Abstract
Introduction
Understanding human mixing patterns relevant to infectious diseases spread through close contact is vital for modelling transmission dynamics and optimisation of disease control strategies. Mixing patterns in low-income countries like Malawi are not well known.
Methodology
We conducted a social mixing survey in urban Blantyre, Malawi between April and July 2021 (between the 2nd and 3rd wave of COVID-19 infections). Participants living in densely-populated neighbourhoods were randomly sampled and, if they consented, reported their physical and non-physical contacts within and outside homes lasting at least 5 min during the previous day. Age-specific mixing rates were calculated, and a negative binomial mixed effects model was used to estimate determinants of contact behaviour.
Results
Of 1201 individuals enroled, 702 (58.5%) were female, the median age was 15 years (interquartile range [IQR] 5–32) and 127 (10.6%) were HIV-positive. On average, participants reported 10.3 contacts per day (range: 1–25). Mixing patterns were highly age-assortative, particularly those within the community and with skin-to-skin contact. Adults aged 20–49 y reported the most contacts (median:11, IQR: 8–15) of all age groups; 38% (95%CI: 16–63) more than infants (median: 8, IQR: 5–10), who had the least contacts. Household contact frequency increased by 3% (95%CI: 2–5) per additional household member. Unemployed participants had 15% (95%CI: 9–21) fewer contacts than other adults. Among long range (>30 m away from home) contacts, secondary school children had the largest median contact distance from home (257 m, IQR 78–761). HIV-positive status in adults >=18 years-old was not associated with changed contact patterns (rate ratio: 1.01, 95%CI: (0.91–1.12)). During this period of relatively low COVID-19 incidence in Malawi, 301 (25.1%) individuals stated that they had limited their contact with others due to COVID-19 precautions; however, their reported contacts were 8% (95%CI: 1–13) higher.
Conclusion
In urban Malawi, contact rates, are high and age-assortative, with little reported behavioural change due to either HIV-status or COVID-19 circulation. This highlights the limits of contact-restriction-based mitigation strategies in such settings and the need for pandemic preparedness to better understand how contact reductions can be enabled and motivated.
Keywords: Social contacts, Transmission, Mixing data, Infectious disease, Malawi, Africa
1. Introduction
Globally, respiratory tract pathogens carry a substantial burden of morbidity and mortality at all ages (Wahl et al., 2018, WHO Health Organisation, 2021, World Health Organization, 2021), with incidence highest in low- and middle-income countries and in populations where human immunodeficiency virus (HIV) prevalence is high. For instance, an estimated 318 000 pneumococcal deaths occured in 2015 of which 23 300 were among HIV-positive individuals (Wahl et al., 2018). In 2019, there were an estimated 1.5 million deaths from tuberculosis (TB), with HIV a major risk factor for the development of active TB and mortality (WHO Health Organisation, 2021a). By 2021 November, 5.2 million deaths from Coronavirus disease (COVID-19) have been reported globally (WHO, 2020), and with detrimental impact on healthcare delivery, particularly in low-income countries (LICs) (Soko et al., 2021).
Transmission of respiratory tract pathogens requires close contact with infectious respiratory droplets, secretions or inhalation, making understanding of human social mixing patterns an essential part of the design of effective disease control strategies (Heesterbeek et al., 2015). Social contact type, frequency, duration and place have shown to vary substantially between different settings (Mousa et al., 2021, Hoang et al., 2019). Local built environment, population characteristics, and social activities such as traditional, political, religious and leisure events are likely to play important roles in explaining contact patterns variations between countries (Johnstone-Robertson et al., 2011, le Polain de Waroux et al., 2018, Neal et al., 2020). However, relatively little is known about social mixing patterns in LICs (Hoang et al., 2019), including how these are affected by age, occupation, household size, HIV status, and non-pharmaceutical interventions (NPIs) during COVID-19 pandemic.
Social mixing studies in middle (MICs) and high (HICs) income countries have shown that individuals in the same age groups tend to have higher contact rates than with other age groups (age-assortative mixing) (Mousa et al., 2021, Hoang et al., 2019), yet not much is known about contact patterns in LICs in the era of fairly high urbanisation (Dodd et al., 2016, Melegaro et al., 2017, Zandvoort et al., 2021). Intergenerational mixing between younger children and adults was evident in Zimbabwe, reflecting parental or guardian roles played by adults (Melegaro et al., 2017), though age-sex mixing patterns are not well known (Dodd et al., 2016, Melegaro et al., 2017). Commonly, people tend to make high number of contacts within a short distance of their homes, with this being most pronounced for people living close to their usual place of work (Johnstone-Robertson et al., 2011, Read et al., 2012). Where commuting long distances to work is common through mass transport, outbreak containment becomes more difficult due to greater ease of wide spatial spread of respiratory infections (Garske et al., 2011).
Physical distancing and lockdown NPIs to limit the number and geographical spread of close contacts have been used extensively during the COVID-19 pandemic, especially in HICs and MICs where relatively; mobility is usually high, per capita hospitalisation and mortality during the first wave was high, and lockdowns were strictly observed (Quaife et al., 2020, Jarvis et al., 2020, Liu et al., 2021a, Gimma et al., 2021). In LICs, the impact of NPIs on social contacts is less clear, with added uncertainty on whether individual contacts are affected by COVID-19 vaccination status due to perceived attitude of being protected from severe COVID-19 (Usherwood et al., 2021). However, this is unlikely due to relatively very low current COVID-19 vaccination coverage in LICs (Duan et al., 2021). Moreover, despite the 3 pandemic waves in a LIC like Malawi, NPIs were only restricted to face mask wearing, rotational working, limited transit vehicles, self-quarantine for returning travellers, restricted gatherings, closure of non-essential businesses, social distancing, and reduced public transportation capacity, without implementation of a formal countrywide lockdown (Mangal et al., 2021).
Unlike in HICs, urban neighbourhoods in LICs are predominantly comprised of high-density informal settlements, relatively larger households (extended families), have low rates of formal employment, and often high HIV prevalence (Dwyer-Lindgren et al., 2019, Thindwa et al., 2020). Contact patterns may then differ substantially having, for example, much higher rates within than outside households, and a higher ratio of skin-to-skin to verbal contacts (Mousa et al., 2021, Melegaro et al., 2017). Despite these anticipated differences, there are only two studies of social contacts from LICs (Uganda and Somaliland) (le Polain de Waroux et al., 2018, Zandvoort et al., 2021) and six studies from LMICs (Kenya, South Africa, Zambia and Zimbabwe) (Johnstone-Robertson et al., 2011, Dodd et al., 2016, Melegaro et al., 2017, Quaife et al., 2020, Kiti et al., 2014, Del Fava et al., 2021). Also, understanding how contact patterns vary by HIV status is important to inform targeted interventions to mitigate risks both to HIV-positive individuals and to their social contacts.
The main objectives of this study in urban Malawi was to (1) estimate age-specific daily rates of social contacts, (2) investigate factors associated with social contact rates, (3) explore the influence of sex, household, community, HIV status and distance of contact event place in social contact patterns and (4) explore self-reported changes in social contact behaviour during the COVID-19 pandemic in the context of Malawi not having had a formal “lockdown” at any stage during the pandemic.
2. Methods
2.1. Study design
A cross-sectional study was undertaken in Ndirande, Blantyre, Malawi between April and July 2021. Ndirande mainly comprises high-density informal residential neighbourhoods, with minimal town-planning, building regulations or access to municipal services such as roads, electricity, water or refuse collection. Buildings tend to be single storey, built by unqualified local artisans using low-cost and sometimes locally-made materials like fired bricks. The road network is extremely limited, making access by motor vehicle impassable for most parts of the suburb.
Our sampling frame was based-on a previous Blantyre household TB prevalence survey, conducted in 2018–2019, where adult HIV status was ascertained using Government-approved rapid diagnostic tests (Ministry of Health, 2018a). The TB prevalence survey mapped high density neighbourhoods in Blantyre city into 72 clusters where 12 865 adults from randomly selected households were recruited proportional to cluster size. A cluster was defined as a physical location whose boundaries were based on existing community health workers combined catchment areas with about 4000 adults aged 18 + years. Ndirande, the site for the present social mixing study, contributed 14 clusters and 2626 adults to the TB prevalence survey.
The targeted sample size for the present social mixing study was ≥ 1080 participants from 393 households across 14 clusters, of whom 490 were adults aged ≥ 18 years-old (y), and 190 were HIV-positive adults taking ART. A randomly sample (proportional to cluster size) of household members from Ndirande clusters of TB prevalence survey was taken, aiming to recruit equal number of participants across six age groups of < 1 y (infants), 1–4 y (preschool children), 5–14 y (primary school children), 15–19 y (secondary school children), 20–49 y (adults), and 50 +y (older adults) to give adequate power of ≥ 80% to measure the number of social contacts with standard deviation of no more than 50% of the mean number of daily social contacts, and to detect the true differences in the mean contact rates between age groups if in excess of 30% (le Polain de Waroux et al., 2018, Field trials of health interventions, 2015Field trials of health interventions : a toolbox. (Oxford University Press, 2015).). Age groups were chosen based on transmission patterns of a typical respiratory infection (Mousa et al., 2021, Dodd et al., 2016, Flasche et al., 2020).
A household was defined as a group of family members or unrelated individuals living in the same compound and sharing food from the same kitchen (Tc et al., 2017). Household size was split into < 4, 4–6, > 6 members during analysis based on interquantile range of measured household size in the study. Using the sampling frame of households in the 14 Ndirande clusters, handheld geographical positioning system (GPS, Garmin ETrex 30) devices were used to identify previously enroled households. After visiting and validating each household, the household head aged ≥ 18 years was invited to participate, along with their < 18 years-old household members. If a household or an adult household member who participated in the TB prevalence survey was not found after two visits, they were replaced by next household on the list. If a particular age-group had reached it’s required sample size, recruitment was restricted to households with at least one member in the age-groups that had not reached saturation.
2.2. COVID-19 dynamics during field study
To date, Malawi has experienced three COVID-19 waves (May to August 2020, January to March 2021, and June to September 2021) (Mangal et al., 2021). Our social mixing study was conducted after the end of the second wave (April-May 2021) and during the first half of the third wave (June-July 2021). During our study period, Malawi had initially implemented Level 1 COVID-19 policies, which included social distancing, recommended face mask wearing, rotational working, limited transit vehicles, self-quarantine for returning travellers, and restrictions of gatherings to 100 people (From April-May 2021). From June-July 2021, Level 3 COVID-19 policies were enacted, including: restricted movements, enforced facemask wearing, closing of non-essential businesses, enforced social distancing, reduced public transportation capacity, and 10 people restricted gatherings. Throughout the study period between April and July 2021, school closures were not implemented.
2.3. Data collection
The study outcomes were retrospectively reported physical (participant’s skin to skin touch with a contact) and non-physical contacts (participant’s two-way close verbal conversation lasting for ≥5 min and with ≥3 words exchanged with a contact) during the time period between waking up in the morning and going to bed in the evening (le Polain de Waroux et al., 2018). The household head was interviewed on household characteristics and composition on behalf of other household members, and also responded to their own individual demographic characteristics, travel history, COVID-19 behavioural change, and social mixing events, and those of younger (<7 years-old) household members. Older children mostly reported their contacts with help of an adult. A house was considered well-ventilated if it had open windows, doors and ventilation within the cooking area (Supplementary Questionnaire).
Survey interviews were conducted across all days of the week to ensure representation of weekdays and weekends. Repeated contacts with the same individual were recorded once, noting the frequency and cumulative time. The age of a contact was not always known, in which case it was guessed and approximated to nearest annual age unit. We defined the "hypothesised" number of contacts as additional number of people the participants would have encountered the day before, had there been no COVID-19 pandemic. Open data kit (ODK, Nafundi, Seattle, USA) was used to capture participant’s responses electronically, with an embedded electronic physical address locator (ePAL, Tripod Software, Salford, UK) for geolocating places of contacts (Thindwa et al., 2020).
2.4. Characteristics and determinants of social contact events
We tabulated the number and distribution of participants and their contacts by age, and the proportion and distribution of contact events by the day of the week when contacts occurred and number of contacts per participant, respectively. The number and proportion of physical and non-physical contact events were also tabulated by contact duration, frequency, location, relationship of participant to contact, and sex of contact.
Social contact patterns were characterised by computing the median and mean number of contact events for a set of potential factors (age, sex, occupation, education, HIV, day of the week, COVID-19 restriction, and household size), with bootstrap confidence intervals based on 1000 bootstrap replicates. Factors associated with the mean number of mixing events at p < 0.10 in univariable analysis were retained in multivariable analysis if they reduced the Akaike Information Criterion (AIC) (Stone, 1977). To investigate factors associated with contact rates, a negative binomial mixed model with household random intercept terms was constructed, and the ratio of mean number of contact events in each category of a factor relative to reference category was calculated (le Polain de Waroux et al., 2018). Detailed methods are described in Supplementary Text 1.
2.5. Age-specific social mixing rates
Age-stratified contact matrices were generated to investigate interactions between age groups. Age-specific contact rates through contact matrices were constructed from the mean number of daily contacts between participants and their contacts using the ‘socialmixr’ R package (Funk et al., 2020). Age-based contact matrices were estimated based on the ratio of the measured probability of a contact event between individuals based on age group to a null model of the probability of that contact event under an assumption of random mixing. Contact probabilities under the null model were determined by proportion of the population in each given age category in Ndirande (Thindwa et al., 2020, Tc et al., 2017). Our analyses were weighted by days of the week as well as reciprocity in contact patterns such that the total number of contacts from age group to were equal to the total number of contacts from age group to (m ij w i,=m ji w j) where the elements make up a contact matrix and is the population size in age group . Thus, is the daily mean contact rate given as (le Polain de Waroux et al., 2018; Funk et al., 2020).
For each contact matrix, the assortativity index Q was computed. The Q index is defined as the coefficient of degree between pairs of linked age groups and quantifies the weight of mixing between individuals of the same age groups. A Q value close to 0 represents little dependence of mixing patterns on age while Q= 1 implies exclusivity of contacts within age groups (Del Fava et al., 2021). Age-specific contact matrices were stratified by physical vs. non-physical contact, sex, within vs. outside household, within vs. outside Ndirande community, and adult HIV-positive vs. HIV-negative status, as well as affected vs. unaffected by COVID-19 and sex interactions.
2.6. Spatial distance distribution of contact events
We used longitude and latitude GPS coordinates to calculate the Euclidian distance in metres between participants’ houses and places of contact events. The inverse cumulative distance distributions for physical and non-physical contacts and for the stratification by age groups in relation to the distances away from homes were estimated (Read Jonathan et al., 2014). All analyses were conducted in R v4.1. Results can be reproduced using the data and code in the GitHub repository (Thindwa, 2021).
2.7. Ethics
Ethical approval was granted by the College of Medicine Research Ethics Committee, University of Malawi (P.01/21/3244), and the London School of Hygiene and Tropical Medicine Research Ethics Committee (#22 913). Informed consent was obtained from each participant aged ≥ 16 years, or from the parent or guardian of each individual aged < 6 years, or from each participant aged 6–15 years with additional informed assent.
3. Results
3.1. Study area, participants and their contacts
A total of 378 (85.9% of all who were approached) households participated in the survey from across 14 clusters in Ndirande, Blantyre City. Non-participation was mostly due to relocation, and with only 3% refusals. The proportion of study households within each cluster (relative to total census-counted cluster households) and across clusters varied between 7.9% and 19.7% and 4.0–10.0%, respectively. The most common number of rooms per house was four, of which two or three were most commonly used as bedrooms. The median household size was 6 members (interquartile [IQR] range 4–7), with 53 (14.2%) households reporting having at least one smoker (cigarette, marijuana or local cigar; chingambwe). Charcoal was predominantly used as source of energy in 366 (96.8%) households, of which 200 (52.9%) reported indoor cooking, and of which 23 (11.5%) were not well ventilated (Fig. S1).
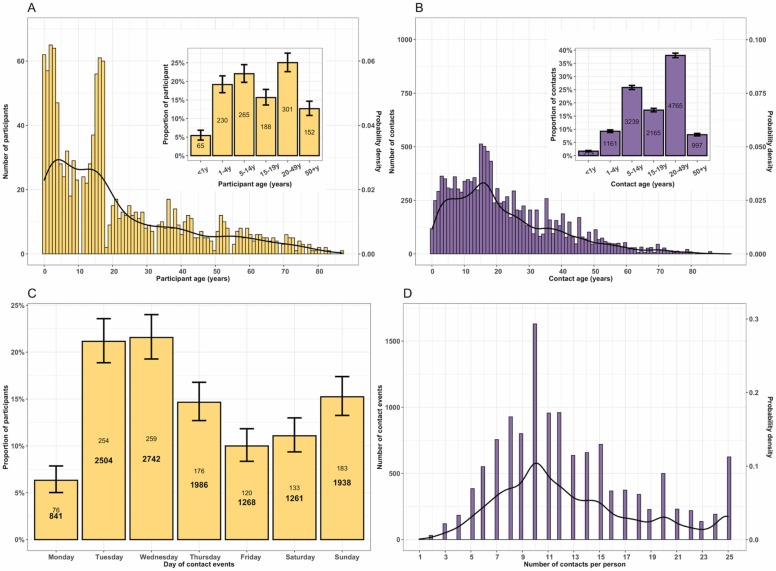
Overall, 1201 participants were recruited, including 65 (5.4%) infants, 230 (19.2%) preschool children, 265 (22.1%) primary school children, 188 (15.7%) secondary school children, 301 (25.1%) adults and 152 (12.7%) older adults. Of enroled participants, 702 (58.5%) were female, 138 (30.0% of adults) were unemployed, and the median age was 15 years (IQR: 5–32). Among all adults aged 18 years or older, 200 (43.3%) had primary education, 18 (3.9%) had no formal education, and 124 (26.8%) self-reported HIV-positive status, all of whom were taking ART. In total, there were 12 540 reported contacts of whom 213 (1.7%) were infants, 1161 (9.3%) preschool children, 3239 (25.8%) primary school children, 2165 (17.3%) secondary school children, 4765 (38.0%) adults, and 997 (7.9%) older adults. The median age of contacts was 18 years (IQR: 10–32). 9341 (74.5%) contacts occurred during Monday to Friday. The average number of contacts per person was 10.43, with 25%, 50% and 75% of participants reporting at most 7, 10 and 13 contacts, respectively ( Fig. 1, Table 1, Table S1).
Fig. 1.
The probability distribution, number and proportion of study participants and reported contact events during the social contact patterns study in urban Blantyre, Malawi between April and July 2021. The frequency and probability distribution of participants, with proportion of participants in age groups of infants (<1 years-old), preschool (1–4 years-old), primary school (5–14 years-old), secondary school (15–19 years-old), adults (20–49 years-old) and older adults (50 + years-old) (insert) (A); The age frequency and probability distribution of contacts, with proportion of contacts by age groups (insert) (B); The number of contacts (bold fontface) and participants (roman fontface), and proportion of participants by day of the week when the contact event occurred (C); and the total number and probability distribution of reported contact events for a given number of contacts reported by the participant (D). Throughout the plots, the black line represents the probability density with values on secondary y-axis whereas yellow and purple colour represent participants and contacts, respectively. The 95% confidence interval for each bar is shown as vertical line.
Table 1.
Characteristics of participants and their reported daily in Blantyre, Malawi, April-July 2021. The relative numbers of mean daily contacts (contacts rate ratio, CRR) were obtained from a negative binomial mixed model.
| Characteristic | Number of participants % | Median number of contacts (95%CI) | Mean number of contacts (95%CI) | Crude CRR (95%CI) | Adjusted CRR# (95%CI) |
|---|---|---|---|---|---|
| Age | (N = 1201) | ||||
| < 1 y | 65 (5.4) | 8.00 (6.39–9.84) | 8.14 (7.26–9.15) | Ref | Ref |
| 1–4 y | 230 (19.1) | 9.00 (8.35–10.26) | 8.63 (8.18–9.08) | 1.14 (1.03–1.25) | 1.13 (1.03–1.25) |
| 5–14 y | 265 (22.0) | 10.00 (9.60–10.51) | 10.24 (9.72–10.82) | 1.29 (1.17–1.42) | 1.23 (1.07–1.41) |
| 15–19 y | 188 (15.7) | 10.00 (9.30–10.83) | 10.88 (10.18–11.61) | 1.40 (1.27–1.54) | 1.31 (1.14–1.50) |
| 20–49 y | 301 (25.1) | 11.00 (10.42–12.38) | 11.75 (11.17–12.38) | 1.50 (1.37–1.63) | 1.38 (1.16–1.63) |
| 50+y | 152 (12.7) | 10.00 (9.38–11.53) | 10.18 (9.43–11.15) | 1.38 (1.24–1.53) | 1.30 (1.08–1.56) |
| Sex | |||||
| Female | 702 (58.5) | 10.00 (9.73–10.32) | 10.45 (10.08–10.8) | Ref | Ref |
| Male | 499 (41.5) | 10.00 (9.44–11.35) | 10.06 (9.64–10.49) | 0.94 (0.90–0.97) | 0.97 (0.94–1.01) |
| Occupation | |||||
| Business‡1 | 206 (17.2) | 10.50 (9.38–11.55) | 12.13 (11.31–12.95) | Ref | Ref |
| Workers‡2 | 79 (6.6) | 11.00 (8.67–13.14) | 11.27 (10.13–12.63) | 0.96 (0.89–1.05) | 1.00 (0.92–1.08) |
| Pre-school child | 267 (22.2) | 9.00 (8.34–9.64) | 8.74 (8.31–9.16) | 0.70 (0.66–0.74) | 0.90 (0.77–1.05) |
| Schoolers | 479 (39.9) | 10.00 (9.42–10.96) | 10.28 (9.87–10.76) | 0.86 (0.82–0.91) | 0.95 (0.85–1.06) |
| Retired | 28 (2.3) | 9.00 (7.60–11.06) | 8.79 (7.26–10.50) | 0.84 (0.73–0.97) | 0.91 (0.78–1.05) |
| Unemployed | 138 (11.5) | 10.00 (9.28–10.79) | 10.34 (9.68–11.08) | 0.85 (0.79–0.91) | 0.85 (0.79–0.91) |
| Other | 4 (0.33) | 7.50 (5.75–12.63) | 9.50 (6.00–15.25) | 0.88 (0.62–1.25) | 0.91 (0.64–1.28) |
| Education level† | |||||
| University/college | 17 (3.7) | 10.00 (6.97–14.57) | 9.76 (7.35–12.23) | Ref | – |
| No education | 18 (3.9) | 9.50 (6.87–11.91) | 11.00 (8.94–14.24) | 1.03 (0.76–1.40) | – |
| Primary school | 200 (43.3) | 11.00 (10.33–12.40) | 11.48 (10.66–12.24) | 1.04 (0.83–1.32) | – |
| Secondary school | 227 (49.1) | 10.00 (9.57–10.36) | 11.22 (10.54–11.99) | 1.03 (0.82–1.29) | – |
| HIV status† | |||||
| Negative | 338 (73.2) | 10.00 (9.19–10.53) | 11.28 (10.70–11.88) | Ref | – |
| Positive on ART | 124 (26.8) | 10.00 (8.95–10.68) | 11.13 (10.26–12.17) | 1.01 (0.91–1.12) | – |
| Interview day | |||||
| Weekday (Mon-Fri) | 885 (73.7) | 10.00 (9.82–10.21) | 10.43 (10.12–10.78) | Ref | – |
| Weekend (Sat-Sun) | 316 (26.3) | 9.00 (7.65–9.73) | 9.90 (9.27–10.45) | 0.99 (0.92–1.06) | – |
| COVID-19 restrictions* | |||||
| No | 900 (74.9) | 10.00 (9.42–11.01) | 10.04 (9.74–10.40) | Ref | Ref |
| Yes | 301 (25.1) | 10.00 (9.78–10.23) | 11.03 (10.50–11.55) | 1.21 (1.16–1.27) | 1.08 (1.01–1.13) |
| Household size | |||||
| 1–3 people | 90 (7.5) | 8.00 (3.35–9.78) | 8.81 (7.70–10.07) | Ref | Ref |
| 4–5 people | 305 (25.3) | 9.00 (7.87–9.67) | 10.40 (9.69–11.24) | 1.20 (1.04–1.39) | 1.24 (1.07–1.43) |
| 6 people | 230 (19.2) | 9.00 (7.60–9.57) | 10.13 (9.50–10.84) | 1.21 (1.03–1.42) | 1.26 (1.07–1.47) |
| 7+ people | 576 (48.0) | 10.00 (9.91–10.09) | 10.80 (10.43–11.19) | 1.29 (1.12–1.49) | 1.36 (1.18–1.57) |
‡1 Business refers to individuals involved in the exchange of goods and services to earn profits usually struggling self-employed traders
‡2 Workers include all persons involved in agricultural, domestic, manual, and office activities
† Only applicable to adults 18 years and older (N = 462)
95%CI - 95% bias-corrected and accelerated bootstrap confidence intervals based on 1000 bootstrap replicates
* Whether or not individual reported that they had modified their social mixing behaviour due to the COVID-19 pandemic
# Estimates in multivariable model were adjusted for age, sex, occupation status, whether social contacts were restricted by COVID-19, and household size.
ART: Antiretroviral therapy; Ref: refers to reference category; Crude CRR: refers to univariable analysis of the relative effect of each variable category on mean # of contacts; Adjusted CRR: refers to multivariable analysis of the relative effect of each variable category on mean # of contacts accounting for other variables simultaneously; Bold font: refers to statistically significant effects at significance level of p < 0.05.
3.2. Travel history and COVID-19 impact
Approximately one quarter of participants (n = 281 23.4%) had travelled more than 5 km outside of Ndirande community in the last 24 h. However, of 1041 (86.7%) participants who travelled outside Ndirande anytime in the past, 81 (7.8%) reported that they made similar travel daily and 161 (15.5%) at least once a week; 840 (80.7%) spent at most 24 h outside Ndirande, and 913 (87.7%) used public transport during similar travels.
A total of 301 (25.1%) participants, almost exclusively adults, reported to have reduced their social contacts due to COVID-19 pandemic, with home (n = 225 74.8%) and market (n = 183 60.8%) being the main localities where contact was reportedly reduced. Among all participants, we estimated that preventive measures against the COVID-19 pandemic contributed 9.1% (95%CI 0–13) to social contacts reduction e.g. from the median of 11 (IQR: 7–18) daily contacts, actual and hypothesised (additional contacts that could have occured in absence of COVID-19 pandemic), to 10 (IQR: 7–13) actual contacts. However, the actual contacts of participants affected by COVID-19 were 8% (95% CI: 1–13) higher than their counterparts who reported no reduced social contacts due to COVID-19 (Table 1, Fig. S2, Fig. S3).
3.3. Characteristics and determinants of reported mixing events
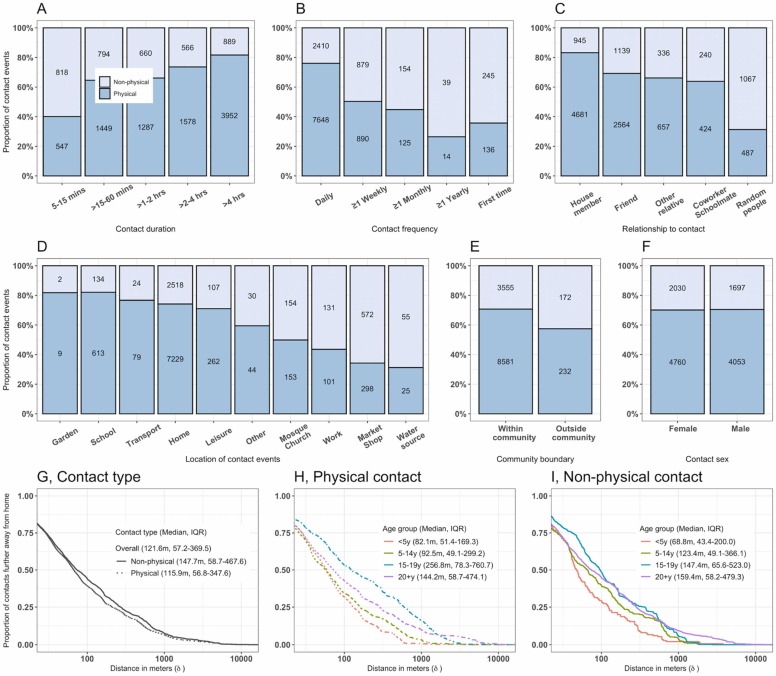
About 74% (8266/11 175) of contacts longer than 15 min involved physical contact whereas 60% (547/1365) of shorter contacts did not. Contacts were likely physical if they occurred on a daily basis (7648/10 058) compared to less frequent contacts. More than 80% (4681/5626) of contacts with family members were physical and only 30% (487/1554) of contacts with unknown (random) people were physical. About 75% (8183/10 966) of contacts at school, home, leisure or in transport were physical whereas less than 50% (552/1409) of contacts at church, market or work were. More than 95% (12 136/12 540) of all contacts happened within than outside the community ( Fig. 2).
Fig. 2.
Characteristics of reported physical and non-physical social contacts in urban Blantyre, Malawi between April and July 2021. The proportion of physical and non-physical contacts by the duration of contacts (A), frequency of contacts (B), relationship of participant to contacts (C), the exact location of contacts (D), contacts within or outside community (E) and contact sex (F). The number in each bar plot indicates absolute number of reported contacts. Physical contact refers to participant’s skin to skin touch with a contact whereas non-physical contact refers to participant’s two-way close verbal conversation lasting for ≥ 5 min and with ≥ 3 words exchanged. The inverse cumulative distance distribution showing the proportion of contacts in relation to the distance (in metres) further away from the participant home by physical and non-physical contact type (G), for physical contact events by age group (H), and for non-physical contact events by age group (I).
Data on spatial distances between participant house and place of mixing was available for 12 449 (99.3%) social mixing events. Overall, the median Euclidian distance metres (m) away from home to where mixing events occurred was 121.6 m (IQR: 57.2–369.5), ranging from 30.0 m to 12 358.8 m. Secondary school children travelled furthest on average for their physical contacts (256.8 m, IQR: 78.3–760.7), primary school children had the most localised physical contacts (82.1 m, IQR: 51.4–169.3). (Fig. 2).
The mean number of contacts varied significantly by age, with infants and preschool children reporting lower contacts (95%CI: 7.26–9.15) than those in older age groups (95%CI: 9.72–12.38). Similarly, households with at most three members had significantly lower contacts (95%CI: 7.70–10.07) than those with at least seven members (95%CI: 10.43–11.19). On the contrary, the mean number of contacts did not significantly differ by sex, education status, HIV status or interview day (Table 1).
In a multivariable analysis, age, occupation status, and household size were significantly associated with daily number of contacts. Adults had the highest contact frequency among all ages, over 30% more than infants (1.38, 95%CI: 1.16–1.63). Unemployed adults (less active community members) had 15% (9−21) fewer contacts than business adults, usually self-employed traders (more active counterparts). Household contact frequency increased by 3% (95%CI: 2–5) per additional household member. Members of households of at least 7 members had 36% (95%CI: 18–57) more contacts than participants from households with 1–3 members (Table 1).
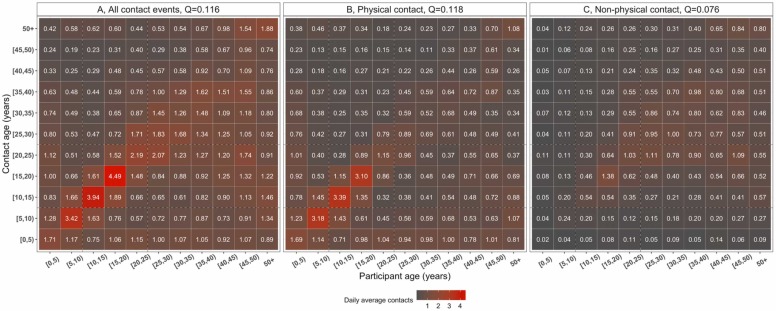
3.4. Age-specific mixing patterns
Social contacts were highly age-assortative, with the most intense contacts clustered among primary school children and secondary school children compared to infants, preschool children or adults. Non-physical contacts were less age-assortative (Q=0.076) than physical contacts (Q=0.118) and were mostly reported among adults, whereas children mostly reported physical contacts ( Fig. 3).
Fig. 3.
The daily mean number of reported contacts between age groups, in urban Blantyre, Malawi between April and July 2021. The number in each cell represents the daily mean number of contacts between two age groups from 1000 bootstrap replicates, corrected for reciprocity between participants and contacts, and weighted for day of the week. The matrices show the daily mean number of all contacts (A), physical contacts (participant’s skin to skin touch with a contact) (B) and non-physical contacts (participant’s two-way close verbal conversation lasting for ≥5 min and with ≥3 words exchanged with a contact) (C).
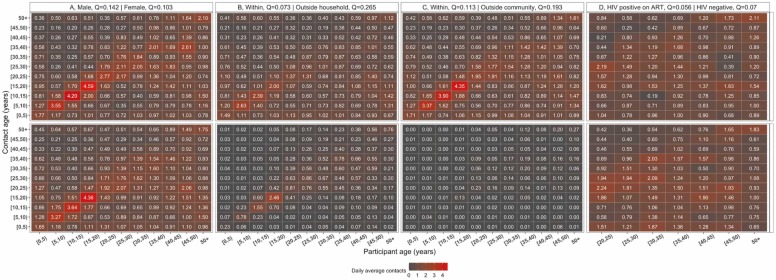
Age-assortativity was particularly pronounced for male-gender mixing patterns (Q =0.142), with women being more involved in intergenerationally mixing (Q=0.103). Participant-contact mixing between male-male (Q=0.420) was more intense and highly assortative than female-female (Q=0.163) or male-female (Q=0.106) or female-male (Q=0.096). Mixing outside household (Q=0.266) and Ndirande community (Q=0.196), was more age-assortative than within household (Q=0.073) and community (Q=0.113). No differential age-assortativity was seen on social contacts behaviour between HIV-positive adults taking ART (Q=0.056) and HIV-negative adults (Q=0.071) ( Fig. 4, Fig. S4, Fig. S5).
Fig. 4.
Daily mean number of reported contacts between age groups, in urban Blantyre, Malawi between April and July 2021. The number in each cell represents the daily mean number of contacts between two age groups from 1000 bootstrap replicates, corrected for reciprocity between participants and contacts, and weighted for day of the week. The top and bottom matrices show the daily mean number of contacts respectively stratified by male and female sex (A), within and outside household contacts (B), within and outside community contacts (C), Human Immunodeficiency Virus (HIV) positive adults (20 + years old) and HIV-negative adults (D). The assortativity index Q quantifies the weight of mixing between individuals of the same age groups and is estimated on the square matrix. For the HIV-stratified matrices, the index Q is estimated on the square matrix between pairs of adult participants and adult contacts aged at least 20 years old.
4. Discussion
Surveying 1201 individuals in high-density informal urban suburb in Malawi, the median number of contacts was higher than previously reported in Zambia (Dodd et al., 2016), but similar to Zimbabwe and Somaliland and many MICs and HICs (Mousa et al., 2021, Melegaro et al., 2017, Zandvoort et al., 2021). Older age, employment, and bigger households were significantly associated with an increased number of contacts in this setting, consistent with previous social contacts studies (Mousa et al., 2021, Hoang et al., 2019). Reported social contacts were strongly age-assortative and intergenerational by sex, mostly physical and occurring within household and geographically localised. Notably, neither HIV status, ART use nor COVID-19 pandemic restrictions significantly reduced population-level social contacts behaviour.
The reported median number of contacts of 10 is similar to reported contacts among internally diplaced people in Digaale camp in Somaliland (Zandvoort et al., 2021), but higher than the median of 4 contacts reported in a LIC in Zambia (Dodd et al., 2016), and lower than the 24 reported in Thailand (Stein et al., 2014) or 18 in rural Kenya (Kiti et al., 2014), highlighting the substantial role local context play in social contacts. The completeness of contacts capture was strengthened by conducting participant’s interview only on second visit with advanced notice to remember contacts on the day, guided interview process collecting only contacts initials to start with and aiding participant’s memory by structuring the interview to prompt participants to the different typical parts of the day. Unlike some LIC, MICs and HICs where the number of daily contacts usually are highest among school age children (Mousa et al., 2021, Melegaro et al., 2017, Zandvoort et al., 2021, Kiti et al., 2014, Mossong et al., 2008), our finding that working age adults had the largest number of increasing daily contacts aligns with results from upper MICs (Mousa et al., 2021). This suggests that schools may play a less prominent role in the transmission of infectious diseases in this setting than they do in other parts of the world; albeit that this may be counter-balanced by children comprising a generally larger share of the population in LICs.
The 15% relatively higher average number of contacts among workers (often self-employed traders in this setting) than among unemployed individuals is in line with evidence from several previous studies irrespective of location (Mousa et al., 2021), and reflects the influence of human mobility and trading activities on social contacts (Garske et al., 2011), which in part has motivated lockdowns in some MICs and HICs during the COVID-19 pandemic (Liu et al., 2021b). Increasing number of contacts with household size has also been reported in some MICs and HICs (Mousa et al., 2021), with household density suggested to be a driving factor particularly in this setting where extended families sharing a compound is common. We also report higher proportion of home contacts similar to other LICs and MICs than those reported in HICs, and by contrast, the proportion of school and work contacts were substantially lower than those in HICs (Mousa et al., 2021). This implies that household may be a key site of transmission for respiratory diseases (Thompson et al., 2021), and public preventive measures may only be efficacious at reducing the speed for spatial spread outside homes. However, the relevance of contact location on transmission will also depend on, among other things, specific pathogen and transmission routes e.g. droplet, fomite or aerosol (Mousa et al., 2021).
The strong age-assortative contacts in this study are consistent with widespread evidence globally (Mousa et al., 2021, Hoang et al., 2019), and support adjusting for age-heterogeneity when calibrating models for predictions (Keeling and Rohani, 2011, Vynnycky and White, 2010). Approximately 80% of contacts in this study were skin-to-skin, falling on the higher side of the reported range of 19–84% globally (Mousa et al., 2021). While the predominance of physical contacts in children compared to adults is similar across all settings, substantial physical contacts in our study align with reports in LICs and MICs but not HICs (Mousa et al., 2021). Social interactions by women were less strongly age-assortative than for men, consistent with a greater role for intergenerational mixing between mothers or female guardians with younger children, as also reported in Zimbabwe and Kenya (Melegaro et al., 2017, Kiti et al., 2014). This finding may imply that shielding older adults as a public health policy during pandemics may substantially reduce the spread of infectious respiratory pathogens. Age-sex interactions showed relatively high age-assortativeness among male-male contacts than other sex combinations, implying that relatively small underlying biological or behavioural difference in susceptibility by sex may be amplified by assortative social networks, as hypothesised for TB (Dodd et al., 2016). Moreover, relatively high (97%) frequency of contacts occurring within rather than outside community in this study may imply that epidemics would mostly be localised. However, adults > 15 years-old reported the highest intense physical contacts including minibus travel with potential to disseminate outbreaks outside of the local community.
Ours is the first study to evaluate whether HIV-positive status modulates contact behaviour. In this high HIV prevalence population with good access to ART, we found no evidence that HIV status per se influences contact rates or age-assortativeness. Given that immunosuppressed individuals may be at increased risk of prolonged COVID-19 infectiousness and generation of new variants as well as increased pneumococcal carriage (Heinsbroek et al., 2015, Corey et al., 2021), this suggests that HIV-positive individuals may have equivalent or increased potential for contributing to respiratory disease transmission in this setting (Thindwa et al., 2021). We have not, however, assessed the other drivers of pathogen transmissibility, such as carriage density and duration of infection, and so cannot confirm a likely disproportionate role in transmission. Of note, reported adult HIV prevalence in this setting is slightly higher (26.8%) compare to that from the impact HIV assessment in Blantyre in 2015–16 (17.7%) (Ministry of Health, 2018b), due to undersampled HIV-negative adults in our study since our sampling procedure was not aimed at estimating HIV prevalence but rather comparing age-specific contact rates from representative sample of HIV-positive and HIV-negative adults.
Contrary to MICs and HICs who reported substantial reduction in social contacts due to NPIs (Quaife et al., 2020, Jarvis et al., 2020, Gimma et al., 2021), only 25% of participants in our study reported to have changed their contact behaviour between the second and third COVID-19 waves in Malawi. However, in this study, behavioural changes are self-reported and entirely hypothetical and the actual changes in social contact behaviour cannot be reliably measured, and that little is known about contact patterns in Malawi prior to the pandemic. Moreover, contacts among younger children reported by their parents or guardians may also be underreported affecting estimation of average contacts in this age group. Nevertheless, we did not find reduced contact rates among participants who reported to have reduced their social contacts due to COVID-19 pandemic restrictions compared to those who reported to not have changed their behaviour. Additional analysis of average contacts between the COVID-19 waves (level 1 policies) compared to during the COVID-19 wave (level 3 policies) consistently showed little contact behavioural change similar to participants responses about their hypothetical contact behaviour (Supplementary Table 2 and Supplementary Table 3). This may suggest sub-optimal adherence to COVID-19 prevention measures such as restricted movements, closing of non-essential businesses, enforced social distancing, reduced public transportation capacity, and limited numbers (recommended 10 people per gathering) in this setting, which may reflect the day-to-day demands in the context of pressing economic hardship (Josephson et al., 2021, Walker et al., 2020, Mzumara et al., 2021). Economic implications of policy options aimed at limiting transmission of respiratory diseases need to be considered in order to make pragmatic recommendations that can be adhered to in the local context. Of note, relatively few per capita hospitalisations and deaths from COVID-19 occurred during the first wave in Malawi despite evidence of widespread transmission, and this may explain low compliance with recommendations to reduce social contacts during the second and third waves of COVID-19 pandemic in urban Malawi.
The strengths of this study include a sufficiently large sample size to detect significant differences in contact rates between age groups, although small numbers limited our precision for infants. We sampled households from 14 clusters making up a high proportion of residential Blantyre. Substantial number of participants were recruited during weekdays (75%) and weekends for representation and contact matrices were weighted for weekdays and weekends and accounted for reciprocity. This study collected novel data on social contacts in Malawi, adding insights to limited primary datasets on contact patterns in LICs in Africa, with only 3 countries represented. Limitations include that reported contacts by participants may be subject to recall bias (Smieszek et al., 2012). This bias was partially addressed, however, by conducting two visits within three days; asking participants on the first visit to remember all their contacts during waking up and going to bed, and on the second visit, asking participants to report those contacts. Prospective reporting of cases reduces bias, but can lead to overreporting (Mikolajczyk and Kretzschmar, 2008). Data on contacts within schools and other indoor spaces were not collected at fine scale, hence are difficult to assess. Social contacts may change over time (Liu et al., 2021a, Gimma et al., 2021), and our cross-sectional design did not include longitudinal sampling of mixing patterns. Our results may not be generalisable to rural settings of Malawi that have relatively low household density and socio-economic activities but large school sizes compared to urban communities (National Statistical Office Malawi, 2019). Thus, it remains uncertain whether or not contacts would be low given mixed results from rural Zimbabwe and Kenya (Melegaro et al., 2017, Kiti et al., 2014).
In conclusion, high rates of physical, age-assortative and localised contacts were observed, particularly among secondary school children and adults. With the demographic shift that many LICs are undergoing this raises the potential for adults in this and similar settings to play a more prominent role in the transmission of respiratory diseases than typically the case in HICs. In addition, the lack of change in contact behaviour in response to the ongoing pandemic highlights specific challenges for mitigation strategies in poor communities with no social protection mechanisms. For pandemic planning, it will be crucial to better understand what factors would enable and encourage poor urban populations to reduce their contacts to slow pandemic spread if the need arises.
Funding source
This manuscript was funded by the UK National Institute for Health and Care Research (NIHR) Global Health Research Unit on Mucosal Pathogens (MPRU) using UK aid from the UK Government (16/136/46). D. Thindwa, J. Ojal, K.E. Gallagher, Robert S. Heyderman, N. French, and S. Flasche are supported by the UK National Institute for Health and Care Research (NIHR) Global Health Research Unit on Mucosal Pathogens (MPRU) using UK aid from the UK Government. RSH is a NIHR Senior Investigator. S. Flasche is also supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome and the Royal Society (Grant number 208 812/Z/17/Z) in the UK. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors have declared that no conflict of interest or competing interests exist.
Acknowledgements
We would like to thank Dr. Emanuele Del Fava of the Carlo F. Dondena Centre for Research on Social Dynamics and Public Policy, Bocconi University, Milan, Italy and Max Planck Institute for Demographic Research, Rostock, Germany, and Kevin van Zandvoort of the London School of Hygiene and Tropical Medicine, in London, United Kingdom, for insightful discussions at the analysis stage of this work. We thank all Social Mixing Patterns (SOMIPA) participants for their participation in the study as well field workers for administering consents and collecting data. We also thank the Blantyre Directorate of Health and Social Services as well as the Blantyre City Council for their support to the study activities.
CRediT authorship contribution statement
DT, SF, NF, AP: Conceptualization. DT: Data curation. DT: Formal analysis. DT, RSH: Funding acquisition. DT: Investigation. DT, SF, AP: Methodology. DT, KJ: Project administration. DT, RSH: Resources. DT: Software. SF, NF, KJ: Supervision. DT, KCJ, JO, PM, MDP, AP, MK, LC, KEG, RSH, ELC, NF, SF: Validation. DT: Visualization. DT: Roles/Writing – original draft. DT, KCJ, JO, PM, MDP, AP, MK, LC, KEG, RSH, ELC, NF, SF: Writing – review & editing. All authors read and approved the final manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.epidem.2022.100590.
Appendix A. Supplementary material
Supplementary material.
.
Data availability
Results can be reproduced using the data and code in the GitHub repository: https://github.com/deusthindwa/social.contact.rates.estimation.hiv.malawi.
References
- Corey L. SARS-CoV-2 variants in patients with immunosuppression. N. Engl. J. Med. 2021;385:562–566. doi: 10.1056/NEJMsb2104756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fava E. Individual’s daily behaviour and intergenerational mixing in different social contexts of Kenya. Sci. Rep. 2021;11:21589. doi: 10.1038/s41598-021-00799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd P.J. Age- and sex-specific social contact patterns and incidence of Mycobacterium tuberculosis infection. Am. J. Epidemiol. 2016;183:156–166. doi: 10.1093/aje/kwv160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y., et al. Disparities in COVID-19 vaccination among low-, middle-, and high-income countries: the mediating role of vaccination policy. Vaccines. 2021;9:905. doi: 10.3390/vaccines9080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer-Lindgren L. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570(189) doi: 10.1038/s41586-019-1200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field Trials of Health Interventions: A Toolbox. Oxford University Press, 2015. [PubMed]
- Flasche S., Lipsitch M., Ojal J., Pinsent A. Estimating the contribution of different age strata to vaccine serotype pneumococcal transmission in the pre vaccine era: a modelling study. BMC Med. 2020;18 doi: 10.1186/s12916-020-01601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk, S., et al., 2020. Social mixing matrices for infectious disease modelling in R. 〈https://cran.r-project.org/web/packages/socialmixr/index.html〉.
- Garske T. Travel patterns in China. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimma, A., et al., 2021. CoMix: changes in social contacts as measured by the contact survey during the COVID-19 pandemic in England between March 2020 and March 2021. 2021.05.28.21257973 〈https://www.medrxiv.org/content/10.1101/2021.05.28.21257973v1〉. doi:10.1101/2021.05.28.21257973. [DOI] [PMC free article] [PubMed]
- Heesterbeek H., et al. Modeling infectious disease dynamics in the complex landscape of global health. Science. 2015;347:aaa4339. doi: 10.1126/science.aaa4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek E. Persisting high prevalence of pneumococcal carriage among HIV-infected adults receiving antiretroviral therapy in Malawi. Aids. 2015;29:1837–1844. doi: 10.1097/QAD.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T. A systematic review of social contact surveys to inform transmission models of close-contact infections. Epidemiology. 2019;30:723–736. doi: 10.1097/EDE.0000000000001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis C.I. Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med. 2020;18:124. doi: 10.1186/s12916-020-01597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone-Robertson S.P. Social mixing patterns within a South African township community: implications for respiratory disease transmission and control. Am. J. Epidemiol. 2011;174:1246–1255. doi: 10.1093/aje/kwr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson A., Kilic T., Michler J.D. Socioeconomic impacts of COVID-19 in low-income countries. Nat. Hum. Behav. 2021;5:557–565. doi: 10.1038/s41562-021-01096-7. [DOI] [PubMed] [Google Scholar]
- Keeling M.J., Rohani P. Princeton University Press; 2011. Modeling Infectious Diseases in Humans and Animals. [Google Scholar]
- Kiti M.C. Quantifying age-related rates of social contact using diaries in a rural coastal population of Kenya. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.Y. Rapid review of social contact patterns during the COVID-19 pandemic. medRxiv. 2021;2021 doi: 10.1101/2021.03.12.21253410. 03.12.21253410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. The impact of non-pharmaceutical interventions on SARS-CoV-2 transmission across 130 countries and territories. BMC Med. 2021;19:40. doi: 10.1186/s12916-020-01872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangal T. Potential impact of intervention strategies on COVID-19 transmission in Malawi: a mathematical modelling study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-045196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melegaro A. Social contact structures and time use patterns in the Manicaland Province of Zimbabwe. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk R.T., Kretzschmar M. Collecting social contact data in the context of disease transmission: prospective and retrospective study designs. Soc. Netw. 2008;30:127–135. [Google Scholar]
- Ministry of Health, 2018a. Malawi Guidelines for Clinical Management of HIV in Children and Adults. 1–128 〈https://differentiatedservicedelivery.org/Portals/0/adam/Content/yb4xSSLvE0SW98_z7wTm_w/File/Malawi%20Clinical%20HIV%20Guidelines%202018%20(1).pdf〉.
- Ministry of Health, Malawi, 2018b. Malawi Population-Based HIV Impact Assessment. 1–306 〈https://phia.icap.columbia.edu/resources/〉.
- Mossong J. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa A., et al. Social contact patterns and implications for infectious disease transmission – a systematic review and meta-analysis of contact surveys. eLife. 2021;10 doi: 10.7554/eLife.70294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mzumara G.W., et al. The health policy response to COVID-19 in Malawi. BMJ Glob. Health. 2021;6 doi: 10.1136/bmjgh-2021-006035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Statistical Office Malawi, 2019. Malawi Population and Housing Census 2018. 1–311 〈http://www.nsomalawi.mw/index.php%3Foption%3Dcom_content%26view%3Darticle%26id%3D226:2018-malawi-population-and-housing-census%26catid%E2%80%89%3D%E2%80%898:reports%26Itemid%E2%80%89%3D%E2%80%896〉.
- Neal E.F.G., et al. Associations between ethnicity, social contact, and pneumococcal carriage three years post-PCV10 in Fiji. Vaccine. 2020;38:202–211. doi: 10.1016/j.vaccine.2019.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Polain de Waroux O., et al. Characteristics of human encounters and social mixing patterns relevant to infectious diseases spread by close contact: a survey in Southwest Uganda. BMC Infect. Dis. 2018;18:172. doi: 10.1186/s12879-018-3073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaife M., et al. The impact of COVID-19 control measures on social contacts and transmission in Kenyan informal settlements. BMC Med. 2020;18:316. doi: 10.1186/s12916-020-01779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read J.M., Edmunds W.J., Riley S., Lessler J., Cummings D.A.T. Close encounters of the infectious kind: methods to measure social mixing behaviour. Epidemiol. Infect. 2012;140:2117–2130. doi: 10.1017/S0950268812000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read Jonathan M., et al. Social mixing patterns in rural and urban areas of southern China. Proc. R. Soc. B Biol. Sci. 2014;281(20140268) doi: 10.1098/rspb.2014.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smieszek T., Burri E.U., Scherzinger R., Scholz R.W. Collecting close-contact social mixing data with contact diaries: reporting errors and biases. Epidemiol. Infect. 2012;140:744–752. doi: 10.1017/S0950268811001130. [DOI] [PubMed] [Google Scholar]
- Soko, R.N., et al., 2021. Effects of Coronavirus Disease Pandemic on Tuberculosis Notifications, Malawi - Volume 27, Number 7—July 2021 - Emerging Infectious Diseases Journal - CDC. doi:〈10.3201/eid2707.210557〉. [DOI] [PMC free article] [PubMed]
- Stein M.L., et al. Comparison of contact patterns relevant for transmission of respiratory pathogens in Thailand and the Netherlands using respondent-driven sampling. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M.An. Asymptotic equivalence of choice of model by cross-validation and Akaike’s criterion. J. R. Stat. Soc. Ser. B Methodol. 1977;39:44–47. [Google Scholar]
- Tc D., et al. The STRATAA study protocol: a programme to assess the burden of enteric fever in Bangladesh, Malawi and Nepal using prospective population census, passive surveillance, serological studies and healthcare utilisation surveys. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016283. e016283–e016283. e016283–e016283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thindwa, D., 2021. R code and data for social mixing patterns relevant to infectious diseases spread by close contact in urban Blantyre, Malawi. 〈https://github.com/deusthindwa/social.contact.rates.estimation.hiv.malawi〉. [DOI] [PMC free article] [PubMed]
- Thindwa D., et al. Electronic data capture for large scale typhoid surveillance, household contact tracing, and health utilisation survey: strategic Typhoid Alliance across Africa and Asia. Wellcome Open Res. 2020;5:66. doi: 10.12688/wellcomeopenres.15811.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thindwa D., et al. Estimating the contribution of HIV-infected adults to household pneumococcal transmission in South Africa, 2016-2018: a hidden Markov modelling study. medRxiv. 2021 doi: 10.1101/2021.05.21.21257622. 2021.05.21.21257622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H.A., et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) setting-specific transmission rates: a systematic review and meta-analysis. Clin. Infect. Dis. 2021;73:e754–e764. doi: 10.1093/cid/ciab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usherwood T., LaJoie Z., Srivastava V. A model and predictions for COVID-19 considering population behavior and vaccination. Sci. Rep. 2021;11:12051. doi: 10.1038/s41598-021-91514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vynnycky E., White R. Oxford University Press; 2010. An Introduction to Infectious Disease Modelling. [Google Scholar]
- Wahl B., et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob. Health. 2018;6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P.G.T., et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science. 2020 doi: 10.1126/science.abc0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2020. Coronavirus disease 2019 (COVID-19). 〈https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200405-sitrep-76-covid-19.pdf?sfvrsn=6ecf0977_2〉.
- WHO Health Organisation, 2021a. Tuberculosis Global Burden. 〈https://www.who.int/news-room/fact-sheets/detail/tuberculosis〉.
- World Health Organization, 2021b. WHO Coronavirus (COVID-19) Burden. 〈https://covid19.who.int〉.
- Zandvoort, K. van, et al., 2021. Social contacts and other risk factors for respiratory infections among internally displaced people in Somaliland. 2021.08.20.21262338 〈https://www.medrxiv.org/content/10.1101/2021.08.20.21262338v1〉. doi:10.1101/2021.08.20.21262338. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Results can be reproduced using the data and code in the GitHub repository: https://github.com/deusthindwa/social.contact.rates.estimation.hiv.malawi.