Abstract
We examined the involvement of Mn(II) in the conversion of phenylalanine to benzaldehyde in cell extracts of lactic acid bacteria. Experiments performed with Lactobacillus plantarum demonstrated that Mn(II), present at high levels in this strain, is involved in benzaldehyde formation by catalyzing the conversion of phenylpyruvic acid. Experiments performed with various lactic acid bacterial strains belonging to different genera revealed that benzaldehyde formation in a strain was related to a high Mn(II) level.
Degradation of amino acids by lactic acid bacteria (LAB) is important for the generation of flavor compounds during cheese ripening. We previously described that the conversion of phenylalanine to benzaldehyde in cell extracts of Lactobacillus plantarum (14) differs from the pathways described for fungi and for Pseudomonas putida (6, 8, 9, 11, 13, 18). In L. plantarum, the conversion of phenylalanine to benzaldehyde involves both an enzymatic step and a chemical reaction. In the cell extract of this strain, phenylalanine is initially converted to phenylpyruvic acid by the action of an aminotransferase. In the presence of oxygen, the keto acid is then oxidized to benzaldehyde in a nonenzymatic reaction. We demonstrated that this oxidation step depended on one or more unidentified, heat-stable components from the cell extract. However, in the absence of cell extract, phenylpyruvic acid was easily converted to benzaldehyde under mild conditions after addition of several metal ions, suggesting that this chemical step may be due to the presence of one or more metal ions in the cell extract.
Metal ions are involved in several functions in the metabolism of LAB, e.g., as the catalytic centers of enzymes (for a review, see reference 4). The so-called micronutrients, which are usually present at very low concentrations in microorganisms, include the metal ions manganese (Mn), iron (Fe), cobalt (Co), and copper (Cu) (1). However, in several LAB, including L. plantarum, the intracellular level of Mn(II) is extremely high compared to the levels of other metal ions (2). This makes Mn(II) a possible candidate for the component in the cell extract involved in the chemical conversion of phenylpyruvic acid. The reported biological effects of Mn(II) are numerous and include structuring and activation of enzymes, detoxification of chemicals harmful to the bacterial cell, and stabilization of subcellular entities (16). Besides contributing to the biological functions described above, Mn(II) can be used by L. plantarum for a different purpose. Archibald and Fridovich (3) demonstrated that L. plantarum can accumulate Mn(II) to high intracellular levels as a defense mechanism against oxygen toxicity. This strain lacks the enzyme superoxide dismutase, which is present in most aerobic and oxygen-tolerant microorganisms. In the present work, we studied the role of manganese in flavor production not only in L. plantarum but also in a number of other LAB.
Addition of metal ions to dialyzed cell extracts.
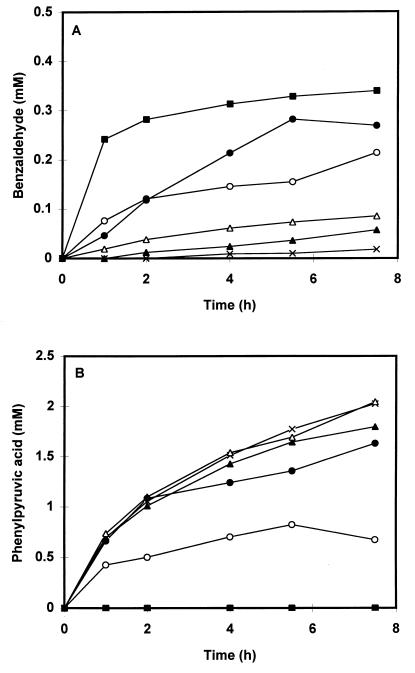
In our previous report, we demonstrated that several metal ions can catalyze the conversion of phenylpyruvic acid to benzaldehyde in the absence of cell extract. However, the availability of the metal ions may be reduced in a cell extract-containing system due to binding of the metal ions to components present in the cell extract. To test the catalyzing properties of the metal ions in the presence of cell extract, we compared the formation of benzaldehyde in both dialyzed and undialyzed extracts and in dialyzed extracts to which either Cu(II), Mn(II), Fe(II), or Co(II) was added. The metal ions were added to a final concentration of 350 μM from filter-sterilized stock solutions of either FeSO4 · 7H2O, MnSO4 · H2O, CuSO4 · 5H2O, or CoSO4 · 7H2O. Preparation of the cell extract from MRS (Merck)-grown cells of L. plantarum LcL1 and incubation of the extracts with phenylalanine were performed as described previously (14). Dialysis of the cell extract was performed overnight at 4°C against 50 mM potassium phosphate buffer (pH 7.0) containing 0.02 mM pyridoxal 5′-phosphate. Cell extracts were stored at −20°C for not more than 1 week until further use. Figure 1 shows that in keeping with our previous report, phenylpyruvic acid and benzaldehyde were formed upon incubation with phenylalanine in the undialyzed extract. However, no benzaldehyde was formed in the dialyzed extract; instead, phenylpyruvic acid accumulated in the extract over time. Addition of either Fe(II), Mn(II), Co(II), or Cu(II) to the dialyzed extracts restored the conversion of phenylpyruvic acid to benzaldehyde, although the rate of benzaldehyde formation depended on which metal ion was added to the extract. Besides benzaldehyde and phenylpyruvic acid, small amounts of mandelic acid (0.16 and 0.12 mM), phenylglyoxylic acid (0.05 and 0.07 mM), and phenylacetic acid (0.1 and 0.05 mM) were formed in the undialyzed cell extract and in the Mn(II)-supplemented cell extract, respectively. We previously observed that in the absence of cell extract, the conversion of phenylpyruvic acid decreased in the order Cu(II) > Mn(II) > Fe(II). However, in the presence of dialyzed cell extract, benzaldehyde formation falls in the order Mn(II) > Fe(II) > Cu(II). This effect may be explained by the stability constants for complex formation of the metal ions with various ligands. These constants follow the order Mn(II) < Fe(II) < Co(II) < Cu(II) (17, 19).
FIG. 1.
Formation of benzaldehyde (A) and phenylpyruvic acid (B) over time. Incubations were performed with both an undialyzed cell extract (○) and dialyzed cell extracts of L. plantarum LcL1 (cells grown in MRS medium) without addition of metal ions (×) and in the presence of either 350 μM Mn(II) (■), Fe(II) (●), Co(II) (▵), or Cu(II) (▴). Incubations were performed at 37°C in 50 mM Tris buffer (pH 8.0) containing 8 mM phenylalanine, 8 mM α-ketoglutaric acid, and 0.02 mM pyridoxal 5′-phosphate. A total of 1.70 mg of protein was present in the reaction mixture. In all cases, SO42− was the counterion of the metal ion added. The values represent the averages of duplicate incubations and generally varied from the means by no more than 10%.
Analysis of the metals ions in the extract.
We analyzed the level of Mn, Fe, Cu, and Co in both the undialyzed and the dialyzed cell extracts of the MRS-grown L. plantarum LcL1 by inductivity-coupled plasma mass spectrometry. These analyses were performed at the Department of Soil Science and Plant Nutrition of the Wageningen University (Wageningen, The Netherlands). The MRS medium used for cultivation of L. plantarum LcL1 contained Mn at 144 μM as determined by inductivity-coupled plasma mass spectrometry. Manganese was present in the undialyzed extract at 8.8 μg/mg of protein, which was extremely high compared to the levels of Fe, Cu, and Co in the same extract. The difference in concentration of these metals was greater than a factor 80. If we assume that the specific internal volume is 3 μl/mg of protein as reported for L. plantarum (7), then it can be calculated that the intracellular Mn(II) concentration is as high as 53 mM. This indicates that, considering the Mn(II) concentration in the medium, this metal must have been transported by an active uptake system. The dialyzed extract contained only 0.05 μg of Mn/mg of protein, while the concentrations of the other metal ions tested were even lower. The amount of Mn present in the undialyzed cell extract of L. plantarum LcL1 accounts for a final concentration of 54 μM in the reaction mixture. For Fe and Cu, this value is below 1 μM, and Co is present in the nanomolar range. Therefore, the latter metal ions are not very likely to have a significant contribution to benzaldehyde formation in the extract compared to that of Mn(II).
Since Mn(II) seems to be important in benzaldehyde formation, we used a chemically defined medium (10) for cultivation of L. plantarum LcL1 to study the role of this metal ion in more detail. In this medium, the concentration of Mn(II) could be varied while the concentrations of Co(II), Fe(II), and Cu(II) were kept at 0.8, 7.5, and 0.01 μM, respectively. Erlenmeyer flasks of 500 ml containing 150 ml of medium, supplemented with either 10, 25, 200, 300, or 500 μM MnSO4, were inoculated with cells grown overnight in medium containing the same concentrations. Cells were cultured overnight at 30°C, and cell extracts were prepared as described previously (14). The protein concentration of the cell extracts were determined by the Bradford method (5), with bovine serum albumin as a standard. The amount of benzaldehyde formed in the cell extracts after 4 h of incubation with phenylalanine increased with increasing levels of Mn(II) in the culture medium, up to a concentration of 200 μM (Table 1). Similarly, the Mn(II) content of the cell extracts increased with increasing levels of Mn(II) in the culture medium, up to a concentration of 200 μM. In the extracts of cells grown in medium containing 10 or 25 μM Mn(II), phenylpyruvic acid accumulated in the medium due to a limited conversion of the keto acid to benzaldehyde. In keeping with this finding, these extracts contained low levels of Mn(II).
TABLE 1.
Effect of Mn(II) concentration in the medium on the amount of benzaldehyde and phenylpyruvic acid formed in cell extracts of L. plantarum LcL1 and the Mn(II) content of these extractsa
| Mn(II) in medium (μM) | Concn in extractb
|
||
|---|---|---|---|
| Benzaldehyde (nmol/ mg of protein) | Phenylpyruvic acid (nmol/mg of protein) | Mn(II) (μg/mg of protein) | |
| 10 | 67 | 1,836 | 1.3 |
| 25 | 241 | 391 | 2.4 |
| 200 | 683 | <300 | 14.5 |
| 300 | 766 | <300 | 11.1 |
| 500 | 767 | <300 | 9.6 |
Cells were grown overnight in chemically defined medium (15) containing MnSO4 at different concentrations. Incubations were performed as described in the legend to Fig. 1. Each value represents the mean of duplicate measurements and varied from the mean by no more than 15%.
Measured after 4 h of incubation.
Inhibition of the aminotransferase by manganese.
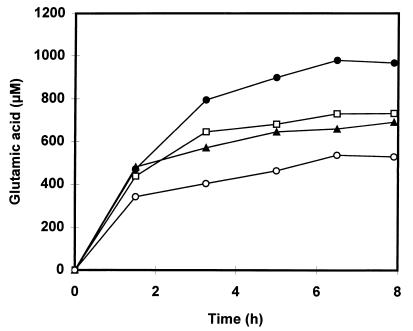
We observed that in the undialyzed cell extract, the amounts of phenylpyruvic acid and the metabolites formed from this compound together accounted for only 59% of the phenylpyruvic acid formed in the dialyzed extract. In the Mn(II)-supplemented cell extract, the proportion was only 29%. We therefore tested to see if either benzaldehyde or Mn(II) had a negative effect on the activity of the aminotransferase. Addition of up to 0.5 mM benzaldehyde to a dialyzed cell extract of L. plantarum NC8 showed no decrease in the formation of phenylpyruvic acid by the enzyme (results not shown). In order to demonstrate an effect of Mn(II) on the aminotransferase activity, we had to determine the formation of glutamic acid, instead of phenylpyruvic acid, over time. Glutamic acid is the transaminated product of α-ketoglutaric acid that arises when phenylalanine is converted to phenylpyruvic acid. Since Mn(II) ions catalyze the conversion of phenylpyruvic acid, it was not possible to study the effect of this metal ion by measuring the amount of phenylpyruvic acid formed. Glutamic acid concentrations were determined by the method of Kunte et al. (12) with a Chromspher5 C18 column (Chrompack, Bergen op Zoom, The Netherlands). Figure 2 demonstrates that addition of Mn(II) to a dialyzed cell extract of L. plantarum NC8 indeed reduces the amount of glutamic acid formed by the aminotransferase. In the dialyzed extracts supplemented with either 25, 100, or 350 μM Mn(II), the amount of glutamic acid formed after 8 h of incubation was reduced by 25, 30, and 50%, respectively, compared to the amount in the manganese-free extract. The inhibition of the aminotransferase by Mn(II) reduces the yield in the Mn(II)-containing extracts compared to that in the dialyzed extract. There was no reduction in yield for the Co(II), Fe(II), or Cu(II) supplemented extract.
FIG. 2.
The formation of glutamic acid over time in a dialyzed cell extract of L. plantarum NC8 without the addition of Mn(II) (●) and in the presence of either 25 μM (□), 100 μM (▴), or 350 μM (○) Mn(II). Incubations were performed under the conditions described in the legend to Fig. 1. The values represent the averages of duplicate incubations and varied from the means by no more than 10%. Cells were grown in MRS medium. A total of 5.56 mg of protein was present in the reaction mixture.
Benzaldehyde formation in cell extracts of other LAB.
We examined whether the relationship between benzaldehyde formation and Mn(II) accumulation was restricted to L. plantarum or whether it could be observed for other LAB strains. Therefore, cell extracts of strains belonging to several different genera were tested for benzaldehyde formation from phenylalanine. For this experiment, cells were grown in chemically defined medium containing 300 μM MnSO4 and 10 g of glucose per liter. The Lactobacillus and Leuconostoc strains and Lactococcus lactis B26 and B27 were grown in the medium described elsewhere (10). The other Lactococcus strains were cultured in the medium described by Poolman and Konings (15). All strains were grown at 30°C, except for Lactobacillus helveticus and Lactobacillus fermentum; these strains were incubated at 37°C. Table 2 shows that benzaldehyde was formed not only in L. plantarum extracts but also in the extracts of several other LAB. Benzaldehyde-forming strains were distributed among the genera Lactobacillus and Leuconostoc. Benzaldehyde formation by a strain correlated with high levels of Mn(II) in the extract. Leuconostoc lactis and Lactobacillus fermentum were exceptions, showing high Mn(II) contents but very poor benzaldehyde formation. In these strains, phenylpyruvic acid generation is limiting due to either low or no activity of the aminotransferase for phenylalanine. Only minor amounts of benzaldehyde were formed in extracts of the four Lactococcus lactis strains. Since the production of phenylpyruvic acid in these extracts was high, the limited benzaldehyde formation can be attributed to the low levels of Mn(II) in these extracts compared to the levels in benzaldehyde-forming strains. Besides Mn, the levels of Co, Fe, and Cu in all the extracts were determined. These values were below 0.02, 0.09, and 0.1 μg/mg of protein for Co, Fe, and Cu, respectively.
TABLE 2.
Amounts of benzaldehyde and phenylpyruvic acid formed in dialyzed and undialyzed cell extracts of LAB and the Mn(II) contents of these extracts
| Strainc | Concn in extracta
|
|||
|---|---|---|---|---|
| Benzaldehyde (nmol/mg of protein), undialyzed | Phenylpyruvic acid (nmol/mg of protein)
|
Mn(II) (μg/mg of protein), undialyzed | ||
| Undialyzed | Dialyzed | |||
| Lactobacillus plantarum LcL1 | 908 | <300 | 2,283 | 11.10 |
| Lactobacillus plantarum NC8 | 582 | <300 | 4,167 | 12.63 |
| Lactobacillus casei subsp. casei DSM 20011 | 1,196 | <300 | 3,570 | 26.10 |
| Leuconostoc paramesenteroides DSM 20288 | 1,266 | <300 | 8,424 | 5.28 |
| Leuconostoc lactis DSM 20192 | <30 | <300 | <300 | 7.54 |
| Lactobacillus helveticus ATCC 15009b | 217 | <300 | 1,697 | 0.43 |
| Lactobacillus fermentum ATCC 9338 | 170 | <300 | 849 | 6.27 |
| Lactococcus lactis subsp. lactis NIZO C17 | <30 | 3,188 | 4,239 | 0.74 |
| Lactococcus lactis subsp. lactis NCDO 712 | 74 | 8,036 | 8,344 | 1.19 |
| Lactococcus lactis subsp. lactis NIZO B26 | <30 | 10,274 | 12,254 | 0.29 |
| Lactococcus lactis subsp. lactis NIZO B27 | <30 | 6,221 | 6,974 | 0.33 |
Measured after 8 h of incubation, except for strains LcL1, B26, and B27; these values were obtained after 7 h of incubation.
L. helveticus was grown in MRS broth.
Strains were obtained from American Type Culture Collection, Rockville, Md. (ATCC); Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany (DSM); National Collection of Dairy Organisms, Reading, United Kingdom (NCDO); NIZO Food Research, Ede, The Netherlands (NIZO); Unilever Research Laboratory, Vlaardingen, The Netherlands (for LcL1); Laboratoire de Génétique Moléculaire, Université Catholique de Louvain, Louvain-la-Neuve, Belgium (for NC8).
The work reported by Archibald and Fridovich (2, 3) showed that manganese accumulation in LAB provide the cells with a defense mechanism against the toxic effects of oxygen. In the present study, we clearly demonstrate that accumulation of Mn(II) has a surprising additional effect. We showed that in LAB that convert phenylalanine to phenylpyruvic acid, benzaldehyde is formed when these strains contain a large Mn(II) pool. The difference between the intracellular Mn(II) concentration and the concentration in the medium suggests that those strains must have a system for the active uptake of Mn(II). Therefore, benzaldehyde formation in LAB by the mechanism we described previously (14) seems to be related to the presence of an active uptake system for Mn(II) in the strain. An interesting perspective on these results could be obtained by transferring the Mn(II) uptake system from L. plantarum into the more widely used Lactococcus lactis, thereby directing phenylpyruvic acid generation in this strain towards benzaldehyde.
Acknowledgments
We thank Jeroen Hugenholtz for critically reading the manuscript and providing the strains Lactococcus lactis subsp. lactis B26 and B27 and Ingeborg Boels for critically reading the manuscript.
REFERENCES
- 1.Archibald F. Manganese: its acquisition by and function in the lactic acid bacteria. Crit Rev Microbiol. 1986;13:63–109. doi: 10.3109/10408418609108735. [DOI] [PubMed] [Google Scholar]
- 2.Archibald F S, Fridovich I. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J Bacteriol. 1981;146:928–936. doi: 10.1128/jb.146.3.928-936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archibald F S, Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145:442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyaval P. Lactic acid bacteria and metal ions. Lait. 1989;69:87–113. [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Fabre C E, Blanc P J, Goma G. Production of benzaldehyde by several strains of Ischnoderma benzoinum. Sci Aliment. 1996;16:61–68. [Google Scholar]
- 7.Glaasker E, Tjan F S B, Ter Steeg P F, Konings W N, Poolman B. Physiological response of Lactobacillus plantarum to salt and nonelectrolyte stress. J Bacteriol. 1998;180:4718–4723. doi: 10.1128/jb.180.17.4718-4723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen K A, Jr, Evans K M C, Kirk T K, Hammel K E. Biosynthetic pathway for veratryl alcohol in the ligninolytic fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1994;60:709–714. doi: 10.1128/aem.60.2.709-714.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawabe T, Morita H. Production of benzaldehyde and benzylalcohol by the mushroom Polyporus tuberaster K2606. J Agric Food Chem. 1994;42:2556–2560. [Google Scholar]
- 10.Kets E P W, Galinski E A, de Bont J A M. Carnitine: a novel compatible solute in Lactobacillus plantarum. Arch Microbiol. 1994;162:243–248. [Google Scholar]
- 11.Krings U, Hinz M, Berger R G. Degradation of [2H]phenylalanine by the basidomycete Ischnoderma benzoinum. J Biotechnol. 1996;51:123–129. [Google Scholar]
- 12.Kunte H J, Galinski E A, Trüper H G. A modified FMOC-method for the detection of amino acid-type osmolytes and tetrahydropyrimidines (ectoines) J Microbiol Methods. 1993;17:129–136. [Google Scholar]
- 13.Lapadatescu C, Ferron G, Vergoignan C, Djian A, Durand A, Bonnarme P. Influence of cell immobilization on the production of benzaldehyde and benzylalcohol by the white-rot fungi Bjerkandera adusta, Ischnoderma benzoinum and Dichomitus squalens. Appl Microbiol Biotechnol. 1997;47:708–714. [Google Scholar]
- 14.Nierop Groot M N, de Bont J A M. Conversion of phenylalanine to benzaldehyde initiated by an aminotransferase in Lactobacillus plantarum. Appl Environ Microbiol. 1998;64:3009–3013. doi: 10.1128/aem.64.8.3009-3013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poolman B, Konings W N. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. J Bacteriol. 1988;170:700–707. doi: 10.1128/jb.170.2.700-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raccach M. Manganese and lactic acid bacteria. J Food Prot. 1985;48:895–898. doi: 10.4315/0362-028X-48.10.895. [DOI] [PubMed] [Google Scholar]
- 17.Sigel H, McCormick D B. On the discriminating behaviour of metal ions and ligands with regards to their biological significance. Accounts Chem Res. 1970;3:201–208. [Google Scholar]
- 18.Simmonds J, Robinson G K. Formation of benzaldehyde by Pseudomonas putida ATCC 12633. Appl Microbiol Biotechnol. 1998;50:353–358. [Google Scholar]
- 19.Vallee B L, Coleman E. Metal coordination and enzyme action. Comp Biochem. 1964;12:165–235. [Google Scholar]