Abstract
Alcohol use dysregulates responsivity to stress, which is mediated by corticotropin-releasing factor (CRF). With repeated cycles of alcohol use, the hypothalamic-pituitary-adrenal axis becomes hyporesponsive, rendering individuals vulnerable to the reinstatement of alcohol-seeking behavior during stressful episodes. Orexin (Orx; also called hypocretin) plays a well-established role in regulating diverse physiological processes, including stress, and interacts with CRF. The infralimbic cortex (IL) is a CRF-rich region. Anatomical evidence suggests that CRF and Orx interact in this area. To test the behavioral implication of CRF and Orx transmission in the IL during the stress-induced reinstatement of alcohol-seeking behavior, male Wistar rats were trained to self-administer 10% alcohol for 3 weeks. The rats then underwent two weeks of extinction training (identical to the alcohol self-administration sessions, but alcohol was withheld). The day after the last extinction session, the rats received a bilateral intra-IL injection of the CRF1 receptor antagonist CP154,526 (0.6 μg/0.5 μl/side), the dual Orx receptor antagonist TCS1102 (15 μg/0.5 μl/side), or their combination and then were tested for the footshock stress-induced reinstatement of alcohol-seeking behavior. CP154,526 significantly prevented reinstatement, but TCS1102 did not produce such an effect. Interestingly, the co-administration of TCS1102 and CP154,526 reversed the effect of CP154,526 alone, and footshock stress induced a significant increase in Crhr1 and Hcrtr2 mRNA expression in the IL. These results demonstrate a functional interaction between Orx receptor and CRF1 receptor signaling and suggest that CRF1 receptor antagonism may ameliorate stress-induced alcohol-seeking behavior.
Keywords: Alcohol; corticotropin-releasing factor; orexin; CP154,526; TCS1102
Introduction
Repeated alcohol use is well known to dysregulate stress responses, including the hypothalamic-pituitary-adrenal (HPA) axis and extrahypothalamic stress systems (Quadros, Macedo, Domingues, & Favoretto, 2016; Stephens & Wand, 2012). Recurrent exposure to alcohol appears to increase extrahypothalamic corticotropin-releasing factor (CRF) activity in several brain structures that play a role in withdrawal-related anxiety and dysphoria (Becker, 2012; Merlo Pich et al., 1995; Olive, Koenig, Nannini, & Hodge, 2002; Winsky-Sommerer et al., 2004) and blunt responsivity of the HPA axis (Koob & Kreek, 2007; Koob, 2008).
Several neuropeptides have been proposed to interact with CRF to either promote or prevent the reinstatement of drug-seeking behavior (Shalev, Erb, & Shaham, 2010). Behavioral and functional studies highlighted that the orexin (Orx; also called hypocretin) system plays an important role in neurobehavioral and motivational effects of drugs of abuse, including alcohol (Bonci & Borgland, 2009; Borgland, Taha, Sarti, Fields, & Bonci, 2006; Harris, Wimmer, & Aston-Jones, 2005; Thompson & Borgland, 2011). Stress has been shown to activate Orx neurons (Ida et al., 2000), and Orx has been proposed to modulate the stress response (for review, see Giardino & de Lecea, 2014). Neuroanatomical studies have shown that CRF terminals from the prefrontal cortex directly contact Orx cells that express CRF1 and CRF2 receptors in the hypothalamus and that CRF-expressing neurons receive contacts from the Orx system (Winsky-Sommerer et al., 2004). Supporting these anatomical studies, some behavioral evidence of an Orx/CRF interaction has been reported. For example, intracerebroventricular Orx administration increased anxiety-like behavior (Suzuki, Beuckmann, Shikata, Ogura, & Sawai, 2005) and elevated intracranial self-stimulation thresholds (Boutrel et al., 2005), an effect that was mediated by CRF (Hata et al., 2011), and reinstated cocaine-seeking behavior in a CRF-dependent manner (Boutrel et al., 2005). Altogether, these findings provide evidence that the Orx modulation of CRF neurons participates in the maintenance and persistence of negative affective states that characterize drug addiction.
Of the many brain structures that contain CRF interneurons (Swanson, Sawchenko, Rivier, & Vale, 1983), the medial prefrontal cortex (mPFC) has emerged as being directly involved in compulsive drug-seeking behavior (Kalivas, 2008; Koob, 2008). There is a dearth of knowledge about the exact function of these neurons, but they were recently identified as γ-aminobutyric acid (GABA)ergic inhibitory interneurons (Chen et al., 2020). In rodents, a functional dichotomy has been described between mPFC subregions, consisting of the dorsal region (i.e., prelimbic cortex [PL]) and ventral region (i.e., infralimbic cortex [IL]). The PL was shown to play a role in executing behaviors, whereas the IL is involved in response inhibition (Moorman & Aston-Jones, 2015; Moorman, James, McGlinchey, & Aston-Jones, 2015). With regard to drug-seeking behavior, earlier studies suggested that the PL promotes drug seeking, whereas the IL suppresses it. Activation of the IL has been associated with the extinction and inhibition of drug-seeking behavior (Moorman et al., 2015; Van den Oever, Spijker, Smit, & De Vries, 2010). Furthermore, chronic alcohol exposure produces greater effects on neuronal transmission and excitability in the IL compared with the PL, indicating a more pronounced role of the IL in alcohol-seeking behavior (Pleil et al., 2015). Additionally, the inactivation of a neuronal ensemble in the IL but not PL increased alcohol-seeking behavior (Pfarr et al., 2015). Evidence suggests that mPFC CRF neurons interact with Orx in the IL. The IL receives Orx projections from the hypothalamus (HYP; Date et al., 1999), and Hcrtr1 and Hcrtr2 mRNA expression has been detected in the IL (Marcus et al., 2001). Altogether, these studies suggest that dysregulation of the IL and functional interactions between the CRF and Orx systems in the IL may play a central role in compulsive alcohol seeking that is exacerbated by stress.
Therefore, to verify the implication of both CRF and Orx transmission in the IL during the stress-induced reinstatement of alcohol-seeking behavior, the present study had a threefold purpose: (1) test the ability of intra-IL administration of the CRF1 receptor antagonist CP154,526 and dual Orx receptor antagonist (DORA) TCS1102 to reverse the stress-induced reinstatement of alcohol-seeking behavior, (2) behaviorally assess whether an interaction between IL CRF1 receptor and Orx receptor signaling exists that could alter the stress-induced reinstatement of alcohol-seeking behavior by co-administering both ligands, and (3) further dissected this interaction by evaluating whether stress alters Crhr1, Hcrtr1, and Hcrtr2 mRNA expression in the IL.
Based on previous research from our laboratory (Matzeu & Martin-Fardon, 2020), we hypothesized that CP154,526, but not TCS1102, would be efficacious in preventing stress-induced reinstatement of alcohol-seeking behavior. Furthermore, we also hypothesized that an interaction between the CRF and Orx system will occur, influencing stress-induced reinstatement by increasing the effects of CP154,526 with a coadministration of TCS1102.
1. Methods
2.1. Animals
Sixty-four male Wistar rats (Charles River Laboratories, Hollister, CA, USA), weighing 150–170 g upon arrival, were housed two per cage in a temperature- and humidity-controlled vivarium on a reverse 12 h/12 h light/dark cycle (lights OFF at 8:00 AM; Lights ON at 8:00 PM) with ad libitum access to food and water. All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Council, 2003) and Animal Research: Reporting In Vivo Experiments Guidelines (Percie du Sert et al., 2020) and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. Before the experiments began, the animals were given a 1-week acclimation period to the housing and handling conditions.
2.2. Drugs
CP154,526 and TCS1102 (Tocris Bioscience, Bristol, UK) were dissolved in 100% dimethylsulfoxide (DMSO; Sigma Aldrich, St. Louis, MO, USA) at doses of 0.6 μg/0.5 μl and 15 μg/0.5 μl, respectively. Control vehicle (VEH)-treated animals received 100% DMSO only.
2.2. Alcohol self-administration training
Alcohol self-administration training was conducted as previously described (Matzeu & Martin-Fardon, 2020). Importantly, no fading (saccharin or sucrose) was required to induce voluntary alcohol intake. After the 1-week housing acclimation period and for the remainder of the alcohol training procedure (Fig. 1A), the rats were given access to standard operant conditioning chambers (29 cm × 24 cm × 19.5 cm; Med Associates, St. Albans, VT, USA) at 2:00 PM in daily 30-min self-administration sessions on a fixed-ratio 1 (FR1) schedule of reinforcement. In these sessions, responses on the right lever resulted in the delivery of 0.1 ml of 10% (w/v) alcohol (prepared in tap water from 95% w/v alcohol) and the brief (0.5 s) illumination of a cue light above the lever. To calculate total intake (g/kg), the total number of rewards (i.e., when active lever presses resulted in the delivery of alcohol) was normalized to the animal’s body weight on the day of that session. To assess whether the animals had drunk, we checked that the feeding wells in the chambers were dry after each self-administration session. Responses on the left inactive lever resulted in no programmed consequences. Responses on both levers were recorded.
Fig. 1.
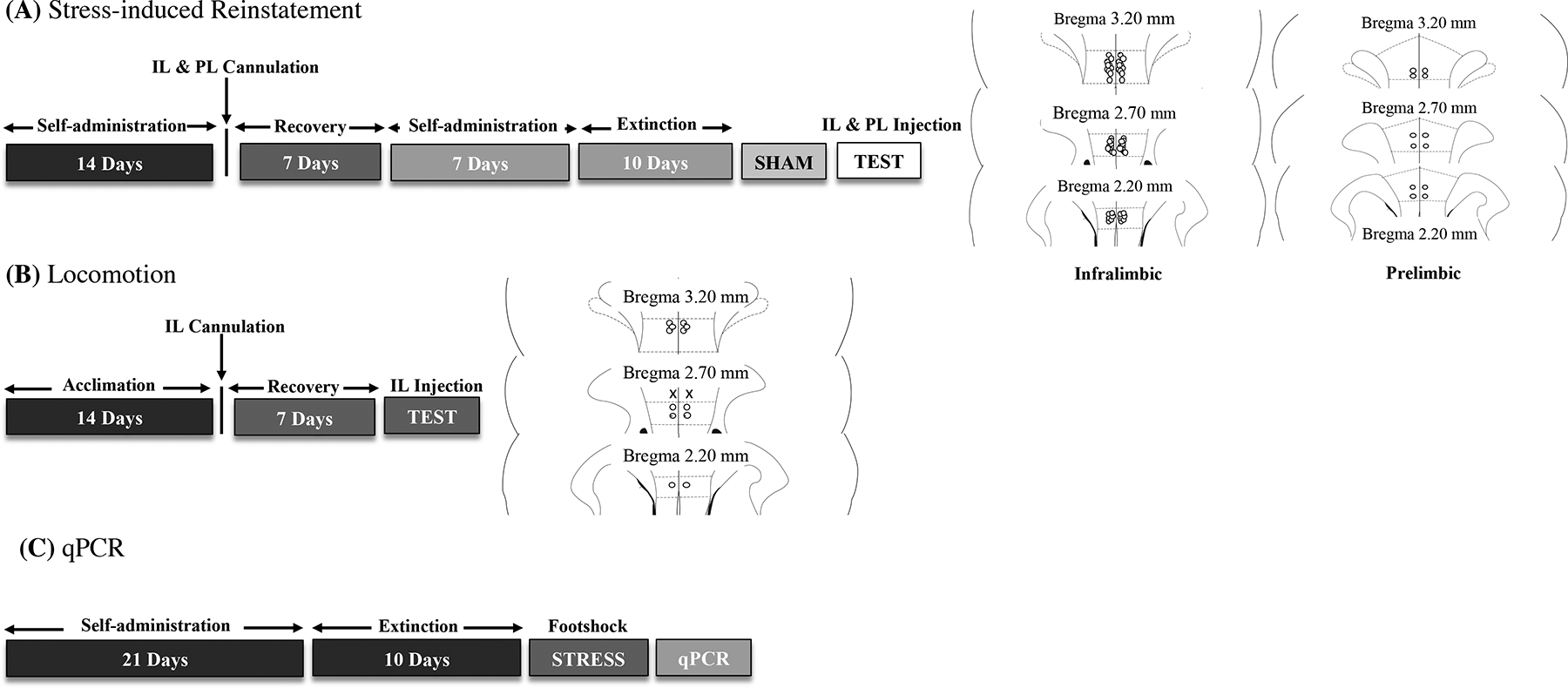
Timeline of the experimental procedures. (A) Behavioral procedure for stress-induced reinstatement and representation of injection sites in animals that received bilateral IL and PL cannulations. (B) Spontaneous locomotion testing procedure and representation of injection sites. o, Rats with correct injection sites. ×, Rats with missed injection sites. (C) Molecular procedure (qPCR).
2.3. Infralimbic and prelimbic cannulation
Two weeks after self-administration training began, the animals were implanted with bilateral guide cannulas (22-gauge, 15 mm, Plastics One, Roanoke, VA, USA) that were aimed at the IL (anterior/posterior, +3.2 mm from bregma; medial/lateral, ±0.75 mm; dorsal/ventral, −2.6 mm from dura; Paxinos & Watson, 1998) and positioned 2 mm above the target injection point. Bilateral guide cannulas were also aimed at the PL (anterior/posterior, +3.2 mm from bregma; medial/lateral, ±0.75 mm; dorsal/ventral, −1.6 mm; Paxinos & Watson, 1998) and positioned 2 mm above the target injection point. After 7 days of recovery from surgery, all rats resumed self-administration training for 1 week (Fig. 1A).
2.4. Extinction
Twenty-four hours after the last alcohol self-administration session, the rats underwent daily 30-min extinction sessions for 10 days (Fig. 1A). Extinction sessions were identical to the alcohol self-administration sessions, but alcohol was withheld. For habituation to the footshock stress procedure, the rats were placed in the operant chambers 15 min before the extinction sessions. At the end of this 15-min period, both levers were extended into the operant chambers, and the sessions began. The rats underwent extinction training for 10 days. Fifteen minutes before the last extinction session, the rats received a sham injection to habituate them to the microinjection procedure. This involved the insertion of an injector (that was left in place for 2 min) in the guide cannula that extended into the IL. After the sham injections, the rats were returned to their home cages for 2 min and then placed in the operant chamber for 15 min. At the end of this 15-min period, both levers were extended into the operant chambers, and the rats were tested under extinction conditions (Fig. 1A).
2.5. Stress-induced reinstatement
Twenty-four hours after the sham injection, separate groups of rats received a central injection of DMSO (VEH), the CRF1 receptor antagonist CP154,526 (0.6 μg/0.5 μl/side; Hwa, Debold, & Miczek, 2013), the DORA TCS1102 (15 μg/0.5 μl/side; Dong, Li, & Kirouac, 2015; Hsiao, Jou, Yi, & Chang, 2012; Matzeu & Martin-Fardon, 2020), or their combination and then were tested for the footshock stress-induced reinstatement of alcohol-seeking behavior via footshock stress. To test specificity of the effect to the IL, a group of rats (n = 6) received an infusion of CP154,526 (0.6 μg/0.5 μl/side) in the PL. Intra-IL and -PL microinjections were performed using of a microinfusion pump (Harvard 22 Syringe Pump, Holliston, MA, USA) and injectors (Plastics One, Roanoke, VA, USA) that extended 2.0 mm beyond the guide cannula. Injections were performed at a flow rate of 0.5 μl/min over 1 min. While the pump was shut off, the injectors were left in the guide canula for an additional 1 min to allow diffusion away from the injector tip. Once the injectors were removed, the animals were returned to their home cages for 2 min and then placed in the operant chambers to undergo footshock stress (15 min; variable intermittent electric footshock, 0.5 mA; duration, 0.5 s; mean shock interval, 40 s; range, 10–70 s; Martin-Fardon, Ciccocioppo, Massi, & Weiss, 2000; Matzeu & Martin-Fardon, 2020; Sidhpura, Weiss, & Martin-Fardon, 2010; Zhao et al., 2006). Two minutes after the termination of footshock, the levers were extended into the chamber, and responses were recorded for 30 min. Each animal was tested only once with vehicle, CP154,526, TCS1102, or CP154,526+TCS1102 according to a between‐subjects design. To verify the injections sites, the rats were euthanized, and their brains were harvested, snap frozen, and cut into 30 μM coronal sections using a cryostat (Leica CM3050S, Leica Biosystems Nussloch GmbH, Heidelberg, Germany). Using an adult rat brain atlas as a reference (Paxinos & Watson, 1998), the injector tips were plotted from clear representative sections to determine their exact location. Off-target cannulations were excluded from the study (Fig. 1A, right).
2.6. Spontaneous locomotion
Given that the co-administration of CP154,526 (0.6 μg/0.5 μl/side) and TCS1102 (15 μg/0.5 μl/side) increased inactive lever responses during stress-induced reinstatement (see Fig. 3B), spontaneous locomotion tests were conducted in a separate group of alcohol- and experimentally naive rats (n = 8) to control for the nonspecific increase in locomotor activity that was induced by intra-IL injections of CP154,526+TCS1102. After recovery from IL cannulation (Fig. 1B), the rats received vehicle or CP154,526+TCS1102 and individually placed in their home cage. Fifteen minutes later (i.e., to match the duration of the footshock stress session), the total distanced traveled and velocity were recorded for 30 min using a standard video camera and EthovisionXT video tracking software (Noldus, Leesburg, VA) to analyze behavior in this task. Injection sites were verified, and off-target cannulations were excluded from the study (see Fig. 1B, right)
2.7. Quantitative polymerase chain reaction procedure
Rats that were used for the gene expression analysis were prepared in parallel and underwent the same behavioral procedure as described above, but they did not undergo IL cannulation, did not receive IL injections, and did not undergo reinstatement testing (Fig. 1C). Twenty-four hours after the last extinction session, the rats were split into three groups: one group (n = 8) that was experimentally naive to all conditions, one group (n = 8) that underwent training and extinction and was euthanized and whose brains were rapidly harvested, snap frozen in methylbutane, and stored at −80°C, and one group (n = 8) that underwent a 15-min footshock stress session in the operant chambers and was euthanized and whose brains were rapidly harvested, snap frozen, and stored at −80°C. Brains from all groups were dissected into serial coronal sections, and the IL was collected using tissue punches. RNA isolation was performed using RNA concentrator-5 kits according to the manufacturer’s instructions (Zymo Research, Irvine, CA, USA). Total RNA was measured using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and then reversed transcribed into cDNA using 5X mix, iScript, reverse transcription, and Supermix for real-time quantitative polymerase chain reaction (RT-qPCR) with the CFX 384 Real-Time System (Bio-Rad, Hercules, CA, USA). The amplification of cDNA was achieved using SYBR, iTap Universal SYBR, and Green Supermix running duplicate samples from each animal. Cycle threshold (Ct) values were determined, and changes in gene expression were assessed using the ΔCq method with β-actin as the housekeeping reference gene. The forward and reverse primer sequences, respectively, of the antisense oligonucleotides were the following: β-actin (5’-ATC TGG CAC ACC TTC-3’ and 5’-AGC CAG GTC CAG ACG CA-3’), Crhr1 (5’-TGC CAG GAG ATT CTC AAC GAA-3’ and 5’-AAA GCC GAG ATG AGG TTC CAG-3’), Hcrtr1 (5’-CCC TCA ACT CCA GTC CTA GC-3’ and 5’-CAG GGA GGG CCT ATA ATT GA-3’), and Hcrtr2 (5’-CCA TGT TGG GGT GCT TA-3’ and 5’-TCC CCC TCT CAT AAA CTT GG-3’).
2.8. Statistical analysis
Alcohol self-administration was analyzed using two-way repeated-measures analysis of variance (ANOVA). The extinction data were analyzed using two-way repeated-measures ANOVA, with time (i.e., session) and lever (active vs. inactive) as independent factors. Stress-induced reinstatement was analyzed using mixed-factors ANOVA, with session (i.e., sham and reinstatement tests) and treatment (i.e., CP154,526, TCS1102, and CP154,526+TCS1102) as within- and between-subjects factors, respectively. Significant interactions and main effects were followed by the Bonferroni post hoc test for the two-way repeated-measures ANOVA. For analyses of spontaneous locomotion data, two-tailed Student’s t-tests were used. The gene expression data were analyzed using one-way ANOVA, followed by the Tukey post hoc test. The data are expressed as mean + SEM. Values of p < 0.05 were considered statistically significant. The statistical analyses were performed using Prism 8 software (GraphPad, San Diego, CA, USA).
3. Results
Among the rats that were designated for the stress-induced reinstatement study (Fig. 1A), four were lost because of health complications. The remaining 28 animals were divided into the VEH group (n = 8), CP154,526 group (n = 8), TCS1102 group (n = 6), and CP154,526+TCS1102 group (n = 6). To test specificity of the effect to the IL, a separate group of rats (n = 6) received an infusion of CP154,526 in the PL. To assess whether the effect of the CRF1 receptor antagonist was confined to the IL, an additional group (n = 6) received an infusion of CP154,526 in the PL. For the spontaneous locomotion study (Fig. 1B), one rat was lost because of health complications, and one rat was removed because of cannula misplacement, reducing the total number of animals for this experiment to n = 6. No rats were lost for the qPCR analysis (n = 24; Fig. 1C).
3.1. Stress-induced reinstatement
Over the 21 sessions of self-administration training (30 min/day), the rats (n = 34) acquired alcohol self-administration (two-way repeated measures ANOVA; main effect of session: F20,1320 = 5.80, p < 0.0001; main effect of lever: F1,66 = 130.45, p < 0.0001; session × lever interaction: F20,1320 = 15.21, p < 0.0001; Fig. 2A). The Bonferroni post hoc test confirmed that beginning in the fifth training session, active lever presses were significantly higher than inactive lever presses (p < 0.05; Fig. 2A). Furthermore, no-differences in self-administration training were observed in animals that received IL cannulation (n = 8) and designated to receive CP154,526, when compared to the ones that received PL cannulation (n = 6) and were designated to receive CP154,526 (three-way factorial ANOVA; main effect of session: F20,480 = 3.05, p = 0.0092; main effect of lever: F1,24 = 51.18, p < 0.0001; main effect of mPFC subregion: F1,24 = 0.0049, p > 0.05; session × lever interaction × mPFC subregion: F20,480 = 1.048, p > 0.05; data not shown).
Fig. 2.
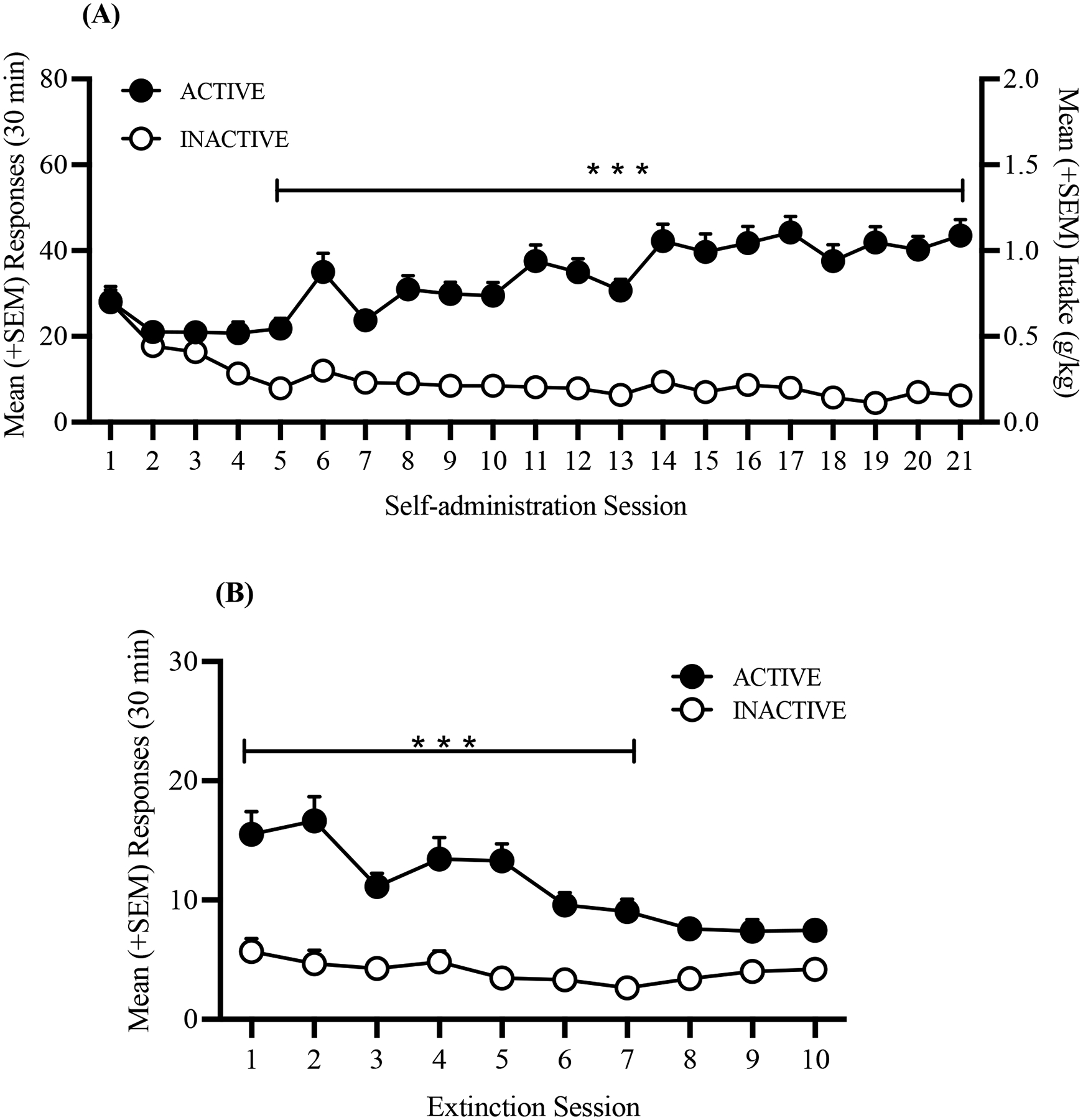
Time course of alcohol self-administration over 21 sessions of training and 10 extinction sessions. (A) Rats acquired alcohol self-administration over the 21 training sessions. (B) Extinction of alcohol-seeking behavior rats over the 10 sessions. The data are expressed as mean + SEM. ***p < 0.001, vs. inactive lever presses.
Over the 10 days of extinction training, the number of responses at the active lever gradually decreased until the total number of responses on the active lever was undistinguishable from the number of responses at the inactive lever (two-way repeated-measures ANOVA; main effect of session: F9,585 = 7.95, p < 0.0001; main effect of lever: F1,65 = 67.79, p < 0.0001; session × lever interaction: F9,585 = 4.26, p < 0.0001; Fig. 2B). The Bonferroni post hoc test revealed a significantly higher number of responses at the active lever from the first to the seventh extinction session (p < 0.05). After the seventh extinction session, the number of active lever presses was the same as the number of inactive lever presses (Fig. 2B).
Following extinction, the rats received a sham injection in the IL and PL to habituate them to the injection procedure. Exposure to stress precipitated the reinstatement of alcohol-seeking behavior in rats that received the VEH injection, which was prevented by the intra-IL injection of CP154,526. Alcohol-seeking behavior was reinstated by stress in rats that received an intra-IL injection of TCS1102 alone, and the intra-IL injection of CP154,526+TCS1102 reversed the effect of CP154,526 alone. Finally, alcohol-seeking behavior was also reinstated in animals that received intra-PL CP154,526 (Bonferroni post hoc test following two-way ANOVA; main effect of session: F1,29 = 69.66, p < 0.0001; main effect of treatment: F4,29 = 3.81, p = 0.013; session × treatment interaction: F4,29 = 4.36, p = 0.018; Fig. 3A). Rats that received CP154,526+TCS1102 emitted a significantly higher number of inactive lever responses (Bonferroni post hoc test following two-way ANOVA; main effect of session: F1,29 = 17.22, p = 0.0003; main effect of treatment: F4,29 = 8.61, p = 0.0001; session × treatment interaction: F4,29 = 10.83, p = 0.0001; Fig. 3B).
Fig. 3.
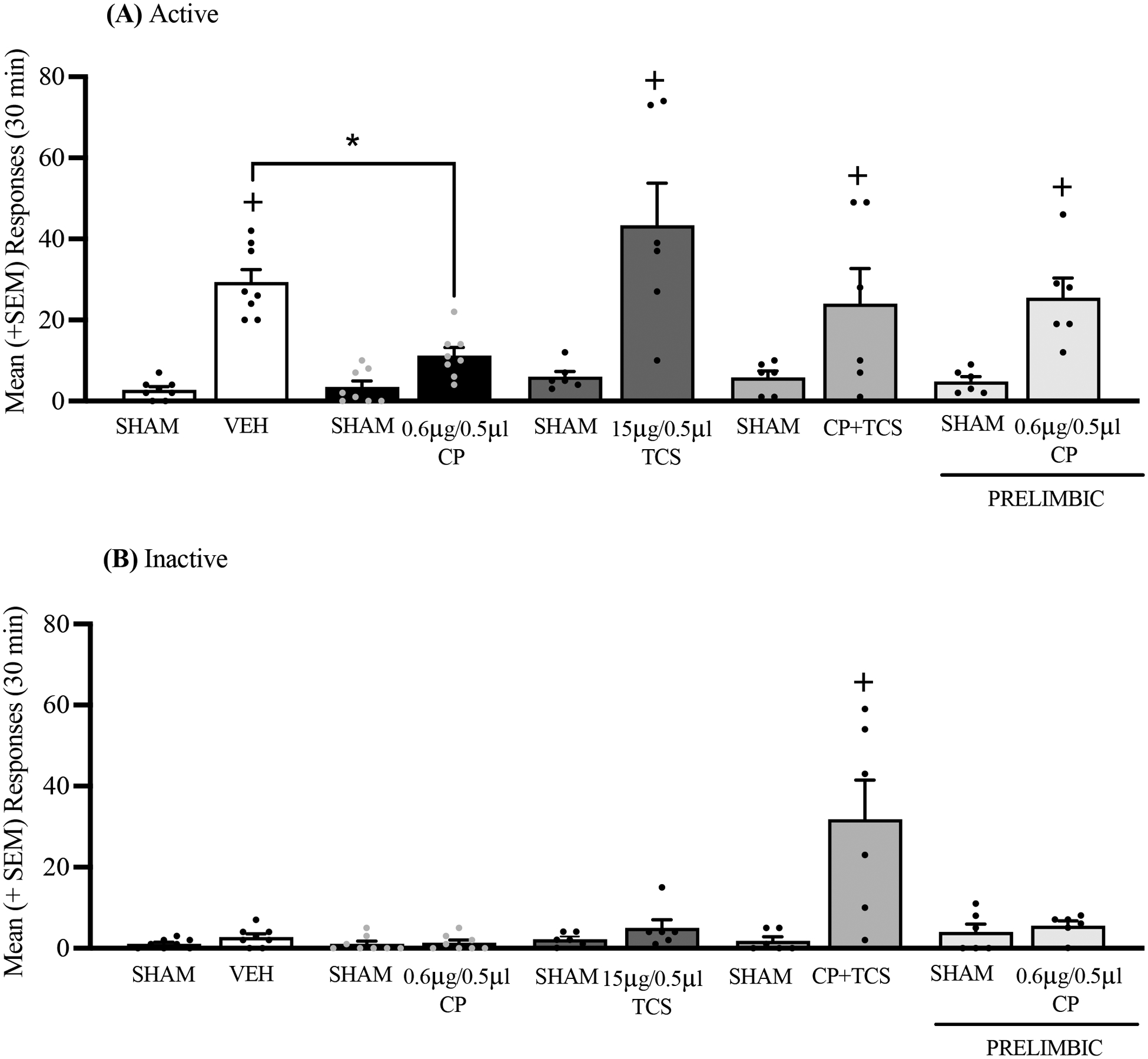
Effects of CP154,526 (CP), TCS1102 (TCS), and CP154,526+TCS1102 (CP+TCS) on the stress-induced reinstatement of alcohol-seeking behavior. (A) Intermittent footshock stress precipitated alcohol-seeking behavior in rats that received vehicle (VEH). The administration of CP154,526 in the IL prevented the stress-induced reinstatement of alcohol seeking, an effect that was blocked by the co-administration of CP154,526+TCS1102. The administration of CP154,526 in the PL had no effect on the stress-induced reinstatement of alcohol-seeking behavior. (B) Co-administration of CP154,256 and TCS1102 increased inactive lever responses following administration in the IL. The data are expressed as mean + SEM. +p < 0.05, vs. respective sham injection; *p < 0.05, vs. VEH.
3.2. Spontaneous locomotion
Given that the number of responses at the inactive lever in the stress-induced reinstatement test was higher in animals that received CP154,526+TCS1102, we conducted an experiment to determine whether this combination affects spontaneous locomotion. The co-administration of CP154,526 and TCS1102 significantly increased the total distance traveled (t10 = 2.34, p = 0.041; Fig. 4A), but no differences in speed were observed (p = 0.60; Fig. 4B).
Fig. 4.
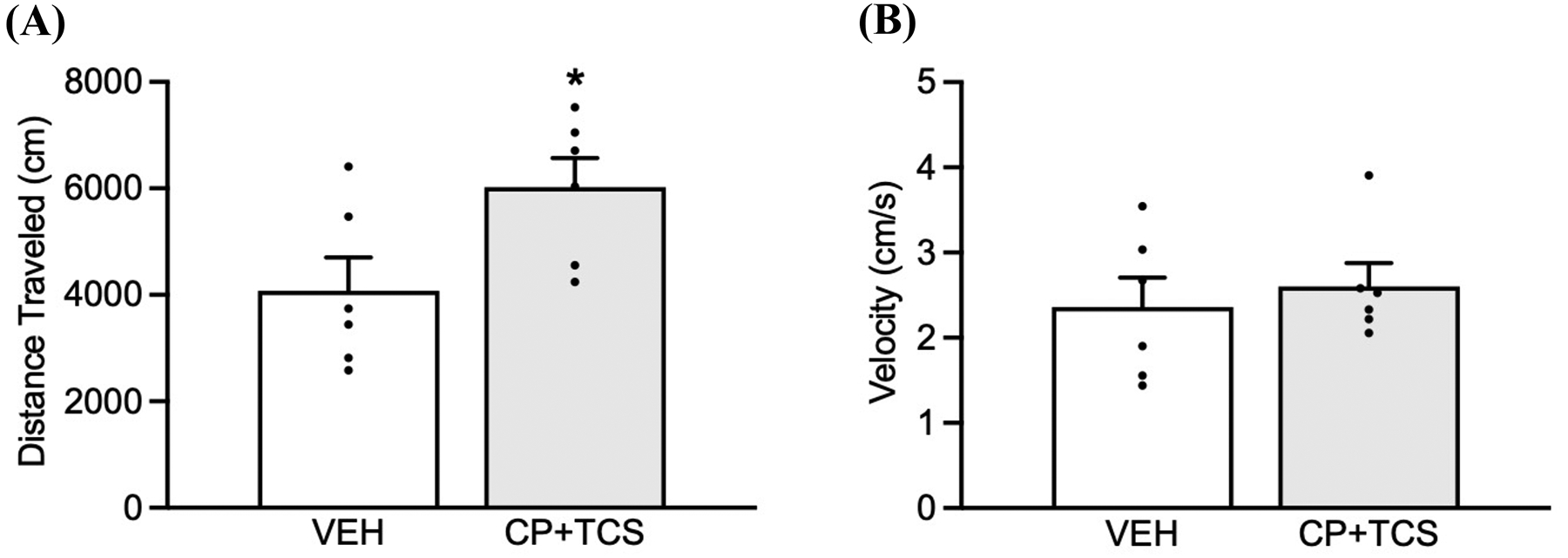
Effects of CP154,526+TCS1102 on spontaneous locomotion. (A) CP154,526+TCS1102 administration increased the total distance traveled compared with VEH administration. (B) No significant difference in speed was found between VEH and CP154,526+TCS1102. The data are expressed as mean + SEM. *p < 0.05.
3.3. qPCR
Over the 21 self-administration training sessions (30 min/day), the rats (n = 16) acquired alcohol self-administration (two-way repeated-measures ANOVA: main effect of session: F20,600 = 4.50, p < 0.0001; main effect of lever: F1,30 = 103.96, p < 0.0001; session × lever interaction: F20,600 = 12.14, p < 0.0001; Fig. 5A). The Bonferroni post hoc test confirmed that beginning in the fifth training session, the number of responses at the active lever was significantly higher than the number of responses at the inactive lever (p < 0.05; Fig. 5A).
Fig. 5.
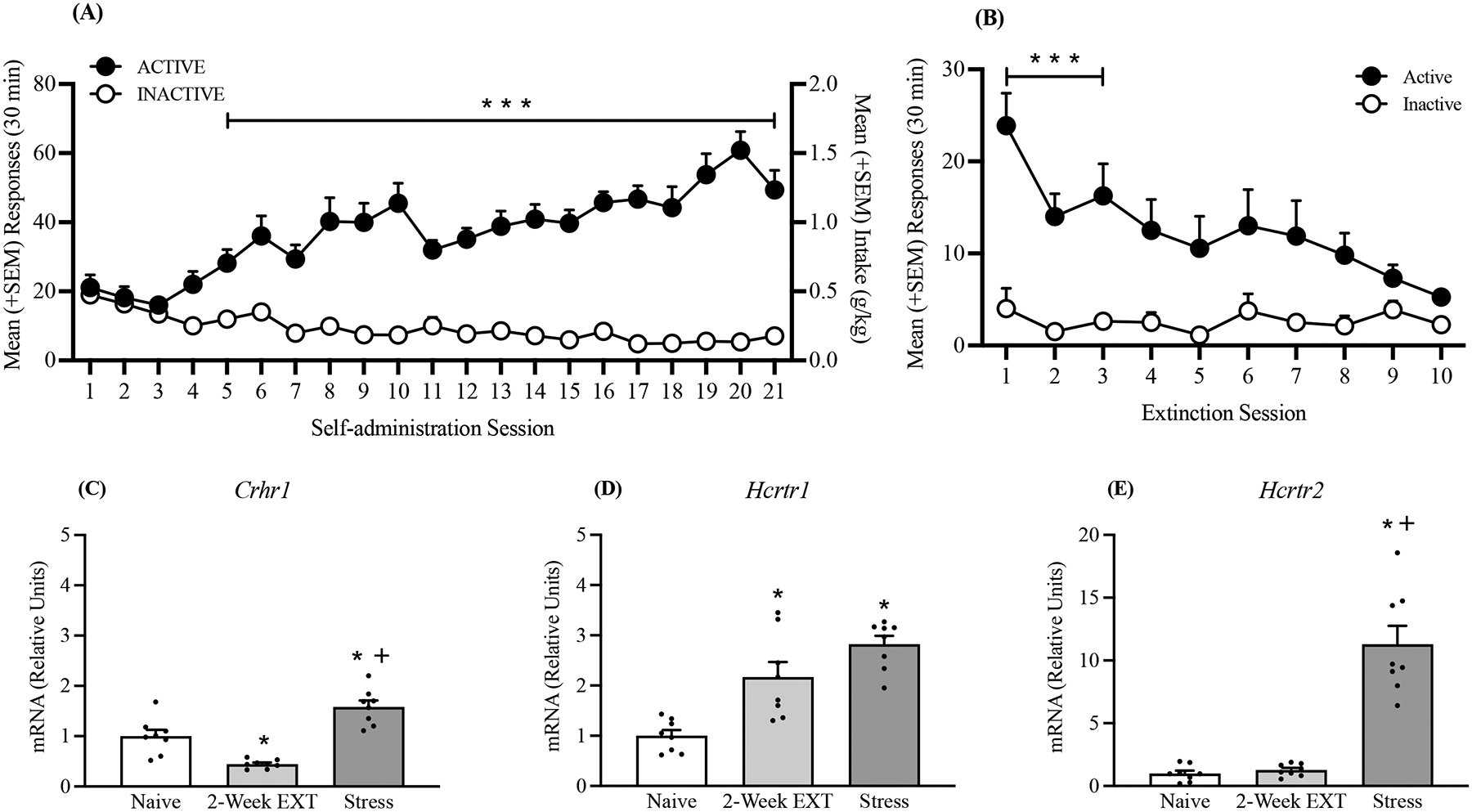
Effects of alcohol extinction and intermittent footshock stress on the gene expression of Crhr1, Hcrtr1, and Hcrtr2. (A) Rats acquired alcohol self-administration over the 21 training sessions. (B) Extinction of alcohol-seeking behavior over the 10 extinction sessions. (C) Stress increased Crhr1 mRNA expression compared with extinction and naive rats but decreased following extinction. (D) Increase in Hcrtr1 mRNA expression following extinction and stress. (E) Increase in Hcrtr2 mRNA expression by stress. The data are expressed as mean + SEM. ***p < 0.001, vs. inactive lever; +p < 0.05, vs. extinction (EXT). *p < 0.05, vs. naive.
Over the 10 extinction sessions, the number of responses at the active lever gradually decreased until they were the same as the number of responses at the inactive lever starting in the fourth session (two-way repeated-measures ANOVA; main effect of session: F9,198 = 3.84, p = 0.046; main effect of lever: F1,22 = 7.67, p = 0.011; session × lever interaction: F9,198 = 3.00, p = 0.0023). The Bonferroni post hoc test revealed a significantly higher number of responses at the active lever from the first to the third extinction session (p < 0.05, Fig. 5B). After the third extinction session, the number of active lever presses was the same as the number of inactive lever presses.
Analyses of mRNA expression in the IL demonstrated that stress significantly increased Crhr1 and Hcrtr2 mRNA expression (separate one-way ANOVAs: Crhr1, F2,21 = 29.41, p < 0.0001, Fig. 5C; Hcrtr1, F2,21 = 19.56, p < 0.0001, Fig. 5D; Hcrtr2, F2,21 = 46.45, p < 0.0001, Fig. 5E). A decrease in Crhr1 mRNA expression was observed following extinction compared with naive rats, whereas stress significantly increased Crhr1 mRNA expression (Tukey post hoc test; p = 0.0021, vs. naive, and p < 0.0001, vs. 2-Week EXT; Fig. 5C). Interestingly, the patterns of Hcrtr1 and Hcrtr2 mRNA expression were different. Although stress increased Hcrtr2 mRNA expression (Tukey post hoc test; p < 0.0001, vs. naive; Fig. 5D, E), a significant increase in Hcrtr1 mRNA expression was also found following extinction (Tukey post hoc test; p = 0.002, vs. naive; Fig. 5D), whereas no change in Hcrtr2 mRNA expression was observed (p = 0.093, vs. naive; Fig. 5E).
4. Discussion
The present study investigated the participation of CRF1 and Orx receptor signaling in the IL and whether an interaction between their transmission occurs during the stress-induced reinstatement of alcohol-seeking behavior. To explore whether stress causes molecular changes, Crhr1, Hcrtr1, and Hcrtr2 mRNA expression were measured in the IL following footshock stress. Consistent with many previous studies (Ahmed & Koob, 1997; Erb & Stewart, 1999; Le et al., 1999; Martin-Fardon et al., 2000; Matzeu & Martin-Fardon, 2020), the present results showed that intermittent footshock stress reinstated extinguished alcohol-seeking behavior. The intra-IL administration of CP154,526 significantly blocked stress-induced reinstatement, whereas TCS1102 did not produce a significant effect. Interestingly, the co-administration of CP154,526 and TCS1102 blocked the effect of TCS1102 alone but also increased spontaneous locomotion. Furthermore, the administration of CP154,526 in the PL did not prevent the reinstatement of alcohol-seeking behavior, suggesting an IL-specific effect. Ultimately, alterations of Crhr1, Hcrtr1, and Hcrtr2 gene expression were apparent following extinction and following the delivery of footshock stress.
Interestingly, the intra-IL administration of CP154,526 significantly reduced the stress-induced reinstatement of alcohol-seeking behavior, suggesting that CRF1 receptor signaling in the IL contributes to the stress-induced reinstatement of alcohol-seeking behavior. Behaviorally, this is consistent with previous studies that reported that acute intraperitoneal pretreatment with CP154,526 attenuated the footshock stress-induced reinstatement of alcohol-seeking behavior in male Wistar rats (Le et al., 2000), decreased both cocaine and heroin self-administration (Shaham, Erb, Leung, Buczek, & Stewart, 1998), and decreased the κ-opioid receptor agonist-induced reinstatement of cocaine-seeking behavior in squirrel monkeys (Valdez, Platt, Rowlett, Ruedi-Bettschen, & Spealman, 2007). Similarly, intraperitoneal administration of the CRF1 receptor antagonist antalarmin significantly decreased established volitional alcohol consumption in high alcohol-preferring Fawn-Hooded rats (Lodge & Lawrence, 2003). Notably, CP154,526 administration in the PL did not prevent the stress-induced reinstatement of alcohol-seeking behavior, providing evidence that the compound’s ability to prevent relapse may be confined to the IL. This is unsurprising because of functional dichotomy between these two regions, in which the PL is involved in executive behavior, while the IL plays a more significant role in response inhibition (Moorman & Aston-Jones, 2015; Moorman et al., 2015). Indeed, others have reported that these regions have opposite functions in the control of stress responsivity. For example, inhibition of the IL decreased activation of the HPA axis and cardiovascular reactivity that were induced by acute aversive stimuli, whereas inhibition of the PL increased these responses (Frysztak & Neafsey, 1994; Radley, Gosselink, & Sawchenko, 2009; Tavares, Correa, & Resstel, 2009). These opposite roles have also been reported for behavioral responsivity to stress (Vidal-Gonzalez, Vidal-Gonzalez, Rauch, & Quirk, 2006). Together with the extant literature, the present data suggest that selectively blocking CRF1 receptors in the IL specifically can prevent stress-induced alcohol craving and relapse and that the IL is a pivotal brain region in mediating this effect.
In recent decades, to study neurobehavioral mechanisms that underlie stress-induced relapse to alcohol seeking, several behavioral neuroscience research groups have used models of reinstatement (Le & Shaham, 2002; Shaham, Shalev, Lu, de Wit, & Stewart, 2003). Indeed, mimicking relapse-like behavior by reinstating alcohol-seeking following the delivery of intermittent footshock has been extensively validated (Cippitelli et al., 2010; Le et al., 1999; Le et al., 1998; Martin-Fardon et al., 2000; Schank et al., 2011), which highlights stress-induced reinstatement as an adequate model for assessing possible therapeutic targets for the prevention of craving and relapse. Notably, previous research from our laboratory (Matzeu & Martin-Fardon, 2020) showed that stress also induces the reinstatement of sweetened condensed milk-seeking behavior in nondependent rats, suggesting that stress affects generalized reward-seeking behavior and not just drug reward. Indeed, using an experimental approach that allowed them to identify two separate reward neuronal networks, Pfarr et al. (2018) found that the cued-induced seeking of both alcohol and saccharin activated neuronal ensembles that were analogous with regard to organization and size, and these networks comprised mostly overlapping cellular populations, indicating that the IL is a hub that integrates general reward-seeking behavior.
Individually, OrxR1 and OrxR2 have been shown to be involved in drug self-administration and reinstatement (Plaza-Zabala, Flores, Maldonado, & Berrendero, 2012; Plaza-Zabala et al., 2013; Uslaner et al., 2014). Administration of the DORA almorexant reduced cocaine- and amphetamine-induced conditioned place preference (Steiner, Lecourt, & Jenck, 2013) and alcohol self-administration in rats (Srinivasan et al., 2012). Additionally, the DORA suvorexant selectively decreased the reinstatement of nicotine-seeking behavior that was induced by an intraperitoneal injection of nicotine but not food-seeking behavior that was induced by the noncontingent delivery of a food pellet (Winrow et al., 2010). In the present study, intra-IL TCS1102 administration did not prevent footshock stress-induced alcohol-seeking behavior. This result is consistent with a previous study from our laboratory that showed that TCS1102 administration in the paraventricular nucleus of the thalamus (PVT) did not prevent stress-induced reinstatement in nondependent rats (Matzeu & Martin-Fardon, 2020). Our results are also consistent with a study that reported that intracerebroventricular TCS1102 administration had no effect on nicotine reinstatement in rats in a short (15-day) nicotine self-administration regimen (Khoo, McNally, & Clemens, 2017). Although differences in methodologies should be considered (i.e., different drugs of abuse and different brain regions), TCS1102’s lack of effect in the present study, together with previous studies, may indicate that although CRF and Orx mechanisms interact with each other, CRF may play a more pronounced role in stress-induced reinstatement than Orx, at least in the IL and in nondependent animals.
One major finding in the present study was that TCS1102 reversed the effects of CP154,526, indicating that an interaction between CRF1 and Orx receptor signaling occurred in the IL in animals that were subjected to footshock stress. This effect was surprising, given that we expected TCS1102 to increase CP154,526’s ability to prevent reinstatement. Nevertheless, accumulating neuroanatomical evidence shows that projections from the lateral hypothalamus innervate the IL (Date et al., 1999), where Orx receptors are expressed (Marcus et al., 2001). Moreover, CRF receptor subtypes are expressed in the lateral hypothalamus (Winsky-Sommerer et al., 2004), which has been shown to receive projections from the IL (Vertes, 2004), forming a potential reciprocal loop and suggesting that an interplay between these two systems may mediate alcohol craving and relapse, given their well-known roles in regulating diverse physiological processes, including stress, motivation, and drug-seeking behavior. Interestingly, previous studies showed that the systemic administration of an OrxR1 antagonist inhibited the stress (yohimbine)-induced reinstatement of alcohol seeking, without affecting locomotor activity, suggesting that Orx receptor blockade can prevent the CRF-mediated stress response, thus supporting the possibility of a functional Orx-CRF interaction (Richards et al., 2008). Supporting this possibility, TCS1102 reversed the effect CP154,526, strongly suggesting a functional interaction between CRF1 and Orx receptors such that Orx receptors exert an inhibitory influence on CRF1 receptor signaling in the IL under stress conditions. The novel findings in the present study further confirm a functional, albeit complex, interaction between CRF1 and Orx receptors in the IL.
The co-administration of CP154,526 and TCS1102 resulted in more inactive lever responses after footshock stress. Thus, an experiment was designed to assess whether this effect was attributable to an overall increase in spontaneous locomotion in animals that received this treatment. The results confirmed that CP154,526+TCS1102 significantly increased the total distance traveled but had no effect on speed during a 30 min locomotion test in the rats’ home cage 15 min after the IL injections. These results are intriguing when considering that central CRF administration increases locomotor activity in rats in a familiar environment (Sutton, Koob, Le Moal, Rivier, & Vale, 1982), an effect that is known to be ameliorated by nonselective CRF receptor antagonists (Britton, Lee, Vale, Rivier, & Koob, 1986; Menzaghi et al., 1994). Indeed, chronic administration of the CRF1 receptor antagonist CRA1000 significantly decreased locomotor activity in the dark phase of the diurnal cycle. Moreover, the DORA almorexant decreased locomotor activity and induced a sedative effect in rats, dogs, and humans (Brisbare-Roch et al., 2007). Interestingly, in the present study, the administration of CP154,526 or TCS1102 alone had no effect on inactive lever responses. Altogether, these results further confirm an interaction between CRF1 and Orx receptor signaling in the IL, in which TCS1102 blocks the effects of CP154,526, resulting in a paradoxical increase in spontaneous locomotion.
Although the molecular mechanisms by which the CRF1/Orx receptor interaction in the IL mediates alcohol craving and relapse are not completely understood, the present study found a decrease in Crhr1 mRNA expression after extinction and an increase following intermittent footshock stress. These results are intriguing and may partially explain CP154,526’s ability to prevent the stress-induced reinstatement of alcohol seeking via a possible increase in CRF1 receptor expression in the IL in the present study. To date, there is no evidence to suggest that the IL receives major inputs from other major CRF regions (e.g., amygdala); therefore, the present results may be attributable to local (i.e., IL) CRF signaling. In this context, using cell morphology, calcium imaging, and electrophysiological approaches, previous research has shown that CRF neurons in the mPFC may be identified as a unique subtype of GABAergic inhibitory interneurons that when inhibited exacerbate responsivity to stressful stimuli, whereas activating them has an opposite effect (Chen et al., 2020). Interestingly, this contrasted with the effect of inhibiting interneurons that expressed vasoactive intestinal polypeptide, which resulted in the amelioration of avoidance behavior (Lee et al., 2019). Thus, CP154,526’s ability to prevent stress-induced alcohol-seeking behavior in the present study may have occurred via a different neuronal ensemble in the IL beyond GABAergic inhibitory interneurons. Given we only measured mRNA and not actual CRF1 receptor density and did not assess the actual function of these interneurons, this hypothesis requires further testing.
Notably, stress increased Hcrtr2 mRNA expression compared with the extinction and naive control rats, whereas no difference in Hcrtr1 mRNA expression was found between extinction and stress groups of rats, suggesting the differential regulation of Hcrtr1 vs. Hcrtr2 transcription by stress. Consistent with these results, previous studies showed that Orx administration increased stress-related behavior, an effect that was blocked by OrxR1 and OrxR2 antagonists (Duxon et al., 2001; Grafe et al., 2017; Ida et al., 2000). Additionally, restraint stress increased calcium signals of Orx neurons (Gonzalez, Iordanidou, Strom, Adamantidis, & Burdakov, 2016), and acute stress increased Orx neuron activation (Panhelainen & Korpi, 2012) and produced long-lasting effects on Hcrt gene expression (Chen et al., 2014). Altogether, these previous studies provide evidence of an interaction between CRF1 and Orx receptor signaling, which we now extend to the IL in the present study. Considering that CRF-containing neurons innervate hypothalamic Orx cells and that CRF depolarizes these cells through CRF1 receptors, CRF may likely be released onto Orx cells during episodes of stress, thereby increasing arousal and mediating the response to stress (Winsky-Sommerer et al., 2004), which may partially explain the molecular findings in the present study.
One limitation of the present study was that female rats were not included, which may limit the generalizability of our results. There are well-established sex differences in alcohol intake and preference in two-bottle choice tests (Li & Lumeng, 1984) and sex differences in reactivity to the rewarding and aversive properties of alcohol (Torres, Walker, Beas, & O’Dell, 2014). Future studies should elucidate the possible sex-dependent relationship between the CRF and Orx systems in mediating the reinstatement of alcohol-seeking behavior after an episode of stress. Furthermore, given that the timing of the microinjections did not align with the timing of the intermittent footshock stress, the exact meaning of our qPCR results is difficult to ascertain. Our original goal was to only assess whether stress alone leads to changes in Crhr1, Hcrtr1, and Hcrtr2 mRNA expression, thereby providing evidence of a functional interaction between CRF and Orx systems. Future studies should assess actual receptor density in groups of animals that receive the pharmacological compounds that were used in the present study.
Overall, the present findings support the hypothesis of a functional interaction between Orx and CRF transmission in the IL and the participation of this interaction in the reinstatement of alcohol use as a function of exposure to stress. The present results highlight the importance of considering multiple pharmacological targets when developing therapies to prevent stress-induced craving and relapse.
Highlights.
The blockade of CRF1 receptors with CP154,526 in the infralimbic cortex decreased the stress-induced reinstatement of alcohol-seeking behavior.
Co-administration of the dual orexin receptor antagonist TCS1102 and CRF1 receptor antagonist CP154,526 in the infralimbic cortex reversed the effect of CP154,526, suggesting a functional interaction between orexin receptor and CRF1 receptor signaling.
Intermittent footshock stress increased Crhr1 and Hcrtr2 mRNA expression.
Acknowledgements
This is publication number 30122 from The Scripps Research Institute. The authors thank Michael Arends for proofreading the manuscript. This work was supported by the National Institute on Alcohol Abuse and Alcoholism (grant no. AA006420, AA026999, and AA028549 to RM-F; T32 AA007456 to FJF-R) and the Instituto de Salud Carlos III (MV19/00060 to LS-M).
References
- Ahmed SH, & Koob GF (1997). Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Psychopharmacology (Berl), 132(3), 289–295. doi: 10.1007/s002130050347 [DOI] [PubMed] [Google Scholar]
- Becker HC (2012). Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res, 34(4), 448–458. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23584111 [PMC free article] [PubMed] [Google Scholar]
- Bonci A, & Borgland S (2009). Role of orexin/hypocretin and CRF in the formation of drug-dependent synaptic plasticity in the mesolimbic system. Neuropharmacology, 56 Suppl 1, 107–111. doi: 10.1016/j.neuropharm.2008.07.024 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, & Bonci A (2006). Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron, 49(4), 589–601. doi: 10.1016/j.neuron.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, & de Lecea L (2005). Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A, 102(52), 19168–19173. doi: 10.1073/pnas.0507480102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, … Jenck F (2007). Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med, 13(2), 150–155. doi: 10.1038/nm1544 [DOI] [PubMed] [Google Scholar]
- Britton KT, Lee G, Vale W, Rivier J, & Koob GF (1986). Corticotropin releasing factor (CRF) receptor antagonist blocks activating and ‘anxiogenic’ actions of CRF in the rat. Brain Res, 369(1–2), 303–306. doi: 10.1016/0006-8993(86)90539-1 [DOI] [PubMed] [Google Scholar]
- Chen P, Lou S, Huang ZH, Wang Z, Shan QH, Wang Y, … Zhou JN (2020). Prefrontal Cortex Corticotropin-Releasing Factor Neurons Control Behavioral Style Selection under Challenging Situations. Neuron, 106(2), 301–315 e307. doi: 10.1016/j.neuron.2020.01.033 [DOI] [PubMed] [Google Scholar]
- Chen X, Wang H, Lin Z, Li S, Li Y, Bergen HT, … Kirouac GJ (2014). Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct Funct, 219(6), 2103–2118. doi: 10.1007/s00429-013-0626-3 [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Karlsson C, Shaw JL, Thorsell A, Gehlert DR, & Heilig M (2010). Suppression of alcohol self-administration and reinstatement of alcohol seeking by melanin-concentrating hormone receptor 1 (MCH1-R) antagonism in Wistar rats. Psychopharmacology (Berl), 211(4), 367–375. doi: 10.1007/s00213-010-1891-y [DOI] [PubMed] [Google Scholar]
- Council N (2003). Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington: National Academy Press. [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, … Nakazato M (1999). Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A, 96(2), 748–753. doi: 10.1073/pnas.96.2.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Li Y, & Kirouac GJ (2015). Blocking of orexin receptors in the paraventricular nucleus of the thalamus has no effect on the expression of conditioned fear in rats. Front Behav Neurosci, 9, 161. doi: 10.3389/fnbeh.2015.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxon MS, Stretton J, Starr K, Jones DN, Holland V, Riley G, … Upton N (2001). Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (OX1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology (Berl), 153(2), 203–209. doi: 10.1007/s002130000550 [DOI] [PubMed] [Google Scholar]
- Erb S, & Stewart J (1999). A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci, 19(20), RC35. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10516337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frysztak RJ, & Neafsey EJ (1994). The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res, 643(1–2), 181–193. doi: 10.1016/0006-8993(94)90024-8 [DOI] [PubMed] [Google Scholar]
- Giardino WJ, & de Lecea L (2014). Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol, 29, 103–108. doi: 10.1016/j.conb.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JA, Iordanidou P, Strom M, Adamantidis A, & Burdakov D (2016). Awake dynamics and brain-wide direct inputs of hypothalamic MCH and orexin networks. Nat Commun, 7, 11395. doi: 10.1038/ncomms11395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Eacret D, Luz S, Gotter AL, Renger JJ, Winrow CJ, & Bhatnagar S (2017). Orexin 2 receptor regulation of the hypothalamic-pituitary-adrenal (HPA) response to acute and repeated stress. Neuroscience, 348, 313–323. doi: 10.1016/j.neuroscience.2017.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, & Aston-Jones G (2005). A role for lateral hypothalamic orexin neurons in reward seeking. Nature, 437(7058), 556–559. doi: 10.1038/nature04071 [DOI] [PubMed] [Google Scholar]
- Hata T, Chen J, Ebihara K, Date Y, Ishida Y, & Nakahara D (2011). Intra-ventral tegmental area or intracerebroventricular orexin-A increases the intra-cranial self-stimulation threshold via activation of the corticotropin-releasing factor system in rats. Eur J Neurosci, 34(5), 816–826. doi: 10.1111/j.1460-9568.2011.07808.x [DOI] [PubMed] [Google Scholar]
- Hsiao YT, Jou SB, Yi PL, & Chang FC (2012). Activation of GABAergic pathway by hypocretin in the median raphe nucleus (MRN) mediates stress-induced theta rhythm in rats. Behav Brain Res, 233(1), 224–231. doi: 10.1016/j.bbr.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Hwa LS, Debold JF, & Miczek KA (2013). Alcohol in excess: CRF(1) receptors in the rat and mouse VTA and DRN. Psychopharmacology (Berl), 225(2), 313–327. doi: 10.1007/s00213-012-2820-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, & Murakami N (2000). Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun, 270(1), 318–323. doi: 10.1006/bbrc.2000.2412 [DOI] [PubMed] [Google Scholar]
- Kalivas PW (2008). Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res, 14(2–3), 185–189. doi: 10.1007/BF03033809 [DOI] [PubMed] [Google Scholar]
- Khoo SY, McNally GP, & Clemens KJ (2017). The dual orexin receptor antagonist TCS1102 does not affect reinstatement of nicotine-seeking. PLoS One, 12(3), e0173967. doi: 10.1371/journal.pone.0173967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, & Kreek MJ (2007). Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry, 164(8), 1149–1159. doi: 10.1176/appi.ajp.2007.05030503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2008). A role for brain stress systems in addiction. Neuron, 59(1), 11–34. doi: 10.1016/j.neuron.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, & Shaham Y (2002). Neurobiology of relapse to alcohol in rats. Pharmacol Ther, 94(1–2), 137–156. doi: 10.1016/s0163-7258(02)00200-0 [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, & Shaham Y (2000). The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl), 150(3), 317–324. doi: 10.1007/s002130000411 [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, & Shaham Y (1999). Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology, 21(3), 435–444. doi: 10.1016/S0893-133X(99)00024-X [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, & Shaham Y (1998). Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl), 135(2), 169–174. doi: 10.1007/s002130050498 [DOI] [PubMed] [Google Scholar]
- Lee AT, Cunniff MM, See JZ, Wilke SA, Luongo FJ, Ellwood IT, … Sohal VS (2019). VIP Interneurons Contribute to Avoidance Behavior by Regulating Information Flow across Hippocampal-Prefrontal Networks. Neuron, 102(6), 1223–1234 e1224. doi: 10.1016/j.neuron.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, & Lumeng L (1984). Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcohol Clin Exp Res, 8(5), 485–486. doi: 10.1111/j.1530-0277.1984.tb05708.x [DOI] [PubMed] [Google Scholar]
- Lodge DJ, & Lawrence AJ (2003). The CRF1 receptor antagonist antalarmin reduces volitional ethanol consumption in isolation-reared fawn-hooded rats. Neuroscience, 117(2), 243–247. doi: 10.1016/s0306-4522(02)00793-5 [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, & Elmquist JK (2001). Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol, 435(1), 6–25. doi: 10.1002/cne.1190 [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, & Weiss F (2000). Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport, 11(9), 1939–1943. doi: 10.1097/00001756-200006260-00026 [DOI] [PubMed] [Google Scholar]
- Matzeu A, & Martin-Fardon R (2020). Blockade of Orexin Receptors in the Posterior Paraventricular Nucleus of the Thalamus Prevents Stress-Induced Reinstatement of Reward-Seeking Behavior in Rats With a History of Ethanol Dependence. Front Integr Neurosci, 14, 599710. doi: 10.3389/fnint.2020.599710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzaghi F, Howard RL, Heinrichs SC, Vale W, Rivier J, & Koob GF (1994). Characterization of a novel and potent corticotropin-releasing factor antagonist in rats. J Pharmacol Exp Ther, 269(2), 564–572. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8182523 [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, & Weiss F (1995). Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci, 15(8), 5439–5447. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7643193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, & Aston-Jones G (2015). Prefrontal neurons encode context-based response execution and inhibition in reward seeking and extinction. Proc Natl Acad Sci U S A, 112(30), 9472–9477. doi: 10.1073/pnas.1507611112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, & Aston-Jones G (2015). Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res, 1628(Pt A), 130–146. doi: 10.1016/j.brainres.2014.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, & Hodge CW (2002). Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav, 72(1–2), 213–220. doi: 10.1016/s0091-3057(01)00748-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhelainen AE, & Korpi ER (2012). Evidence for a role of inhibition of orexinergic neurons in the anxiolytic and sedative effects of diazepam: A c-Fos study. Pharmacol Biochem Behav, 101(1), 115–124. doi: 10.1016/j.pbb.2011.12.011 [DOI] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (1998). The Rat Brain in Stereotaxic Coordinates: Academic Press. [DOI] [PubMed] [Google Scholar]
- Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, … Wurbel H (2020). The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMJ Open Sci, 4(1), e100115. doi: 10.1136/bmjos-2020-100115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schonig K, … Sommer WH (2015). Losing Control: Excessive Alcohol Seeking after Selective Inactivation of Cue-Responsive Neurons in the Infralimbic Cortex. J Neurosci, 35(30), 10750–10761. doi: 10.1523/JNEUROSCI.0684-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr S, Schaaf L, Reinert JK, Paul E, Herrmannsdorfer F, Rossmanith M, … Sommer WH (2018). Choice for Drug or Natural Reward Engages Largely Overlapping Neuronal Ensembles in the Infralimbic Prefrontal Cortex. J Neurosci, 38(14), 3507–3519. doi: 10.1523/JNEUROSCI.0026-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Maldonado R, & Berrendero F (2012). Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biol Psychiatry, 71(3), 214–223. doi: 10.1016/j.biopsych.2011.06.025 [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Martin-Garcia E, Saravia R, Maldonado R, & Berrendero F (2013). A role for hypocretin/orexin receptor-1 in cue-induced reinstatement of nicotine-seeking behavior. Neuropsychopharmacology, 38(9), 1724–1736. doi: 10.1038/npp.2013.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, … Kash TL (2015). Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology, 99, 735–749. doi: 10.1016/j.neuropharm.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros IM, Macedo GC, Domingues LP, & Favoretto CA (2016). An Update on CRF Mechanisms Underlying Alcohol Use Disorders and Dependence. Front Endocrinol (Lausanne), 7, 134. doi: 10.3389/fendo.2016.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, & Sawchenko PE (2009). A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci, 29(22), 7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, & Bartlett SE (2008). Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl), 199(1), 109–117. doi: 10.1007/s00213-008-1136-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, … Heilig M (2011). Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl), 218(1), 111–119. doi: 10.1007/s00213-011-2201-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, & Stewart J (1998). CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl), 137(2), 184–190. doi: 10.1007/s002130050608 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, & Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl), 168(1–2), 3–20. doi: 10.1007/s00213-002-1224-x [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, & Shaham Y (2010). Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res, 1314, 15–28. doi: 10.1016/j.brainres.2009.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, & Martin-Fardon R (2010). Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry, 67(9), 804–811. doi: 10.1016/j.biopsych.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, … Bartlett SE (2012). The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PLoS One, 7(9), e44726. doi: 10.1371/journal.pone.0044726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Lecourt H, & Jenck F (2013). The dual orexin receptor antagonist almorexant, alone and in combination with morphine, cocaine and amphetamine, on conditioned place preference and locomotor sensitization in the rat. Int J Neuropsychopharmacol, 16(2), 417–432. doi: 10.1017/S1461145712000193 [DOI] [PubMed] [Google Scholar]
- Stephens MA, & Wand G (2012). Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res, 34(4), 468–483. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23584113 [PMC free article] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, & Vale W (1982). Corticotropin releasing factor produces behavioural activation in rats. Nature, 297(5864), 331–333. doi: 10.1038/297331a0 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Beuckmann CT, Shikata K, Ogura H, & Sawai T (2005). Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res, 1044(1), 116–121. doi: 10.1016/j.brainres.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, & Vale WW (1983). Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology, 36(3), 165–186. doi: 10.1159/000123454 [DOI] [PubMed] [Google Scholar]
- Tavares RF, Correa FM, & Resstel LB (2009). Opposite role of infralimbic and prelimbic cortex in the tachycardiac response evoked by acute restraint stress in rats. J Neurosci Res, 87(11), 2601–2607. doi: 10.1002/jnr.22070 [DOI] [PubMed] [Google Scholar]
- Thompson JL, & Borgland SL (2011). A role for hypocretin/orexin in motivation. Behav Brain Res, 217(2), 446–453. doi: 10.1016/j.bbr.2010.09.028 [DOI] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, & O’Dell LE (2014). Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcohol Clin Exp Res, 38(1), 108–115. doi: 10.1111/acer.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Winrow CJ, Gotter AL, Roecker AJ, Coleman PJ, Hutson PH, … Renger JJ (2014). Selective orexin 2 receptor antagonism blocks cue-induced reinstatement, but not nicotine self-administration or nicotine-induced reinstatement. Behav Brain Res, 269, 61–65. doi: 10.1016/j.bbr.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, & Spealman RD (2007). Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther, 323(2), 525–533. doi: 10.1124/jpet.107.125484 [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, & De Vries TJ (2010). Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev, 35(2), 276–284. doi: 10.1016/j.neubiorev.2009.11.016 [DOI] [PubMed] [Google Scholar]
- Vertes RP (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse, 51(1), 32–58. doi: 10.1002/syn.10279 [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, & Quirk GJ (2006). Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem, 13(6), 728–733. doi: 10.1101/lm.306106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winrow CJ, Tanis KQ, Reiss DR, Rigby AM, Uslaner JM, Uebele VN, … Renger JJ (2010). Orexin receptor antagonism prevents transcriptional and behavioral plasticity resulting from stimulant exposure. Neuropharmacology, 58(1), 185–194. doi: 10.1016/j.neuropharm.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, … de Lecea L (2004). Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci, 24(50), 11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, & Weiss F (2006). Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci, 26(39), 9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]


