Abstract
Background:
Over the past decade, minimally invasive (MI) mitral valve surgery has grown in popularity. The purpose of this study was to compare both short and long-term outcomes of mitral valve repair and replacement performed through a MI versus traditional sternotomy (ST) incision using a propensity analysis approach to account for differences in baseline risk.
Methods:
From January 2000 – December 2008, a total of 1121 isolated mitral valve operations were performed at our institution (548 ST, 573 MI). Data were retrospectively collected on all patients and a logistic regression model created to predict selection to a MI versus ST approach. Propensity scores were then generated based on the regression model and matched pairs created using 1:1 nearest neighbor matching. There were 382 matched pairs in the analysis for a total sample size of 764, or 68.2% of the original cohort. Major outcomes of interest included: cardiopulmonary bypass time (CPB), cross clamp time (XCT), hospital length of stay (LOS), major in-hospital complications, and both short- and long-term survival.
Results:
CPB was 117.1 ± 2.0 minutes in the ST and 139.7 ± 2.6 minutes in the MI group (p<0.0001), and XCT was 79.6 ± 1.5 minutes in the ST and 83.7 ± 1.9 in the MI group (p=0.106). The average LOS was 9.81 ± 0.61 days among ST and 7.76 ± 0.37 days among MI patients (p=0.0043). There was no significant difference in the frequency of major in-hospital complications between groups. The mean duration of survival follow-up was 4.2 ± 2.4 years. There was no significant difference in mortality at 30 days (p=0.622) or 1 year (p=0.599). In addition, there was no significant difference in long-term survival between groups (p=0.569).
Conclusions:
Although minimally invasive mitral valve surgery required a slightly longer CPB, there was no difference in XCT, morbidity, or mortality, and LOS was significantly shorter when compared to matched sternotomy controls.
Keywords: mitral valve, minimally invasive surgery, outcomes
INTRODUCTION
The field of minimally invasive cardiothoracic surgery (MICS) continues to grow in popularity due to improvements in technology, surgical technique, and a growing acceptance of minimally invasive approaches for operations previously performed through a traditional median sternotomy [1–4]. While minimally invasive approaches have been integrated into many areas of cardiac surgery, minimally invasive mitral valve surgery has been particularly influenced by minimally invasive techniques [5–6].
Beginning in the early 1990s, a variety of minimally invasive incision types have been developed for mitral valve surgery including partial sternotomy, parasternal incisions, mini-thoracotomy, and totally endoscopic approaches [7–10]. While the technical aspects of each approach differ, the overall goals are similar – development of a safe and effective mitral valve repair or replacement with minimal surgical trauma. The proposed benefits of a minimally invasive approach include decreased post-operative pain, improved cosmesis and patient satisfaction, improved post-operative recovery, decreased hospital length of stay, decreased resource utilization, and ultimately faster return to normal activities [11–15].
While several single-institution studies have shown that these described benefits can be achieved with low perioperative morbidity and short-term mortality [16–20], no prospective, randomized clinical trials exist that compare minimally invasive mitral valve surgery to the traditional sternotomy approach. Moreover, significant differences in baseline characteristics between groups create a challenge for comparing outcomes. Thus the goal of this study was to compare minimally invasive and sternotomy approaches using a propensity matched approach to control for differences in baseline risk.
MATERIAL AND METHODS
Study population
From January 1, 2000 to December 31, 2008, a total of 1121 patients underwent isolated mitral valve surgery at our institution. Isolated mitral valve surgery was defined as mitral valve repair or replacement in the absence of a major concomitant procedure such as coronary artery bypass grafting or aortic valve surgery. There were 294 patients (26.2%) who underwent minor concomitant procedures including: atrial fibrillation ablation (n=198, 17.7%), atrial septal defect repair (n=75, 6.7%), and tricuspid valve repair (n=21, 1.9%). There were 438 mitral valve replacements (39.1%) and 683 mitral valve repairs (60.9%) in the cohort. A traditional median sternotomy approach was used in 548 (48.9%) patients and a minimally invasive approach was used in 573 (51.1%) patients. After obtaining Institutional Review Board approval, data on patient demographics, operative parameters, and both short- and long-term morbidity and mortality were retrospectively gathered using data combined from the New York State Cardiac Surgery Database [21] and institutional medical records and operative reports that comprise our internal cardiac surgery registry.
Operative Technique
For the series, minimally invasive mitral valve surgery was defined as any mitral valve repair or replacement performed through an incision other than a full median sternotomy. Of the minimally invasive cases, 569 (99.3%) were performed through a right mini-thoracotomy [Figure 1] and 4 (0.7%) were performed through a hemi-sternotomy. The operative procedure for cases performed through a mini-thoracotomy is presented here. Briefly, after induction of general anesthesia, the endotracheal tube is replaced by a double lumen tube. The patient is then placed in a left lateral decubitus position and a 6- to 8-cm skin incision is made in the right chest along the fifth rib, lateral to the midclavicular line. After establishment of single (left) lung ventilation, the chest is entered in the fourth intercostal space. A small chest retractor is then inserted and the pericardium entered. After ACT-guided heparinization, aortic and venous cannulation is carried out. For the series, aortic cannulation was most commonly performed in a central fashion and venous drainage was most commonly achieved through a percutaneous femoral vein approach with a single multistage venous cannulation. Central aortic cannulation is performed through the initial minimally invasive incision. After placing the patient on cardiopulmonary bypass, antegrade and retrograde cardioplegia catheters are placed and Sondergaard’s groove is dissected. The retrograde cardioplegia is placed directly by the surgeon through the right atrium. The patient’s pressure is temporarily reduced to 50 mmHg and a transthoracic aortic cross clamp (Chitwood) is passed through a stab wound in the right axilla, and applied to the ascending aorta. Cold blood (4:1) cardioplegia is then given through an antegrade cardioplegia catheter, and repeated every 20 minutes via antegrade and/or retrograde catheters. The left atrium is opened, and a transthoracic left atrial retractor positioned. The mitral valve is then inspected, and repair or replacement carried out. The left atriotomy is closed and the patient de-aired and the left atrium closed in the standard fashion.
Figure 1:
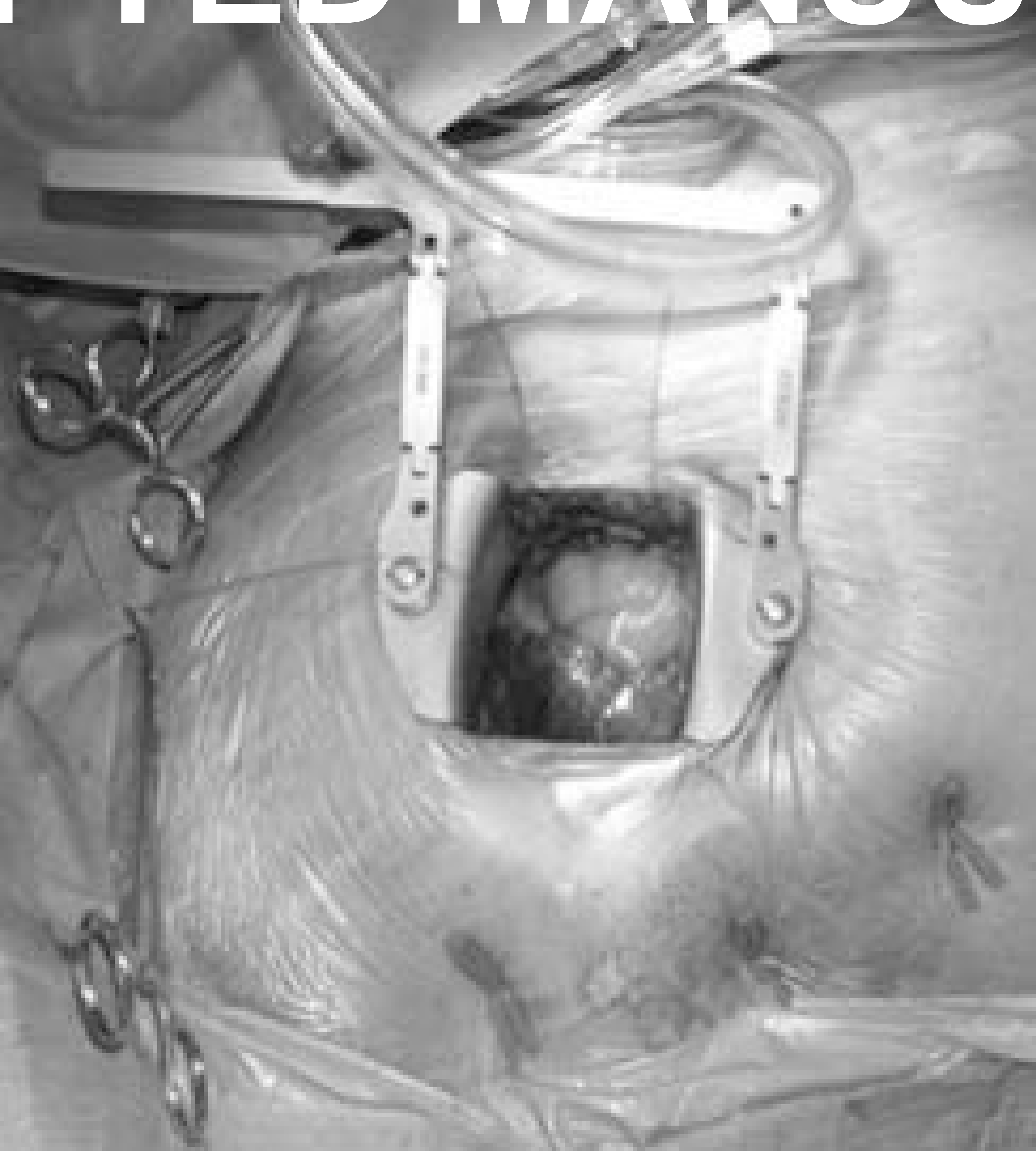
Right mini-thoracotomy approach for mitral valve surgery
Propensity analysis
To account for differences in baseline characteristics between groups, a propensity analysis approach was used for data analysis. A parsimonious model of risk factors for selection to a minimally invasive versus sternotomy approach was created using baseline patient characteristics (Appendix 1). A stepwise logistic regression approach (backward, remove p>0.20) was used for variable selection to create the final model (Appendix 2). From this model, propensity scores were generated for each patient. Propensity scores were then nearest neighbor matched 1:1 to create sternotomy and minimally invasive matched pairs for analysis.
Outcome measures
Major outcomes of interest included: cardiopulmonary bypass time (CPB), cross clamp time (XCT), hospital length of stay (LOS), major in-hospital complications, and both short- and long-term survival. Major complications included: intubation > 72 hours, renal failure, sepsis, re-operation for bleeding, stroke < 24 hours after surgery, stroke ≥ 24 hours after surgery, gastrointestinal bleeding, and transmural myocardial infarction. Multivariable logistic regression (backward stepwise, remove p>0.20) was used to assess the simultaneous effect of multiple variables (Appendix 1) on in-hospital complications.
Long-term survival rates were calculated using the Kaplan–Meier method, and statistical significance was calculated by the log-rank test. Multivariable Cox proportional hazards regression (backward stepwise, remove p>0.20) was used to assess the simultaneous effect of multiple variables on survival (in addition to variables in Appendix 1, the major complications described above were also included in the multivariate model). Survival data was obtained from the New York State Cardiac Surgery Database and supplemented with data from the Social Security Death Index [22]. Follow-up survival data was provided through December 14, 2009. Continuous variables were reported as mean ± standard deviation and were compared using the Student’s t test or Wilcoxon-rank sum test when noted. Categorical variables were reported as percentages and compared using the chi-squared or Fisher’s exact test when appropriate. For all analyses, the conventional p-value of 0.05 or less was used to determine level of statistical significance. All reported p-values are two-sided. All data were analyzed using the statistical software package, Stata 10 (Stata Corp, College Station, TX).
RESULTS
Study population
A total of 1121 patients underwent isolated mitral valve surgery between January 1, 2000 and December 31, 2008. Baseline characteristics of the original study cohort are shown in Table 1. There were statistically significant differences among most major baseline characteristics between minimally invasive (MI) and sternotomy (ST) groups.
Table 1:
Baseline characteristics of original study population
| ST (n=548) |
MI (n=573) |
p-value | |
|---|---|---|---|
|
| |||
| Age | 61.6 ± 0.67 | 57.8 ± 0.58 | <0.0001 |
| BMI | 26.5 ± 0.25 | 25.2 ± 0.17 | <0.0001 |
| Cerebrovascular accident | 49 (8.9%) | 23 (4.0%) | 0.001 |
| COPD | 45 (8.2%) | 26 (4.5%) | 0.012 |
| Creatinine | 1.24 ± 0.06 | 0.98 ± 0.02 | <0.0001 |
| Current congestive heart failure | 162 (29.6%) | 88 (15.4%) | <0.001 |
| Current smoker | 21 (3.83%) | 22 (3.84%) | 0.995 |
| Diabetes | 54 (9.9%) | 32 (5.6%) | 0.007 |
| Ejection fraction % | 48.3 ± 0.55 | 52.0 ± 0.40 | <0.0001 |
| Gender (male) | 256 (46.7%) | 309 (53.9%) | 0.016 |
| Hepatic failure | 2 (0.36%) | 1 (0.17%) | 0.646 |
| History of endocarditis | 6 (1.09%) | 16 (2.79%) | 0.051 |
| History of ventricular arrhythmias | 5 (0.91%) | 4 (0.70%) | 0.748 |
| Immune system deficiency | 30 (5.5%) | 12 (2.1%) | 0.003 |
| Intra-aortic balloon pump | 6 (1.09%) | 0 | 0.013 |
| Peripheral vascular disease | 11 (2.01%) | 4 (0.70%) | 0.070 |
| Previous myocardial infarction | 7 (1.28%) | 2 (0.35%) | 0.101 |
| Previous surgery | 145 (26.5%) | 34 (5.9%) | <0.001 |
| Renal failure or dialysis | 26 (4.7%) | 2 (0.35%) | <0.001 |
| Vasodilatory shock (pre-operative) | 10 (1.82%) | 1 (0.17%) | 0.005 |
MI: minimally invasive; ST: sternotomy
Propensity analysis
Twenty baseline variables (Appendix 1) were used to create a parsimonious logistic regression model (Appendix 2) for selection to an MI versus ST approach. The area under the receiver operating characteristic (ROC) curve for the model was 0.75 ± 0.015, demonstrating good model discrimination. After nearest neighbor matching, a total of 382 matched pairs were generated for a total sample size of 764 patients or 68.2% of the original cohort. Matched pairs were created across a range of propensity scores (Figure 2). Once matched, there were no longer significant differences among major baseline characteristics between groups (Table 2).
Figure 2:
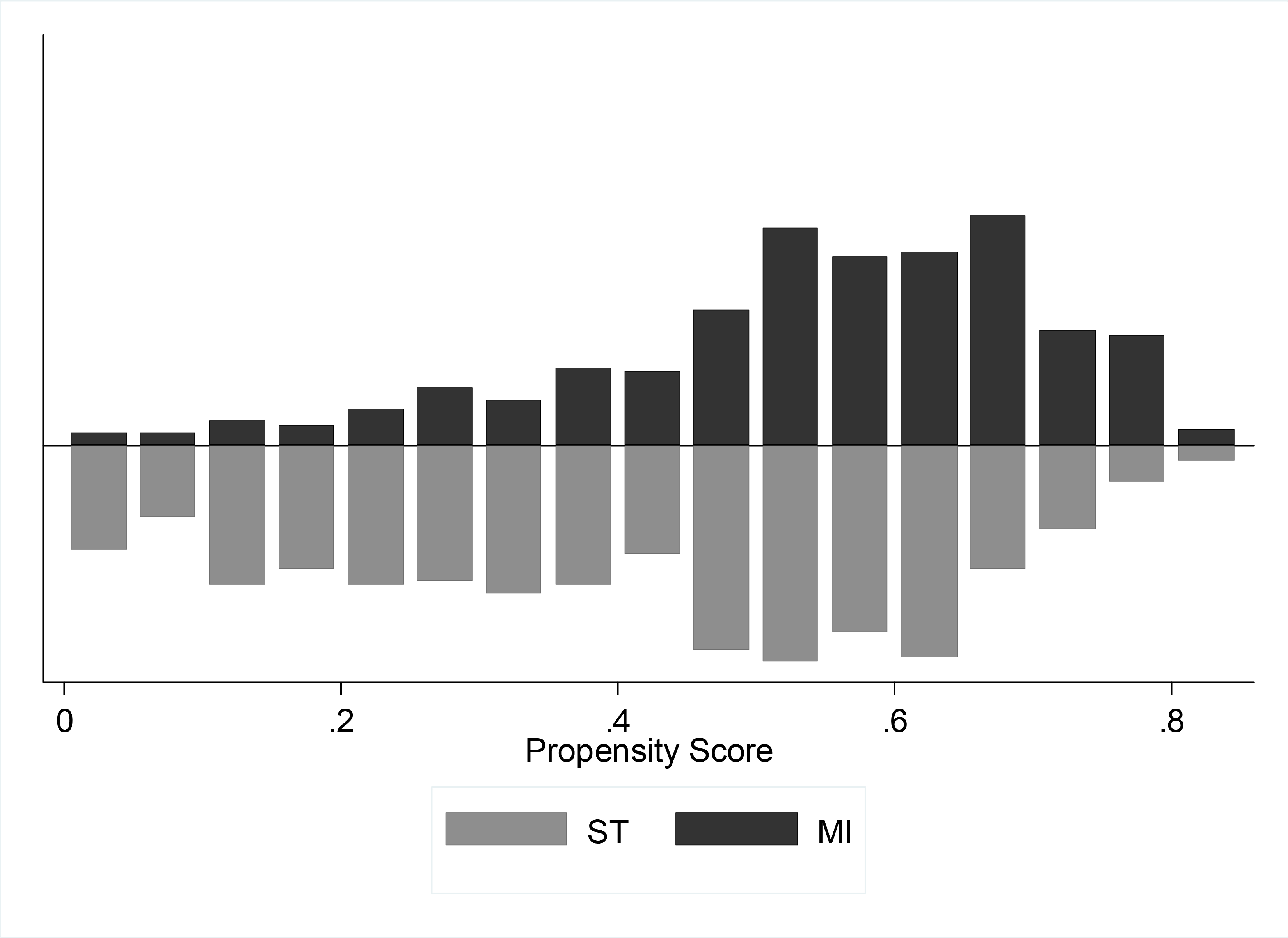
Histogram of propensity score distribution by matched pairs
Table 2:
Baseline characteristics of matched pairs
| ST (n=382) |
MI (n=382) |
p-value | |
|---|---|---|---|
|
| |||
| Age | 60.7 ± 0.81 | 59.1 ± 0.71 | 0.140 |
| BMI | 25.9 ± 0.27 | 25.5 ± 0.22’ | 0.264 |
| Cerebrovascular accident | 27 (7.1%) | 21 (5.5%) | 0.371 |
| COPD | 28 (7.3%) | 22 (5.8%) | 0.380 |
| Creatinine | 1.05 ± 0.03 | 0.99 ± 0.03 | 0.092 |
| Current congestive heart failure | 88 (23.0%) | 79 (20.7%) | 0.431 |
| Current smoker | 15 (3.93%) | 13 (3.40%) | 0.848 |
| Diabetes | 25 (6.5%) | 26 (6.8%) | 0.885 |
| Ejection fraction % | 49.8 ± 0.62 | 51.3 ± 0.50 | 0.070 |
| Gender (male) | 177 (46.3%) | 184 (48.2%) | 0.612 |
| Hepatic failure | 1 (0.26%) | 1 (0.26%) | 1.0 |
| History of endocarditis | 5 (1.31%) | 10 (2.62%) | 0.297 |
| History of ventricular arrhythmias | 4 (1.05%) | 3 (0.79%) | 1.0 |
| Immune system deficiency | 14 (3.7%) | 12 (3.1%) | 0.690 |
| Intra-aortic balloon pump | 5 (1.31%) | 0 | 0.062 |
| Peripheral vascular disease | 2 (0.52%) | 3 (0.79%) | 1.0 |
| Previous myocardial infarction | 2 (0.52%) | 2 (0.52%) | 1.0 |
| Previous surgery | 31 (8.1%) | 28 (7.3%) | 0.684 |
| Renal failure or dialysis | 8 (2.1%) | 2 (0.52%) | 0.107 |
| Vasodilatory shock (pre-operative) | 6 (1.57%) | 1 (0.26%) | 0.123 |
MI: minimally invasive; ST: sternotomy
Operative outcomes
The distributions of incision and cannulation configurations for MI patients are shown on Table 3. All but four of the MI cases were performed through a mini-thoracotomy. The aorta was most commonly cannulated in a central fashion (n=342, 89.5%) and venous drainage was most commonly achieved through a percutaneous femoral drainage of the superior and inferior vena cavae (n=212, 55.5%). Operative outcomes are shown on Table 4. Although there was an even distribution of repairs (n=195, 51.1%) and replacements (n=187, 49.0%) among ST patients, the majority of MI patients (n=287, 75.1%) underwent a mitral valve repair (p<0.001). Among the three concomitant procedures in the series, atrial septal defect repairs and atrial fibrillation ablation procedures were evenly distributed between groups, however, there were significantly more tricuspid valve procedures among MI patients (p<0.001). Regarding operative times, the cardiopulmonary bypass time was longer among MI patients by 22.5 ± 3.2 minutes (p<0.001), however, the cross clamp time did not differ between groups (p=0.106). Among the MI group, there were no conversions to the median sternotomy approach.
Table 3:
Incision and cannulation configurations for MI patients.
| MI (n=382) | |
|---|---|
|
| |
| Incision location | |
| Mini-thoracotomy | 379 (99.2%) |
| Hemisterotomy | 3 (0.79%) |
| Aortic cannulation | |
| Central | 342 (89.5%) |
| Femoral | 37 (9.7%) |
| Axillary | 3 (0.79%) |
| Venous drainage | |
| pSVC, pIVC | 212 (55.5%) |
| dSVC, pIVC | 127 (33.2%) |
| dSVC, dIVC | 30 (7.9%) |
| pSVC, dIVC | 8 (2.1%) |
| dRA | 5 (1.3%) |
d = direct, IVC = inferior vena cava, MI = minimally invasive, p = percutaneous, RA = right atrium, SVC = superior vena cava
Table 4:
Operative outcomes of matched pairs
| ST (n=382) |
MI (n=382) |
p-value | |
|---|---|---|---|
|
| |||
| Mitral valve surgery type | |||
| Replacement | 187 (49.0%) | 95 (24.9%) | <0.001 |
| Repair | 195 (51.1%) | 287 (75.1%) | |
| Concomitant procedures | |||
| Atrial septal defect repair | 24 (6.3%) | 32 (8.4%) | <0.001 |
| Atrial fibrillation ablation | 74 (19.4%) | 67 (17.5%) | |
| Tricuspid valve repair | 0 | 16 (4.5%) | |
| Operative times (minutes) | |||
| Cardiopulmonary bypass time | 117.1 ± 2.0 | 139.7 ± 2.6 | <0.0001 |
| Cross clamp time | 79.6 ± 1.5 | 83.7 ± 1.9 | 0.106 |
MI: minimally invasive; ST: sternotomy
Clinical outcomes
There was a significant difference in mean length of stay by incision type. The MI group had a shorter overall hospital stay by 2.05 ± 0.72 days when compared to the ST group (MI=7.76 ± 0.37 days; ST=9.81 ± 0.61 days; p=0.0043). The median lengths of stay for ST and MI groups were 7 and 6 days, respectively which was statistically significant by Wilcoxon rank-sum test (p<0.0001).
In-hospital complications are summarized in Table 5. Although the observed proportion of patients requiring intubation > 72 hours was less among MI patients, the trend was not statistically significant (p=0.093). Overall, no significant differences existed among major in-hospital complications between groups. There were 3 (0.79%) sternal wound infections among patients in the ST group. There was no significant difference in survival at 30-days (p=0.622) or 1-year (p=0.599) between groups. In multivariable logistic regression, risk factors significant for in-hospital complications among MI patients included only CHF (OR = 2.02 [1.13–3.59], p=0.017).
Table 5:
In-hospital complications and short-term survival
| ST (n=382) |
MI (n=382) |
p-value | |
|---|---|---|---|
|
| |||
| Complications | |||
| Gastrointestinal bleed | 3 (0.79%) | 2 (0.52%) | 1.0 |
| Intubation > 72 hours | 29 (7.6%) | 17 (4.5%) | 0.093 |
| Renal failure | 7 (1.8%) | 5 (1.3%) | 0.773 |
| Re-operation for bleeding | 11 (2.9%) | 1 4(3.7%) | 0.685 |
| Sepsis | 7 (1.8%) | 4 (1.1%) | 0.546 |
| Stroke (< 24 hours) | 5 (1.3%) | 1 (0.26%) | 0.217 |
| Stroke (≥ 24 hours) | 5 (1.3%) | 3 (0.79%) | 0.725 |
| Transmural myocardial infarction | 1 (0.26%) | 0 | 1.0 |
| Mortality | |||
| 30-day mortality | 7 (1.8%) | 7 (1.8%) | 0.622 |
| 1-year mortality | 19 (5.0%) | 15 (3.9%) | 0.599 |
MI: minimally invasive; ST: sternotomy
The mean duration of survival follow-up was 4.2 ± 2.4 years. There was no significant difference in long-term survival between groups in Kaplan-Meier analysis (p=0.569) (Figure 3). In multivariable Cox proportional hazards regression, risk factors significant for mortality among MI patients included: age (HR = 1.04 [1.01–1.08], p=0.017), post-operative stroke (HR = 4.46 [1.23–16.1], p<0.001), and post-operative renal failure (HR = 5.56 [1.52–19.6], p=0.01).
Figure 3:
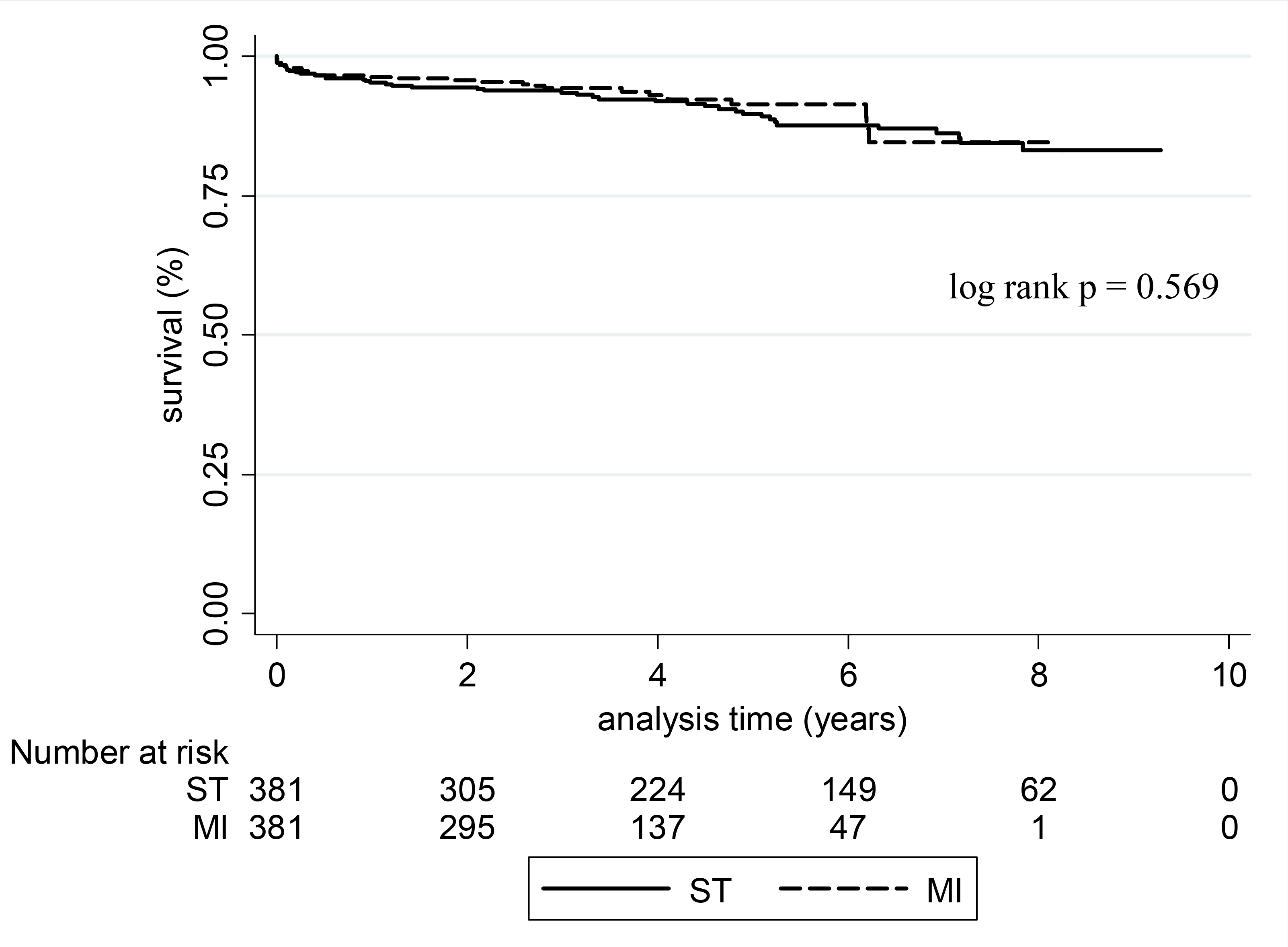
Kaplan-Meier analysis of long-term survival by incision type
COMMENT
Over the past decade, the field of minimally invasive cardiothoracic surgery has seen rapid growth and an increasing number of investigators have described positive outcomes with minimally invasive approaches. Mitral valve surgery has been particularly amenable to minimal access approaches, and the described benefits in the literature include improved patient satisfaction, improved cosmesis, decreased length of hospitalization, and decreased overall resource utilization. Unfortunately, previous studies have been limited either by descriptions of minimally invasive outcomes with no reference group for comparison, or a sternotomy reference group with significant differences in baseline risk. To address these challenges, we use a propensity matched comparison technique to simulate outcomes after pseudo-randomization of patients to a sternotomy versus MI approach for mitral valve surgery.
As previous studies have shown, baseline characteristics of sternotomy patients differed significantly from MI patients, with sternotomy patients having increased peri-operative risk. As our institutional experience has grown with minimally invasive surgery, we have offered the approach to increasingly higher risk patients. At our institution, the decision to pursue a minimally invasive approach is not surgeon-specific, but rather involves an overall assessment of the feasibility and safety of such an approach based on the patient’s pre-operative risk. As such, among unmatched pairs, the majority of re-operative cases were performed through a sternotomy approach and, on average, minimally invasive patients had a lower BMI than sternotomy patients. Despite the significant baseline differences in the original study cohort, the propensity matching in our analysis was particularly strong with matched pairs generated across a range of propensity scores, accounting for nearly 70% of the original study cohort, and having no statistically significant differences in baseline risk.
In adopting a MI approach for mitral valve surgery, there was early concern in the literature about the potential trade-off of limited exposure in minimally access surgery versus safety and operative times using a traditional sternotomy approach [23]. While cardiopulmonary bypass times were slightly longer in our analysis, there was no significant increase in cross clamp times among the MI group. In addition, there was no significant difference in major perioperative complications between groups and no conversions to median sternotomy among the MI patients. Importantly, stroke rates were particularly low among our MI patients which may be a reflection of our preference to cannulate the aorta centrally and use a transthoracic clamp rather than endo-aortic balloon occlusion. This approach also avoids potential groin complications associated with femoral access [24–25].
With regard to survival, there was no difference in 30-day or 1-year mortality between groups, and both MI and sternotomy patients had excellent short-term survival. Long-term survival was also similar between groups, with both groups achieving survival rates above 85% at 4 years after surgery. Thus, in our series, a minimal access approach for mitral valve surgery does not appear to compromise morbidity or mortality when compared to matched sternotomy controls.
In addition to providing equivalent safety to a conventional sternotomy approach, MI surgery has the added potential benefit of reduction in overall length of hospitalization. In our analysis, MI patients had, on average, a 2 day shorter length of stay, and when median length of stay was analyzed there was still a significant difference between groups. One of the drivers of the decreased length of stay among MI patients may be improved inpatient functional status. At our institution, we have observed that MI patients appear to achieve major physical therapy milestones at shorter time intervals than sternotomy patients. Moreover, the improved length of stay may be the result of less post-operative pain and improved post-operative respiratory function [26–27]. Additional studies have demonstrated that a MI approach is associated with decreased procedural pain, faster return to work, and overall improved patient satisfaction [28]. In our analysis, there was a trend towards a decreased proportion of MI patients intubated > 72 hours, although the results did not achieve statistical significance. It should be noted that the greatest advantages of the MI approach, with respect to functional status, may occur after the patient has left the hospital. Although the exact cause of improved length of stay is not entirely evident from our study, in an era with growing national attention to comparative effectiveness of both medical and surgical treatments and as endpoints in clinical trials [29–31], a MI approach that results in decreased length of hospitalization would likely translate into decreased overall resource utilization.
Limitations
There are several limitations to our analysis. First, in this analysis we do not include data on mitral valve pathology due to limitations of our internal cardiac surgery registry. Inclusion of such data could partially explain why the valve repair rate is different between groups. It is unlikely however, that differences in the distribution of repairs or replacements between groups would have significantly confounded the major clinical efficacy endpoint of this study – length of stay. Second, our analysis is retrospective and subject to multiple potential biases. Selection bias cannot be completely eliminated through a propensity matching approach, however nearly 70% of the original cohort was matched and matched pairs were generated across a range of propensity scores. Third, long-term echocardiographic data was not available due to the retrospective nature of this analysis and the fact that many of our patients receive follow-up echocardiograms at the offices of their local referring physician. Despite this limitation, other reports on large series of MI mitral valve surgery have failed to demonstrate differences in incidence of mitral regurgitation at long-term follow-up when compared to the sternotomy approach [16, 18]. Fourth, the results of this study with regard to parameters such as cross clamp and bypass time do not highlight the potential effect of learning curves for the minimally invasive technique.
Conclusions and Implications
In this series, we demonstrate through propensity matching that a minimally invasive approach for mitral valve surgery is associated with slightly increased cardiopulmonary bypass times but equivalent cross clamp times when compared to a sternotomy approach. Moreover, we show that a minimally invasive approach is associated with equivalent rates of morbidity and mortality with the benefit of decreased hospital length of stay, which likely translates into decreased resource utilization. With advancements in percutaneous valve technology, minimally invasive outcomes should serve as a benchmark when analyzing outcomes such as length of stay, hospital costs, and quality of life in prospective trials. In conclusion, minimally invasive mitral valve surgery represents a safe and effective surgical technique that we feel should be used more routinely in the surgical management of mitral valve disease.
ACKNOWLEDGMENTS
This work was supported in part by NIH Training Grant 5T32HL007854-13 (Dr. Iribarne).
Appendix 1: Univariate analysis of risk factors considered for selection into logistic regression model to predict selection to MI versus ST approach
| Risk factor | Odds Ratio MI: ST | 95% CI | p-value | |
|---|---|---|---|---|
|
| ||||
| Age | 0.982 | 0.974 | 0.990 | <0.001 |
| BMI | 0.951 | 0.928 | 0.975 | <0.001 |
| Cerebrovascular accident | 0.426 | 0.256 | 0.709 | 0.001 |
| COPD | 0.531 | 0.323 | 0.874 | 0.013 |
| Creatinine | 0.511 | 0.368 | 0.709 | <0.001 |
| Current congestive heart failure | 0.432 | 0.323 | 0.579 | <0.001 |
| Current smoker | 1.01 | 0.545 | 1.84 | 0.995 |
| Diabetes | 0.541 | 0.344 | 0.852 | 0.008 |
| Ejection fraction | 1.03 | 1.02 | 1.04 | <0.001 |
| Gender (male) | 0.749 | 0.592 | 0.947 | 0.016 |
| Hepatic failure | 0.477 | 0.043 | 5.28 | 0.546 |
| History of endocarditis | 2.59 | 1.01 | 6.68 | 0.048 |
| History of ventricular arrhythmias | 0.763 | 0.204 | 2.86 | 0.689 |
| Immune system deficiency | 0.369 | 0.187 | 0.729 | 0.004 |
| Intra-aortic balloon pump | 0.094 | 0.012 | 0.737 | 0.024 |
| Peripheral vascular disease | 0.343 | 0.107 | 1.08 | 0.068 |
| Previous myocardial infarction | 0.271 | 0.056 | 1.31 | 0.104 |
| Previous surgery | 0.175 | 0.118 | 0.260 | <0.001 |
| Renal failure or dialysis | 0.070 | 0.017 | 0.298 | <0.001 |
| Vasodilatory shock (pre-operative) | 1.92 | 0.350 | 10.5 | 0.453 |
MI: minimally invasive; ST: sternotomy
Appendix 2: Final regression model used to generate propensity scores
| Risk factor | Odds Ratio MI:ST | 95% CI | p-value | |
|---|---|---|---|---|
|
| ||||
| Age | 0.985 | 0.976 | 0.994 | 0.001 |
| BMI | 0.953 | 0.927 | 0.979 | 0.001 |
| Creatinine | 0.782 | 0.571 | 1.071 | 0.125 |
| Current congestive heart failure | 0.698 | 0.498 | 0.977 | 0.036 |
| Cerebrovascular accident | 0.459 | 0.255 | 0.828 | 0.01 |
| Ejective fraction (%) | 1.023 | 1.015 | 1.040 | <0.001 |
| Gender (male) | 0.665 | 0.502 | 0.880 | 0.004 |
| Immune system deficiency | 0.419 | 0.203 | 0.866 | 0.019 |
| Previous surgery | 0.179 | 0.114 | 0.279 | <0.001 |
| Renal failure | 0.171 | 0.035 | 0.825 | 0.028 |
MI: minimally invasive; ST: sternotomy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Iribarne A, Karpenko A, Russo MJ, et al. Eight-year experience with minimally invasive cardiothoracic surgery. World J Surg. 2009. Oct 17. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Totaro P, Carlini S, Pozzi M, et al. Minimally invasive approach for complex cardiac surgery procedures. Ann Thorac Surg 2009;88(2):462–6. [DOI] [PubMed] [Google Scholar]
- 3.Kypson AP. Recent trends in minimally invasive cardiac surgery. Cardiology 2007;107(3):147–58. [DOI] [PubMed] [Google Scholar]
- 4.Mack MJ. Minimally invasive cardiac surgery. Surg Endosc 2006;20 Suppl 2:S488–92. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin JC. Minimally invasive mitral valve surgery: current status. Curr Surg 2002;59(3):241–5. [DOI] [PubMed] [Google Scholar]
- 6.Greelish JP, Cohn LH, Leacche M, et al. Minimally invasive mitral valve repair suggests earlier operations for mitral valve disease. J Thorac Cardiovasc Surg 2003;126(2):365–71. [DOI] [PubMed] [Google Scholar]
- 7.Vanermen H, Farhat F, Wellens F, et al. Minimally invasive video-assisted mitral valve surgery: from Port-Access towards a totally endoscopic procedure. J Card Surg 2000;15(1):51–60. [DOI] [PubMed] [Google Scholar]
- 8.Tatooles AJ, Pappas PS, Gordon PJ, Slaughter MS. Minimally invasive mitral valve repair using the da Vinci robotic system. Ann Thorac Surg 2004;77(6):1978–82. [DOI] [PubMed] [Google Scholar]
- 9.Mishra YK, Malhotra R, Mehta Y, Sharma KK, Kasliwal RR, Trehan N. Minimally invasive mitral valve surgery through right anterolateral minithoracotomy. Ann Thorac Surg 1999;68(4):1520–4. [DOI] [PubMed] [Google Scholar]
- 10.Karagoz HY, Bayazit K, Battaloglu B, et al. Minimally invasive mitral valve surgery: the subxiphoid approach. Ann Thorac Surg 1999;67(5):1328–32. [DOI] [PubMed] [Google Scholar]
- 11.Modi P, Hassan A, Chitwood WR Jr. Minimally invasive mitral valve surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2008;34(5):943–52. [DOI] [PubMed] [Google Scholar]
- 12.Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226(4):421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada T, Ochiai R, Takeda J, Shin H, Yozu R. Comparison of early postoperative quality of life in minimally invasive versus conventional valve surgery. J Anesth 2003;17(3):171–6. [DOI] [PubMed] [Google Scholar]
- 14.Grossi EA, Galloway AC, Ribakove GH, et al. Impact of minimally invasive valvular heart surgery: a case-control study. Ann Thorac Surg 2001;71(3):807–10. [DOI] [PubMed] [Google Scholar]
- 15.de Vaumas C, Philip I, Daccache G, et al. Comparison of minithoracotomy and conventional sternotomy approaches for valve surgery. J Cardiothorac Vasc Anesth 2003;17(3):325–8. [DOI] [PubMed] [Google Scholar]
- 16.McClure RS, Cohn LH, Wiegerinck E, et al. Early and late outcomes in minimally invasive mitral valve repair: an eleven-year experience in 707 patients. J Thorac Cardiovasc Surg 2009;137(1):70–5. [DOI] [PubMed] [Google Scholar]
- 17.Galloway AC, Schwartz CF, Ribakove GH, et al. A decade of minimally invasive mitral repair: long-term outcomes. Ann Thorac Surg 2009;88(4):1180–4. [DOI] [PubMed] [Google Scholar]
- 18.Aybek T, Dogan S, Risteski PS, et al. Two hundred forty minimally invasive mitral operations through right minithoracotomy. Ann Thorac Surg 2006;81(5):1618–24. [DOI] [PubMed] [Google Scholar]
- 19.Svensson LG, Atik FA, Cosgrove DM, et al. Minimally invasive versus conventional mitral valve surgery: A propensity-matched comparison. J Thorac Cardiovasc Surg. 2009. Nov 26. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Mihaljevic T, Cohn LH, Unic D, Aranki SF, Couper GS, Byrne JG. One thousand minimally invasive valve operations: early and late results. Ann Surg 2004;240(3):529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apolito R, Greenberg M, Menegus M, et al. Impact of the New York State Cardiac Surgery and Percutaneous Coronary Intervention Reporting System on the management of patients with acute myocardial infarction complicated by cardiogenic shock. Am Heart J 2008;155(2):267–73. [DOI] [PubMed] [Google Scholar]
- 22.Wentworth DN, Neaton JD, Rasmussen WL. An evaluation of the Social Security Administration master beneficiary record file and the National Death Index in the ascertainment of vital status. Am J Public Health 1983;73(11):1270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Segesser LK, Westaby S, Pomar J, Loisance D, Groscurth P, Turina M. Less invasive aortic valve surgery: rationale and technique. Eur J Cardiothorac Surg 1999;15(6):781–5. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz C, Ashraf O, Bimmel D, Welz A. Direct aortic cannulation in minimally invasive mitral-valve operations. Heart Surg Forum 2002;5(4):370–2. [PubMed] [Google Scholar]
- 25.Aklog L, Adams DH, Couper GS, Gobezie R, Sears S, Cohn LH. Techniques and results of direct-access minimally invasive mitral valve surgery: a paradigm for the future. J Thorac Cardiovasc Surg 1998;116(5):705–15. [DOI] [PubMed] [Google Scholar]
- 26.Walther T, Falk V, Metz S, et al. Pain and quality of life after minimally invasive versus conventional cardiac surgery. Ann Thorac Surg 1999;67(6):1643–7. [DOI] [PubMed] [Google Scholar]
- 27.Vleissis AA, Bolling SF. Mini-reoperative mitral valve surgery. J Card Surg. 1998; 13(6):468–70. [DOI] [PubMed] [Google Scholar]
- 28.Casselman FP, Van Slycke S, Wellens F, et al. Mitral valve surgery can now routinely be-performed endoscopically. Circulation 2003;108 Suppl 1:II48–54. [DOI] [PubMed] [Google Scholar]
- 29.Iribarne A, Russo MJ, Moskowitz AJ, Ascheim DD, Brown LD, Gelijns AC. Assessing technological change in cardiothoracic surgery. Semin Thorac Cardiovasc Surg 2009. Spring;21(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson ED. Innovation and comparative-effectiveness research in cardiac surgery. N Engl J Med 2009;361(19):1897–9. [DOI] [PubMed] [Google Scholar]
- 31.Gibbons RJ, Gardner TJ, Anderson JL, et al. The American Heart Association’s principles for comparative effectiveness research: a policy statement from the American Heart Association. Circulation 2009;119(22):2955–62. [DOI] [PubMed] [Google Scholar]


