Abstract
This study was undertaken to identify baseline conditions and triggering factors for skeletal‐related events (SRE) in multiple myeloma (MM) patients treated with denosumab. During the median follow‐up of 17 months, SRE occurred in 6 out of 52 newly diagnosed patients and in 5 out of 23 relapsed/refractory patients. Bone fractures occurred by falling down due to orthostatic hypotension and/or muscle weakness in three out of four cases with amyloid light‐chain (AL) amyloidosis. A loss of balance and falling down appear to be triggering factors for SRE, especially in frail MM patients with AL amyloidosis, indicating the importance of retaining physical functions to prevent SRE.
Keywords: amyloidosis, bone disease, denosumab, myeloma, skeletal‐related events
1. INTRODUCTION
Multiple myeloma (MM) develops and expands almost exclusively in the bone marrow and generates devastating bone destruction. Zoledronic acid is widely used for the prevention of skeletal‐related events (SRE) in MM [1, 2]. A large phase 3 study demonstrated the noninferiority of denosumab to zoledronic acid in newly diagnosed MM (NDMM) patients with at least one bone lesion in terms of the prevention of SRE [3]. Overall survival was similar between the denosumab and zoledronic acid arms; of note, progression‐free survival (PFS) was longer with denosumab than zoledronic acid [3]. According to the subgroup analysis of this study, most of MM patients were treated with proteasome inhibitor (PI)‐based regimens, and PFS benefits with denosumab were observed in NDMM who underwent autologous stem cell transplantation and those who received PI‐based regimens, suggesting that denosumab may prolong anti‐MM efficacy in combination with PI‐based induction therapy [4]. PI‐based regimens combined with denosumab might provide a potentially synergistic anti‐MM effects and prevent the occurrence of SRE. Although MM tumor progression is generally accepted to be among the major causative factors for SRE in MM patients, baseline conditions and triggering factors for SRE in MM patients remain largely unknown. The present study was undertaken to identify triggering factors or the underlying physical functions associated with SRE in MM patients treated with denosumab.
1.1. Patients and methods
We retrospectively analyzed 75 MM patients who received a subcutaneous injection of 120 mg denosumab for bone disease between June 2012 and December 2020 in Tokushima University Hospital. The present study was approved by the Institutional Review Board of Tokushima University (permission number 3086‐2). The bone scale was determined as previously reported [5]. The documentation of a lytic bone lesion was based on radiographic (X‐ray or CT) evidence of at least one lytic bone lesion. SRE were defined as one or more of the following: a pathologic fracture (vertebral or nonvertebral), radiation therapy to bone, surgery to bone, or spinal cord compression. We assessed the time to the first SRE after the denosumab treatment using medical charts and showed Kaplan–Meier estimates of the time to the first SRE in our cohort. Univariate and multivariate analyses of risk factors for SRE were performed using Fisher's exact test and a logistic regression analysis. The proportion of MM patients without SRE was estimated using the Kaplan–Meier method and analyzed using the Log‐rank test. Data were analyzed using JMP13.0 (SAS Institute Inc.). p values < 0.05 were considered statistically significant.
2. RESULTS AND DISCUSSION
Patient characteristics at the first administration of denosumab are shown in Table 1. Fifty‐two patients with NDMM and 23 with relapsed/refractory MM (RRMM) were enrolled. The severity of bone disease at baseline was as follows: 13, 45, and 17 patients were scored as bone scale 1, 2, and 3, respectively, according to the Durie and Salmon criteria [5]. Fifteen were in poor performance status (PS) with Eastern Cooperative Oncology Group (ECOG) 3 and more, and 4 were combined with amyloid light‐chain (AL) amyloidosis. All patients were treated with PI‐based regimens at least one line in their clinical courses. The number of times denosumab was administered ranged between 1 and 35 (median 7). At the median follow‐up of 17 months (interquartile range [IQR]: 1–86), SRE occurred in 6 out of 52 patients with NDMM and 5 out of 23 with RRMM. Patients with RRMM had a significantly shorter time to the first occurrence of SRE than those with NDMM (Figure 1). The proportion of patients without SRE at 3 years was 89.6% in NDMM and 55.7% in RRMM (Log‐rank test, p = 0.02) (Figure 1). We next exploited the baseline physical conditions and triggering factors for SRE. A univariate analysis revealed that PS with ECOG 3 and more (odds ratio: 4.50, 95% CI: 1.15–17.63, p = 0.037) and the coexistence of AL amyloidosis (odds ratio: 23.63, 95% CI: 2.19–255.21, p = 0.009) correlated with the occurrence of SRE (Table 2, upper panel). In the multivariate analysis, the coexistence of AL amyloidosis remained an independent risk factor for SRE occurrence (odds ratio: 14.62, 95% CI:1.20–177.50, p = 0.035) (Table 2, lower panel).
TABLE 1.
Patient characteristics
| Sex (male/female) | 38/37 |
|---|---|
| Median age (range), years | 69 (44‐88) |
| Newly diagnosed | 52 (69%) |
| Relapsed/refractory | 23 (31%) |
| Immunoglobulin subtype | |
| IgG | 46 (61%) |
| IgA | 12 (16%) |
| IgD | 3 (4%) |
| Light chain only | 13 (17%) |
| Nonsecretory | 1 (1%) |
| PS (ECOG) | |
| 0 | 26 (35%) |
| 1 | 24 (32%) |
| 2 | 10 (13%) |
| 3 | 10 (13%) |
| 4 | 5 (7%) |
| Durie and Salmon stage | |
| Ⅰ | 0 (0%) |
| Ⅱ | 23 (31%) |
| III | 52 (69%) |
| A | 61 (81%) |
| B | 14 (19%) |
| ISS stage | |
| 1 | 24 (32%) |
| 2 | 28 (37%) |
| 3 | 23 (31%) |
| Bone scale | |
| 1 | 13 (17%) |
| 2 | 45 (60%) |
| 3 | 17 (23%) |
| Previous bisphosphonate treatment | 16 (21%) |
| History of DM | 17 (23%) |
| History of SRE | 34 (45%) |
| AL amyloidosis | 4 (5%) |
| Anti‐myeloma treatment | |
| Proteasome inhibitors | 75 (100%) |
| IMiDs | 51 (68%) |
| ASCT | 25 (33%) |
ASCT, autologous stem cell transplantation; DM, diabetes mellitus; ECOG, Eastern Cooperative Oncology Group; IMiDs, immunomodulatory drug; ISS: international staging system; PS, performance status; SRE, skeletal‐related events,
FIGURE 1.
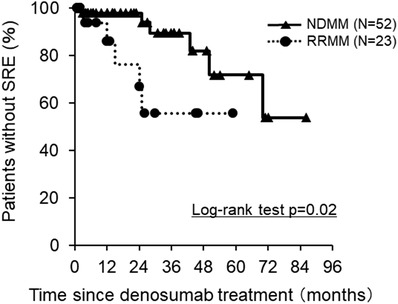
Kaplan–Meier estimates of the time to the first SRE on this study. The proportion of patients without SRE according to the disease status at the first administration of denosumab, NDMM (N = 52, solid line) and RRMM (N = 23, dotted line)
TABLE 2.
Risk factors for SRE
| Univariate analysis | |||||
|---|---|---|---|---|---|
| Variables | SRE− | SRE+ | 95% C.I./Odds raio | p‐value | |
| Age |
<65 ≥65 |
19 45 |
3 8 |
0.27‐4.71 1.13 |
1.0000 |
| Sex |
male female |
31 33 |
7 4 |
0.14‐2.01 0.54 |
0.5161 |
| Previous BP treatment |
no yes |
51 13 |
8 3 |
0.34‐6.33 1.47 |
0.6922 |
| Bone scale |
0, 1 2, 3 |
12 52 |
1 10 |
0.93‐17.30 4.00 |
0.1009 |
| History of DM |
no yes |
52 12 |
6 5 |
0.94‐13.83 3.61 |
0.1110 |
| History of SRE |
no yes |
35 29 |
6 5 |
1.25‐24.43 1.01 |
1.0000 |
| PS (ECOG) |
0, 1, 2 3, 4 |
54 10 |
6 5 |
1.15‐17.63 4.50 |
0.0370 |
| AL amyloidosis |
no yes |
63 1 |
8 3 |
2.19‐255.21 23.63 |
0.0090 |
| Multivariate analysis | ||||
|---|---|---|---|---|
| Variables | 95% C.I. | Odds ratio | p‐value | |
| PS (ECOG) | 3, 4 | 0.58 – 12.90 | 2.74 | 0.2030 |
| AL amyloidosis | yes | 1.20 – 177.50 | 14.62 | 0.0352 |
BP, bisphosphonate; CI, confidence interval; DM, diabetes mellitus; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
SRE occurred in 11 MM patients in our cohort. Among 8 patients without documented AL amyloidosis, 7 showed progressive disease and 1 stable disease (Table 3, patients # 1–8). Falling down and a loss of balance caused bone fractures in 3 patients whose PS were ECOG 2 and more (Table 3, patients # 1, 3, and 7). Importantly, 3 MM patients with AL amyloidosis developed SRE triggered by falling down and/or a loss of balance, although they achieved very good partial response (Table 3, patient # 9–11). Two of these patients suffered from cardiac amyloidosis with orthostatic hypotension and muscle weakness or peripheral neuropathy (Table 3, patients # 9 and 11).
TABLE 3.
Characteristics of MM patients when SRE occurred
| No. | Age/Sex | Amyloidosis | Details of SRE | Treatment response | Triggers | Complications/Comorbidities | PS (ECOG) |
|---|---|---|---|---|---|---|---|
| 1 | 76/M | None | Femoral fracture | SD |
Loss of balance Falling down |
Peripheral neuropathy | 2 |
| 2 | 47/M | None |
Rib fracture Spinal cord compression |
PD | None | None | 2 |
| 3 | 56/M | None | Pelvic fracture | PD |
Loss of balance Falling down |
DM type 2 Orthostatic hypotension |
4 |
| 4 | 55/M | None |
Vertebral fracture Spinal cord compression |
PD | None | Peripheral neuropathy | 4 |
| 5 | 78/M | None |
Sacral fracture Spinal cord compression |
PD | None | None | 3 |
| 6 | 77/M | None |
Vertebral fracture Rib fracture |
PD | None |
DM type 2 Orthostatic hypotension |
1 |
| 7 | 74/F | None | Vertebral fracture | PD | Loss of balance | Peripheral neuropathy | 3 |
| 8 | 80/F | None | Vertebral fracture | PD | None |
DM type 2 Muscle weakness |
3 |
| 9 | 73/F | Heart, Tongue, Skin | Femoral fracture | VGPR | Falling down |
Muscle weakness Orthostatic hypotension |
2 |
| 10 | 78/F | GI tract, Skin, Muscle | Vertebral fracture | VGPR |
Loss of balance Falling down |
Muscle weakness | 1 |
| 11 | 70/M |
Heart, Kidney, GI tract |
Rib fracture | VGPR | Falling down |
DM type 2 Peripheral neuropathy Orthostatic hypotension |
0 |
DM, diabetes mellitus; ECOG, Eastern Cooperative Oncology Group; F, female; GI tract, gastrointestinal tract; M, male; PD, progressive disease; PS, performance status; SD, stable disease, VGPR, very good partial response.
The occurrence of new SRE was documented in more than 60% of NDMM patients within the first 3 months in a pivotal phase 3 study [3]. Achieving quick and deep response is needed to prevent the early occurrence of SRE and the progression of bone disease in MM. However, a loss of balance and falling down due to ill physical functioning, including muscle weakness, sarcopenia, orthostatic hypotension, and peripheral neuropathy, appear to be triggering factors for SRE, indicating the importance of retaining physical functions in MM patients with bone disease. We need to pay much attention to avoid falling down and a loss of balance especially in frail MM patients and those with AL amyloidosis, although avoiding falling down is a trite and obvious remark.
However, there are some limitations in the present study that include (1) a small sample size especially with the very small number of cases with AL amyloidosis, (2) a retrospective analysis in a single center, and (3) no objective estimation of physical functions. A large multicenter prospective study should be conducted to further elucidate the importance of physical functions to prevent the occurrence of SRE especially in frail MM patients including those with AL amyloidosis.
AUTHOR CONTRIBUTIONS
H.M., S.N., I.E., and M.A. designed the study and wrote the manuscript. All authors were involved in the analyses and interpretation of data. All authors approved the submission of the manuscript.
CONFLICT OF INTEREST
M.A. received honoraria for lectures from Daiichi Sankyo Company Limited. Other authors declare no competing financial interests related to this work.
ACKNOWLEDGMENT
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Miki H, Nakamura S, Oura M, Nakamura M, Sumitani R, Sogabe K, et al. The importance of retaining physical functions to prevent skeletal‐related events in multiple myeloma patients with bone disease. eJHaem. 2022;3:480–483. 10.1002/jha2.402
REFERENCES
- 1. Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, et al. First‐line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Cook G, et al. Long‐term follow‐up of MRC myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res. 2013;19:6030–6038. [DOI] [PubMed] [Google Scholar]
- 3. Raje N, Terpos E, Willenbacher W, Shimizu K, Garcia‐Sanz R, Durie B, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double‐blind, double‐dummy, randomised, controlled, phase 3 study. Lancet Oncol, 2018;19:370–381. [DOI] [PubMed] [Google Scholar]
- 4. Terpos E, Raje N, Croucher P, Garcia‐Sanz R, Leleu X, Pasteiner W, et al. Denosumab compared with zoledronic acid on PFS in multiple myeloma: exploratory results of an international phase 3 study. Blood Adv. 2021;5:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. [DOI] [PubMed] [Google Scholar]


