Abstract
In hybrid striped bass aquaculture ponds, dinoflagellate blooms were found on 10 of 14 occasions to co-occur with concentrations of urea in excess of 1.5 μM nitrogen. When urea levels were <1.5 μM nitrogen, on seven occasions, no evidence of dinoflagellate blooms was observed in these ponds.
Phytoplankton ecologists have long grappled with the fundamental question of what factors determine the differential growth of species in phytoplankton communities which may result in the dominance, or bloom, of one particular species at a particular time. The availability of different forms of nitrogen and their relative rates of utilization are important factors contributing to the relative success and productivity of different phytoplankton (2, 12, 18). Typically, fast-growing diatoms have been found to be highly correlated with large and/or frequent additions of NO3− (16, 20, 33). By contrast, microflagellates (including dinoflagellates) have been correlated with low nitrate concentrations and high rates of NH4+ or dissolved organic nitrogen (DON) supply (2, 7, 11, 28). Recent studies of enriched coastal areas also suggest that while phytoplankton production may increase quantitatively with overall nitrogen availability, the DON component may contribute disproportionately to the alteration of phytoplankton succession and the triggering of harmful algal blooms (2, 7, 28). In the past few years, notable advances have been made in the study of the DON pool. The fluxes and composition of this large and complex pool are now characterized to a much better extent than just a few years ago (4, 5, 24, 25).
The factors contributing to the differential growth of phytoplankton species are important issues for aquaculturists as well. Aquaculturists aim to have stable phytoplankton blooms, and unfavorable blooms may cause off flavors or may threaten the survival and marketability of the cultured species (32). In recent years, harmful algal blooms, in particular those of the icthyotoxic dinoflagellates Gyrodinium galatheanum and Pfiesteria piscicida, have caused massive mortality of fish in natural riverine systems along the east coast of the United States and in cultured fish ponds (6). Aquaculture ponds are typically eutrophic or hypereutrophic, as fertilizer and feed additions are made routinely (10). Indeed, it has been suggested that aquaculture systems, due to their enriched conditions, may actually promote the growth of harmful algal species not previously detected in the source water body (15).
In early 1997 we began an investigation of the nitrogen dynamics and phytoplankton succession in a commercial hybrid striped bass aquaculture facility to determine the role that nitrogen, and in particular DON, plays in development of dinoflagellate blooms in these ponds. Our hope was to identify either specific components of the nitrogen pool or other characteristics of the nitrogen supply that were related to the dinoflagellate blooms and thus could serve as predictors of such harmful blooms.
Samples were collected from commercial ponds located at HyRock Farms on the Manokin River, a tributary of Chesapeake Bay. At the time of sampling, the ponds contained commercial densities of 1-year-old hybrid striped bass (striped bass, Morone saxitilis, and white bass, Morone chrysops). Sampling was conducted biweekly from 6 June to 15 August 1997, and in addition, one sample was collected in December 1997. In total, three ponds were sampled on seven occasions each, six times on a biweekly basis during the summer and on one occasion in early winter. Samples were collected with acid-cleaned buckets and immediately returned to the laboratory on ice, where they were filtered through precombusted (550°C for 2 h) Whatman GF/F filters, and the filtrates were frozen for later determination of nutrient content (within 2 weeks). Inorganic-nutrient concentrations were determined by standard autoanalyzer methods (29), while concentrations of urea were determined in triplicate by the urease method (23). The filters were retained for analysis of pigment and particulate composition (29). Samples for phytoplankton identification and enumeration were preserved in Lugol’s solution and enumerated with a hemacytometer under phase-contrast microscopy (14).
Water temperatures ranged from 19 to 33°C during the midsummer growing season and from 3 to 7°C in early winter. Salinity ranged from 6 to 11.5 PSU. Of the 21 sampling periods, 9 in summer and 1 in fall had dinoflagellate blooms sufficiently developed to require water quality treatment by the grower. Dinoflagellate blooms were classified as a minimum of 5 × 103 cells ml−1. The summer dinoflagellate blooms were largely composed of G. galatheanum, Gymnodinium nelsonii, and Prorocentrum minimum, and the winter bloom was composed of Katodinium sp. When dinoflagellates were not abundant, the dominant phytoplankton group was diatoms.
The range of water quality factors observed over the grow-out cycle of 1997 (Table 1) reflect the “boom-bust” cycles of hypereutrophic systems, in which phytoplankton blooms develop and crash over time scales of several days. Concentrations of NH4+, while typically being <2 μM, did exceed 70 μM on two occasions, and NO3− also became elevated above 50 μM on several occasions. Concentrations of chlorophyll a typically exceeded 100 μg liter−1, reflecting the high phytoplankton density typical of these systems.
TABLE 1.
Phytoplankton biomass (as chlorophyll a) and nutrient ranges in striped bass aquaculture ponds during the 1997 grow-out period in midsummer and early winter
| Analyte | Midsummer range | Early-winter range |
|---|---|---|
| Chlorophyll a (μg liter−1) | 10–338 | 20–83 |
| Urea (μM N) | 0.4–9.9 | 0.4–3.6 |
| Total dissolved nitrogen (μM N) | 33–252 | 31–59 |
| DON (μM N) | 24–180 | 29–45 |
| NH4+ (μM N) | 0.6–71 | 1.6–9.4 |
| NO3− + NO2− (μM N) | <0.03–70 | 0.3–4.5 |
| PO43− (μM N) | 0.04–0.72 | 0.11–0.45 |
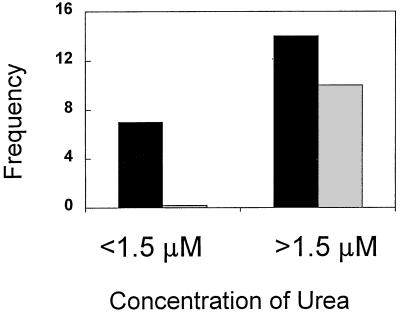
All dinoflagellate blooms were observed to co-occur with elevated levels of urea (>1.5 μM nitrogen). In total, we found 14 instances of elevated urea levels, of which 10 co-occurred with dinoflagellate blooms. In all cases where urea was <1.5 μM nitrogen (seven occasions), no dinoflagellate blooms were found (Fig. 1). The occurrence and/or abundance of dinoflagellate blooms was not found to be correlated significantly with any other nutrient parameter.
FIG. 1.
Frequency diagram of number of sampling periods in which urea concentrations were <1.5 or >1.5 μM N (solid bars) and number of sampling periods in which dinoflagellate blooms developed (shaded bars) in commercial hybrid striped bass (M. saxitilis and M. chrysops) aquaculture ponds in Maryland in 1997.
Urea constitutes only a small percentage of total DON in eutrophic and oligotrophic waters; in estuarine fish ponds it is typically <1% of the DON pool. However, urea has been shown to contribute from 60 to 80% of the nitrogen utilized much of the year in the plume of the Chesapeake Bay and up to 50% of the nitrogen utilized in many other coastal regions (13, 17, 19).
The correlation between urea availability and the development of dinoflagellate blooms has implications for phytoplankton ecology in general as well as for the management of aquaculture fish ponds. First, these results are consistent with observations suggesting that some flagellates may have a preference for the uptake of urea over NO3− (21, 28). In addition, the strong correlation between dinoflagellate blooms and elevated levels of urea may also suggest that release of urea may be stimulated directly, or indirectly, by the presence of dinoflagellates themselves. Dinoflagellates are known to irritate the gills of finfish, which may lead to the loss of nitrogenous waste products other than ammonia. In teleost fish exposed to other stressors, blood urea nitrogen levels have been found to increase substantially (27). Urea regeneration also occurs from zooplankton as well as bacteria, and rates are dependent on both DON concentration and bacterial activity and/or growth rate (3, 8, 9, 26, 30). The combination of high capacity for urea uptake by dinoflagellates and the potential for enhanced release of urea by the fish exposed to high concentrations of dinoflagellates, or to enhanced bacterial regeneration, could lead to conditions favorable for sustaining a dinoflagellate bloom if other environmental conditions remain favorable.
There is some evidence that urea may directly stimulate toxin production in dinoflagellates. Gymnodinium breve, for example, has been shown to increase its production of brevetoxin to up to six times that of the control when exposed to urea levels of 0.5 to 1.0 mM nitrogen in culture (31).
Finally, aquaculturists have an important interest in maintaining high water quality and minimizing the development of harmful algal blooms. After-the-fact treatment of dinoflagellate blooms is very costly, in terms of loss of marketable product and also in the cost of chemical treatment, often permanganate. These results suggest that an early warning signal for aquaculturists, and perhaps coastal managers as well, may be available by monitoring urea concentrations. By monitoring urea levels and aggressively treating blooms when they appeared during the 1998 growth season at the study site, dinoflagellate blooms similar to those observed in 1997 were avoided and levels of urea were maintained at <1.5 μM nitrogen. A chemical test to detect elevated levels of urea would be more feasible to implement in the field than microscopic analyses of phytoplankton composition, particularly when dealing with the small, poorly known dinoflagellates that are of increasing concern in aquaculture operations.
These results further underscore the need to incorporate urea, and likely other organic nitrogen compounds, into our models of nitrogen flow and our estimates of nitrogen incorporation by primary producers (1). This is especially true for coastal and estuarine ecosystems, which receive nutrient inputs from river flow, sewage, and runoff, including aquaculture pond effluent. The appearance of harmful dinoflagellates, especially P. piscicida, in the Pocomoke River, a tributary of the Chesapeake Bay, during the summer of 1997 was correlated with increased organic loading (22). Nutrient reduction efforts in coastal waters will need to take these forms of nitrogen, among other factors, into consideration if a reduction in the occurrence of harmful algal blooms is to be achieved.
Acknowledgments
This work was supported by USDA grant 95-37101-1699 to P.M.G. and University of Maryland Agricultural Experiment Station and Maryland Sea Grant awards to D.E.T.
We thank Tony Mazzaccaro for access to HyRock Farms and Michael Lomas for technical assistance and critical review of the manuscript.
Footnotes
This is contribution number 3240 from the Center for Environmental Science.
REFERENCES
- 1.Antia N J, Harrison P J, Oliveira L. The role of dissolved organic nitrogen in phytoplankton nutrition, cell biology and ecology. Phycologia. 1991;30:1–89. [Google Scholar]
- 2.Berg G M, Glibert P M, Lomas M L, Burford M. Organic nitrogen uptake and growth by the Chrysophyte Aureococcus anophagefferens during a brown tide event. Mar Biol. 1997;129:377–387. [Google Scholar]
- 3.Billen G. Heterotrophic utilization and regeneration of nitrogen. In: Hobbie J E, Williams P J L, editors. Heterotrophic activity in the sea. New York, N.Y: Plenum Press; 1984. pp. 313–355. [Google Scholar]
- 4.Bronk D A, Glibert P M. Application of a method for the measurement of dissolved organic nitrogen during spring and summer in Chesapeake Bay. Mar Biol. 1993;115:501–508. [Google Scholar]
- 5.Bronk D A, Glibert P M, Malone T C, Banahan S, Salsten E. Inorganic and organic nitrogen cycling in Chesapeake Bay: autotrophic versus heterotrophic processes and relationships to carbon flux. Aquat Microb Ecol. 1998;15:177–189. [Google Scholar]
- 6.Burkholder J M, Glasgow H B., Jr Pfiesteria piscicida and Pfiesteria-like dinoflagellates: behavior, impacts, and environmental controls. Limnol Oceanogr. 1997;42:1052–1075. [Google Scholar]
- 7.Carlsson P, Edling H, Béchemin C. Interactions between a marine dinoflagellate (Alexandrium catenella) and a bacterial community utilizing riverine humic substances. Aquat Microb Ecol. 1998;16:65–80. [Google Scholar]
- 8.Cho B C, Azam F. Urea decomposition by bacteria in the Southern California Bight and its implications for the mesopelagic nitrogen cycle. Mar Ecol Prog Ser. 1995;122:21–26. [Google Scholar]
- 9.Cho B C, Park M G, Shim J H, Azam F. Significance of bacteria in urea dynamics in coastal surface waters. Mar Ecol Prog Ser. 1996;142:19–26. [Google Scholar]
- 10.Daniels H V, Boyd C E. Chemical budgets for polyethylene-lined brackish water ponds. J World Aquacult. 1989;20:53–60. [Google Scholar]
- 11.Davis C O. The importance of understanding phytoplankton life strategies in the design of enclosure experiments. In: Grice G D, Reeve M R, editors. Marine mesocosms: biological and chemical research in experimental ecosystems. New York, N.Y: Springer-Verlag; 1982. pp. 323–332. [Google Scholar]
- 12.Eppley R W, Peterson B J. Particulate organic matter flux and planktonic new production in the deep ocean. Nature. 1979;282:677–680. [Google Scholar]
- 13.Glibert P M, Garside C, Fuhrman J A, Roman M R. Time-dependent coupling of inorganic and organic nitrogen uptake and regeneration in the plume of the Chesapeake Bay estuary and its regulation by large heterotrophs. Limnol Oceanogr. 1991;36:895–909. [Google Scholar]
- 14.Guillard R R L. Division rates. In: Stein J R, editor. Handbook of phycological methods: culture methods and growth measurements. New York, N.Y: Cambridge University Press; 1973. pp. 289–311. [Google Scholar]
- 15.Hallegraeff G M. A review of harmful algal blooms and their global increase. Phycologia. 1993;32:79–99. [Google Scholar]
- 16.Harrison P J, Turpin D H. The manipulation of physical, chemical, and biological factors to select species from natural phytoplankton communities. In: Grice G D, Reeve M R, editors. Marine mesocosms: biological and chemical research in experimental ecosystems. New York, N.Y: Springer-Verlag; 1982. pp. 275–289. [Google Scholar]
- 17.Harrison W G, Head E J D, Conover R J, Longhurst A R, Sameoto D D. The distribution and metabolism of urea in the eastern Canadian Arctic. Deep-Sea Res. 1985;32:23–42. [Google Scholar]
- 18.Kokkinakis S A, Wheeler P A. Uptake of ammonium and urea in the northeast Pacific: comparison between netplankton and nanoplankton. Mar Ecol Prog Ser. 1988;43:113–124. [Google Scholar]
- 19.Kristansen S. The temperature optimum of the nitrate reductase assay for marine phytoplankton. Limnol Oceanogr. 1983;28:776–780. [Google Scholar]
- 20.Lomas M W, Glibert P M. Temperature regulation of nitrate uptake: a novel hypothesis about nitrate uptake and reduction in cool-water diatoms. Limnol Oceanogr. 1999;44:556–572. [Google Scholar]
- 21.Margalef R. Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanologia Acta. 1978;1:493–509. [Google Scholar]
- 22.Maryland Department of Natural Resources. Water quality, habitat and biological conditions of river systems affected by Pfiesteria or Pfiesteria-like organisms on the Lower Eastern Shore of Maryland. 1997 technical summary. Annapolis, Md: Maryland Department of Natural Resources; 1998. [Google Scholar]
- 23.McCarthy J J. A urease method for urea in seawater. Limnol Oceanogr. 1970;15:309–312. [Google Scholar]
- 24.McCarthy M D, Pratum T, Hedges J I, Benner R. Chemical composition of dissolved organic nitrogen in the ocean. Nature. 1997;390:150–154. [Google Scholar]
- 25.McCarthy M D, Hedges J I, Benner R. Major bacterial contribution to marine dissolved organic nitrogen. Science. 1998;281:231–234. doi: 10.1126/science.281.5374.231. [DOI] [PubMed] [Google Scholar]
- 26.Miller C A, Glibert P M. Nitrogen excretion by the calenoid copepod Acartia tonsa: results of mesocosm experiments. J Plankton Res. 1998;20:1767–1780. [Google Scholar]
- 27.Nelson, K., J. Jones, S. Jacobsen, and R. Reimschuessel. Elevated BUN levels in goldfish as an indicator of gill dysfunction. J. Aquat. Anim. Health, in press.
- 28.Paerl H W. Nuisance phytoplankton blooms in coastal, estuarine, and inland waters. Limnol Oceanogr. 1988;33:823–847. [Google Scholar]
- 29.Parsons T R, Maita Y, Lalli C M. A manual of chemical and biological methods for seawater analysis. New York, N.Y: Pergamon Press; 1984. [Google Scholar]
- 30.Price N M, Cochlan W P, Harrison P J. Time course of uptake of inorganic and organic nitrogen by phytoplankton in the Strait of Georgia: comparison of frontal and stratified communities. Mar Ecol Prog Ser. 1985;27:39–53. [Google Scholar]
- 31.Shimizu Y, Watanabe N, Wrensford G. Biosynthesis of brevetoxins and heterotrophic metabolism in Gymnodinium breve. In: Watanabe N, Wresford G, Lassus P, Arzul G, Erard-Le Denn E, Gentien P, Marcailloi-Le Baut C, editors. Harmful marine algal blooms. New York, N.Y: Lavoisier Publishing Inc.; 1995. pp. 351–358. [Google Scholar]
- 32.Shumway S. A review of the effects of algal blooms on shellfish and aquaculture. J World Aquacult Soc. 1990;21:65–104. [Google Scholar]
- 33.Takahashi M, Koike I, Iseki K, Bienfang P K, Hattori A. Phytoplankton species responses to nutrient changes in experimental enclosures and coastal waters. In: Grice G D, Reeve M R, editors. Marine mesocosms: biological and chemical research in experimental ecosystems. New York, N.Y: Springer-Verlag; 1982. pp. 333–340. [Google Scholar]