To the Editor
Nonspecific myelodysplastic features (MDF) due to hematopoietic effects of the human immunodeficiency virus (HIV) were frequent before the antiretroviral therapy (ART) era. Pronounced hypocellularity, plasmacytosis, and eosinophilia were observed and are referred to as HIV myelopathy [1, 2]. These MDF alterations generally resolve with the institution of ART. They should be separated from myelodysplastic syndrome (MDS), a heterogeneous group of myeloid clonal diseases. Myelodysplastic syndromes can have a primary cause, also called de novo MDS, or secondary (sMDS), both with the risk of progression to acute myeloid leukemia (AML). Some case reports and small case series of MDS in well‐controlled HIV patients were described [3, 4, 5, 6]. Herein, a new case of a long‐stand well‐controlled HIV infected patient with MDS is reported. The MDS status evolved with clonal karyotype associated with trisomy 8 and ASXL1 mutation.
A 61‐year‐old black woman was diagnosed with HIV in 2002, and ART—Zidovudine, Lamivudine (3TC), and Efavirenz—was initiated. She remained on this treatment for 16 years with long‐term suppressed viral load, and CD4 cell counts >600 cells/mm3. In January 2018, she presented with progressive anemia and mental depression and the scheme was switched to Tenofovir, 3TC, and Dolutegravir. In September 2018, she developed severe anemia and thrombocytopenia (hemoglobin [Hb] 4.3 g/dL, leucocyte 8.7 × 109/L, neutrophils 2.2 × 109/L, and platelets 45 × 109/L, reticulocyte 0.13%, and serum ferritin 1225 μg/L) with red blood cell transfusion dependency. The polymerase chain reaction for Parvovirus B19 was negative on the blood sample. Bone marrow (BM) aspiration and biopsy were performed. The morphology disclosed a BM hypercellular with multilineage dysplasia (80%) and blast cells (5%). The histology was characterized by erythroid reduction and granulocytic hyperplasia with increased precursor cells and abnormal localization of immature precursors (ALIP). No acid‐fast or fungal microorganisms were observed. The G‐banding of BM cells identified the karyotype: 47,XX,+8[5]/46,XX[9] (Figure 1A), and the fluorescence in situ hybridization (FISH) analysis confirmed the trisomy 8 (Figure 1B). ASXL1 and DNMT3A somatic mutations were tested by Sanger sequencing [7, 8]. DNMT3A was wild type, and ASXL1 mutation in exon 12 (c.1772dup; p.Y591*A) was detected (Figure 1C). Additionally, two nonpathogenic single nucleotide polymorphisms were identified in exon 12 of ASXL1 (c.3973C>A p.L1325F; c.1965 C>T p.T655T) (Figure 1C). The patient was diagnosed with refractory anemia with excess of blasts 1 (MDS‐EB‐1) and at very high risk according to the revised International Prognostic Scoring System [9, 10]. The patient was under supportive treatment care with ART, red blood cell and platelet transfusions, erythropoietin, and one cycle of Azacitidine (75 mg/m2/day for 7 days) without improvement. Her renal function was preserved, and 7 months later the karyotype of the BM cells demonstrated a clonal evolution: 47,XX,+8[7]/48,XX,+8,+21[6]/49,XX,+8,+10,+21[9]/46,XX[4] (Figure 1D). FISH analysis using the probe LSI RUNX1 break apart (RUNX1 21q22 spectrum green/RUNX1T1 8q21 spectrum orange) identified cells with both trisomy 8 and trisomy 21 (Figure 1E). BM analysis maintained the MDS‐EB‐1 pattern, showing a pleomorphic and hyperplastic bone marrow, absence of megakaryocytic sector, erythroid aplasia, hyperplasia myeloid with dysplasia, and 5% of blasts. The biopsy of bone marrow depicted ALIP and erythroid aplasia. Despite all efforts, the hematological parameters disclosed low Hb level (3.5 g/dL), increasing white blood cell count (19.8 × 109/L), and deficient platelet levels (7 × 109/L) with circulating myeloblasts (3%) and the patient expired in July 2019.
FIGURE 1.
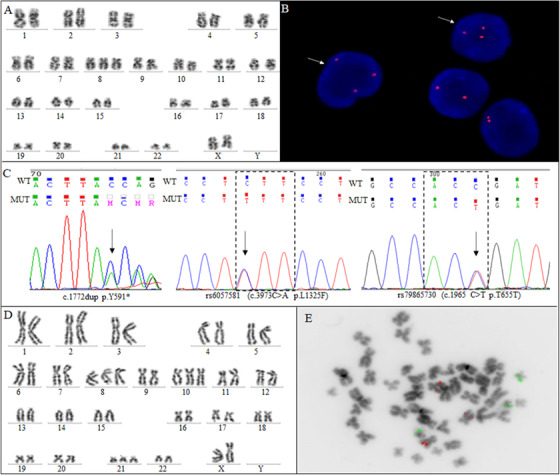
Cytogenetic and molecular alterations in a well‐controlled HIV patient with MDS. A, G‐banded showing the karyotype 47,XX,+8. B, FISH analysis using LSI MYC SpectrumOrange, red signal, with interphase nuclei counterstain with DAPI, Vysis. The arrows show an extra copy of the c‐myc gene, showing the gain of chromosome 8. C, Electropherogram of ASXL1 sequencing showing a frameshift mutation in exon 12 (c.1772dup; p.Y591*A) and two single nucleotide polymorphisms identified in exon 12 of ASXL1 (c.3973C>A p.L1325F; c.1965 C>T p.T655T). Arrows indicate the point of the mutation and polymorphisms. D, G‐banded showing the karyotype 49,XX,+8,+10,+21. E, FISH analysis using the probe LSI RUNX1 break apart (RUNX1 21q2 spectrum green/RUNX1T1 8q21spectrum orange) showing a metaphase with three red signals demonstrating the trisomy 8 and three green signals showing the trisomy 21
A broad scientific review disclosed that only 23 cases of well‐controlled HIV patients with MDS were described (Table 1). HIV portends a poor prognosis in MDS and patients have an increased prevalence of complex karyotypes characterized by monosomy 7 or deletion 7q at cytogenetic level, whereas at the molecular level, the most frequent alterations are somatic mutations in the ASXL1 exons11‐ 12, P53, and DNMT3A [3, 4, 5, 6]. Our patient developed a very high‐risk MDS with ASXL1 mutation and trisomy 8 followed by clonal karyotypic evolution after 16 years of well‐controlled HIV infection. Age and time to HIV diagnosis are compatible with what was previously described in HIV/MDS patients [6] and she had no history of classically therapy‐related MDS drugs.
TABLE 1.
Review of clinical, cytogenetic, and molecular features: Treatment and outcome of well‐controlled HIV‐positive patients with MDS
| HIV | MDS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age/Sex | Time from HIV (years) | ART | CD4+ count (cells/mm3) | Viral load | MDS Subtypes | IPSS | Karyotype | Gene mutations | Progression to AML | MDS treatment | Reference a |
| 1 | 63/ M | 13 | DDC, AZT, 3TC, ABC, EFV, TDF, TFC | 296 | < 40 | RAEB2/AML | >Int 2 | Complex with monosomy 7 | NA | Yes | Palliative | Rieg et al., 2009 [3] |
| 2 | 56/F | NA | ABC, 3TC, EFV | 206 | ND | RAEB | ≥Int 2 | Complex with del(5q) and monosomy 7 | NA | Yes | NA | Takahashi et al., 2012 [4] |
| 3 | 60/M | NA | ABC, 3TC, LPV | 500 | NA | RAEB (t‐MDS) | ≥Int 2 | Complex with del(5q) and monosomy 7 | NA | Yes | NA | Takahashi et al., 2012 [4] |
| 4 | 43/M | NA | NA | 223 | ND | RAEB | Int‐1 | 46,XY | NA | No | NA | Takahashi et al., 2012 [4] |
| 5 | 55/M | 23 | RTV, DRV, TFC, TDF | 500 | ND | RA | Int‐2 | Complex with monosomy 7 | NA | Yes | NA | Takahashi et al., 2012 [4] |
| 6 | 65/M | 26 | EFV, TFC, TDF | 300 | ND | RA | Int‐2 | Complex with monosomy 5 and 7 | NA | No | NA | Takahashi et al., 2012 [4] |
| 7 | 50/M | NA | ABC, 3TC, TFC, TDF, LPV | 231 | NA | RA | Int‐2 | 46,XY,del (20)(q11) | NA | No | NA | Takahashi et al., 2012 [4] |
| 8 | 51/M | NA | T20, FTC, TDF, RTV, CRV | 254 | >75,000 | RAEB | Int‐2 | 47,XY,+ 21 | NA | Yes | NA | Takahashi et al., 2012 [4] |
| 9 | 56/F | NA | ATV, TFC, TDF | 1,310 | <40 | RCMD | Int‐2 | del(5q), del(7q), dic(9;12) | NA | Yes | Aza | Williamson et al., 2016 [5] |
| 10 | 66/F | 28 | NA | 573 | 52 | MDS | Int‐1 | 46,XX | ASXL1, DNMT3A | No | Len, Elt | Kaner et al., 2019 [6] |
| 11 | 45/M | 4 | NA | 840 | ND | t‐ MDS | Int‐2 | NA | NA | Yes | Aza | Kaner et al., 2019 [6] |
| 12 | 66/M | 13 | NA | 383 | 73 | MDS | Int‐2 | del(7q) | ASXL1 | No | Aza | Kaner et al., 2019 [6] |
| 13 | 70/M | 20 | NA | 76 | 247 | t‐ MDS/ AML | Int‐2 | −5, del(7q),−2 | TP53 | Yes | Aza | Kaner et al., 2019 [6] |
| 14 | 49/M | 11 | NA | 40 | ND | MDS/ AML | Int‐2 | NA | NA | Yes | Aza | Kaner et al., 2019 [6] |
| 15 | 54/M | NA | NA | NA | NA | MDS | Int‐2 | del(7q) | NA | Yes | Aza, Dec | Kaner et al., 2019 [6] |
| 16 | 58/F | 18 | NA | 484 | ND | MDS | >Int 2 | −7 | NA | Yes | 7+3/ HiDAC | Kaner et al., 2019 [6] |
| 17 | 66/M | NA | NA | 304 | ND | t‐ MDS/ AML | Int‐2 | del(20q) | ASXL1 | No | Aza | Kaner et al., 2019 [6] |
| 18 | 66/F | 17 | NA | 781 | ND | MDS | >Int 2 | −7, −5, +8, +9 | ASXL1, DNMT3A, | Yes | Aza | Kaner et al., 2019 [6] |
| 19 | 52/F | 12 | NA | 140 | NA | t‐ MDS/ AML | Int‐2 | NA | TP53 | Yes | Aza | Kaner et al., 2019 [6] |
| 20 | 56/M | NA | NA | 390 | ND | MDS | Int‐1 | Normal | NA | No | Len, Elt | Kaner et al., 2019 [6] |
| 21 | 64/F | 15 | NA | 931 | <40 | MDS/ AML | Int‐2 | del(7q) | ASXL1, TET2, DNMT3A, U2AF1 | Yes | 7+3 | Kaner et al., 2019 [6] |
| 22 | 55/M | 24 | NA | 275 | <40 | t‐ MDS/ AML | >Int 2 | Complex with ‐7 | ASXL1, TP53, ETV6 | Yes | 7+3/ HiDAC, Dec | Kaner et al., 2019 [6] |
| 23 | 65/F | 2 | NA | 148 | 5,599 | MDS | Int‐1 | del(13q) | ND | No | Palliative | Kaner et al., 2019 [6] |
| 24 | 61/F | 16 | AZT, 3TC, EFV | 929 | ND | MDS‐EB‐1 | Int‐2 | +8 with clonal karyotypic evolution hyperdiploidy with +8,+10,+21 | ASXL1 | No | Aza | This report |
Abbreviations: 3TC, Lamivudine; ABC, Abacavir; AML, Acute myeloid leukemia; ART, Antiretroviral treatment; Aza, Azacitidine; AZT, Zidovudine; Dec, Decitabine, del, deletion; EFV, Efavirenz; Elt, Eltrombopag; F, Female; FTC, Emtricitabine; Int, Intermediate; IPSS, International Prognostic Scoring System; Len, Lenalidomide; LVR, Lopinavir; M, Male; MDS‐EB‐1, myelodysplastic syndrome with excess of blasts 1; NA, Not available; ND, Not detected; RA, Refractory anemia; RAEB, Refractory anemia with excess of blasts; RCMD, Refractory cytopenia with multilineage dysplasia; RTV, Ritonavir; T20, Enfuvirtide; t‐MDS, Therapy‐related myelodysplastic syndrome.
In this table, the patient collection included 2 case reports and 2 case series, beyond our case.
Hypothetically, MDS development in HIV patients can be related to chronic viral infection, with the inflammatory cytokines environment affecting immune system regulation. ART may also accelerate BM aging leading the acquisition of somatic mutations followed by clonal hematopoiesis [6]. Besides, ART is related to genotoxic effects [11] with genomic instability and loss of heterozygosity [12]. Other previous studies also linked ART to hematopoietic precursor cell dysplastic abnormalities [1, 13, 14]. We hypothesized that the coexistence of trisomy 8 and ASXL1 mutation in our patient would be a consequence of long‐standing exposition to ART, leading to a sMDS. Mutations in ASXL1 are detected in 11‐14% of MDS; most of them occur as heterozygous exon 12 frameshift or nonsense mutations and predict inferior prognosis [15]. Isolated trisomy 8 is found in about 7% of MDS cases and is considered a secondary or late event in the MDS evolution [15].
The chromosomes are frequently missegregated during mitosis in cancer cells. This process is known as whole‐chromosome instability (W‐CIN) and leads to aneuploidy. W‐CIN induces tumorigenesis and treatment resistance. The mitotic stress associated with W‐CIN is generally induced by oncogenes and suppressor tumor genes rather than mutations in genes involved in chromosome segregation [16]. Our patient was characterized cytogenetically by an aneuploidy. The ASXL1 mutation and an extra copy of c‐myc, located at 8q24 seem to be possible factors associated with the increase in W‐CIN, leading to clonal evolution and refractoriness to treatment.
Our case report and the literature review highlight the importance of cytogenetic and molecular tests to monitoring HIV‐positive patients under long‐stand treatment and the occurrence of MDS. Often, these patients are not eligible for hematopoietic stem cell transplantation due to their poor clinical condition [6]. Because of the lack of specific treatment for HIV/MDS patients, it is essential to unveil the mechanisms involved in its pathogenesis to led promissory therapy.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Informed consent was obtained from the case in accordance with the Declaration of Helsinki, and ethics was approved by the Ethics and Research Committee of Evandro Chagas Institute for Clinical Research (reference number CAAE #0032.0.009.000‐10).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
DPMA and MTS attended the patient, collected and analysed clinical data. TSF, EAAK, and VLL performed cytogenetic and FISH analysis. FVSB, FGA. and VLL performed genetic tests. EPN and BGJG revised the manuscript. MSPO and TSF supervised the study and reviewed the manuscript. MSPO and TSF provided funding. All authors contributed significantly to the work and have seen and approved the manuscript and its submission.
ACKNOWLEDGMENTS
The authors thank the patient who made samples available. This study was supported by the Brazilian Ministry of Health (National Institute of Cancer/INCA, Brazil) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de134 Nível Superior ‐ Brasil (CAPES) ‐ Finance Code 001; M.S.P.O. is supported by Conselho 135 Nacional de Desenvolvimento Científico e Tecnológico‐ CNPq [#301594/2015‐5]. The authors also thank the director of Evandro Chagas National Institute of Infectious Diseases‐ FIOCRUZ Dr. Valdiléia Veloso for publication support and Michelle Roesler for the English revision. We also are grateful to Alexandre Vizzoni for the transfusion and laboratory support, and the many physicians who attended the patient, especially Adriana Pinto and Juliana Netto.
DATA AVAILABILITY STATEMENT
The documentation is available on a reasonable request to the corresponding author.
REFERENCES
- 1. Ryu T, Ikeda M, Okazaki Y, Tokuda H, Yoshino N, Honda M, Kimura S, Miura Y. Myelodysplasia associated with acquired immunodeficiency syndrome. Intern Med. 2001;40:795–801. [DOI] [PubMed] [Google Scholar]
- 2. Katsarou O, Terpos E, Patsouris E, Peristeris P, Viniou N, Kapsimali V, Karafoulidou A. Myelodysplastic features in patients with long‐term HIV infection and haemophilia. Haemophilia. 2001;7:47–52. [DOI] [PubMed] [Google Scholar]
- 3. Rieg S, Lübbert M, Kern WV, Timme S, Gärtner F, Rum JA. Myelodysplastic syndrome with complex karyotype associated with long‐term highly active antiretroviral therapy. Br J Haematol. 2009;145:670–3. [DOI] [PubMed] [Google Scholar]
- 4. Takahashi K, Yabe M, Shapira I, Pierce S, Garcia‐Manero G, Varma M. Clinical and cytogenetic characteristics of myelodysplastic syndrome in patients with HIV infection. Leuk Res. 2012;36:1376–9. [DOI] [PubMed] [Google Scholar]
- 5. Williamson BT, Leitch H. Higher risk myelodysplastic syndromes in patients with well‐controlled HIV infection: clinical features, treatment, and outcome. Case Rep Hematol. 2016;2016:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaner JD, Thibaud S, Jasra S, Wang Y, Janakiram M, Sharma A, et al. HIV portends a poor prognosis in myelodysplastic syndromes. Leuk Lymphoma. 2019;60:3529–35. [DOI] [PubMed] [Google Scholar]
- 7. Gelsi‐Boyer V, Trouplin V, Adélaïde J, Bonansea J, Cervera N, Carbuccia N, et al. Mutations of polycomb‐associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2009;145:788–800. [DOI] [PubMed] [Google Scholar]
- 8. Pezzi A, Moraes L, Valim V, Amorin B, Melchiades G, Oliveira F, et al. DNMT3A mutations in patients with acute myeloid leukemia in South Brazil. Adv Hematol. 2012;2012:697691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia‐Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. [DOI] [PubMed] [Google Scholar]
- 11. Moraes Filho AV, Carvalho CJ, Carneiro CC, et al. Genotoxic and cytotoxic effects of antiretroviral combinations in mice bone marrow. PLoS One. 2016;11:e0165706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guimarães NN, de Andrade HHR, Lehmann M, Dihl RR, Cunha KS. The genetic toxicity effects of lamivudine and stavudine antiretroviral agents. Expert Opin Drug Saf. 2010;9:771–81. [DOI] [PubMed] [Google Scholar]
- 13. Inoue T, Cronkite EP, Hirabayashi Y, Bullis JE, Mitsui H, Umemura T. Lifetime treatment of mice with azidothymidine (AZT) produces myelodysplasia. Leukemia. 1997;11(Suppl 3):123–7. [PubMed] [Google Scholar]
- 14. Bayram S, Topaktaş M. Confirmation of the chromosome damaging effects of lamivudine in in vitro human peripheral blood lymphocytes. Environl Mol Mutagen. 2008;49:328–33. [DOI] [PubMed] [Google Scholar]
- 15. Hosono N. Genetic abnormalities and pathophysiology of MDS. Int J Clin Oncol. 2019;24:885–92. [DOI] [PubMed] [Google Scholar]
- 16. Duijf PHG, Benezra R. The cancer biology of whole‐chromosome instability. Oncogene. 2013;32:4727–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The documentation is available on a reasonable request to the corresponding author.


