Abstract
The relationship between periodontitis (or periodontal disease) with Alzheimer’s disease has been reported by various primary sources in the past decade, but not with a solid secondary research statement. A systematic review and meta-analysis was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and registered (Reference number: CRD42020185264) with PROSPERO (International prospective register for systematic reviews). A literature search was conducted on specific databases for suitable articles in English language. Out of 612 studies selected, 41 underwent full-text analysis; five studies were eligible for systematic review, and 3 for meta-analysis. Meta-analysis was performed with tests for sensitivity and statistical heterogeneity followed by calculation of summary effect measures in terms of odds ratio (OR) and 95% confidence interval (CI). The results of this review showed a significant association between periodontitis and Alzheimer’s disease in the meta-analysis [OR 1.67 (1.21–2.32)].
Keywords: Alzheimer’s disease, meta-analysis, neurology, periodontitis, psychiatry
Introduction
Periodontitis (‘peri’= around; ‘odont’= related to tooth; ‘itits’= inflammation) is a disorder of tooth supporting structures, namely gingiva and periodontium. Thus, the term periodontal disease can be applied to gingivitis and periodontitis [1]. The clinical presentation often is like gingivitis (a painful inflammation/swelling of gums leading to bleeding) which later, if not treated, develops to inflammation of the periodontium or periodontitis. The hallmark of periodontitis is the loss of pocket formation, clinical attachment loss (CAL) and loss supporting bone [1]. The periodontal disease (PD) ranks 11th, among the topmost prevalent conditions in the world with an estimated prevalence ranging as high as 20% to 50% [1]. The impact of the periodontitis is understood from fact that it accounted for disability in 3.5 million patients in the year 2016. Also, the global loss of productivity owing to PD related disability was reported to be 54 billion USD per annum [2,3]. The overall prevalence rates of PD are expected to raise pertaining to factors such as survival of older individuals/aging population and increased awareness/availability of dental treatments saving natural teeth in these older population [4].
The periodontitis mainly contributes to masticatory disabilities leading to impaired intake of food, thus negatively affecting nutrition, general health and also predisposing or associating with morbid systemic disorders [1,3,5]. The main periodontitis - ‘associated health conditions’ (PAHC) are global burdens themselves, such as diabetes mellitus (DM), inflammatory cardiovascular diseases, adverse pregnancy outcomes, autoimmune joint disorders, and inflammatory obstructive lung diseases to name a few [1,3,5]. The disseminated or metastatic spread of microbes or associated microbial products/inflammatory mediators from dental plaque/biofilms of periodontal tissues to distant organs was explained previously as the basis of the PAHC [5]. The association of Alzheimer’s disease with periodontitis was studied in recent times, some studies being true observations, based on indirect hypothesis, or still unclear [6–9].
Alzheimer’s disease (AD) is the a advanced neurodegenerative disorder, accounting for 75% of all dementia cases in the world [10]. The global prevalence of dementia was estimated to be 3.9 % (more than 25 million people) aged 60 years or above, of which, AD adds with around 5 million new cases [10,11]. The AD is a burden for families, societies, and countries as a whole. Given the improvement of survival rates of aged population, the disease numbers are high in developed countries [12]. The AD is not just a morbid condition, it is also a leading cause of mortality. The official death certificates record reported AD to be the sixth important cause of demise in the United States [11,12].
The chronic / severe periodontitis and AD both occur in geriatric population having other comorbidities. The association of AD with periodontal disease has been investigated in last decade with varying conclusions [6–9]. A partially casual association, role of the APOE4ɛ, the existence of elevated immunoglobulin G (IgG) levels against Prophyromonas gingivalis [(P gingivalis), an established periodontal pathogen] to possibility of cognitive impairment have been shown in literature [7–9]. A noteworthy association between dementia and raised IgG, corresponding to P. gingivalis has been reported but, a positive correlation between PD and degenerative disorder of nervous system, like AD is not established clearly from studies [7,8]. A research lacunae noted here led to the conduct of this review for further understanding of these associations.
The objective of this study is to evaluate the association between periodontitis and Alzheimer’s disease based on a systematic review form observational studies conducted in last decade. The following PICO based research question was applied: ‘Are individuals with periodontitis (or those exposed to periodontal pathogens) more likely to develop Alzheimer’s disease?’
P (Patients): Subjects with periodontitis
I (Intervention): periodontal pathogens (Porphyromonalis gingivalis)
C (Comparison): controls (subjects without known periodontitis)
O (Outcome): Occurrence of Alzheimer’s disease/cognitive impairment.
Methods
The review was performed with reference to standards laid down by Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (PRISMA) [13] and registered in the International Prospective Register of Systematic Reviews (PROSPERO Registration number: CRD42020185264).
The observational (cohort/case-control both prospective and retrospective) studies that were in line with research question having data of interest were eligible for inclusion. Studies were carried out in adults subjects and had evaluated the presence of periodontitis in AD patients; those which reported periodontal parameters and AD as per universally acceptable standards were only taken into inclusion for keeping up value to the meta-analysis. The standards were defined as clinical parameters of periodontitis as reported according to criteria I: Armitage classification [14] or criteria II: periodontitis patients with specific isolation corresponding to P gingivalis plus a non-specific periodontal inflammation based marker; AD diagnosis as per acceptable international standards [i.e. Criteria I: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA); Criteria II: Diagnostic and Statistical Manual of Mental disorders (DSM-IV) [15,16] or Criteria III: population form studies diagnosed with reliable cognitive tests [Mini-Mental State Examination (MMSE)] [17].
Studies were defining periodontitis by self-reported/unstandardized criteria or without a conducting a clinical examination; studies that defined dementia due to non-AD etiology and randomised control trials in the topic (if any) were excluded. The type of studies namely narrative reviews, letters, commentaries, viewpoints, and editorials were excluded. The titles published in languages other than English were considered for exclusion.
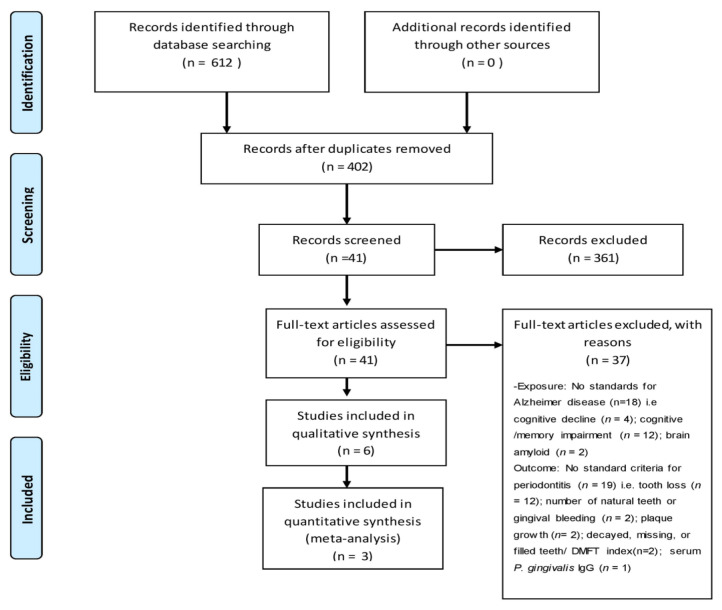
The titles and abstracts of all articles identified through search were evaluated individually by two review authors (A1, A2) for eligibility. The following databases were searched: MEDLINE via PUBMED, Google Scholar, Web of science, EMBASE and SCOPUS between timelines of January 2010 to March 31st 2020. The search strategy used for a data base was key words or near matches to the key words separated by boolean operator; PUBMED database the strategy was: “Alzheimer’s” (All fields) AND “periodontitis” (MESH Term) OR “Alzheimer’s” (All fields) AND “Dental infection” (All fields) OR “Alzheimer’s” (All fields) AND “Dentistry” (MESH Term) AND “Prevention” (All fields) OR “periodontitis” (All fields) OR “periodontal disease” OR ”edentulous” AND “cognition” OR “memory’” OR “dementia” OR “cognitive decline” OR “cognitive loss” OR “cognitive impairment” OR “poor cognitive function”. The medical subject headings (MeSH) and free text were used in search bar. The keyword based search strategy led to some uniform resource locators (URLs) as shown in figure 1.
Figure 1.
Search strategy applied in PubMed database search.
The full texts of articles that met the stated criteria was obtained and were individually assessed by the next two authors (A3, A4) to confirm the inclusion of articles based on the established criteria. The final settlements on inclusion or exclusion were made after discussion between these authors (A1–A4) and conflicts in this regard were resolved by fifth author (A5). The sixth author had acted as guarantor and monitored the quality and standards of overall process. The data was extracted by two authors (A1, A2) independently, overseen as and when needed by guarantor (A6). In the case of insufficient data/unpublished article, the corresponding authors were contacted for full texts via emails/message in social research site (Research gate).
The Newcastle-Ottawa scale was used for analyzing the quality of the studies included for review [18]. The score ranges from 0 to 9 points allotted to selected studies by two authors (A1, A2). The quality of the included studies was evaluated independently by next two authors (A3, A4). The conflicts with scores assigned for included studies were resolved by authors (A5/A6) available. The results are depicted as appraisals of the specific/adjusted log odds ratios (OR) by the inverse of their variance to derive a pooled OR with its 95% CI) using the software HEpiMA 2.1.3 [19].
Results
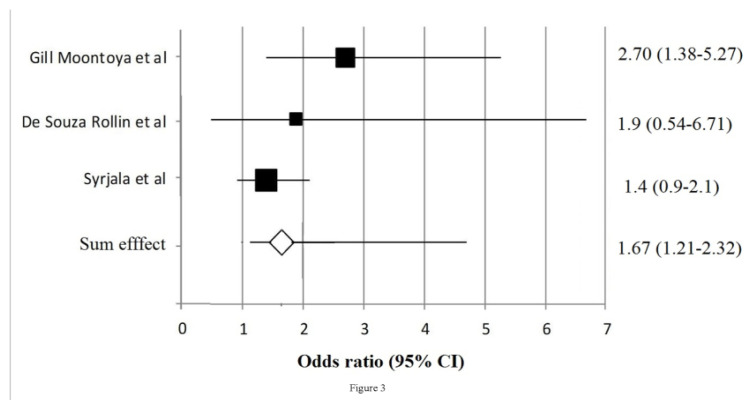
The stated strategy of search provided us with 612 citations of which 402 titles (with abstracts) were screened (in duplicate) by authors A1 and A2. The next stage of process involved exclusion of 360 titles based on the opinion of the authors and criteria set for the study, leaving 41 full-text articles, which were again subjected to screening individually (in duplicate) based on established criteria. Finally 6 titles were selected for systematic review (table II), 3 of which were considered for meta-analysis. The detailed flow of process is shown in figure 2. The summaries of the studies included for systemic review [20–25] are presented in table I. The meta-analysis had revealed a significant association between PD and AD with the pooled OR 1.67 (95% CI 1.21–2.32). See table III. The final studies considered [20,23,24], for meta-analysis and their sum effects OR (95% CI) is shown in figure 3.
Table II.
Quality assessment criteria for cohort study through Newcastle-Ottawa Scale for selected studies.
| Studies Study design | Criteria for scoring | Gil-Montoya et al. | de Souza Rolim et al. | Martande et al. | Syrjälä et al. | Ide et al. | Sparks Stein et al. |
|---|---|---|---|---|---|---|---|
| Selection just one star (*) given for each question | 1) Is the case definition adequate? a) yes, with independent validation* b) yes, record linkage or based on self-reports c) no description |
a* | a* | a* | a* | a* | a* |
| 2) Representativeness of the cases a) consecutive or obviously representative series of cases* b) potential for selection biases or not stated |
a* | a* | a* | a* | a* | a* | |
| 3) Selection of controls a) community controls* b) hospital controls no description |
b | a* | b | a | b | a | |
| 4) Definition of controls a) no history of disease (endpoint)* b) no description of source |
a* | a* | b | b | b | b | |
| Comparability: to 2 stars (*) given for each question | 1) Comparability of cases and controls on the basis of the design or analysis a) study controls for age* b) study controls for duration of hospitalization* |
a*b | a*b* | ||||
| Exposure: up to 1 star (*) given for each question |
1) Ascertainment of exposure a) secure record (e.g. surgical records)* b) structured interview blinded to case/control status c) interview not blinded to case/control status d) written self-report or medical record only e) no description |
a* | a* | a* | a* | a* | a* |
| 2) Same method of ascertainment for cases and controls a) yes* b) no |
a* | a* | a* | a* | a* | a* | |
| 3) Nonresponse rate a) same rate for both groups* b) nonrespondents described rate different and no designation |
a* | a* | b | c | c | c | |
| Score | 8 | 7 | 5 | 7 | 6 | 5 |
Figure 2.
The PRISMA methodological flow diagram.
Table I.
Characteristics of included studies.
| Author (year) | Study design | AD Sample size | PD diagnosis | AD diagnosis | Essential findings | Risk factors adjusted |
|---|---|---|---|---|---|---|
| Syrjälä et al (2012) [20] | Cross-sectional | n = 49 (AD) ; 274 (non-AD or controls) | PD Criteria I (degree of PD : PPD ≥4 mm) | AD criteria I (DSM-IV ) | PD was noted in AD patients, but not significant when taking PPD of 4mm+ as criteria [ RR (95% CI): 1.4 (0.9–2.1)] | Age, Sex, education, number of teeth present smoking status, dementia severity, type of dwelling |
| Sparks Stein et al (2012) [21] | Longitudinal study | AD/MCI cases=81; controls=77 | PD Criteria I, (AAP with PPD >4 mm, CAL >3) | AD criterion III (BRAINS participants, MMSE) | An association between antibody levels [F. nucleatum and P. intermedia, were significantly increased (α = 0.05)] with an onset and progression of AD | Age, sex, education, occupation/profession, smoking history, baseline MMSE scores, Apo-E4, and Diabetes. |
| Martande et al (2014) [22] | Cohort study | AD Cases=60 n=13 mild AD); n=18 (moderate AD); n=18 (severe AD) |
PD Criteria II | AD criteria I (NINCDS-ADRDA ) II and III (MMSE) | There were significant differences in mean periodontal parameters (GI, PI, PD, CAL, and %BOP) between all the groups and that periodontal parameters were higher in individuals as AD progressed to severe stage. | Age, sex, number of teeth, MMSE scores and oral hygiene status. |
| Gil-Montoya et al (2015) [23] | Case-control study | n = 21( MCI); n = 159 (dementia) with 70% of these diagnosed with AD | PD Criteria I (Degree of PD: % of sites with CAL >3 mm) | AD criteria I (NINCDS-ADRDA) | Significant correlation between PD and AD parameters with Overall PD: 2.70 (1.38–5.27) (p < 0.05) and 2.89 (1.46–5.73) (p < 0.05) for severe PD. | Age, sex, number of teeth, oral hygiene habits and hyperlipidaemia |
| de Souza Rolim et al (2014) [24] | Case-control | mild AD (n = 29); n = 30 (controls) | PD Criteria I (AAP ; degree of PD : CAL >3 mm) | AD criterion I, III (NINCDS-ADRDA with MMSE for disease severity) | Significant correlation between PD and AD parameters with an overall OR (95% CI): for PD of 1.90 (0.54–6.71) (p > 0.05) and 3.57 (0.65–19.59) (p > 0.05) for severe PD | Age, gender, marital status, race, occupation and systemic disorders (such as atrial hypertension, Diabetes mellitus etc) |
| Ide et al (2016) [25] | Cohort study | Patients with dementia (n= 60) | PD Criteria I (CDP/ AAP) | AD criterion I, III (NINCDS-ADRDA with MMSE and ADS-cog for disease severity) | A direct relationship between periodontitis and cognitive decline with an MMSE change of −1.8 (−3.6 to −0.03), p = 0.04; −1.8 (−3.6 to 0.04), p = 0.06 Also, an ADAS-COG change of 5.2 (1.7 to 8.8), p = 0.005; 4.9 (1.2 to 8.6), p = 0.01 was additionally reported |
Age, gender, number of teeth, P gingivalis antibody units, base line ADOS-cog / MMSE scores. |
%BOP, % sites with with bleeding on probing; ADOS-cog, Alzheimer’s Disease Assessment Scale–Cognitive Subscale; Apo-E4, apolipoprotein epsilon 4; MCI, mild-cognitive impairment; BRAINS, Biologically Resilient Adults in Neurological Studies; MMSE, Mini-Mental State Exam; RR, relative risk; CI, 95% confidence interval; CAL, clinical attachment loss; PPD, probing pocket depth; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; GI, gingival index, PI, plaque index; CDC/AAP, Centre for Disease Control/American Academy of Periodontology.
Table III.
Pooled RR and 95% CIs of periodontitis and Alzheimer’s disease.
| Number of Studies taken for meta-analysis | RR (95% CI) fixed effects | RR (95% CI) random effects | Ri* | Q test p value |
|---|---|---|---|---|
| 03 | 1.67 (1.21–2.32) | 1.78 (1.12–2.74) | 0.34 | 0.30 |
RR, relative risks; PD, periodontal disease; CI, 95% confidence interval; Ri* Proportion of total variance due to between-study variance.
Figure 3.
Forest plot showing the sum effects of studies included.
Discussion
The periodontal disease (PD) ranks 11th amongst the most prevalent condition in the world [1]. As stated previously, the world-wide prevalence of PD is likely to upsurge in the near future, thus necessitating further understanding of its associated outcomes on systemic health [4]. Alzheimer’s disease (AD) is an advanced neurodegenerative disorder characterized by dementia and cognitive decline/impairment, often leading to severe morbidity and mortality in elderly [10,12]. The association of AD with periodontal disease has been carried out in the last decade, emphasizing the need for further evaluation [6–9]. A substantial connection between immunoglobulin G (IgG), for P. gingivalis and different types of dementia is reported in literature, but a positive correlation between PD and AD is not definitively established by sound studies [7,8].
A significant relationship between PD and AD was noted in the current study [OR 1.67,(1.21–2.32)]. The evidence comes from observational studies which have shown similar associations. A previous study had evaluated for severity of PD with AD, and reported a AD patients (prevalence= 20.7%) had severe PD as opposed to controls (prevalence= 6.7%) [24]. Likewise, the other studies which qualified by standards for the meta analysis showed that to PD was greater in subjects with diagnosed AD than those who had no AD/controls [22,23], however this was not in case of study by Syrjala et al. (2012). They showed no significant association considering PPD ≥4 mm as the criteria (RR 1.4, 95% CI 0.9–2.1), after adjusting for known confounding factors namely age, sex, history of smoking or related habits, grade of dementia, and existing number of remaining teeth [20].
The review of literature puts forward inflammation as key connector for of AD with PD. The role of inflammatory mediators and response of host immune mechanism in these two diseases had provided basis of PD–AD associations. The periodontal microbes and altered host defense leads to secretion of inflammatory mediators [like interleukin -1β/6, C-reactive protein, or tumor necrosis factor-alpha] as noted in AD [26,27]. This can be furthered by the concept that cytokines and mediators of inflammation producing local destruction in PD are distributed systemically, affect the primed glial cells and contribute to the progression of AD [28]. The concept may also be furthered in a different directional curve, that the amyloid metabolism might be influenced by these inflammatory mediators. The amyloid dispositions and neuro-tangles are features of AD patients [10,11].
As with many other systemic inflammatory disorders like atherosclerotic cardiovascular disease [29], diabetes mellitus [30], or chronic inflammatory lung disease [31], a ‘bi-directional pathogenesis’ can be also be explained in case of AD-PD association. There is evidence that reported an association of cerebral vessel inflammation or ischemic stroke and lacunar infarcts (as in vascular dementia which is often seen overlapping in AD) endorsing on this bidirectional relationship [32,33]. From more practical point of view, the AD patients have difficulty in brushing or maintaining oral hygiene due to reduced manual dexterity in early – moderate stages. The visiting for dental care is often not a choice in advanced AD as patient may need caretaker for basic functions or for those under palliative care. Many opportunistic and non-opportunistic (infections inclusive of P gingivalis or other periodontal pathogens) appear easily in this AD patients and PD associated tooth loos is no surprise as reported in studies [34]. The patients with dementia (as in AD) may need home based oral and regular care. The caregiver-reported dementia is a reported risk factor for poor hygiene and prompts a need for dental management in advancing AD patients [35]. On the other hand institutionalized patients (may be done for dementia patients where home care is not possible) with advanced age need planned funds for oral or any healthcare in general [36]. In a scenario of developing nations, early detection, control of early PD and recognition of severity of PD with concurrent evaluation for AD symptoms may be ideally practiced.
The results of the current study are in line with other secondary research conducted. A recent meta-analysis by Qui C et al showed that patients with periodontitis had a higher risk of AD (with a RR=1.22, 95%CI: 1.13–1.33, p<0.00001). They reported that no significant difference in the risk of AD in patients with moderate periodontitis (RR=1.19, 95% CI: 0.98–1.44, p=0.07) but a higher risk in severe PD [37]. This finding is endorsed by anther meta-analysis which also showed that severe forms of PD had a significant association (OR 2.98, 95% CI 1.58–5.62) with AD [38]. Also, as per these meta-analyses, the clinical periodontal parameters (PPD, CAL, plaque index and bleeding on probing) and number of teeth were significantly different in AD as opposed to controls [37] or those with dementia and without dementia [39]. Another meta-analysis by Nadim R et al associating dementia with PD, showed that pooled RR of dementia in relation to PD from all high quality studies was 1.38 (95% CI 1.01–1.90), agreeing with the current study. They had estimated a 50% reduction in the current prevalence of 20% of PD could save 850,000 patients with dementia in the world [39].
The number of studies selected for final consideration varied in existing meta-analyses [37–39], but a positive association was almost reported from observational studies. Also, they had pointed to a need for more epidemiological studies with more participants or implementing exact definitions both for terminology with adjustments for confounders. The scope of studying this topic is further warranted by recent interesting findings reported form basic murine models to human studies with changes in young or early PD [40–44]. The association of specific molecules between PD and AD [40], a direct possible role of P gingivalis [41] crossing blood-brain barrier [42], and leading to induced AD like condition in diabetic mice [43], are recently documented. The cognitive decline in young people with PD [44] and dementia/AD signs in earliest stage of PD (gingivitis) [45] are also being studied.
The strength of this study is considering recent studies (of the last decade), while the important limitation of this study is the small number of studies included for meta-analysis. This may be addressed by more studies with acceptable research standards. The establishment of the PD-AD association can be further endorsed by randomized control trials (RCTs) evaluating the effectiveness of various periodontal treatments in AD patients with standard dementia / cognitive impairment outcome assessments. Such prospective clinical trials can only aid in proposing treatment directives for AD patients with progressive PD and are future implications of this study. However, since the association is significant, routine dental hygiene practices, professional periodontal treatments and patient awareness /education are needed at this point. The assisted oral care provision (home or care giver based) may be indicated in the early stages of AD, when professional help cannot be given at the dental office.
Conclusion
An affirmative association between periodontal disease and Alzheimer’s disease was obtained from evidence of standard observational studies conducted in last decade. Individuals with periodontitis were more likely to develop dementia/cognitive loss pertaining to Alzheimer’s disease than those without periodontitis. The evidence from more experimental clinical trials adjusting possible confounders shall throw further light on this association and propose periodontal therapeutic needs for patients with Alzheimer’s disease.
References
- 1.Nazir M, Al-Ansari A, Al-Khalifa K, Alhareky M, Gaffar B, Almas K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. ScientificWorldJournal. 2020;2020:2146160. doi: 10.1155/2020/2146160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin LJ, Lamster IB, Greenspan JS, Pitts NB, Scully C, Warnakulasuriya S. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis. 2016;22:609–619. doi: 10.1111/odi.12428. [DOI] [PubMed] [Google Scholar]
- 3.Tonetti MS, Bottenberg P, Conrads G, Eickholz P, Heasman P, Huysmans MC, et al. Dental caries and periodontal diseases in the ageing population: call to action to protect and enhance oral health and well-being as an essential component of healthy ageing - Consensus report of group 4 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44(Suppl 18):S135–S144. doi: 10.1111/jcpe.12681. [DOI] [PubMed] [Google Scholar]
- 4.Listl S, Galloway J, Mossey PA, Marcenes W. Global Economic Impact of Dental Diseases. J Dent Res. 2015;94:1355–1361. doi: 10.1177/0022034515602879. [DOI] [PubMed] [Google Scholar]
- 5.Nagpal R, Yamashiro Y, Izumi Y. The Two-Way Association of Periodontal Infection with Systemic Disorders: An Overview. Mediators Inflamm. 2015;2015:793898. doi: 10.1155/2015/793898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orr ME, Reveles KR, Yeh CK, Young EH, Han X. Can oral health and oral-derived biospecimens predict progression of dementia? Oral Dis. 2020;26:249–258. doi: 10.1111/odi.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dioguardi M, Crincoli V, Laino L, Alovisi M, Sovereto D, Mastrangelo F, et al. The Role of Periodontitis and Periodontal Bacteria in the Onset and Progression of Alzheimer’s Disease: A Systematic Review. J Clin Med. 2020;9:495. doi: 10.3390/jcm9020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart R, Sabbah W, Tsakos G, D’Aiuto F, Watt RG. Oral health and cognitive function in the Third National Health and Nutrition Examination Survey (NHANES III) Psychosom Med. 2008;70:936–941. doi: 10.1097/PSY.0b013e3181870aec. [DOI] [PubMed] [Google Scholar]
- 9.Chen CK, Wu YT, Chang YC. Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study. Alzheimers Res Ther. 2017;9:56. doi: 10.1186/s13195-017-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11:111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020;16:391–460. doi: 10.1002/alz.12068. [DOI] [PubMed] [Google Scholar]
- 12.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) Fifth Edition. Arlington, USA: American Psychiatric Association; 2013. [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res”. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 19.Costa-Bouzas J, Takkouche B, Cadarso-Suárez C, Spiegelman D. HEpiMA: software for the identification of heterogeneity in meta-analysis. Comput Methods Programs Biomed. 2001;64:101–107. doi: 10.1016/s0169-2607(00)00087-0. [DOI] [PubMed] [Google Scholar]
- 20.Syrjälä AM, Ylöstalo P, Ruoppi P, Komulainen K, Hartikainen S, Sulkava R, et al. Dementia and oral health among subjects aged 75 years or older. Gerodontology. 2012;29:36–42. doi: 10.1111/j.1741-2358.2010.00396.x. [DOI] [PubMed] [Google Scholar]
- 21.Sparks Stein P, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement. 2012;8:196–203. doi: 10.1016/j.jalz.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martande SS, Pradeep AR, Singh SP, Kumari M, Suke DK, Raju AP, et al. Periodontal health condition in patients with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2014;29:498–502. doi: 10.1177/1533317514549650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gil-Montoya JA, Sanchez-Lara I, Carnero-Pardo C, Fornieles F, Montes J, Vilchez R, et al. Is periodontitis a risk factor for cognitive impairment and dementia? A case-control study. J Periodontol. 2015;86:244–253. doi: 10.1902/jop.2014.140340. [DOI] [PubMed] [Google Scholar]
- 24.de Souza Rolim T, Fabri GM, Nitrini R, Anghinah R, Teixeira MJ, de Siqueira JT, et al. Oral infections and orofacial pain in Alzheimer’s disease: a case-control study. J Alzheimers Dis. 2014;38:823–829. doi: 10.3233/JAD-131283. [DOI] [PubMed] [Google Scholar]
- 25.Ide M, Harris M, Stevens A, Sussams R, Hopkins V, Culliford D, et al. Periodontitis and Cognitive Decline in Alzheimer’s Disease. PLoS One. 2016;11:e0151081. doi: 10.1371/journal.pone.0151081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan Y, Huo YB, Yu X, Zeng H, Leung WK, Watt RM. Complete Genome Sequence of Human Oral Phylogroup 1 Treponema sp. Strain OMZ 804 (ATCC 700766), Originally Isolated from Periodontitis Dental Plaque. Microbiol Resour Announc. 2020;9:e00532–20. doi: 10.1128/MRA.00532-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Visser MB, Ellen RP. New insights into the emerging role of oral spirochaetes in periodontal disease. Clin Microbiol Infect. 2011;17:502–512. doi: 10.1111/j.1469-0691.2011.03460.x. [DOI] [PubMed] [Google Scholar]
- 28.Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun study. J Am Dent Assoc. 2007;138:1314–1322. doi: 10.14219/jada.archive.2007.0046. quiz 1381–1382. [DOI] [PubMed] [Google Scholar]
- 29.Winning L, Patterson CC, Linden K, Evans A, Yarnel J, McKeown PP, et al. Periodontitis and risk of prevalent and incident coronary heart disease events. J Clin Periodontol. 2020;47:1446–1456. doi: 10.1111/jcpe.13377. [DOI] [PubMed] [Google Scholar]
- 30.Jain A, Chawla M, Kumar A, Chawla R, Grover V, Ghosh S, et al. Management of periodontal disease in patients with diabetes-good clinical practice guidelines: A joint statement by Indian Society of Periodontology and Research Society for the Study of Diabetes in India. J Indian Soc Periodontol. 2020;24:498–524. doi: 10.4103/jisp.jisp_688_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sapey E, Yonel Z, Edgar R, Parmar S, Hobbins S, Newby P, et al. The clinical and inflammatory relationships between periodontitis and chronic obstructive pulmonary disease. J Clin Periodontol. 2020;47:1040–1052. doi: 10.1111/jcpe.13334. [DOI] [PubMed] [Google Scholar]
- 32.Leira Y, Seoane J, Blanco M, Rodriguez-Yáñez M, Takkouche B, Blanco J, et al. Association between periodontitis and ischemic stroke: a systematic review and meta-analysis. Eur J Epidemiol. 2017;32:43–53. doi: 10.1007/s10654-016-0170-6. [DOI] [PubMed] [Google Scholar]
- 33.Leira Y, Carballo Á, Orlandi M, Aldrey JM, Pías-Peleteiro JM, Moreno F, et al. Periodontitis and systemic markers of neurodegeneration: A case–control study. J Clin Periodontol. 2020;47:561–571. doi: 10.1111/jcpe.13267. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Jornet P, Zamora Lavella C, Pons-Fuster Lopez E, Tvarijonaviciute A. Oral Health Status in Older People with Dementia: A Case-Control Study. J Clin Med. 2021;10:477. doi: 10.3390/jcm10030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi S, Horigome Y, Endo K, Komagata M, Komai S, Komaki K, et al . Caregiver-reported dementia as a predictor of oral health among patients receiving home-visit dental treatment: A retrospective cohort study. Clin Exp Dent Res. 2021;7:49–55. doi: 10.1002/cre2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werbrouck A, Schmidt M, Annemans L, Duyck J, Janssens B, Simoens S, et al. Oral healthcare delivery in institutionalised older people: A health-economic evaluation. Gerodontology. 2021 Jan 25; doi: 10.1111/ger.12530. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Leira Y, Domínguez C, Seoane J, Seoane-Romero J, Pías-Peleteiro JM, Takkouche B, et al. Is Periodontal Disease Associated with Alzheimer’s Disease? A Systematic Review with Meta-Analysis. Neuroepidemiology. 2017;48:21–31. doi: 10.1159/000458411. [DOI] [PubMed] [Google Scholar]
- 38.Maldonado A, Laugisch O, Bürgin W, Sculean A, Eick S. Clinical periodontal variables in patients with and without dementia-a systematic review and meta-analysis. Clin Oral Investig. 2018;22:2463–2474. doi: 10.1007/s00784-018-2523-x. [DOI] [PubMed] [Google Scholar]
- 39.Qiu C, Zhou W, Shi WT, Song ZC. [Association between periodontitis and Alzheimer disease: a meta analysis]. Shanghai Kou Qiang Yi Xue. 2020;29:661–668. [PubMed] [Google Scholar]
- 40.Jin J, Guang M, Ogbuehi AC, Li S, Zhang K, Ma Y, et al. Shared Molecular Mechanisms between Alzheimer’s Disease and Periodontitis Revealed by Transcriptomic Analysis. Biomed Res Int. 2021;2021:6633563. doi: 10.1155/2021/6633563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costa MJF, de Araújo IDT, da Rocha Alves L, da Silva RL, Dos Santos Calderon P, Borges BCD, et al. Relationship of Porphyromonas gingivalis and Alzheimer’s disease: a systematic review of pre-clinical studies. Clin Oral Investig. 2021;25:797–806. doi: 10.1007/s00784-020-03764-w. [DOI] [PubMed] [Google Scholar]
- 42.Olsen I. Possible effects of Porphyromonas gingivalis on the blood-brain barrier in Alzheimer’s disease. Expert Review of Anti-infective Therapy. 2021;19:1367–1371. doi: 10.1080/14787210.2021.1925540. [DOI] [PubMed] [Google Scholar]
- 43.Bahar B, Kanagasingam S, Tambuwala MM, Aljabali AAA, Dillon SA, Doaei S, et al. Porphyromonas gingivalis (W83) infection induces Alzheimer’s disease like pathophysiology in obese and diabetic mice. J Alzheimers Dis. 2021;82:1259–1275. doi: 10.3233/JAD-210465. [DOI] [PubMed] [Google Scholar]
- 44.Hategan SI, Kamer SA, Craig RG, Sinescu C, de Leon MJ, Jianu DC, et al. Cognitive dysfunction in young subjects with periodontal disease. Neurological Sciences. 2021:1–9. doi: 10.1007/s10072-021-05115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsen I. Can Porphyromonas gingivalis Contribute to Alzheimer’s Disease Already at the Stage of Gingivitis? J Alzheimers Dis Rep. 2021;5:237–241. doi: 10.3233/ADR-210006. [DOI] [PMC free article] [PubMed] [Google Scholar]