Abstract
We observed anastomosis between hyphae originating from the same spore and from different spores of the same isolate of the arbuscular mycorrhizal fungi Glomus mosseae, Glomus caledonium, and Glomus intraradices. The percentage of contacts leading to anastomosis ranged from 35 to 69% in hyphae from the same germling and from 34 to 90% in hyphae from different germlings. The number of anastomoses ranged from 0.6 to 1.3 per cm (length) of hyphae in mycelia originating from the same spore. No anastomoses were observed between hyphae from the same or different germlings of Gigaspora rosea and Scutellospora castanea; no interspecific or intergeneric hyphal fusions were observed. We monitored anastomosis formation with time-lapse and video-enhanced light microscopy. We observed complete fusion of hyphal walls and the migration of a mass of particles in both directions within the hyphal bridges. In hyphal bridges of G. caledonium, light-opaque particles moved at the speed of 1.8 ± 0.06 μm/s. We observed nuclear migration between hyphae of the same germling and between hyphae belonging to different germlings of the same isolate of three Glomus species. Our work suggests that genetic exchange may occur through intermingling of nuclei during anastomosis formation and opens the way to studies of vegetative compatibility in natural populations of arbuscular mycorrhizal fungi.
Arbuscular mycorrhizal (AM) fungi are obligate symbionts that live in association with the roots of most land plants and play a major role in nutrient uptake and in interplant nutrient transfer (8, 27, 39). In experimental microcosms and in the field, AM hyphal connections between roots are important for the maintenance of stability and biodiversity in plant communities (32, 36). Little is known about the dynamics of hyphal growth during presymbiotic and symbiotic phases (24, 30, 31), and virtually nothing is known of the ability of these fungi to form the hyphal networks through which nutrients are proposed to flow. Although anastomoses occur widely between vegetative hyphae of ascomycetes and basidiomycetes (1, 3, 17, 21), they are believed to be lacking or rare in zygomycetes (5, 16), to which AM fungi belong. The occurrence of anastomosis in AM fungi has been mentioned by some authors (11, 15, 26, 40), but no quantitative data are available on the frequency of hyphal fusions in the different species, and, to our knowledge, no information has been published on the cytological events involved.
In this study we monitored anastomosis between hyphae derived from individually germinated spores of AM fungi via a combination of time-lapse and video-enhanced light microscopy, image analysis, and epifluorescence microscopy. Our main objectives were (i) to monitor anastomosis formation in living hyphae; (ii) to detect cytoplasmic flow and nuclear exchange between anastomosing hyphae; (iii) to determine the occurrence and frequency of anastomosis between hyphae belonging to the same and to different germinated spores of the same isolate of Glomus mosseae, Glomus caledonium, Glomus intraradices, Gigaspora rosea, or Scutellospora castanea; and (iv) to determine the kind of interaction occurring between hyphae belonging to different genera (Glomus versus Gigaspora and Gigaspora versus Scutellospora) and to different species of the same genus (Glomus).
MATERIALS AND METHODS
Fungal material.
Spores of AM fungi were obtained from pot cultures maintained in the collection of the Department of Chemistry and Agricultural Biotechnology, University of Pisa, Pisa, Italy. The AM fungi used were G. mosseae (Nicolson et Gerdemann) Gerdemann et Trappe (Kent isolate) (Banque Européenne des Glomales [BEG] code 12), G. caledonium (Nicolson et Gerdemann) Trappe et Gerdemann (Rothamsted isolate) (BEG 20), G. intraradices Schenck et Smith, isolated from Bourgogne, France (LPA 8), G. intraradices Schenck et Smith, isolated from Liguria, Italy (IMA 5), Glomus viscosum Nicolson (BEG 27), G. rosea Nicolson et Gerdemann (BEG 9), and S. castanea Walker (BEG 1). Inocula of the G. intraradices French isolate, G. rosea, and S. castanea were kindly provided by V. Gianinazzi-Pearson, Laboratoire de Phytoparasitologie, Dijon, France.
Dynamics of anastomosis formation in living hyphae.
Spores of G. caledonium and G. mosseae were surface sterilized with 2% chloramine T supplemented with streptomycin (400 μg ml−1) for 20 min and then rinsed five times in sterile distilled water. The spores were germinated on 20- by 20-mm cellophane membranes (Hoefer, San Francisco, Calif.) and placed on a thin layer of 1% water agar in 5.5-cm-diameter petri dishes for direct observations of living hyphae under a Reichert-Jung (Vienna, Austria) Polyvar microscope equipped with differential interference contrast optics and epifluorescence optics. We monitored anastomosis formation in hyphal tips showing directed growth towards nearby hyphae, which were growing at a distance of about 70 μm. To visualize the establishment of protoplasmic continuity and the viability of anastomosed hyphae, succinate dehydrogenase (SDH) activity was assessed on germinated spores, which were stained, mounted on microscope slides, and observed for the presence of formazan salt depositions in hyphal bridges (38).
Nuclear migration and cytoplasmic flow through anastomoses.
We mounted cellophane membranes bearing germinated spores of G. caledonium and G. mosseae on microscope slides in sterile distilled water. The coverslips were sealed with 1% water agar, which was periodically wetted with sterile distilled water. The microchambers obtained were transferred to the Polyvar microscope and observed for 2 h. Bright contrast images were acquired after regulation of the condenser diaphragm. The experiments were carried out at room temperature (19 to 21°C). To monitor nuclei in hyphae during anastomosis formation, 7- to 14-day-old germinated spores were mounted on microscope slides in diaminophenylindole (DAPI) (Sigma, St. Louis, Mo.) at 5 μg/ml of microscopy 1:1 water-glycerol and were observed under epifluorescence by using the filter combination U1 (BP 330-380, LP 418, DS 420). Images were acquired with a Reichert 100× oil immersion lens with a 1.25 numerical aperture. Time-lapse and video-enhanced light microscopy was used to obtain images, which were captured with a 3 charge-coupled device color video camera connected to a videocassette recorder (Hi 8, EV-C2000E; SONY, Tokyo, Japan). Selected frames were printed on a video graphic printer (UP-890 CE; SONY).
Occurrence and frequency of anastomoses.
Spores were rinsed five times in sterile distilled water and were allowed to germinate individually in sterile distilled water in microliter plates (Sigma). Germinated spores were transferred to Millipore membranes (0.45-μm-diameter pores), which were placed on moist, sterile quartz sand in 9-cm-diameter petri dishes. After 7 to 14 days of incubation in the dark at 28°C, mycelium growing on the membranes was stained with Trypan Blue (0.05% in lactic acid), mounted on microscope slides, and observed under the Polyvar light microscope. Hyphal length was assessed by using Quantimet 500 image analysis software (Leica, Milan, Italy). Pairings of individually germinated spores were made by placing germinated spores approximately 1 cm apart on the membrane filter. Findings are based on at least 10 germinated spores for anastomoses within the same germling, 9 pairings for anastomoses between different germlings from the same isolate, and 9 sets of pairings for interspecific and intergeneric anastomoses. Replicate experiments were carried out on spores produced in different pot cultures (Table 1). The frequency of anastomosis was calculated by determining the proportion of hyphal contacts which had anastomosed. Chi-square analysis was used to determine the homogeneity of anastomosis frequency data, and the chi-square test of independence was performed to detect significant differences in anastomosis frequency between hyphae from the same spores and hyphae from different spores.
TABLE 1.
Anastomosis rate in mycelia originating from the same spore or from different spores of the same isolate in five species of AM fungi and numbers of anastomoses and hyphal contacts per centimeter (length) of hypha in mycelia originating from the same spore
| Fungal species | % Anastomosisa (mean and 95% exact confidence limits) between:
|
No. of anastomoses/cm (length) of hypha (mean ± SE) | No. of hyphal contacts/cm (length) of hypha (mean ± SE) | |
|---|---|---|---|---|
| HSS | HDS | |||
| G. mosseae BEG 12 | ||||
| Culture 14.93 | 57 (48–66) | 0.65 ± 0.11 | 1.2 ± 0.19 | |
| Culture 44.93 | 51 (42–61) | 40 (31–49) | 0.95 ± 0.23 | 2.2 ± 0.76 |
| G. caledonium BEG 20 | ||||
| Culture 156.92 | 55 (46–63) | 34 (27–41)** | 0.62 ± 0.06 | 1.2 ± 0.14 |
| Culture 5.91 | 35 (30–39) | 1.1 ± 0.1 | 3.4 ± 0.37 | |
| Culture 163.92 | 48 (43–53) | 34 (24–44)* | 1.1 ± 0.3 | 2.3 ± 0.59 |
| G. intraradices | ||||
| French isolate LPA 8 | 59 (51–66) | 1.3 ± 0.23 | 2.3 ± 0.34 | |
| Italian isolate IMA 5 | 69 (62–75) | 90 (81–99)** | 1.1 ± 0.18 | 1.7 ± 0.29 |
| G. rosea | 0 | 1.7 ± 0.14 | ||
| S. castanea | 0 | 3.2 ± 0.23 | ||
Anastomosis as a percentage of contacts. HSS, hyphae from the same spore. HDS, hyphae from different spores. Within the same fungal species, values of HDS marked with one or two asterisks are significantly different from the corresponding values of HSS at P < 0.05 or P < 0.01, respectively.
RESULTS
Dynamics of anastomosis formation in living hyphae.
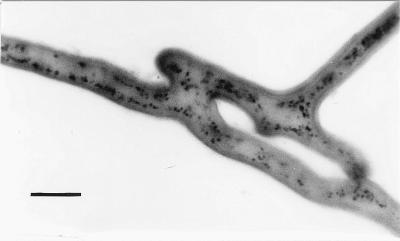
Spatiotemporal studies made it possible to monitor anastomosis formation, which took about 35 min after a hyphal tip showed directed growth towards another hypha, in G. caledonium and G. mosseae mycelia. Complete fusion of hyphal walls and cytoplasmic flow between fused hyphae were observed. We observed protoplasmic continuity, the characteristic feature of true vegetative hyphal fusion, as SDH activity in all the anastomoses. Depositions of formazan salt were detected in anastomosing hyphae and in hyphal bridges (Fig. 1).
FIG. 1.
Micrograph showing complete fusion of hyphal walls and the establishment of protoplasmic continuity in two anastomosing hyphae of G. mosseae, visualized by histochemical localization of SDH activity. Depositions of formazan salt are evident in hyphal bridges. Bar, 10 μm.
Nuclear migration and cytoplasmic flow through anastomoses.
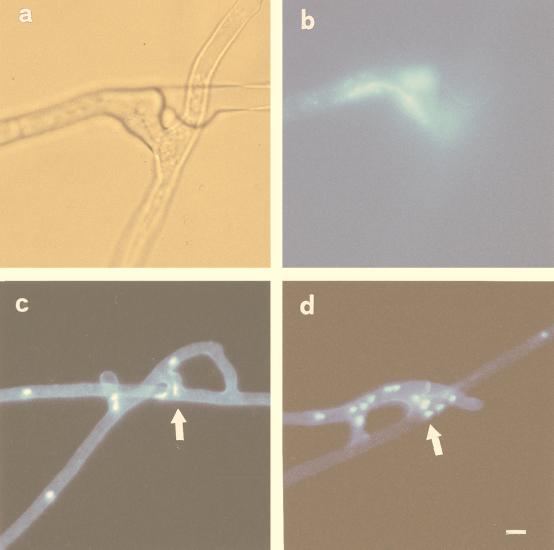
DAPI staining and epifluorescence microscopy showed that hyphal anastomosis involved the migration of nuclei via the fusion bridge. Migration occurred between hyphae belonging to the same germling (Fig. 2a and b) and between hyphae belonging to different germlings of the same isolate (Fig. 2c and d).
FIG. 2.
Localization of nuclear migration between two anastomosing hyphae of G. caledonium belonging to the same germling (a and b) and to different germlings of the same isolate (c and d). (a) Light micrograph illustrating the site of hyphal fusion. (b) Epifluorescence image of the same field, showing an elongated nucleus (stained with DAPI) in the middle of the fusion bridge. Bar, 7 μm. (c and d) Epifluorescence microscopy of the nuclei within fusion bridges (arrows). Bar, 12 μm.
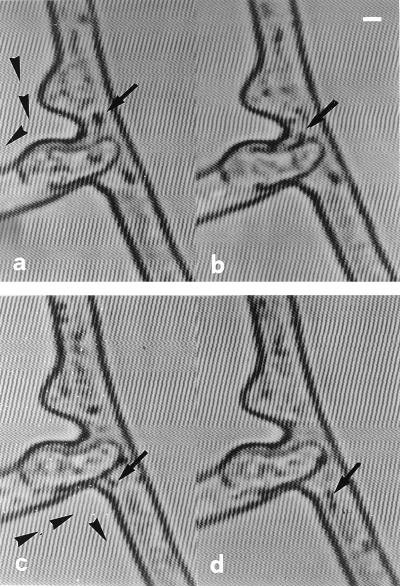
We used time-lapse and video-enhanced microscopy to detect protoplasmic streaming inside the hyphae and to monitor the trajectory of a mass of particles (e.g., vacuoles, mitochondria, nuclei, and fat droplets) migrating in both directions within the protoplasm and through anastomoses (Fig. 3). The mass streaming of particles occurred at a speed of 1.8 ± 0.06 μm/s (mean ± standard error of the mean [SEM]) in hyphal bridges of G. caledonium.
FIG. 3.
Protoplasmic flow subsequent to anastomosis in G. caledonium, visualized over time by video-enhanced light microscopy. Cytoplasmic continuity is established between two fused hyphae, evidenced by the bidirectional movement of particles (arrowheads). (a and b) A large, light-opaque particle migrating from one hypha to the other via the fusion bridge (arrows). Time sequence: 0 (a) and 2 (b) s. (c and d) Two coupled light-opaque particles migrating in the opposite direction, via the fusion bridge (arrows). Time sequence: 0 (c) and 2 (d) s. Bar, 3.8 μm.
Occurrence and frequency of anastomoses.
We observed anastomoses between hyphae belonging to the same germinated spore and between different spores of the same isolate in all three Glomus species. The percentage of anastomoses between hyphae from the same germling ranged from 35% in G. caledonium to 69% in the Italian isolate of G. intraradices (Table 1), and the number of anastomoses ranged from 0.62 ± 0.06 (mean ± SEM) to 1.3 ± 0.23 per cm (length) of hyphae (Table 1). Hyphal fusions also occurred readily between hyphae belonging to different germlings from the same isolate. The anastomosis rate ranged from 34% in G. caledonium to 90% in the Italian isolate of G. intraradices (Table 1).
No anastomoses were observed between hyphae belonging to the same germling of G. rosea or S. castanea (Table 1). No fusions were detected in more than 220 hyphal contacts between germlings of G. rosea. No fusions were detected in more than 460 hyphal contacts between germlings of S. castanea.
No anastomoses were observed in pairings between germlings of different species of AM fungi: G. mosseae and G. caledonium (0 fusions, 90 contacts), G. mosseae and G. rosea (0 fusions, 140 contacts), G. caledonium and G. rosea (0 fusions, 232 contacts), and G. rosea and S. castanea (0 fusions, 98 contacts).
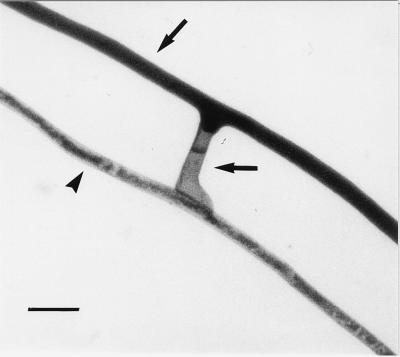
During interspecific and intergeneric hyphal interactions, the responses ranged from no contact interference, when hyphae simply appeared to overgrow each other, to contact responses such as formation of hyphal swellings devoid of protoplasm and septate (Fig. 4) or growth of one hypha along the other without anastomosis formation.
FIG. 4.
Interactions between hyphae originating from two different species of AM fungi, G. mosseae (upper arrow) and G. viscosum (arrowhead). Note the formation of a hyphal swelling which becomes empty and septate without any anastomosis formation (lower arrow). Bar, 20 μm.
DISCUSSION
Anastomosis formation and the cytological events involved have been studied extensively in ascomycetes and basidiomycetes (1, 3, 17, 21, 25). Anastomoses are believed to be lacking or rare in zygomycetes (5, 16), although their occurrence in AM fungi has been mentioned by others (11, 15, 26, 40). In this report, we present evidence for anastomosis formation between hyphae originating from the same spore and from different spores in one isolate of G. mosseae, one isolate of G. caledonium, and two different isolates of G. intraradices. The frequency of anastomoses per contacts in hyphae from the same germling was comparable to that found within self-anastomosing isolates of Rhizoctonia solani—more than 50% (19). The number of anastomoses per centimeter (length) of hypha ranged from 0.62 to 1.3, corresponding to 0.22 to 0.45 fusions per mm2, as worked out from hyphal density data (13). These values are lower than those observed in fungi that are able to grow saprophytically, such as Gibberella fujikuroi—6.9 to 8.1 fusions per mm2 in self-compatible strains (6)—but still significant when the poor growth ability of the presymbiotic mycelium of AM fungi is considered (2, 24).
In the three Glomus species, hyphal fusions occur regularly in mycelia originating from different spores. In G. mosseae the frequency of anastomosis formation between hyphae from different germlings was not statistically different from that for mycelia originating from the same germling, whereas G. intraradices and G. caledonium showed significantly higher and lower frequencies of anastomosis per contact, respectively, in hyphae originating from different spores. No anastomoses were observed between hyphae from the same or different germlings of G. rosea and S. castanea, and this characteristic may be another difference between the Glomineae and Gigasporineae families (37).
During interspecific and intergeneric interactions, no hyphal fusions were detected, suggesting that hyphae recognize species-level differences and confirming previous qualitative findings (40). The ability to discriminate self from other at the intraspecific level, however, remains to be demonstrated. The use of tests based on vegetative compatibility may lead to the identification of genetically different isolates in population studies of pathogenic, saprophytic, and ectomycorrhizal fungi (3, 7, 9, 20–22, 29, 35). Our work opens the way to studies of vegetative compatibility in natural populations of AM fungi. For example, different isolates of G. mosseae, a species of worldwide distribution, could be paired with known tester strains to determine if they are genetically isolated as indicated by vegetative compatibility.
AM fungi are ancient obligate biotrophs (18) which, although lacking host-regulated spore germination (14), have survived for 400 million years (28, 33, 37). We suggest that the ability of germlings to form anastomoses with compatible hyphae may affect the fitness of Glomus species; the young mycelium produced by spores germinating in the absence of the host may connect to a mycelial network as soon as the germ tube contacts a compatible mycelium, increasing its chance to colonize host roots. The formation of anastomoses also can restore protoplasmic continuity in damaged hyphae (10).
We obtained direct evidence for nuclear exchange between hyphae from the same germling and between hyphae of different germlings of the same isolate in three Glomus species. Nuclei from single spores of Glomus species may be heterogeneous, based on variation in molecular characters (23, 34, 41). Since Glomus spores are multinucleate and may contain 1,000 to 5,000 nuclei per spore (4, 12, 42), some researchers have suggested that these spores are heterokaryotic (23, 34). Our results suggest that genetic exchange may occur through intermingling of nuclei during anastomosis formation between different germlings of the same isolate. This exchange could result in information flow, as well as physiological and genetic integration, between mycelia belonging to different germlings.
Lack of knowledge of the genetics of AM fungi and our inability to culture them in axenic culture make it difficult to discern the mechanism by which genetic exchange occurs. Further studies are needed in order to understand how these obligate symbionts maintain genetic diversity within spores.
ACKNOWLEDGMENT
This work was partly supported by a University of Pisa grant (Fondo di Ateneo).
REFERENCES
- 1.Ainsworth A M, Rayner A D M. Responses of living hyphae associated with self and non-self fusions in the basidiomycete Phanerochaete velutina. J Gen Microbiol. 1986;132:191–201. [Google Scholar]
- 2.Becard G, Piché Y. Fungal growth stimulation by CO2 and root exudates in vesicular-arbuscular mycorrhizal symbiosis. Appl Environ Microbiol. 1989;55:2320–2325. doi: 10.1128/aem.55.9.2320-2325.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brasier C. A champion thallus. Nature. 1992;356:382–383. [Google Scholar]
- 4.Burggraaf A J P, Beringer J E. Nuclear division and VA-mycorrhizal in vitro culture. In: Sylvia D M, Hung L L, Graham J H, editors. Mycorrhizae in the next decade. Gainesville: University of Florida; 1988. p. 190. [Google Scholar]
- 5.Carlile M J. The success of the hypha and mycelium. In: Gow N A R, Gadd G M, editors. The growing fungus. London, United Kingdom: Chapman & Hall; 1995. pp. 3–19. [Google Scholar]
- 6.Correll J C, Klittich C J R, Leslie J F. Heterokaryon self-incompatibility in Gibberella fujikuroi (Fusarium moniliforme) Mycol Res. 1989;93:21–27. [Google Scholar]
- 7.Dahlberg A, Stenlid J. Size, distribution and biomass of genets in populations of Suillus bovinus (L.: Fr.) Roussel revealed by somatic incompatibility. New Phytol. 1994;128:225–234. doi: 10.1111/j.1469-8137.1994.tb04006.x. [DOI] [PubMed] [Google Scholar]
- 8.Francis R, Read D J. Direct transfer of carbon between plants connected by vesicular-arbuscular mycorrhizal mycelium. Nature. 1984;307:53–56. [Google Scholar]
- 9.Fries N. Somatic incompatibility and field distribution of the ectomycorrhizal fungus Suillus luteus (Boletaceae) New Phytol. 1987;107:735–739. [Google Scholar]
- 10.Gerdemann J W. Wound-healing of hyphae in a phycomycetous mycorrhizal fungus. Mycologia. 1955;47:916–918. [Google Scholar]
- 11.Giovannetti M, Avio L, Sbrana C, Citernesi A S. Factors affecting appressorium development in the vesicular-arbuscular mycorrhizal fungus Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe. New Phytol. 1993;123:115–122. [Google Scholar]
- 12.Giovannetti M, Gianinazzi-Pearson V. Biodiversity in arbuscular mycorrhizal fungi. Mycol Res. 1994;98:705–715. [Google Scholar]
- 13.Giovannetti M, Sbrana C, Avio L, Citernesi A S, Logi C. Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during preinfection stages. New Phytol. 1993;125:587–593. doi: 10.1111/j.1469-8137.1993.tb03907.x. [DOI] [PubMed] [Google Scholar]
- 14.Giovannetti M, Sbrana C, Logi C. Early processes involved in host recognition by arbuscular mycorrhizal fungi. New Phytol. 1994;127:703–709. doi: 10.1111/j.1469-8137.1994.tb02973.x. [DOI] [PubMed] [Google Scholar]
- 15.Godfrey R M. Studies on British species of Endogone. III. Germination of spores. Trans Br Mycol Soc. 1957;40:203–210. [Google Scholar]
- 16.Gregory P H. The fungal mycelium—an historical perspective. In: Jennings D H, Rayner A D M, editors. The ecology and physiology of the fungal mycelium. Cambridge, United Kingdom: Cambridge University Press; 1984. pp. 1–22. [Google Scholar]
- 17.Gregory P H. The fungal mycelium: an historical perspective. Trans Br Mycol Soc. 1984;82:1–11. [Google Scholar]
- 18.Hepper C M. Limited independent growth of a vesicular-arbuscular mycorrhizal fungus in vitro. New Phytol. 1983;93:537–542. [Google Scholar]
- 19.Hyakumachi M, Ui T. Non-self-anastomosing isolates of Rhizoctonia solani obtained from fields of sugarbeet monoculture. Trans Br Mycol Soc. 1987;89:55–159. [Google Scholar]
- 20.Legrand P, Ghahari S, Guillaumin J J. Occurrence of genets of Armillaria spp. in four mountain forests in Central France: the colonization strategy of Armillaria ostoyae. New Phytol. 1996;133:321–332. doi: 10.1111/j.1469-8137.1996.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 21.Leslie J F. Fungal vegetative compatibility. Annu Rev Phytopathol. 1993;31:27–150. doi: 10.1146/annurev.py.31.090193.001015. [DOI] [PubMed] [Google Scholar]
- 22.Leslie J F. Mating populations in Gibberella fujikuroi (Fusarium section Liseola) Phytopathology. 1991;81:1058–1060. [Google Scholar]
- 23.Lloyd-MacGilp S A, Chambers S M, Dodd J C, Fitter A H, Walker C, Young J P W. Diversity of the ribosomal internal transcribed spacers within and among isolates of Glomus mosseae and related mycorrhizal fungi. New Phytol. 1996;133:103–111. [Google Scholar]
- 24.Logi C, Sbrana C, Giovannetti M. Cellular events involved in survival of individual arbuscular mycorrhizal symbionts growing in the absence of the host. Appl Environ Microbiol. 1998;64:3473–3479. doi: 10.1128/aem.64.9.3473-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCabe P A, Gallagher M P, Deacon J W. Microscopic observation of perfect hyphal fusion in Rhizoctonia solani. Mycol Res. 1999;103:487–490. [Google Scholar]
- 26.Mosse B. The regular germination of resting spores and some observations on the growth requirements of an Endogone sp. causing vesicular-arbuscular mycorrhiza. Trans Br Mycol Soc. 1959;42:273–286. [Google Scholar]
- 27.Newman E I, Eason W R. Rates of phosphorus transfer within and between ryegrass (Lolium perenne) plants. Funct Ecol. 1993;7:242–248. [Google Scholar]
- 28.Phipps C J, Taylor T N. Mixed arbuscular mycorrhizae from the Triassic of Antarctica. Mycologia. 1996;88:707–714. [Google Scholar]
- 29.Rayner A D M. The challenge of the individualistic mycelium. Mycologia. 1991;83:48–71. [Google Scholar]
- 30.Read D J. The structure and function of the vegetative mycelium of mycorrhizal roots. In: Jennings D H, Rayner A D M, editors. The ecology and physiology of the fungal mycelium. Cambridge, United Kingdom: Cambridge University Press; 1984. pp. 215–240. [Google Scholar]
- 31.Read D J. The mycorrhizal mycelium. In: Allen M F, editor. Mycorrhizal functioning. An integrative plant-fungal process. New York, N.Y: Chapman & Hall; 1984. pp. 102–133. [Google Scholar]
- 32.Read D J. The ties that bind. Nature. 1997;388:517–518. [Google Scholar]
- 33.Remy W, Taylor T N, Hass H, Kerp H. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc Natl Acad Sci USA. 1994;91:11841–11843. doi: 10.1073/pnas.91.25.11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders I R, Alt M, Groppe K, Boller T, Wiemker A. Identification of ribosomal DNA polymorphisms among and within spores of the Glomales: application to studies on the genetic diversity of arbuscular mycorrhizal fungal communities. New Phytol. 1995;130:419–427. [Google Scholar]
- 35.Sen R. Intraspecific variation in two species of Suillus from Scots pine (Pinus sylvestris L.) forests based on somatic incompatibility and isozyme analyses. New Phytol. 1990;114:603–612. [Google Scholar]
- 36.Simard S W, Perry D A, Jones M D, Myrold D D, Durall D M, Molina R. Net transfer of carbon between ectomycorrhizal tree species in the field. Nature. 1997;388:579–582. [Google Scholar]
- 37.Simon L, Bousquet J, Levesque R C, Lalonde M. Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature. 1993;363:67–69. [Google Scholar]
- 38.Smith S E, Gianinazzi-Pearson V. Phosphate uptake and arbuscular activity in mycorrhizal Allium cepa L.: effects of photon irradiance and phosphate nutrition. Aust J Plant Physiol. 1990;17:177–188. [Google Scholar]
- 39.Smith S E, Read D J. Mycorrhizal symbiosis. San Diego, Calif: Academic Press; 1996. [Google Scholar]
- 40.Tommerup I C. The vesicular-arbuscular mycorrhizas. Adv Plant Pathol. 1988;6:81–91. [Google Scholar]
- 41.Vandenkoornhuyse P, Leyval C. SSU rDNA sequencing and PCR-fingerprinting reveal genetic variation within Glomus mosseae. Mycologia. 1998;90:791–797. [Google Scholar]
- 42.Viera A, Glenn M G. DNA content of vesicular-arbuscular mycorrhizal fungal spores. Mycologia. 1990;82:263–267. [Google Scholar]