOACs are not superior to warfarin in IS/SE prevention but may have lower major bleeding risks compared to warfarin.
The overall quality of this systematic review and meta-analysis is low and the effect estimate is of very low certainty. The effect estimate is consistent because CIs of studies in the meta-analysis overlapped widely. However, the effect estimate is imprecise as the CIs of most studies included statistically significant and non-significant effects, and the total number of outcomes in both groups was small.
Discussion of findings
Anticoagulation reduces the risk of IS/SE in people with TAF. This conclusion is biologically plausible as thyrotoxicosis induces hypercoagulability and reduces fibrinolysis. The mean risk reduction for IS/SE with anticoagulation in TAF is 3% (95% CI: reduction by 1–6%). In the best-case scenario, there is a 6% risk reduction whilst in the worst-case scenario, there is a 1% risk reduction of IS/SE. The risk for IS/SE was 9% in the control group. The minimal clinically important difference (MCID) for stroke prevention using warfarin for AF was an absolute risk reduction of 2.01% over 2 years, 95% CI (1.60–2.42), assuming a baseline risk for stroke of 10%, or 1%/year in the study by Mon-Son-Hing et al. (32). Howitt et al. reported the MCID for stroke prevention using warfarin for AF as a risk reduction by 2.4%/year to 4.1%/year (33). The risk reduction for IS/SE in our meta-analysis exceeds the MCID for stroke prevention, so anticoagulation is worthwhile in people with TAF.
Anticoagulation does not appear to increase bleeding risk in TAF. Chan et al. (20) reported lower HRs for major bleeding in people with TAF treated with DOACs compared to those on warfarin. However, this benefit was not seen in the subgroup analysis of the RCT by Goldstein et al. (14).
Overall completeness and applicability of evidence
Subjects from the included studies were seen in specialist and general practice clinics. Thus, the results of this study are generalizable to tertiary and general healthcare settings. Participants were recruited from European, North American, South American, and Asian Pacific countries so the results can be generalized to people of most ethnicities. The results of this study are not applicable to people below 18 years old.
The duration of AF was not stated in the studies included in this meta-analysis so there was no data to guide the duration of anticoagulation in TAF, especially in patients without cardiac structural abnormalities or intra-cardiac clots. Staffurth et al. reported embolic events accounting for 11.5% of events after patients reverted to sinus rhythm and 42.3% of events occurred after patients became euthyroid (25). There was insufficient data to compare anticoagulation vs antiplatelet therapy, or antiplatelet vs no antiplatelet therapy in people with TAF. There was no data for subgroup analysis of anticoagulation in people with renal impairment.
Agreement and disagreement with other studies or systematic review
Our results are consistent with the meta-analysis by Joundi et al. (34) who evaluated the efficacy of IS prevention using anticoagulation in people with AF and CHA2DS2-VASc scores above 1. They found that DOACs may be beneficial in these patients. Our results are consistent with the network meta-analysis by Hirschl et al. who reported lower major bleeding and GIB risks with DOACs (35).
Strengths of this study
This is the first systematic review on the efficacy and safety of anticoagulation in people with TAF. An extensive search of multiple databases and systematic review registers was performed and the risks of errors in study selection and data extraction are reduced as article screening and data extraction were conducted independently.
We minimized the impact of reporting bias by identifying and including unpublished data of relevant outcomes. We searched the Cochrane prospective trial register and reference lists of published studies and review articles for potential data. We asked experts regarding unpublished data that are relevant to our study.
Weaknesses of this study
The meta-analysis included several small studies that were at high ROB. Random-effects analysis gives more weight to smaller studies compared to fixed-effects analysis, so the smaller studies with serious ROB (6, 12) may introduce error in the effect estimate. Results are imprecise because the CIs of most studies were wide and included clinically important and unimportant effects. Total number of outcomes in both groups was small. Reporting bias is very likely to be present. Consequently, the evidence derived from our analysis is of very low certainty.
Conclusions
Implications for practice
Anticoagulation prevents IS/SE in people with TAF whose CHA2DS2-VASc score are 1 or more and when AF persists beyond 7 days. It is uncertain whether patients below age 18 years will benefit from anticoagulation. DOACs may be safer in patients with high bleeding risks.
Implications for research
There is a paucity of research in the efficacy and safety of anticoagulation and antiplatelet therapy in preventing stroke and thromboembolism in people with TAF and RCTs should focus on this population. Research should evaluate the optimal duration of treatment in these people.
Supplementary materials
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data
The datasets generated and/or analyzed during the current study are available in the Zenodo repository https://doi.org/10.5281/zenodo.4855376.
Author contribution statement
E L T designed the study, devised the search strategies, conducted literature searches, conducted analysis, and wrote the review. Y S T and A T A performed screening of titles, abstracts, and full-texts. N C Y Y and Z W extracted data and performed risk-of-bias assessment. All authors have reviewed and revised the manuscript for its intellectual and technical content.
References
- 1.Auer J, Scheibner P, Mische T, Langsteger W, Eber O, Eber B. Subclinical hyperthyroidism as a risk factor for atrial fibrillation. American Heart Journal 2001142838–842. ( 10.1067/mhj.2001.119370) [DOI] [PubMed] [Google Scholar]
- 2.Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Archives of Internal Medicine 20041641675–1678. ( 10.1001/archinte.164.15.1675) [DOI] [PubMed] [Google Scholar]
- 3.Osman F, Franklyn JA, Holder RL, Sheppard MC, Gammage MD. Cardiovascular manifestations of hyperthyroidism before and after antithyroid therapy: a matched case-control study. Journal of the American College of Cardiology 20074971–81. ( 10.1016/j.jacc.2006.08.042) [DOI] [PubMed] [Google Scholar]
- 4.Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson PW, Benjamin EJ, D’Agostino RB. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. New England Journal of Medicine 19943311249–1252. ( 10.1056/NEJM199411103311901) [DOI] [PubMed] [Google Scholar]
- 5.Shimizu T, Koide S, Noh JY, Sugino K, Ito K, Nakazawa H. Hyperthyroidism and the management of atrial fibrillation. Thyroid 200212489–493. ( 10.1089/105072502760143863) [DOI] [PubMed] [Google Scholar]
- 6.Wong CL, Tam HV, Fok CV, Lam PE, Fung LM. Thyrotoxic atrial fibrillation: factors associated with persistence and risk of ischemic stroke. Journal of Thyroid Research 201720174259183. ( 10.1155/2017/4259183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q, Yan Y, Zhang L, Cheng K, Liu Y, Zhu W. Effect of hyperthyroidism on the hypercoagulable state and thromboembolic events in patients with atrial fibrillation. Cardiology 2014127176–182. ( 10.1159/000356954) [DOI] [PubMed] [Google Scholar]
- 8.Stuijver DJ, van Zaane B, Romualdi E, Brandjes DP, Gerdes VE, Squizzato A. The effect of hyperthyroidism on procoagulant, anticoagulant and fibrinolytic factors: a systematic review and meta-analysis. Thrombosis and Haemostasis 20121081077–1088. ( 10.1160/TH12-07-0496) [DOI] [PubMed] [Google Scholar]
- 9.Franchini M, Montagnana M, Manzato F, Vescovi PP. Thyroid dysfunction and hemostasis: an issue still unresolved. Seminars in Thrombosis and Hemostasis 200935288–294. ( 10.1055/s-0029-1222607) [DOI] [PubMed] [Google Scholar]
- 10.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks Jet al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European Heart Journal 2016372893–2962. ( 10.1093/eurheartj/ehw210) [DOI] [PubMed] [Google Scholar]
- 11.Poulin MF, Doukky R. Hyperthyroid atrial fibrillation: does it matter for stroke risk? Cardiology 201412851–53. ( 10.1159/000357613) [DOI] [PubMed] [Google Scholar]
- 12.Chan PH, Hai J, Yeung CY, Lip GY, Lam KS, Tse HF, Siu CW. Benefit of anticoagulation therapy in hyperthyroidism-related atrial fibrillation. Clinical Cardiology 201538476–482. ( 10.1002/clc.22427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siu CW, Pong V, Zhang X, Chan YH, Jim MH, Liu S, Yiu KH, Kung AW, Lau CP, Tse HF. Risk of ischemic stroke after new-onset atrial fibrillation in patients with hyperthyroidism. Heart Rhythm 20096169–173. ( 10.1016/j.hrthm.2008.10.023) [DOI] [PubMed] [Google Scholar]
- 14.Goldstein SA, Green J, Huber K, Wojdyla DM, Lopes RD, Alexander JH, Vinereanu D, Wallentin L, Granger CB, Al-Khatib SM. Characteristics and outcomes of atrial fibrillation in patients with thyroid disease (from the Aristotle Trial). American Journal of Cardiology 20191241406–1412. ( 10.1016/j.amjcard.2019.07.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gundlund A, Kümler T, Bonde AN, Butt JH, Gislason GH, Torp-Pedersen C, Køber L, Olesen JB, Fosbøl EL. Comparative thromboembolic risk in atrial fibrillation with and without a secondary precipitant-Danish nationwide cohort study. BMJ Open 20199 e028468. ( 10.1136/bmjopen-2018-028468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SMet al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019366 l4898. ( 10.1136/bmj.l4898) [DOI] [PubMed] [Google Scholar]
- 17.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron Iet al. Robins-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016355 i4919. ( 10.1136/bmj.i4919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods 20211255–61. ( 10.1002/jrsm.1411) [DOI] [PubMed] [Google Scholar]
- 19.The Cochrane Collaboration (ed.). Review Manager (RevMan) [Computer Program]. Version 5.4. The Cochrane Collaboration, 2020. [Google Scholar]
- 20.Chan YH, Wu LS, See LC, Liu JR, Chang SH, Chao TF, Yeh YH, Kuo CT, Lee HF, Lip GYH. Direct oral anticoagulants in atrial fibrillation patients with concomitant hyperthyroidism. Journal of Clinical Endocrinology and Metabolism 2020105dgaa050. ( 10.1210/clinem/dgaa050) [DOI] [PubMed] [Google Scholar]
- 21.Chen ZC, Wu NC, Chang CL, Ho CH, Liao CT, Chiang CY, Chang WT. Risk of ischaemic stroke in thyrotoxic atrial fibrillation. Clinical Endocrinology 201991561–570. ( 10.1111/cen.14061) [DOI] [PubMed] [Google Scholar]
- 22.Fauchier L, Clementy N, Bisson A, Stamboul K, Ivanes F, Angoulvant D, Babuty D, Lip GY. Prognosis in patients with atrial fibrillation and a presumed ‘temporary cause’ in a community-based cohort study. Clinical Research in Cardiology 2017106202–210. ( 10.1007/s00392-016-1040-7) [DOI] [PubMed] [Google Scholar]
- 23.Bar-Sela S, Ehrenfeld M, Eliakim M. Arterial embolism in thyrotoxicosis with atrial fibrillation. Archives of Internal Medicine 19811411191–1192. ( 10.1001/archinte.1981.00340090087019) [DOI] [PubMed] [Google Scholar]
- 24.Petersen P, Hansen JM. Stroke in thyrotoxicosis with atrial fibrillation. Stroke 19881915–18. ( 10.1161/01.str.19.1.15) [DOI] [PubMed] [Google Scholar]
- 25.Staffurth JS, Gibberd MC, Fui SN. Arterial embolism in thyrotoxicosis with atrial fibrillation. BMJ 19772688–690. ( 10.1136/bmj.2.6088.688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuen RW, Gutteridge DH, Thompson PL, Robinson JS. Embolism in thyrotoxic atrial fibrillation. Medical Journal of Australia 19791630–631. ( 10.5694/j.1326-5377.1979.tb119428.x) [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, Zhang R, Chakraborty P, Farooq F & Alford SH. Comparative effectiveness of edoxaban and warfarin in prevention of stroke and systemic embolism in non-valvular atrial fibrillation using observational healthcare data. Value in Health 201821(Supplement 1)S56. [Google Scholar]
- 28.Ruiz Ortiz M, Esteve-Pastor MA, Rana Miguez P, Marin F, Martinez-Selles M, Roldan I, Muniz J, Cequier A, Bertomeu V, Anguita M.Effectiveness and safety of direct anticoagulants versus vitamin K antagonists in octogenarians patients with atrial fibrillation in a ‘real world’ nationwide registry. European Heart Journal 2018391309. ( 10.1093/eurheartj/ehy566.P6284) [DOI] [Google Scholar]
- 29.Gulløv AL, Koefoed BG, Petersen P. Bleeding during warfarin and aspirin therapy in patients with atrial fibrillation: the AFASAK 2 study. Atrial fibrillation aspirin and anticoagulation. Archives of Internal Medicine 19991591322–1328. ( 10.1001/archinte.159.12.1322) [DOI] [PubMed] [Google Scholar]
- 30.Ridha E, Lacoin L, Lefevre C, Donaldson R, Ramagopalan S, Halcox J.Aspirin, not without bleeding risk in the real world: results of a UK cohort study evaluating the use of antiplatelet therapy for stroke prevention in atrial fibrillation (AF). European Heart Journal 201637510–519.26726043 [Google Scholar]
- 31.Rutherford OCW, Jonasson CJ, Ghanima WG, Halvorsen SH.Effectiveness and safety of apixaban, dabigatran and warfarin compared to rivaroxaban in non-valvular atrial fibrillation; a Norwegian Nationwide Cohort Study. European Heart Journal 201940 1846. ( 10.1371/journal.pone.0221500) [DOI] [Google Scholar]
- 32.Man-Son-Hing M, Laupacis A, O’Connor A, Wells G, Lemelin J, Wood W, Dermer M. Warfarin for atrial fibrillation. The patient’s perspective. Archives of Internal Medicine 19961561841–1848. ( 10.1001/archinte.1996.00440150095011) [DOI] [PubMed] [Google Scholar]
- 33.Howitt A, Armstrong D. Implementing evidence based medicine in general practice: audit and qualitative study of antithrombotic treatment for atrial fibrillation. BMJ 19993181324–1327. ( 10.1136/bmj.318.7194.1324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joundi RA, Cipriano LE, Sposato LA, Saposnik G. & Stroke Outcomes Research Working Group. Ischemic stroke risk in patients with atrial fibrillation and CHA2DS2-VASc score of 1: systematic review and meta-analysis. Stroke 2016471364–1367. ( 10.1161/STROKEAHA.115.012609) [DOI] [PubMed] [Google Scholar]
- 35.Hirschl M, Kundi M. Safety and efficacy of direct acting oral anticoagulants and vitamin K antagonists in nonvalvular atrial fibrillation – a network meta-analysis of real-world data. Vasa: Zeitschrift Fur Gefasskrankheiten 201948134–147. ( 10.1024/0301-1526/a000746) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the Zenodo repository https://doi.org/10.5281/zenodo.4855376.



 This work is licensed under a
This work is licensed under a