Abstract
Background
Scarce information is available regarding the long-term immunogenicity of the Sputnik V vaccine. Here Sputnik V vaccinated subjects were evaluated 6 months after receiving the 2-dose prime-boost schedule.
Methods
Eighty-six hospital workers from Venezuela, 32 with a previous COVID-19 infection and 54 SARS-CoV-2 naïve subjects, were enrolled. IgG antibodies levels against the wild-type Receptor Binding Domain (RBD) were measured in an ELISA and with an in vitro ACE2-surrogate RBD binding inhibition assay at day 42 and day 180 after receiving the second dose. IgG levels were expressed in BAU/ml. Binding inhibition antibodies were expressed in IU/ml.
Results
On average, RBD-IgG levels decreased by approximately 50% between the two time-points in the COVID-19 naïve cohort (geometric mean concentration (GMC) 675 BAU/mL vs. 327 BAU/ml) and decreased by approximately 25% in the previously infected cohort (GMC 1209 BAU/mL vs 910 BAU/ml). Within our cohort, 94% showed a “good to excellent” neutralizing activity measured with the in vitro test 6 months after vaccination.
Conclusions
The Sputnik V vaccine provided long-term and durable humoral immunity in our cohort specially if a person has been both vaccinated and had a previous infection with SARS-CoV-2.
Keywords: Sputnik V vaccine, IgG antibodies, Receptor Binding Domain (RBD), Nucleocapsid protein (NP), Binding antibody units or BAU/ml, RBD binding inhibition assay, International units or IU/ml, Hybrid COVID-19 immunity
Introduction
The introduction of SARS-CoV-2 vaccines has played a crucial role in reducing the spread of SARS-CoV-2 and the severity of COVID-19. These vaccines induce viral-specific humoral and cellular immunity that protect against serious illness, hospitalization, or death. Most vaccines target the spike protein as it has been demonstrated that this glycoprotein can induce a protective immune response (Yang et al., 2020). Consequently, most immunity studies have focused on the role of anti-spike binding antibodies in vaccine-induced protection, while the role of T-cell immunity is less well characterized, although pre-existing T-cell immunity to SARS-CoV-2 has been documented (Sette and Croty, 2020; Grifoni et al., 2020; Echeverria et al., 2021). Antibody responses against the receptor binding domain (RBD) of the spike protein are considered the most important antibodies because they neutralize the virus and impair the virus in its attempt to bind to cell receptors and consequentially play an essential role in protection against reinfection (Wagner et al., 2021; Cromer et al., 2022; Pallett et al., 2021; Bergwerk et al., 2021; Khoury et al., 2021). For efficient protection the duration of the antibody response is of major importance, however, early after the introduction of vaccines, it was shown that over time the humoral response to vaccines begins to wane. In a study in Estonia, individuals who received the Pfizer vaccine showed RBD IgG levels at six months that were only from 2 to 25% of their peak levels, detected after the second dose (Naaber et al., 2021). A study in the USA showed that the binding titers of the RBD protein of the Moderna vaccine assessed with an enzyme-linked immunosorbent assay (ELISA), six months after receiving the second dose, had decreased by approximately a factor of 10 (Doria-Rose et al., 2021). Another study in Israel showed that at 8 months after Pfizer and Moderna vaccination, the RBD-specific binding antibody titers elicited by the vaccines were respectively a factor of 29 and 17 lower than the peak titers (Collier et al., 2021).
Directly associated with the declining humoral immune response of the vaccines, it has been demonstrated in several studies that there is a growing risk of breakthrough infections (Khoury et al., 2021; Feng et al., 2021) and a decrease in RBD titers over time increases the risk of reinfection. In Israel, among fully vaccinated health care workers, SARS-CoV-2 breakthrough infections were documented in those with lower antibody levels (Bergwerk et al., 2021). In US veterans, vaccine effectiveness declined, decreasing from 87.9% to 48.1% in approximately 6 months after vaccination. Declines were greatest for the Janssen vaccine followed by Pfizer–BioNTech and Moderna (Cohn et al., 2021). These findings were consistent with the better neutralizing antibody response observed following vaccination with Moderna or Pfizer-BioNtech compared to Janssen vaccines (Tada et al., 2022). Moreover, the antibody response to SARS-CoV-2 vaccination is related to the immune status of the vaccinated subject and declined rapidly in persons receiving dialysis, with higher odds for breakthrough infection in subjects with a lower antibody response against the RBD (Anand et al., 2021).
Here, we aimed to evaluate the long-term IgG antibody response against the nucleocapsid protein (NP) and the receptor binding domain (RBD) of the spike protein of SARS-CoV-2 in a cohort of 86 hospital workers from Venezuela at 6 weeks and 6 months after their second doses of the Sputnik V vaccine. With serology, we divided this population into individuals with previous SARS-CoV-2 seroconversion (presence of IgG against NP), individuals with no previous history of SARS-CoV-2 infection or disease (IgG negative against NP), and breakthrough infection (NP converters after the second vaccine dose). We compared previously SARS-CoV-2-infected with NP antibody-negative individuals and we described the durability of vaccine-induced antibodies against wild-type SARS-CoV-2. A WHO standard serum pool was used to normalize antibody levels measured in an ELISA in Binding Antibody Units (BAU/ml) and in International Units (IU) for a SARS-CoV-2 surrogate virus RBD binding inhibition assay (Kristiansen et al., 2021; Tan et al., 2020)
Material and methods
Participants
The 86 hospital workers in this study were vaccinated with two doses of Sputnik V vaccine in the period between February and March 2021. The demographic data of 84 of the subjects can be found in a previous publication from our laboratory. Also, IgG responses against SARS-CoV-2 anti-NP and the RBD of the Spike protein on the day of the first vaccine dose, at day 21 after receiving the second dose, and day 42 after receiving the second dose were reported previously (Claro et al., 2021). About 180 days (6 months) after the second vaccine dose, blood samples were taken from these subjects and the serum samples were used to quantify again the IgG concentration against NP and RBD of the spike protein and compared with the IgG levels 42 days after the second vaccine dose. Previous infection with SARS-CoV-2 in our subjects was serologically defined as NP positive (> 40 BAU/ml) at any time point or anti-RBD positive before vaccination.
IgG antibodies against RBD and NP
An in-house ELISA was used that has been described earlier (Claro et al., 2021) using as capture antigen the native SARS-CoV-2 Receptor Binding Domain (RBD) and the Nucleocapsid protein (NP) of the SARS-CoV-2 virus (MyBioSource cat. numbers MBS8574742 and MBS8574741, respectively). Although in the earlier publication the ELISA results were expressed in S/P values, after the release of the WHO standard serum for SARS-CoV-2 IgG antibodies, the ELISA was standardized against the first WHO international standard for anti-SARS-CoV2 immunoglobulin (NIBSC code: 20/136), and the results in the present study are expressed in Binding Antibody Units per milliliter (BAU/ml) (Kristiansen et al., 2021).
Determination of neutralizing antibodies
The serum samples at day 42 and day 180 after vaccination were also used to quantify binding inhibition antibodies developed upon vaccination using a commercial kit (ACE2-RBD Neutralization Assay DIA.PRO, Italy). This ELISA determines the inhibition of the binding of antibodies present in the serum samples between the ACE2 receptor and RBD. For this purpose, the microtiter plates are coated with SARS-CoV-2 wild-type recombinant glycosylated RBD.
The first step was to incubate the samples, allowing anti-RBD-Spike antibodies, if present, to bind to the antigen. After washing, free glycosylated RBD on the plate was determined by the addition of recombinant ACE2 biotinylated antigen that will bind to the antigen only when RBD-specific antibodies do not block the antigen. After washing and incubating with Streptavidin-HRP, a color was generated with a TMB-H2O2 substrate. A strong yellow color indicates no or few neutralizing antibodies present. No color development means that the whole antigen has been blocked by antibodies and consequently a high titer of neutralizing antibodies. The assay has been calibrated against the WHO international standard for SARS-CoV-2 antibodies and neutralizing antibody levels are expressed as International Units per milliliter (IU/ml)
Statistical analysis
Violin plots and statistical calculations were performed with the R software package. A Welch two sample t-test was performed to compare IgG-RBD antibodies between different groups. A linear regression model was fitted between RBD binding inhibition antibody concentrations (IU/ml) and anti-RBD IgG concentrations (BAU/ml) to find the equivalent classification cutoffs for IU/ml in terms of BAU/ml.
Ethical considerations
Hospital Vargas de Caracas’ ethics committee approved the study and participants gave oral and written permission for an interview and to use their blood samples for serological studies regarding SARS-CoV-19 infection and the immune response against the Sputnik V vaccine.
Results
Study Participants
The 86 participants in this study had been fully vaccinated with two doses of the Sputnik vaccine between February and March 2021. Their ages ranged from 21 to 76 with a mean age of 41±13.2, and 49 (57%) were women. At least one chronic condition was reported by 30% of individuals: hypertension (17 individuals), diabetes (4), and asthma (5).
The study participants could be divided into three groups based on interviews and serology for the NP antigen; never infected (47 individuals, all negative for NP antibodies), infected before the second dose (32 individuals, all NP antibodies positives), and individuals who got infected during the period between 45- and 180-days post-vaccination (7 individuals, seroconverts for NP antibodies). The presence of antibodies against NP indicated previous infection as all participants were vaccinated with Sputnik, which only induces antibodies against the spike protein. Moreover, interviews with these participants revealed that 47 never showed any signs or symptoms compatible with COVID-19 or tested negative with an RT-PCR. The other 32 had been diagnosed with COVID-19 before vaccination and had NP antibodies before vaccination or when the second dose of the vaccine was applied. The 7 breakthrough COVID-19 cases, NP negative 42 days after the second vaccine dose, reported mild COVID-19 symptoms.
Assessment of SARS-CoV-2 antibodies at day 42 and day 180 after vaccination
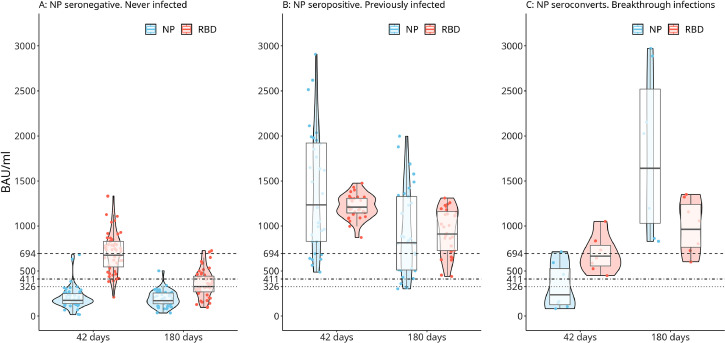
See Figure 1 for the distribution of IgG antibody levels against RBD and NP and expressed in BAU/ml at day 42 and day 180. IgG-RBD antibodies were higher in the previously infected participants in comparison with the COVID-19 naïve cohort (Figure 1B and 1A respectively). In both groups, a decline in IgG-RBD antibodies occurred 6 months after vaccination, though antibody values remained significantly higher in the previously infected cohort (p < 0.01). Considering the 7 patients that got infected somewhere between day 45 and day 180 after vaccination (Figure 1C), a considerable rise in RBD and NP antibodies levels is seen at day 180.
Figure 1.
Violin Plots showing the distribution of the IgG levels in subgroups A (never infected or naïve for COVID-19; 47 individuals), B (previously infected: 32 individuals), and C (breakthrough infection after the second dose; 7 individuals) at day 42 and day 180 after the second vaccine dose with the Sputnik V vaccine. IgG levels against the NP and RBD protein are expressed in BAU/ml. A positive antibody response or a good virus neutralization activity (dotted line) is defined as a titer with a IU/ml of at least 100 IU. Excellent virus neutralization is defined as 400 BAU/ml (dashed line). See also the subsection “Determination of neutralizing antibodies.”
On average, RBD IgG levels decreased by approximately 50% between these two time-points in the “never infected” cohort (geometric mean concentration [GMC] 675 BAU/mL [95% CI, 610-747] vs. 327 BAU/ml [95% CI, 283-377]) and with approximately 25% in the previously infected cohort (GMC 1209 BAU/mL [95% CI,1161-1259] vs. 910 BAU/ml [95% CI, 816-1051]). The break-through cases showed an increase in antibody response in both IgG against NP and IgG against RBD (GMC 910 BAU/ml [95% CI, 816-1051] vs. GMC 1209 BAU/mL [95% CI, 1161-1259].
Determination of neutralizing antibodies
We compared anti-SARS-CoV-2 spike RBD IgG antibody concentrations with an ACE2-RBD Neutralization Assay (DIA.PRO, Italy). This in vitro surrogate virus RBD binding inhibition assay detects total neutralizing anti-S1 SARS-CoV-2 antibodies (IgG, IgM, and IgA). The first international standard WHO 20/136 for anti-SARS-CoV-2 was used to quantify binding inhibition antibodies to RBD, and these antibody levels were expressed in International Units per milliliter (IU/ml).
We performed a linear regression to model the relationship between binding inhibition antibody concentrations (IU/ml) and anti-RBD IgG concentrations (BAU/ml). The resulting model was a fairly good fit for values lower than 900 IU (adjusted-R²=0.8365). For strongly positive samples with a result higher than 900 BAU/ml, the sample should be diluted for a more accurate diagnostic determination of antibody levels. For vaccine studies, this pseudo neutralizing antibody test considers 10-100 WHO IU/ml to be a moderate neutralizing activity, 100-400 IU as good, and >400 as an excellent neutralizing activity. We determined the equivalent cutoffs corresponding to 100 and 400 IU/ml in terms of BAU/ml using the model we fitted, the resulting cutoffs were 326-411 BAU/ml for a moderate neutralizing activity, 411-694 BAU for good neutralizing activity and > 694 BAU for an excellent neutralizing activity. These values are indicated in Figure 1 with dotted and dashed lines. In the uninfected participants at day 42, 91% had good to excellent neutralizing activity (>411 BAU/ml) with 3 participants showing moderate neutralizing activity (>326 BAU/ml) and one showing poor neutralizing activity with 211 BAU/ml. After 180 days post-vaccination, the neutralizing activity for COVID-19 naive participants was at least "moderate" for 55% of the participants, with 38% being “good” or “excellent”. 45% of the participants had BAU/ml levels under 326. For the previously infected cohort and breakthrough infections, the test classified all individuals 180 days post-vaccination as having good or excellent neutralization activity (>411 IU/ml).
Discussion
This longitudinal study determined the humoral immunity induced by Sputnik V vaccination during a 6-month follow-up. We showed that IgG levels declined over a period of 6 months. We also showed a significant difference in anti-SARS-CoV-2 antibody levels between COVID-19-naïve subjects and subjects who had recovered from COVID-19 prior to vaccination. IgG levels 6 months after vaccination in most disease-naïve subjects stayed above 100 BAU/m (only two participants saw their antibody levels lower to 98 and 96 BAU/ml). Moreover, 19 (40%) of the COVID-19 naïve participants in this study had IgG-RBD antibodies above the 400 BAU/ml level. After 6 months, all of the previously COVID-19 infected subjects had IgG-RBD antibodies above that same level. We compared anti-SARS-CoV-2 RBD-IgG antibody concentrations and antibody-mediated surrogate neutralization of spike-angiotensin-converting enzyme (ACE2) receptor binding in vitro and we found a good agreement between IU/ml obtained by this assay and BAU/ml results of our COVID-19 RBD IgG kit for values between 50 and 900 BAU/ml with a linear regression analysis. With this correlation study, we confirmed high levels of agreement between results obtained by a pseudo-virus RBD binding inhibition assay and our COVID-19 RBD IgG kit. We therefore assumed that our ELISA can be used to determine if the concentration of IgG-RBD antibodies, the immunogenic protein measured with our ELISA, has a virus neutralizing effect.
Correlates of protection against SARS-CoV-2 infection in humans have not yet been established and are arbitrarily assigned in the commercial kit as 100 and 400 BAU/ml being a “good” and “excellent” RBD binding inhibition activity respectively. In a study from France, 141 BAU/ml is thought to be high enough for a protection/vaccine efficacy against SARS-CoV-2 infection of approximately 90% (Dimeglio et al., 2022). Out of our 47 Sputnik V vaccinated COVID-19 naïve subjects, after 6 months, only 2 had IgG-BRD antibodies under this threshold. As suggested in another study, 165 BAU/ml and 506 BAU/ml respectively are thought to be high enough for a vaccine efficacy of 70% and 80% against symptomatic infection (Feng et al., 2021;). Out of the 47 COVID naïve participants, after 6 months 3 had had antibody levels of < 165 BAU/ml in this study and 8 had antibody levels of > 506 BAU/ml. Concerning the previously infected cohort, all had antibody levels of > 165 BAU/ml and only 2 out of the 32 had BRD antibody levels of < 506 BAU/ml (respectively 440 and 454 BAU/ml). All breakthrough infections (7 subjects) showed BRD-IgG levels of > 600 BAU/ml.
To the best of our knowledge, only two other studies have explored the waning or persistence of antibodies elicited in time to the Sputnik V vaccine, both in Argentina. Comparable with our study, a longitudinal analysis of 118 volunteers vaccinated with the two-dose regimen of Sputnik V also showed that IgG levels declined over a period of 6 months. The GM of IgG anti-spike antibodies for the group that was NP seronegative (COVID-19 naïve) at baseline (N = 88) in that study declined from 758 (CI95%, 574–1001) at day 42 (comparable with our study) to 73 (CI95%, 50–108) at day 180 (lower than in our study) after the initial vaccination (Chahla et al., 2022). We did not evaluate the immune response for SARS-CoV-2 variants but another study in Argentina, using a cohort of 118 volunteers, evaluated the humoral response for 6 months after Sputnik V vaccination and showed that the neutralizing potency of antibodies was maintained for all SARS-CoV-2 variants analyzed (Gonzales et al., 2022).
Conclusions
Our results showed that despite an expected decline in binding titers and neutralizing antibodies, the Sputnik V vaccine has the potential to provide durable humoral immunity for at least 6 months. For the COVID-19 naïve cohort, after 6 months, the GMC of RBD-IgG antibodies was 327 BAU/ml and 40% had RBD-IgG levels above 400 BAU/ml. The GMC of-RBD-IgG antibodies in previously infected people was 910 BAU/ml with all subjects with an BAU of > 400. Moreover, 94% of both cohort showed a good to excellent neutralizing activity in an in vitro test.
Limitations of this study
Insufficient data regarding comorbid conditions of the participants were available and were not included in the study, which could have resulted in confounding the interpretation of results regarding waning humoral immunity. SARS-CoV-2 variants have been classified by the WHO based on increased transmissibility and/or pathogenicity (Technical Advisory group, WHO, 2022). These variants have not been tested in the ELISA or the RBD binding inhibition assay. Also, our study did not access cell-mediated immunity and all of the subjects were hospital workers at relatively high risk of infection and reinfection. Moreover, the numbers in our study are small and the results need to be confirmed in larger, more diverse populations, with more power regarding sample size and across demographic and clinical subgroups (immune suppressed) that are known to exhibit variation in antibody response following vaccination.
The added value of this study
Although long-term follow-up of COVID-19 vaccines has been evaluated in several studies, most of the research efforts were dedicated to the Pfizer, AstraZeneca, Jansen, and Moderna vaccines. Regarding the Sputnik V vaccine, there is scarce information in the scientific literature. With this publication, we intend to fill this gap in knowledge. Our research adds descriptive data concerning the anti-RBD IgG immune response after Sputnik V vaccination in the context of both natural infection with COVID-19 as well as vaccination-induced immunity. We present data for a prolonged follow-up period of 180 days of serology with IgG levels expressed in BAU/ml, as is currently recommended by the WHO for universal comparison.
Authors’ contributions
FECA, DS, and JHdW designed the study, developed the ELISA assay, and, with the participation of RR, recruited the participants for this study. FC, DS performed the laboratory experiments. JAP was responsible for data analysis. All authors had full access to all data, searched for relevant literature, participated in writing the first draft, and read and approved the final manuscript. Both FECA and DS can be considered as co-first authors as they contributed equally. All authors declare no competing interests.
Funding
This study was funded by the “Fondo Nacional de Ciencia y Tecnología” (FONACIT, Venezuela). JhdW is supported by the Universidad de Las Americas, in Quito, Ecuador. The funders had no role in study design, data collection, or the decision to publish this article.
Ethical considerations
Hospital Vargas de Caracas’ ethics committee approved the study. Participants gave oral permission for an interview and signed an informed consent document for the use of their blood samples for serological studies regarding previous SARS-CoV-19 infection and the serological responses to the Sputnik V vaccine. Participants were individually informed about their serological status induced by the vaccine or induced by natural infection.
Declaration of competing interest
The authors declare no conflict of interest.
References
- Anand S, Montez-Rath ME, Han J, Garcia P, Cadden L, Hunsader P, et al. SARS-CoV-2 Vaccine Antibody Response and Breakthrough Infection in Patients Receiving Dialysis. Ann Intern Med. 2021:M21–4176. doi: 10.7326/M21-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahla RE, Tomas-Grau RH, Cazorla SI, Ploper D, Vera Pingitore E, López MA, et al. Long-term analysis of antibodies elicited by SPUTNIK V: A prospective cohort study in Tucumán, Argentina. Lancet Reg Health Am. 2022 doi: 10.1016/j.lana.2021.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claro F, Silva D, Rodriguez M, Rangel HR, de Waard JH. Immunoglobulin G antibody response to the Sputnik V vaccine: previous SARS-CoV-2 seropositive individuals may need just one vaccine dose. Int J Infect Dis. 2021;111:261–266. doi: 10.1016/j.ijid.2021.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2021 doi: 10.1126/science.abm0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AY, Yu J, McMahan K, Liu J, Chandrashekar A, Maron JS, et al. Differential Kinetics of Immune Responses Elicited by Covid-19 Vaccines. N Engl J Med. 2021;385(21):2010–2012. doi: 10.1056/NEJMc2115596. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer D, Steain M, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3(1):e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimeglio C, Herin F, Martin-Blondel G, Miedougé M, Izopet J. Antibody titers and protection against a SARS-CoV-2 infection. J Infect. 2022;84(2):248–288. doi: 10.1016/j.jinf.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose N, Suthar MS, Makowski M, O'Connell S, McDermott AB, Flach B, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021;384(23):2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverría G, Guevara Á, Coloma J, Ruiz AM, Vásquez MM, Tejera E, et al. Pre-existing T-cell immunity to SARS-CoV-2 in unexposed healthy controls in Ecuador, as detected with a COVID-19 Interferon-Gamma Release Assay. Int J Infect Dis. 2021;105:21–25. doi: 10.1016/j.ijid.2021.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al. Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González López Ledesma MM, Sanchez L, Ojeda DS, Oviedo Rouco S, Rossi AH, Varese A, et al. Longitudinal Study after Sputnik V Vaccination Shows Durable SARS-CoV-2 Neutralizing Antibodies and Reduced Viral Variant Escape to Neutralization over Time. mBio. 2022;13(1) doi: 10.1128/mbio.03442-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Kristiansen PA, Page M, Bernasconi V, Mattiuzzo G, Dull P, Makar K. I WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–1348. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallett SJC, Heskin J, Groppelli E, Mazzella A, Moore LSP. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants. Lancet Microbe. 2021 doi: 10.1016/S2666-5247(21)00337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A, Crotty S. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat Rev Immunol. 2020;20(8):457–458. doi: 10.1038/s41577-020-0389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Zhou H, Samanovic MI, Dcosta BM, Cornelius A, Mulligan MJ, et al. Neutralization of SARS-CoV-2 Variants by mRNA and Adenoviral Vector Vaccine-Elicited Antibodies. Frontiers in Immunology. 2022:13. doi: 10.3389/fimmu.2022.797589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- Wagner A., Guzek A., Ruff J., Jasinska J, Scheikl U, Zwazl I, et al. Neutralising SARS-CoV-2 RBD-specific antibodies persist for at least six months independently of symptoms in adults. Commun Med. 2021;1:13. doi: 10.1038/s43856-021-00012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO technical advisory group 2020. https://www.who.int/groups/technical-advisory-group-on-sars-cov-2-virus-evolution. Last time accessed 28-02- 2022
- Yang J, Wang W, Chen Z, Lu S, Yang F, Bi Z, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020;586(7830):572–577. doi: 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]