Abstract
Vibrio cholerae is a biofilm-forming pathogen with various virulence phenotypes and antimicrobial resistance traits. Phenotypic characteristics play a critical role in disease transmission and pathogenesis. The current study elucidated antibiofilm formation activity, profiled antibiotic-resistant genes and virulence factors of toxigenic Vibrio cholerae isolates from the cholera outbreak in Kisumu County, Kenya. Vibrio cholerae O1 isolates collected during the 2017 cholera outbreak in Kisumu County, Kenya, were utilized. Biofilm and virulence factors were profiled using standard procedures. The study confirmed 100 isolates as Vibrio cholerae , with 81 of them possessing cholera toxin gene (ctxA). Additionally, 99 of the isolates harboured the toxR gene. The study further revealed that 81 and 94 of the isolates harboured the class I integron (encoded by inDS gene) and integrating conjugative element (ICE), respectively. Antibiotic resistance assays confirmed tetracycline resistance genes as the most abundant (97 isolates). Among them were seven isolates resistant to commonly used antibiotics. The study further screened the isolates for antibiofilm formation using various antibiotics. Unlike the four strains (03/17–16, 02/17–09, 04/17–13), three of the strains (04/17–07, 06/17–14 and 05/17–03) did not form biofilms. Further, all the seven isolates that exhibited extensive antibiotic resistance produced haemolysin while 71.42%, 85.71 and 71.42 % of them produced protease, phospholipases and lipase, respectively. This study provides and in-depth understanding of essential features that were possibly responsible for V. cholerae outbreak. Understanding of these features is critical in the development of strategies to combat future outbreaks.
Keywords: antibiofilm activity, antibiotics, resistant genes profiling, Vibrio cholerae, virulence factors
Introduction
Vibrio cholerae is a causative agent of cholera, an acute diarrheal infection caused by ingestion of contaminated food or water. Vibrio cholerae belongs to genus vibrio, family Vibrionaceae [1]. There are two biotypes of Vibrio cholerae, classical and El Tor. The two biotypes differ in severity of clinical symptoms, as well as expression and regulation of major virulence factors [2]. Vibrio cholerae has several factors, which help reach and colonize the epithelium of the small intestine, leading to the production of a variety of extracellular products that have deleterious effects to eukaryotic cells [3].
Cholera has unique epidemiology features, the intriguing ones being the predisposition to cause epidemics with pandemic potential and the ability to remain endemic in all affected areas [4]. The bacteria are transmitted between humans through the faecal–oral route; a bite of contaminated food or a sip of contaminated water can cause infection [5]. Globally, the burden of cholera is highest in sub-Saharan Africa where between 2012 and 2015, six pandemics of cholera have been recorded [6]. In 2016 alone, 132 121 cholera cases with 2420 deaths were reported to the World Health Organization (WHO) worldwide. Overall, 13 % of cases were reported from the USA, 17 % from Asia, 45 % from Africa and 25 % from Kenya [7].
The two major virulence factors expressed by V. cholerae O1 and O139 are cholera toxin (CT), an AB5 family ADP-ribosyltransferase that is responsible for the profuse rice-watery diarrhoea typical of this disease [2], and the toxin-coregulated pilus (TCP), a type IV pilus that mediates adherence and microcolony formation and is required for intestinal colonization in neonate mice and humans [8, 9]. The genes encoding the CT subunits ctxA and ctxB constitute an operon within the prophage form of the filamentous phage CTXF [10]. The genes required for TCP biogenesis form a large cluster known as the V. cholerae pathogenicity island (VPI) or TCP island [11]. Within this cluster, tcpA encodes the major pilus subunit [12].
Other V. cholerae genes are corregulated in the same manner including the tcp operon, which is responsible for fimbrial synthesis and assembly. The ctx operon and the tcp are part of regulon, whose expression is controlled by the same environmental signals [13]. The proteins involved in the control of this regulon expression have been identified as ToxR, ToxS and ToxT. ToxR is a trans membranous protein with about two thirds of its amino terminal part exposed to the cytoplasm. ToxS is a periplasmic protein. It is thought that ToxS can respond to environmental signals, change conformation and can influence dimerization of ToxR, which activates transcription of the operon. Expression of ToxT is activated by ToxR, while ToxT in turn activates transcription of tcp genes for synthesis of tcp pili [14].
These factors included other potential toxins, accessory colonization factors, outer membrane proteins, proteases, haemolysins, haemagglutinins (HAs), and in some strains, a capsular polysaccharide, all of which may contribute to survival and multiplication within the host [10, 15]. The genes that encode the cholera toxin subunits ctxA and ctxB are localized at a CTX genetic element, which is made up of a 4.6 kbp central core region 2.4 kbp repititive sequence termed RS2. Similar RS sequences called RS1 may flank the CTX element [16]. Zonula occludens toxin increases the permeability of enterocytes and is encoded by zot gene, which is within the core region of CTXф [17]. A third toxin encoded by ace affects intestinal secretion [16]. HylA for haemolysin damages cells by acting as pore-forming toxin and studies have shown purified haemolysin is enterotoxic [10]. Outer membrane proteins, which include OmpU, OmpT, OmpS, OmpV and others, are major cell-envelope proteins. OmpU is thought to contribute to bile resistance and functions as a colonizing factor [18]. Bile salts facilitate survival of V. cholerae in the intestine [19]. Toxin co-regulated pilus is clearly required for intestinal colonization [20].
The V. cholerae biofilm life cycle occurs in four stages (graphical Fig. 1), starting with the initial attachment of cells to a surface, formation of microcolonies, maturation of the microcolonies into an established biofilm, and dispersal of planktonic bacteria from the biofilm [19].
Fig. 1.
Gel electrophoresis for confirmation of various genes extracted from the seven isolates of V. cholerae that were resistant to various commonly used antibiotics with plate A representing the ctxA gene in clinical V. cholerae , plate B representing inDS, toxR and int genes in clinical V. cholerae isolate and plate C representing tetA and Ery genes in clinical V. cholerae isolates. The legends: lane 1 (05/17–03 isolate*); lane 2 (06/17–14 isolate*); lane 3 (03/17–16 isolate*); lane 4 (04/17–07 isolate*); lane 5 (02/17–09 isolate*); lane 6 (04/17-13Isolate*) and lane 7 (06/17–07 isolate*). Lane M1 for plate A represents 2 kb DNA Size Marker- Hyper ladder I ctxA band 289 bp; lane M1 for plate B represents 2 kb DNA Size Marker- Hyper ladder I inDS 869 bp, toxR 779 bp and int 481 bp and lane M1 for plate C represents 2 kb DNA Size Marker- Hyper ladder I, tetA 862 bp and Ery 564 bp. * The figures in brackets represent strain numbers.
In all these phases of biofilm formation, quorum-sensing (QS) system is involved in the regulation of population density and metabolic activities. QS system is a central component of bacterial cell-to-cell communication [21], acting as a language for the interaction among the neighbouring bacteria that collectively and genetically respond to the extracellular, diffusible small molecule signals released in a cell-density-dependent manner [22]. As such, the production of molecule signals – autoinducing peptides (AIP) such as AgrD peptide – can be controlled and helps the bacteria in overwhelming the host defences by secreting exotoxins after sufficient colonization in the host has taken place [23].
Microbial cells embedded in a matrix containing polysaccharides, proteins and extracellular microbial DNA are known as biofilm [24–26]. The biofilm-forming pathogens like Vibrio cholerae and drug-resistant pathogens like MRSA can cause fatal diseases to human beings and become resistant to most of the antimicrobial drugs [27]. This is because biofilms are less susceptible to antibiotics, and provide a reservoir for microbial cells, which when dispersed enhances the risk of chronic and persistent infections. It may also promote the reinfection of colonized sites hence increasing the management cost and hospital stay [28, 29]. Likewise, the matrix confers a protection against biocides, immune system activity and drugs and has environmental promoters that induce biofilm formation that contributes to drug resistance development [30, 31]. All these factors contribute to biofilm cells being 1000-fold more resistant to antimicrobial agents than planktonic cells [24]. Moreover, treatment and control of biofilm is complicated because the current antimicrobials have been developed for planktonic cells that are metabolically active [32].
Other virulence factors are also produced by different strains of V. cholerae and may be related to the hydrolysis of lipid barrier in intestinal epithelial cells [33]. While Neuraminidase, is secreted to cause an increase in the number of receptors in the gut [34]. Therefore, it is against this background that this study focused on deducing biofilm formation and inhibition ability, virulence and resistant gene profiling of the V. cholerae isolates obtained from the 2017 cholera outbreak at Kisumu County, Kenya. An in-depth understanding of essential features of V. cholerae strains involved in cholera outbreaks will help in the development strategies to combat future outbreaks.
Methods
(i) The V. cholerae isolates' source
A total of 119 isolates were obtained from the Kenya medical research Institute (KEMRI – Kisumu) where cultures stocks have been kept following the 2017 cholera outbreak at −80 °C. Long-term storage of specimens at −80 °C was done in 10 % glycerol. These cultures were obtained from stool samples randomly selected from patients with severe diarrhoea suspected of having cholera during the outbreak. Consent was obtained from the patients prior to collection of stool samples.
(ii) Culturing of the V. cholerae isolates
Diarrhoeal stool samples were thawed and cultured on alkaline peptone water followed by 6 h incubation at 37 °C using established procedures as used before [35]. The cultures were then plated on thiosulfate citrate bile salts sucrose (TCBS) agar following the manufacturer’s instructions. Agar plates were incubated at 37 °C for 18–24 h. Typical yellow colonies, which were presumed to be V. cholerae isolates [36], were selected and subjected to biochemical, serological and genotypic analyses as previously outlined [35].
(iii) Biofilm formation inhibition assay
As described by Omwenga et al. [37], microtitre plate assay was performed to quantify the effect of commonly used antibiotics on the biofilm formation of V. cholerae strains. The test bacteria were first inoculated on Luria-Bertani medium (LB) agar and incubated at 37 °C overnight. Then a colony was identified, picked and inoculated in 10 ml of LB broth and incubated at 37 ˚C overnight while shaking at 100 r.p.m. for 18 h. By use of a parafilm the flat-bottomed polystyrene tissue culture microplate was sealed for purposes of preventing medium evaporation. After 48 h incubation, the wells were carefully rinsed with double-distilled water to remove loosely attached cells. The microplate was air-dried for 1 h before adding 200 µl per well of 0.4 % crystal violet (CV) solution to the adhered cells in the wells and then stand at room temperature for 15 min. Excess stain was removed by rinsing the wells gently with 200 µl per using distilled water. This was repeated thrice. The microtitre plate was then air-dried for 1 h after, followed by addition of 200 µl of absolute ethanol to each well to solubilize the dye (Data S1, available in the online version of this article). The OD was measured at OD590nm using a Safire Tecan-F129013 Microplate Reader (Tecan, Crailsheim, Germany). For each experiment, background staining was corrected by subtracting the crystal violet bound to un-treated controls (Blank) from those of the tested sample. The experiments were done in triplicate and average OD590nm values were calculated. To estimate the antibiofilm activity (Abf A) of a given antibiotic the following equation was used
Abf A (%) = (1-(ODTest sample - ODBlank)/ (OD Untreated sample – OD Blank)×100.
(iv) Molecular biology techniques/assays – PCR and gel electrophoresis
Confirmation of identification of V. cholerae was performed using a conventional PCR assay targeting the ctxA gene. Additionally, Vibrio spp. were screened for the presence of CtxA, toxR, inDS, int, tetA and Ery gene to determine the specificity of the assay. The specific primers (Data S2) selected for PCR analysis of the ompW gene, according to [3]. These primers are synthesized by the Alpha DNA Company, Canada. PCR analysis was conducted in 50 µl of a reaction mixture containing 24 µl of GoTaq Green Master, 2 µl of 25 mmol l−1 MgCl2, 2 µl of (100 pmol) primer, and 10 µl of distilled water. Amplification was conducted using a master cycler (Eppendorf) programmed with 1 cycle at 95 °C for 1 min, 40 cycles of 95 °C for 1 min, 64 °C for 1 min, 72 °C for 1 min, 72 °C for 10 min. The amplified product was subjected to 1.8 % agarose gel electrophoresis and visualized under UV after ethidium bromide staining [8].
(v) Detection of other virulence factors of V. cholerae
Detection of haemolysin
Haemolysin production by the V. cholerae isolates was detected following protocols by Benson et al. [38]. The β-haemolytic activity was tested for on base agar (Himedia, India) supplemented with 7 % sheep erythrocytes for 18–24 h. Pure isolates were cultured on TSA, before streaking on blood agar and further incubated for 24 h at 37 °C. Zones of haemolysis around the colonies indicated the ability of these bacteria to haemolyse RBCs [39].
Detection of protease
To detect protease production by the V. cholerae isolates skim milk agar was used and the protocol that was described in [38]. Briefly, two solutions (A and B) were made and used in this study. Solution A was prepared by adding 10 g skim milk to 90 ml of distilled water then volume was completed to 100 ml gently heated at 50 °C, then autoclaved and cooled to 50–55 °C. And solution B was also prepared by adding 2 g of agar powder to 100 ml of distilled water, mixed thoroughly, then autoclaved and cooled to 50–55 °C. Aseptically, 100 ml of solution A was mixed with 100 ml of solution B. Then the mixture was poured into sterile petri dishes, and then stored at 4 °C until use. This media used to detect the ability of the bacteria to produce protease [30]. The appearance of a cleared hydrolysis zone indicates a positive test [39].
Detection of lipase
Lipase production ability by V. cholerae isolates was determined by methods outlined by Elliot et al. [39]. Briefly, a single colony of an overnight growth was cultured on Rhan medium (Data S3), and then incubated for 1–5 days at 37 °C. The appearance of a turbid zone around colonies indicates a positive result [38].
Detection of lecithinase (phospholipase)
To detect lecithinase, we followed a standard procedure [40]. One pure colony was cultured on medium of phospholipase activity assay (Data S4) followed by incubation for 1–3 days at 37 °C using established procedures [35]. The appearance of a white to brown colour elongated precipitated zone around colonies is considered a positive result [1].
Statistical analysis
Statistical analyses were carried out using Graph pad prism (version 8; La Jolla, CA, USA). Means were presented as mean±sem as all tests were carried out in triplicates.
Results
V. cholerae O1 virulence genes
Eighty-one (80.2 %) isolates possessed the cholera toxin gene (ctxA). Analysis of the toxR gene revealed that 99 (98.0 %) harboured the toxR gene. Using PCR, a variety of pathogenic and antimicrobial resistance genes were detected (Table 1). It was also revealed that 81 (80.2 %) of the isolates harboured the class I integron (encoded by inDS gene). The majority, 94 (93.1 %) were confirmed to possess the SXT integrating conjugative element (ICE). The tetracycline resistance gene was present in 97 (96.0 %) of the isolates. Seven isolates were confirmed to be resistant against commonly used antibiotics.
Table 1.
Analysis of pathogenic and antimicrobial resistance genes (Data S3) by PCR in V. cholerae isolates from cholera outbreaks in Kisumu County, 2017 (n=101)
|
Primer |
Target gene |
Positive N (%) |
Negative N (%) |
|---|---|---|---|
|
ctxA |
Cholera toxin |
81 (80.2) |
20 (19.8) |
|
toxR |
Regulatory gene |
99 (98.0) |
2 (2.0) |
|
inDS |
Class one intergron |
81 (80.2) |
20 (19.8) |
|
int |
SXT intergrase |
94 (93.0) |
7 (7.0) |
|
tetA |
Tetracycline resistance |
4 (4.0) |
97 (96.0) |
|
Ery |
Erythromycin resistance |
90 (90.0) |
11 (10.0) |
Based on PCR analysis, the ace gene was revealed in all seven isolates as shown in plate A (Fig. 1). Also, inDS, toxR and int genes were revealed to be present as presented in plate B (Fig. 1). Additionally, PCR genotyping also did show the presence of ctxA and tcpI genes from clinical V. cholerae isolates as represented in plate C in Fig. 1 above.
Biofilm formation inhibitory effects of selected antibiotics against the V. cholerae strains
The most resistant drugs towards the seven isolates (tetracycline, ampicillin, amoxicillin, cotrimoxazole, erythromycin and nalidixic acid) [36] were used as treatments for the antibiofilm formation assay in a 96-well microtitre plate. Different concentrations of the drugs were prepared using twofold serial dilution (with dosage ranging from 0.5 to 0.03125 mg ml−1) and the wells were inoculated with 10 µl of V. cholerae isolates. P. aeruginosa ATCC10145 was used as positive control. The results showed that the biofilm formation inhibitory effects of the various concentrations (0.5, 0.25, 0.125, 0.0625 and 0.03125 mg ml−1) were significantly lower than that of the positive control, an indication that biofilm formation was inhibited at these concentrations (Figs. 2–7). As much as such inhibitory effects were recorded these findings clearly demonstrate that the seven isolates that proved to be resistant to commonly used antibiotics have the ability of forming biofilms.
Fig. 2.
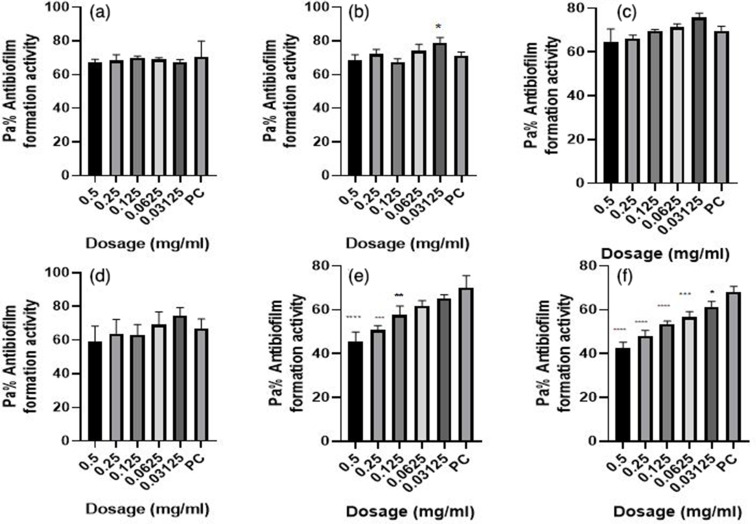
Antibiofilm formation activity against isolate 03/17–16 of V. cholerae against various antibiotics: (a) tetracycline (b) ampicillin, (c) amoxicillin, (d) cotrimoxazole, (e) erythromycin and (f) nalidixic acid; PC=P. aeruginosa – Positive control (n=3, ANOVA Dunnett’s multiple comparisons test; *P=0.05; **P=0.01; ***P=0.001; ****P=0.0001).
Fig. 3.
Antibiofilm formation activity against isolate 02/17–09 of V. cholerae against various antibiotics: (a) tetracycline, (b) ampicillin, (c) amoxicillin, (d) cotrimoxazole, (e) erythromycin and (f) nalidixic acid; PC=P. aeruginosa – positive control (n=3, Anova Dunnett’s multiple comparisons test; *P=0.05; **P=0.01; ***P=0.001; ****P=0.0001).
Fig. 4.
Antibiofilm formation activity against isolate 04/17–13 of V. cholerae against various antibiotics: (a) tetracycline (b) ampicillin, (c) amoxicillin, (d) cotrimoxazole, (e) erythromycin and (f) nalidixic acid; PC=P. aeruginosa – positive control (n=3, Anova Dunnett’s multiple comparisons test; *P=0.05; **P=0.01; ***P=0.001; ****P=0.0001). Against this test isolate 04/17–13, inhibitory activities were observed to be more at lower dosages than at high dosages.
Fig. 5.
Antibiofilm formation activity against isolate 05/17–07 of V. cholerae against various antibiotics: (a) tetracycline (b) ampicillin, (c) amoxicillin, (d) cotrimoxazole, (e) erythromycin and (f) nalidixic Acid; PC=P. aeruginosa – positive control (n=3, Anova Dunnett’s multiple comparisons test; *P=0.05; **P=0.01; ***P=0.001; ****P=0.0001).
Fig. 6.
Antibiofilm formation activity against isolate 05/17–03 of V. cholerae against various antibiotics: (a) tetracycline, (b) ampicillin, (c) amoxicillin, (d) cotrimoxazole, (e) erythromycin and (f) nalidixic acid; PC=P. aeruginosa – positive control (n=3, Anova Dunnett’s multiple comparisons test; *P=0.05; **P=0.01; ***P=0.001; ****P=0.0001).
Fig. 7.
Antibiofilm formation activity against isolate 06/17–14 of V. cholerae against various antibiotics: (a) tetracycline, (b) ampicillin, (c) amoxicillin, (d) cotrimoxazole, (e) erythromycin and (f) nalidixic acid; PC=P. aeruginosa – positive control (n=3, Anova Dunnett’s multiple comparisons test; *P=0.05; **P=0.01; ***P=0.001; ****P=0.0001).
Antibiofilm formation activity against isolate 03/17–16 of V. cholerae against various antibiotics like tetracycline, ampicillin, amoxicillin, cotrimoxazole, erythromycin and nalidixic acid was observed in all the antibiotics (Fig. 2). Against ampicillin, a concentration of 0.03125 mg ml−1 yielded significant biofilm formation inhibition (P=0.05), while for erythromycin obtained significant differences on the biofilm formation inhibition at a concentration of 0.5 mg ml−1 (P=0.0001), 0.25 mg ml−1 (P=0.001), 0.125 mg ml−1 (P=0.01). Nalidixic acid treatment yielded significant biofilm inhibition at concentrations of 0.5, 0.25, 0.125, 0.0625 (P=0.0001) and 0.03125 mg ml−1 (P=0.05). It is more worrying that tetracycline, which was the commonly used antibiotic in the outbreak, had less inhibitory effects against biofilm formation.
Antibiofilm formation activity against isolate 02/17–09 of V. cholerae against various antibiotics was seen in all the antibiotics (Fig. 3), with some significant differences at various dosages. Amoxicillin did not have good inhibitory effects against isolate 02/17–09. On the other hand, cotrimoxazole showed a reverse activity with high inhibitory effects being observed at low dosages as compared with higher concentrations.
In Fig. 4 above antibiofilm formation activity against isolate 04/17–13 of V. cholerae against various antibiotics was seen in all the antibiotics with most antibiotics producing significant differences as compared with the positive control at various dosages as shown in Fig. 4 above. With this test isolate also inhibitory activities were observed more at lower dosages as compared at high dosages in all antibiotics bioassayed.
Isolate 05/17–07 was more prone to the inhibitory effects of the various dosages of the various antibiotics as compared to other isolates. Significant differences were also observed in most of the antibiotics used compared with the positive control (Fig. 5).
Antibiofilm formation activity against isolate 05/17–03 of V. cholerae for various antibiotics was low (Fig. 6). Interestingly, the concentrations of 0.5, 0.25, 0.125, 0.0625 and 0.03125 mg ml−1 were able to inhibit biofilm formation much more as compared with the positive control.
Antibiofilm formation activity against isolate 06/17–14 of V. cholerae against various antibiotics was seen in all the antibiotics, with no significance differences on tetracycline at all the concentration as compared with the positive control (Fig. 7). The rest of the antibiotics produced significant antibiofilm activity. Erythromycin, tetracycline and nalidixic acid also showed significant inhibitory activity against this isolate 06/17–14 at lower concentrations.
Detection of some virulence factors of V. cholerae
The study investigated the production of various virulence enzymes like protease, phospholipase, lipase and haemolysin (Fig. 8) on the seven isolates, which were found to be resistant to all drugs as examined from our previous study [36]. It was revealed that 5/7 (71.42 %) of these isolates of V. cholerae produced protease enzyme (Fig. 9 – plate A; Fig. 8). Also, it was confirmed that six out seven isolates (85.71%) produce phospholipases (Fig. 9 – plate B; Fig. 8). Further findings also indicate that out of the seven isolates five of them (71.42%) had the ability to produce lipase (Fig. 9 – plate D; Fig. 8). Lastly, it was determined that all the seven isolates were able to produce the haemolysin by haemolysing the sheep red blood cells causing beta (β) haemolysis (Fig. 9 – plate C; Fig. 8) above. Haemolysin therefore was the most produced virulence factor by these isolates (100%) and it was followed by phospholipase (85.71%) and lastly lipase and protease (71.42%). Out of the seven isolates four isolates (03/17–16, 02/17–09, 04/17–13 and 06/17–07) produced all the virulence traits studied, two isolates (06/17–14 and 05/17–07) produced at least three virulence traits and one isolate (05/17–03) produced at least one of the virulence traits.
Fig. 8.
Showing the distribution of the seven isolates verses the various virulence traits they produced.
Fig. 9.
Representation of the various virulence traits produced by the V. cholerae isolates (a). The distribution of Protease enzyme production of V. cholerae (b). The Phospholipase enzyme production of V. cholerae (c). The haemolysin enzyme production of V. cholerae – haemolytic activity (d). Lipase enzyme production of V. cholerae .
Discussion
From this study, it was revealed that seven isolates that had been obtained from our previous study were resistant to various commonly used antibiotics [36] indeed possess various factors that enable them either to be resistant or induce various infections in humans. Some of these traits are controlled at the gene level and as such, they can be passed on from one bacteria cell to another through the pilli [27]. Antimicrobial drug resistance in Vibrio species for instance, may arise through mutation or through acquisition of resistance genes on mobile genetic elements like plasmids, transposons integrons and integrating conjugative elements [41]. Isolates analysed in this study possessed the class I integron and the SXT integrating conjugative element. Genetic elements like the class I integron (inDS) and the integrating conjugative elements such as SXT have been associated with the spread of genetic determinants, encoding for antimicrobial resistance in V. cholerae [42]. The SXT element has been reported to harbour genes encoding for resistance to chloramphenicol (encoded by floR), streptomycin (encoded by strA and strB), trimethoprim (encoded by dfrA18) and sulfamethoxazole (encoded by sul2) [43]. The class 1 integron has also been reported to harbour aminoglycoside-resistant gene cassettes in V. cholerae O1 isolates [44]. As such therefore, resistance to erythromycin in the analysed isolates could be attributed to the presence of the class 1 integron gene that was found to be present in some strains isolated.
The finding of susceptible isolates towards streptomycin but still amplifying a 383 bp fragment of the strA gene suggests that this gene is not an intrinsic feature of this family of integrase, but rather appears to have been inserted into these elements, becoming transmissible in bacterial populations, as reported by elsewhere [45]. Nalidixic acid resistance observed in this study could be attributed to mutations in the gyrA gene. Studies have reported gyrA gene mutations in fluoroquinolone-resistant clinical isolates of V. cholerae since it contains the active site tyrosine that forms a transient covalent intermediate with DNA hence making it to be resistance to the drug [46]. However, more studies need to be done to confirm this.
Some studies have also suggested that erythromycin and nalidixic acid do inhibit growth at high concentrations [12, 32] but from our study, it was deduced that they inhibit biofilm formation at lower concentrations. The same scenario was also observed in most antibiotics like tetracycline, amoxicillin, cotrimoxazole and others with such ‘Goldilocks’ effect. A possible explanation to the less activity observed at greater doses could be associated to the aggregation effects of the antibiotics at site of entry into bacteria cell especially at high dosages something that is not observed at lower dosages. It is likely that aggregation may favour biofilm formation as antibiotics struggle to reach at the point of action and hence bacteria will continue to thrive and hence form more biofilms [47]. This finding agrees with the previous studies on biofilm inhibitions by Taganna et al. [48], which showed higher biofilm inhibitory at lower dosage concentration against the positive control. However, it did not concur with the previous study on biofilm inhibition by [49], which showed that Erythromycin growth inhibitory at high concentration but also biofilm inhibitory at high concentration as compared with positive control [49]. On the other hand, tetracycline, ampicillin, amoxicillin and cotrimoxazole were found to inhibit biofilm formation at higher concentration in some isolates and against the positive control. However, it should be noted that they did not fully inhibit biofilm formation ability of the test isolates a clear indication that proper antibiotics should be used in management of conditions caused by these isolates.
Isolates 05/17–07 and 05/17–03 of V. cholerae seemed to be very sensitive to the antibiotics screened at various dosages. This clearly demonstrates that the biofilm formation by these isolates can easily be managed by these antibiotics. Since they proved to be resistant when they were subjected to antimicrobial tests following standard methods [36]. It is possible that these isolates could be possessing other mechanisms of activity against the antibiotics screened. One of such possible mechanisms could be the presence of multi-drug efflux pumps in them as they have been found to be present in some strains of V. cholerae [38]. This makes the bacteria to be resistant to antibacterial agents and other toxic compounds by a mechanism known as active efflux, where the integral membrane transporters known as drug efflux pumps, prevent the accumulation of drugs inside the bacterial cells [48].
Further, the study investigated the ability of these test strains to produce various virulence factors, which may play a role in their pathogenicity. Among the virulence traits examined include detection of proteases, lipases, haemolysin and phospholipase. The study revealed that 71.42 % of the isolates of V. cholerae produced protease. These findings confirm the findings of a previous study [50], which showed that most isolates were protease positive, and that protease enzyme have limited effect on the pathogenesis of this bacteria. Findings from the current study also agree with a study that documented that all isolates had the ability for protease production [14]. Proteases produced by V. cholerae have a critical role in pathogenicity, as they are responsible for hydrolysis of several physiologically important proteins such as mucin, fibronectin and lactoferrin [51]. It could also proteolytically activate cholera toxin A subunit, El Tor cytolycin and haemolysin, hence making this pathogen more virulent [52].
For phospholipases, 85.71 % of the isolates were found to be positive. These findings further confirm previous study findings where out of 20 isolates, 13 (67 %) isolates were found to have phospholipase production potential [13]. Our findings are also in tandem with Chung and Toh's [53] study findings for phospholipase presence. As previously mentioned [44], the role of this enzyme in the cholera disease by the release of arachidonic acid from the phospholipid found in the cell membranes of the lumen cells, this plays an important role in the prostaglandin E2 (PGE2) production, which is responsible for the increase of liquid secretion from the lumen cells. Therefore, its presence makes the V. cholerae isolate more virulent in watery diarrhoea production – a key symptom of cholera [54].
Also, out of the seven isolates five (71.42 %) had the ability to produce lipase. Our findings also concur with other studies, which showed that all isolates obtained in the study had the ability to produce lipase [14]. Lipases enzymes catalyse the hydrolysis of the ester bonds of triacylglycerols and may have a critical role in V. cholerae pathogenicity or nutrition acquisition. The production of an excess amount of lipases allows bacteria to penetrate fatty tissue with the consequent formation of abscesses [2]. The production of these enzymes by the isolates may reflect the presence of genetic organization of a discrete genetic element, which encodes three genes responsible to produce proteases, lipases and phospholipase. This organization could be a possible part of pathogenic island, encoding a product capable of damaging host cells and being involved in nutrient acquisition [51].
In this study, all isolates were able to produce the haemolysin. A finding that conforms with a previous study [50], where 100 % of isolates were haemolysin positive. As stated before, purified haemolysin can cause fluid accumulation [1], in contrast to the watery fluid produced in response to CT, the accumulated fluid produced in response to haemolysin with invariably bloody with mucous [1].
Conclusion
From this study, it can be concluded that most clinical isolates had resistant genes and produced various virulence factors such as haemolysin, lipase, protease and phospholipase. As much as inhibitory effects were recorded, these findings clearly demonstrate that the isolates proved to be resistant to commonly used antibiotics and they do form biofilms. These findings, therefore, add value to our previous findings on these seven isolates that proved to be resistant against commonly used antibiotics in the management of cholera during a outbreak [36]. To the best of our knowledge, this is the first time such data has been documented from the outbreak region, that could help in combating future outbreak events.
Supplementary Data
Funding information
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgements
Authors thank KEMRI-Kisumu, Kenya for provision of specimens from the 2017 cholera outbreak for use in this study. Authors also thank KEMRI Laboratories-Busia, Kenya for providing laboratory space and other resources used this study.
Author contributions
All authors contributed equally to this work.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
The study was cleared by Board of Post graduate Studies (BPS) of Kisii University (S8), and Ethical approval to conduct the study was sought from the Institutional Research Ethics Committee (IREC) (S9) at Moi University/ Moi Teaching and Referral Hospital (MTRH), the National Commission of Science Technology and Innovations (NACOSTI) (S10), County Director Department Of Health and Sanitation-Kisumu County (S11), County Commissioner Ministry of Interior and Coordination of National Government-Kisumu County (S13) and County Director of Education Department of Early Learning and Basic Education-Kisumu County (S14)
Footnotes
Abbreviations: Abf A, antibiofilm activity; ADP, adenosine diphosphate; AIP, autoinducing peptides; ATCC, American type culture collections; CV, crystal violet; DNA, deoxyribonucleic acid; Has, haemagglutinins; IREC, Institutional Research Ethics Committee; KEMRI, Kenya medical research Institute; LB, Luria-Bertani medium; MRSA, methicillin resistant Staphylococcus aureus; MTRH, Moi Teaching and Referral Hospital; NACOSTI, National Commission of Science Technology and Innovations; OD, optical density; PCR, polymerase chain reaction; PGE2, Prostaglandin E2; QS, quorum-sensing; RBCs, red blood cells; TCBS, thiosulfate citrate bile salts sucrose; TCP, toxin-coregulated pilus; TSA, trypticase soy agar; UV, ultraviolet; VPI, V. cholerae pathogenicity island; WHO, World Health Organisation.
Supplementary material is available with the online version of this article.
References
- 1.Halpern M, Izhaki I. Fish as hosts of Vibrio cholerae . Front Microbiol. 2017;8:282. doi: 10.3389/fmicb.2017.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakib SN, Reddi G, Almagro-Moreno S, DiRita VJ. Vibrio JB special issue. minireview environmental role of pathogenic traits in Vibrio cholerae . J Bacteriol. 2018;200:17. doi: 10.1128/JB.00795-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthey N, Blokesch M. The DNA-Uptake process of naturally competent Vibrio cholerae . Trends Microbiol. 2016;24:98–110. doi: 10.1016/j.tim.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Cairncross S, Feachem R. Environmental Health Engineering in the Tropics. Vol. 134. Routledge; 2018. Water, sanitation and disease control; pp. 865–871. vol. [DOI] [Google Scholar]
- 5.Craig RK. Cholera and climate change: pursuing public health adaptation strategies in the face of scientific debate. Houst J Health Law Policy. 2018;3:964–999. doi: 10.1015/S22211691(14)60185-9. [DOI] [Google Scholar]
- 6.Lessler J, Moore SM, Luquero FJ, McKay HS, Grais R, et al. Mapping the burden of cholera in sub-Saharan Africa and implications for control: an analysis of data across geographical scales. Lancet. 2018;391:1908–1915. doi: 10.1016/S0140-6736(17)33050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Cholera Fact sheet Number 107. 2016. http://www.who.int/mediacentre/factsheets/fs107/en/index.html
- 8.Siriphap A, Leekitcharoenphon P, Kaas RS, Theethakaew C, Aarestrup FM, et al. Characterization and genetic variation of Vibrio cholerae Isolated from Clinical and Environmental Sources in Thailand. PLoS One. 2017;12:e0169324. doi: 10.1371/journal.pone.0169324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AL-Fatlawy HNK, Aldahhan HA, Alsaadi AH. Phylogenetic of DNA fingerprinting and new sequencing of Aeromonas species and V. cholerae DNA. Am J Appl Sci. 2017;14:955–964. doi: 10.3844/ajassp.2017.955.964. [DOI] [Google Scholar]
- 10.Moore S, Thomson N, Mutreja A, Piarroux R. Widespread epidemic cholera caused by a restricted subset of Vibrio cholerae clones. Clin Microbiol Infect. 2014;20:373–379. doi: 10.1111/1469-0691.12610. [DOI] [PubMed] [Google Scholar]
- 11.Kirn TJ, Lafferty MJ, Sandoe CM, Taylor RK. Delineation of pilin domains required for bacterial association into microcolonies and intestinal colonization by Vibrio cholerae . Mol Microbiol. 2000;35:896–910. doi: 10.1046/j.1365-2958.2000.01764.x. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hadrawy HAN. A Comparative study of Bacteriological and Molecular Vibrio Cholera Isolated from the Tigris and Euphrates. PhD Thesis. University of Kufa in Arabic; Iran: 2012. [Google Scholar]
- 13.Jabik NA. Study of some Genetic Aspects of Isolated V. cholerae in Babylon. M.Sc. Thesis. University of Babylon; Iraq: 2000. [Google Scholar]
- 14.Al-Khafaji KAA. Identification of Some Virulence Factors in Toxigenic Clinical and Environmental Isolates of Vibrio cholerae. MSc. Thesis. University of Baghdad; Iraq: 2007. [Google Scholar]
- 15.AL-Fatlawy HNK, Al-Ammar MH. Study of some virulence factors of aeromonas hydrophila isolated from clinical samples (iraq) Int J Sci Eng Invest. 2013;2:22113–22116. [Google Scholar]
- 16.Jawetz E, Melnick JI, Adelberg EA. Medical Microbiology. 27th Edn. Appleton and Lange U.S.A; 2016. [Google Scholar]
- 17.Yang QH, Zhou C, Lin Q, Lu Z, He LB, et al. Draft Genome Sequence of Aeromonas sobria Strain 08005, Isolated from Sick Rana catesbeiana. Genome Announc. 2017;5:e01352-16. doi: 10.1128/genomeA.01352-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Provenzano D, Schuhmacher DA, Barker JL, Klose KE. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect Immun. 2000;68:1491–1497. doi: 10.1128/IAI.68.3.1491-1497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fasano A. Bacterial infections: small intestine and colon. Curr Opin Gastroenterol. 2001;17:4–9. doi: 10.1097/00001574-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Reguera G, Kolter R. Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin. J Bacteriol. 2005;187:3551–3555. doi: 10.1128/JB.187.10.3551-3555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asad S, Opal SM. Bench-to-bedside review: Quorum sensing and the role of cell-to-cell communication during invasive bacterial infection. Crit Care. 2008;12:236. doi: 10.1186/cc7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 23.Pan J, Ren D. Quorum sensing inhibitors: a patent overview. Expert Opin Ther Pat. 2009;19:1581–1601. doi: 10.1517/13543770903222293. [DOI] [PubMed] [Google Scholar]
- 24.Taraszkiewicz A, Fila G, Grinholc M, Nakonieczna J. Innovative strategies to overcome biofilm resistance. Biomed Res Int. 2013;2013:150653. doi: 10.1155/2013/150653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simões M, Simões LC, Vieira MJ. A review of current and emergent biofilm control strategies. LWT - Food Science and Technology. 2010;43:573–583. doi: 10.1016/j.lwt.2009.12.008. [DOI] [Google Scholar]
- 26.Lazar V. Quorum sensing in biofilms – How to destroy the bacterial citadels or their cohesion/power? Anaerobe. 2011;17:280–285. doi: 10.1016/j.anaerobe.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Herrington DA, Hall RH, Losonsky G, Mekalanos JJ, Taylor RK, et al. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Wen Y. The role of bacterial biofilm in persistent infections and control strategies. Int J Oral Sci. 2011;3:66–73. doi: 10.4248/IJOS11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan S-E, Chew SC, Tan S-Y, Givskov M, Yang L. Emerging frontiers in detection and control of bacterial biofilms. Curr Opin Biotechnol. 2014;26:1–6. doi: 10.1016/j.copbio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Poole K. Bacterial stress responses as determinants of antimicrobial resistance. J Antimicrob Chemother. 2012;67:2069–2089. doi: 10.1093/jac/dks196. [DOI] [PubMed] [Google Scholar]
- 32.Ammons MCB. Anti-biofilm strategies and the need for innovations in wound care. Recent Pat Antiinfect Drug Discov. 2010;5:10–17. doi: 10.2174/157489110790112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pride AC, Guan Z, Trent MS. Characterization of the Vibrio cholerae VolA surface-exposed lipoprotein lysophospholipase. J Bacteriol. 2014;196:1619–1626. doi: 10.1128/JB.01281-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AL-Fatlawy HNK, Al-Ammar MH. Study of some virulence factors of aeromonas hydrophila isolated from clinical samples (iraq) Int J Sci Eng Invest. 2013;2:22113–22116. [Google Scholar]
- 35.CLSI Wayne, PA: Clinical Laboratory Standards Institute; 2010. Performance standards for antimicrobial sensitivity testing. 30th ed. CLSI supplement M100. [Google Scholar]
- 36.Awuor SO, Omwenga EO, Daud II. Geographical distribution and antibiotics susceptibility patterns of toxigenic Vibrio cholerae isolates from Kisumu County, Kenya. Afr J Prim Health Care Fam Med. 2020;12:e1–e6. doi: 10.4102/phcfm.v12i1.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omwenga EO, Hensel A, Pereira S, Shitandi AA, Goycoolea FM. Antiquorum sensing, antibiofilm formation and cytotoxicity activity of commonly used medicinal plants by inhabitants of Borabu sub-county, Nyamira County, Kenya. PLoS One. 2017;12:e0185722. doi: 10.1371/journal.pone.0185722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benson HJ. Microbiological Applications: Laboratory Manual in General Microbiology. 8th edn. Complete version. U.S.A: McGraw-Hill; 2002. [Google Scholar]
- 39.Elliot EL, Kaysner CA, Jackson L, Tamplin ML. In: Food and Drug Administration: Bacteriological Analytical Manual, Chapter 9. 8th ed. Merker R, editor. Gaithersburg, MD: AOAC International; 2001. Valnificus and other Vibrio spp. [Google Scholar]
- 40.Dogan B, Boor KJ. Genetic diversity and spoilage potentials among Pseudomonas spp. isolated from fluid milk products and dairy processing plants. Appl Environ Microbiol. 2003;69:130–138. doi: 10.1128/AEM.69.1.130-138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjölund-Karlsson M, Reimer A, Folster JP, Walker M, Dahourou GA, et al. Drug-resistance mechanisms in Vibrio cholerae O1 outbreak strain, Haiti, 2010. Emerg Infect Dis. 2011;17:2151–2154. doi: 10.3201/eid1711.110720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalsgaard A, Forslund A, Sandvang D, Arntzen L, Keddy K. Vibrio cholerae O1 outbreak isolates in Mozambique and South Africa in 1998 are multiple-drug resistant, contain the SXT element and the aadA2 gene located on class 1 integrons. J Antimicrob Chemother. 2001;48:827–838. doi: 10.1093/jac/48.6.827. [DOI] [PubMed] [Google Scholar]
- 43.Beaber JW, Hochhut B, Waldor MK. Genomic and functional analyses of SXT, an integrating antibiotic resistance gene transfer element derived from Vibrio cholerae. J Bacteriol. 2002;184:4259–4269. doi: 10.1128/JB.184.15.4259-4269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliver JD, Kaper JB. In: Food Microbiology: Fundamentals and Frontiers. Doyl MP, Beuchat LR, Montville J, editors. Washington, DC; USA: ASM press; 2007. Vibrio Species; pp. 228–260. [Google Scholar]
- 45.Hochhut B, Lotfi Y, Mazel D, Faruque SM, Woodgate R, et al. Molecular analysis of antibiotic resistance gene clusters in vibrio cholerae O139 and O1 SXT constins. Antimicrob Agents Chemother. 2001;45:2991–3000. doi: 10.1128/AAC.45.11.2991-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baranwal S, Dey K, Ramamurthy T, Nair GB, Kundu M. Role of active efflux in association with target gene mutations in fluoroquinolone resistance in clinical isolates of Vibrio cholerae. Antimicrob Agents Chemother. 2002;46:2676–2678. doi: 10.1128/AAC.46.8.2676-2678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu KD, McFeters GA, Stewart PS. Biofilm resistance to antimicrobial agents. Microbiology. 2000;146:547–549. doi: 10.1099/00221287-146-3-547. [DOI] [PubMed] [Google Scholar]
- 48.Taganna JC, Quanico JP, Perono RMG, Amor EC, Rivera WL. Tannin-rich fraction from Terminalia catappa inhibits quorum sensing (QS) in Chromobacterium violaceum and the QS-controlled biofilm maturation and LasA staphylolytic activity in Pseudomonas aeruginosa. J Ethnopharmacol. 2011;134:865–871. doi: 10.1016/j.jep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 49.Vasavi HS, Arun AB, Rekha PD. Inhibition of quorum sensing in Chromobacterium violaceum by Syzygium cumini L. and Pimenta dioica L. Asian Pac J Trop Biomed. 2013;3:954–959. doi: 10.1016/S2221-1691(13)60185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbass NBM. Effectiveness of some physical and chemical factors on the morphological changes of Vibrio cholerae isolated from environment. Ph.D. Thesis. University of Al Mustansyria; Iraq: 2006. [Google Scholar]
- 51.Namdari H, Klaips CR, Hughes JL. A Cytotoxin-Producing Strain of Vibrio choleraeNon-O1, Non-O139 as a Cause of Cholera and Bacteremia after Consumption of Raw Clams. Journal of Clinical Microbiology. 2000;38:3518–3519. doi: 10.1128/JCM.38.9.3518-3519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Booth BA, Boesman-Finkelstein M, Finkelstein RA. Vibrio cholerae hemagglutinin/protease nicks cholera enterotoxin. Infect Immun. 1984;45:558–560. doi: 10.1128/iai.45.3.558-560.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung PY, Toh YS. Anti-biofilm agents: recent breakthrough against multi-drug resistant Staphylococcus aureus . Pathog Dis. 2014;70:231–239. doi: 10.1111/2049-632X.12141. [DOI] [PubMed] [Google Scholar]
- 54.O’Neill E, Pozzi C, Houston P, Humphreys H, Robinson DA, et al. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol. 2008;190:3835–3850. doi: 10.1128/JB.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.