Abstract
Background and aim
Photodynamic therapy, PDT, is a promising option among the local treatments with oncolytic potential. Although the basic principle is simple, its intricate mechanisms allow for a broad range of optimization methods. The purpose of this study was to assess the effects of Resveratrol and Curcumin as adjuvants of PDT on experimental tumors.
Methods
Sixty-six Wistar male rats were divided into 11 groups: control, Curcumin (CUR), Resveratrol (RES) alone or followed by irradiation (IR) (CUR+IR and RES+IR, respectively), 5,10,15,20-tetra-sulphonato-phenyl-porphyrin (TSPP), TSPP+IR (PDT), and CUR or RES administered prior to or after PDT (CUR+TSPP+IR, RES+TSPP+IR, TSPP+IR+CUR, TSPP+IR+RES).
Results
Both CUR and RES significantly decreased lipid peroxidation, while RES also showed an increase in glutathione (GSH) levels, especially when it was administered before PDT (p<0.01). Both antioxidants decreased cyclooxygenase (COX)2 expression, to a minimum when they administered prior to PDT (p<0.001 and p<0.01) while nitric oxide synthase (NOS)2 expression diminished in the combined regimen, particularly in RES associated with PDT. CUR and RES induced similar changes in terms of cell death, but CUR seemed to be more efficient on tumor necrosis and showed a higher apoptotic index when was administered after PDT (p<0.001).
Conclusion
Both RES and CUR in association with PDT decreased oxidative stress, diminished the COX2 and NOS2 expressions and increased cell death by positively influencing the necrotic rate and apoptotic index, particularly when CUR was administered after PDT. The results show that CUR is a promising class to study in PDT optimization and further invites to exploit its promises.
Keywords: photodynamic therapy, ROS, cancer, Curcumin, Resveratrol
Background and aims
Photodynamic therapy is a known oncolytic treatment in which the administration of a photosensitizing agent causes a sequence of photochemical and photobiological processes, which result in selective damage to the target tissue [1–3]. Although it was initially used for benign diseases, PDT has lately been added to the resources mounted to fight the burden of cancer [4] showing promise in this struggle mainly due to its advantages in terms of diminished side-effects over other therapies. Specificity, healing with little or no scarring, and non-invasiveness further add to this [5].
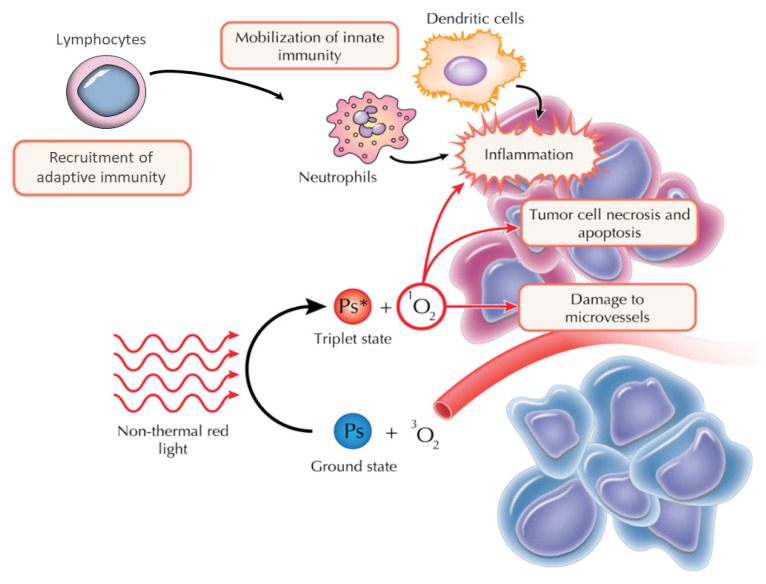
PDT relies on three components: a locally-applied/systemically-administered inactive substance (photosensitizer, PS), its activation by light of appropriate wavelength and oxygen. The specific accumulation of the photosensitizer in pathologic tissues and the activation of its molecules generate intense oxidative and nitrosative stress in the tumor tissue. The reactive oxygen species that are consequently formed (2) exert their action by direct cytotoxic effects, innate and adaptive immunity recruitment and activation, and by tumor vessels damage [6,7] (Figure 1). Subsequently, cells die by apoptosis, necrosis and autophagy.
Figure 1.
Principles of photodynamic therapy (PDT). Upon injecting the inactive photosensitizing agent and its accumulation in the tumor, light of an appropriate wavelength is used to activate it (here, red light). Therefore, a change in state ensues and a triplet state is reached, with a greater energy, but shorter lifespan. The subsequent photochemical and photobiological reactions exert their effects by: (1) innate immunity recruitment and generation of a systemic inflammatory response with adaptive immunity activation, (2) direct cytotoxic effects, and (3) tumor vessels damage. Their synergistic effect results in cell death, either apoptosis, necrosis or autophagy (original illustration reproduced by courtesy of Tiberiu Popescu adapted from Castano, 2006).
Despite promising results, lack of phase three trials cannot make PDT an independent competitor to surgery, chemo- or radiotherapy. For this reason, numerous attempts have been made to improve on the therapy by finding new photosensitizers, combining them, discovering new ways of enhancing their accumulation in the tissue, altering administration by metronomic PDT, cytoprotective blockade, combining PDT with other therapies or adding new drugs/substances as adjuvants to the therapy [8,9].
Such an adjuvant could be Curcumin, a natural compound extracted from the Turmeric plant (Curcuma longa), which has been described as having antimicrobial, antiinflammatory and antitumor characteristics [10–12]. It enhances PDT due to its internal molecular resonance stability, which confers radical chain inducing potential [13]. Curcumin can also scavenge nitric oxide directly and inhibit its biosynthesis [14,15]. Some studies on Curcumin therapy in combination with PDT, performed with UVA or blue light, underlined its antitumoral in vitro activity on tumor cells. Another study presented the effects of Curcumin with phototherapy (visible light) in a murine model of a human subcutaneous tumor [16]. Among the most important actions of Curcumin though seem to be the inhibition of induction pathways of fibroblast growth factor (FGF)-1 [17].
Resveratrol (3,5,40-trihydroxy-trans-stilbene) is another natural compound, a stilbenoid found in blueberries, raspberries, red wine, grapes, peanuts, pomegranates, or soy beans. It is known to be a biologically active substance against diabetes, inflammation, obesity [18], but also a potent growth inhibitor in many types of cancer [19] including thyroid [20], lung [21], breast [22] and gastric cancer [23]. While it can protect normal cells from ROS-induced cytotoxicity due to its antioxidant property, Resveratrol can also help in differentiating cancer cells and hindering their growth [24].
Based on these data, our study investigated an alternative method of PDT optimization by using Resveratrol and Curcumin, individually, both before and after PDT. The effects of the combined therapeutic regimens were evaluated by assessment of oxidative and nitrosative stress parameters (malondialdehyde - MDA, glutathione reduced - GSH, glutathione oxidized - GSSG, NOS2), level of inflammation (COX2 expression) and cell death (histopathological and immunohistochemical quantification of apoptosis).
Methods
Reagents
O-phthalaldehyde, reduced glutathione, Bradford reagent, Curcumin and Resveratrol were provided by Sigma-Aldrich Chemicals GmbH (Munich, Germany), while EDTA-Na2 and 2-thiobarbituric acid were purchased from Merck KGaA (Darmstadt, Germany). Absolute ethanol, hydrogen peroxide and n-butanol were obtained from Chimopar (Bucharest, Romania) and TUNEL assay kit was supplied by Roche (Mannheim, Germany). Polyclonal anti-NOS2 antibody (1:200) and anti-COX2 antibody (1:300) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and LSAB-HRP kit from Dako, North America (Inc. CA, USA, K0679). 5,10,15,20-tetra-sulphonato-phenyl-porphyrin (TSPP) was received as a donation from Mrs. Rodica-Mariana Ion, from National Institute of R&D for Chemistry and Petrochemistry – ICECHIM, Bucharest, Romania [25].
Experimental design
Sixty-six Wistar albino rats (190±10 g) provided by the Animal Facility of the Experimental Medicine and Practical Skills Center, Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca, were used in the experiments. The animals were housed for ten days in the Animal Facility of our Department for acclimatization before treatment. Food was supplied twice daily (standard pellet) and water was provided as libitum. Prior to starting any animal experiment, the Ethical Comittee on Animal Welfare of Iuliu Haţieganu University of Medicine and Pharmacy, approved the study protocol as of decree no 143/30.03.2017, in accordance with the Romanian legislation enforced by European Union (EU) directive 2010/63/EU, and with the National Veterinary and Food Safety Authority.
Each rat was inoculated subcutaneously (s.c.) in the right thigh with Walker 256 carcinosarcoma fragments, by means of a trocar and under anesthesia. Walker carcinosarcoma is a transplantable carcinoma that originally appeared spontaneously in the mammary gland of the pregnant rat. After multiple transplantation the tumor become sarcomatous respectively a mixture of carcinoma and sarcoma. The tumor cells transplanted can induce primary tumors, both subcutaneously (solid) and intraperitoneally (ascitic) and may metastasize after intravenous injection.
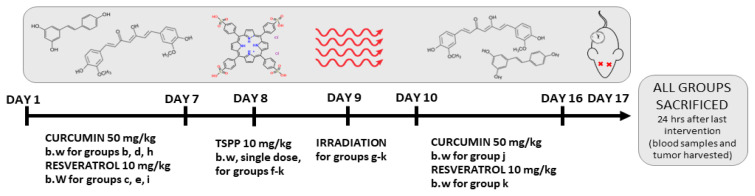
Treatment started when the tumor volume reached 1 cm3. The animals were randomly assigned into eleven groups (n=6) as follows (Figure 2):
Figure 2.
Experimental design.
group a (control) – untreated group;
group b – Curcumin (CUR) 50 mg/kg body weight (b.w.), dissolved in 0.5 ml carboxymethyl cellulose (CMC) 0.5% administered through oral gavage for 7 days;
group c – Resveratrol (RES) 10 mg/kg b.w. in 0.5 ml CMC 0.5% through oral gavage for 7 days;
group d – Curcumin + Irradiation (CUR+IR);
group e – Resveratrol + Irradiation (RES+IR);
group f – TSPP (10 mg/kg b.w., single dose, oral gavage);
group g – TSPP + Irradiation (TSPP+IR), or the actual photodynamic therapy (PDT) regimen;
group h – Curcumin 50 mg/kg b.w. administered for 7 days before PDT (CUR+TSPP+IR);
group i – Resveratrol 10 mg/kg b.w. administered for 7 days before PDT (RES+TSPP+IR);
group j – Curcumin 50 mg/kg b.w. administered for 7 days after PDT (TSPP+IR+CUR);
group k – Resveratrol 10 mg/kg b.w. administered for 7 days after PDT (TSPP+IR+RES).
Irradiation (IR) was performed under anesthesia 24 hrs after CUR or RES or TSPP administration and was delivered by means of a D-58 Therapeutic Laser model (irradiation parameters: λ=685 nm, mean power: 25 W/cm2, dose: 50 J/cm2, frequency: 10 Hz, spot diameter: 10 mm, duration: 15 min). General anesthesia was acquired by means of a 10% ketamine and 2% xylasine combination. Blood samples and tumor tissue fragments were harvested 24 hrs after the last treatment for histopathology, immunohistochemistry (IHC) and biochemical analysis. All parameters quantified in the tumor tissue were mentioned with ”t” before the name and the parameters evaluated in serum samples were noted with ”s” before the name of parameter.
Isolation of tumor samples
In order to perform the biochemical analyses, the harvested tumors were stripped of the adjacent connective tissue and minced on ice with a Polytron homogenizer (Brinkman Kinematica, Switzerland) for 3 min, in PBS (pH 7.4), ratio 1:4 mass/volume [26]. For obtaining the cytosolic fraction, the subsequent suspension was centrifuged for 5 min at 3000 rot/min and 4°C. Protein measurement in the homogenates followed the Bradford protocol [27].
For pathology examination, part of the harvested tumors was fixed in 10% neutral buffered formalin and kept for 18–24 hours at 4°C. Fixed tissue was subsequently dehydrated in growing concentrations of ethanol (70%, 95%, 100%), washed in xylene and paraffin-embedded. Serial 5 μm sections were obtained and processed for conventional hematoxylin–eosin staining, immunohistochemistry for COX2, NOS2 and the TUNEL assay.
Oxidative and nitrosative stress markers
In order to quantify the redox imbalance induced by PDT and the impact of CUR and RES on the PDT efficiency, the malondialdehyde (MDA) levels and glutathione reduced/glutathione oxidized ratio (GSH/GSSG) were assessed. MDA levels were determined using the fluorimetric method with 2-thiobarbituric acid [28] and the results were expressed as nmoles/mg protein [29]. Reduced and oxidized glutathione (GSH and GSSG, respectively) were determined using the fluorimetrical method based on o-phthalaldehyde reactive [30].
Histopathology and immunohistochemistry evaluation of COX2 and iNOS2
Five μm sections were incubated overnight at 4°C with primary polyclonal goat anti-NOS2 and anti-COX2 IgG antibodies (Santa Cruz, CA, USA) in concentrations of 1:200 and 1:300, respectively. The sections were then prepared with a LSAB® peroxidase kit (K0679)-HRP kit (Dako Agilent, CA, USA) and the reaction products were detected by a DAB reaction. IHC reactions were scored according to a system based on the extent and intensity of the positive stain as previously published [31,32] as follows: 0, negative reaction; 0.5–1+, low reaction; 2+, moderate reaction; 3+, intense reaction; 4+, very intense reaction. To check the specificity of the immunohistochemistry tests, tissues in which primary antibodies were omitted from the initial incubation were used as a negative control. All samples were examined by two morphopathologists blinded for treatment conditions by using Optika B-383LD2 microscope, with a CCD sensor camera. The percentage of TUNEL positive cells divided by the total number of all cells from each section were used to appreciate the apototic index (%). For the detection of necrosis, conventional hematoxylin-eosin staining was performed. The Weibel scale was used to dimension the microscopic field. Each field had a surface of 7.56 mm2. The percentage of necrotic cells within the total number of cells on 3 randomly chosen microscopic fields was calculated and the % of necrotic cells/mm2 at ×200 magnification was determined [33,34].
Statistical analysis
Descriptive statistical analysis was done for quantitative variables using means ± standard deviations and column charts. The necrotic and apoptotic indexes were presented in percentages ±standard deviations and column charts. One-way ANOVA was used to check statistically significant difference between group means, followed by multiple comparisons using a pairwise Tukey’s Post-hoc test since all groups’ size was the same. Kolmogorov-Smirnov and Shapiro-Wilk were used to test the normality of data using statistical software SPSS 17.0. The values were statistically processed using GraphPad Prism version 5.03 for Windows, Graph-Pad Software, (San Diego, CA, USA). The threshold significance level was p<0.05 (***p<0.001; **p<0.01; *p<0.05 compared to the control group; ##p<0.01 and ###p<0.001 compared to TSPP+IR group or PDT group)
Results
Oxidative and nitrosative stress markers
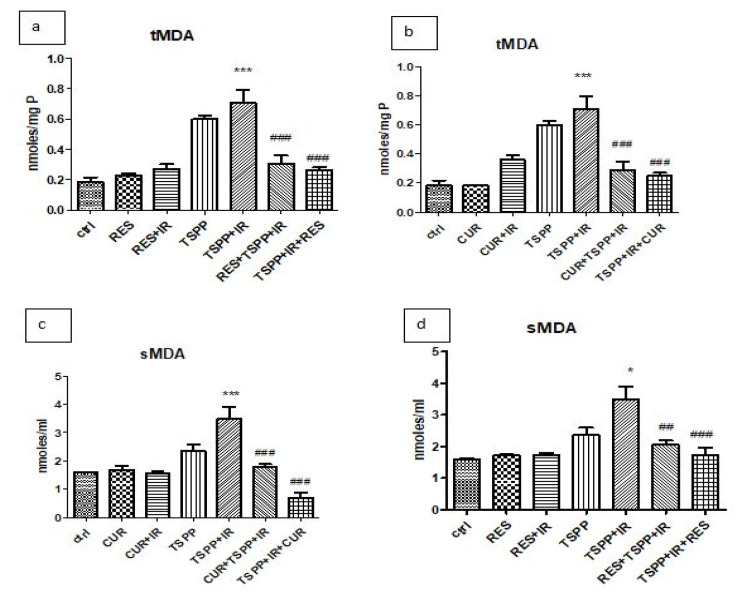
MDA levels express the action of reactive oxygen species on lipids (Figure 3). Lipid peroxidation increased in tumor tissue of the PDT group compared to the control, untreated group (tMDA: 0.70±0.19 nmoles/mg protein vs. 0.18±0.07 nmoles/mg protein; p<0.001) (Figure 3a). MDA levels were invariably lower in the groups subjected to combined therapy than PDT alone (TSPP+IR), irrespective of the sequence of CUR or RES administration. Indeed, the addition of RES prior to PDT significantly lowered the tumor MDA (tMDA) levels to 0.30±0.11 nmoles/mg protein (p<0.001), as did its administration afterwards (2.26±0.04 nmoles/mg protein; p<0.001) when compared to those in the PDT group (0.70±0.19 nmoles/mg protein) (Figure 3a). The same was observed for the serum MDA levels (sMDA) (Figure 3c). PDT induced lipid peroxides formation (3.49±0.92 nmoles/ml vs. 1.58±0.07 nmoles/ml in control group) while the combination regimens significantly lowered the values to 2.04±0.33 nmoles/ml (RES+PDT) and 1.72±0.55 nmoles/ml (PDT+RES), in comparison to the PDT alone (p<0.01 and p<0.001 respectively).
Figure 3.
MDA levels in tumor tissue and serum in groups treated with RES and CUR associated with PDT. PDT with TSPP increased MDA levels in tumor tissue both in regimen which associated RES (a) or CUR (b) to PDT. In addition, PDT enhanced MDA levels in serum in the same groups (c, d). RES administration prior to PDT or after PDT diminished lipid peroxidation in tumor tissue (p<0.001) and serum (p<0.01). The regimen which associated CUR before PDT reduced also MDA levels in tumor and serum (p<0.001). Data are depicted as mean ± SD. Statistical analysis was performed by a one-way ANOVA, with Tukey’s multiple comparisons posttest (*p<0.05, ***p<0.001 as compared to control group and ##p<0.01, ###p<0.001 as compared to PDT group).
A similar tendency was noted in the Curcumin regimens, where pre-PDT tMDA and sMDA decreased compared to the PDT group (0.28±0.12 nmoles/mg protein in tumor and 1.78±0.26 nmoles/ml in serum vs. 0.18±0.07 nmoles/mg protein in tumor and 1.58±0.07 moles/ml in serum of PDT group; p<0.001) (Figures 3b and Figure 3d). However, for the post-PDT tMDA (0.24±0.05 nmoles/mg protein) and sMDA (0.68±0.41 nmoles/ml) levels, a marked lower value was observed in the latter, when Curcumin was administered after PDT (p<0.001).
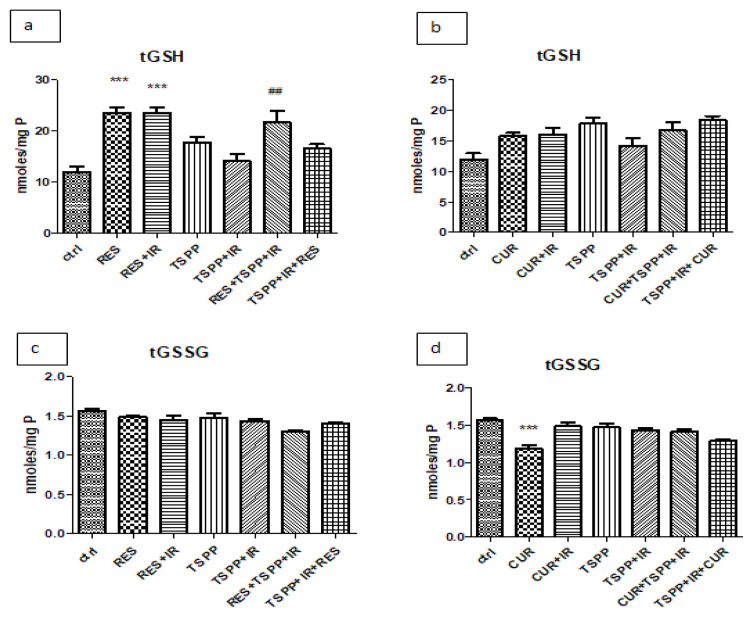
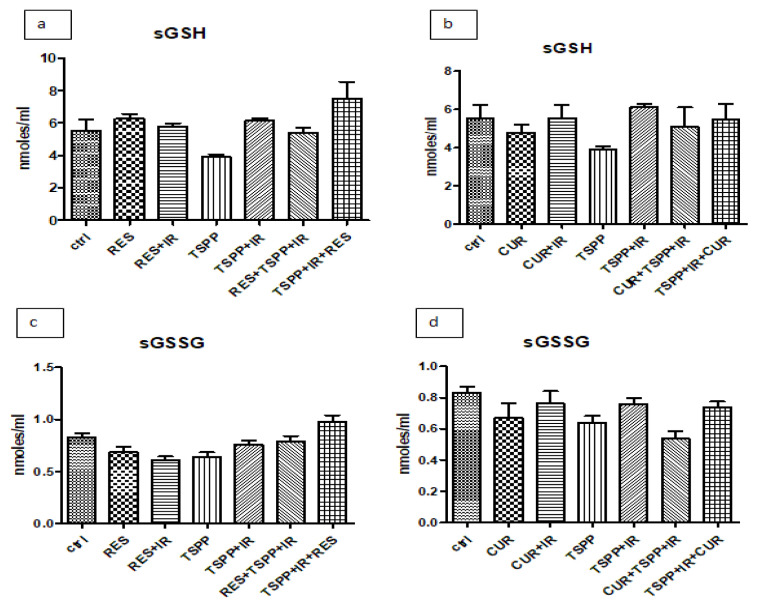
Reduced glutathione (GSH), one of the most important ROS scavengers, and its oxidized counterpart (GSSG), are non-enzymatic parameters used to evaluate antioxidant capacity and assess the redox status (GSH/GSSG ratio). As shown in Figure 4, RES and RES+IR increased the glutathione reduced levels in tumor tissue (23.50±2.41 nmoles/ml) when compared to the control, untreated group (12.01±2.11 nmoles/ml; p<0.001) (Figure 4a). The administration of RES before PDT increased the GSH levels in tumor homogenates (21.64±5.26 nmoles/ml) in comparison to animals subjected PDT (14.14±3.07 nmoles/ml; p<0.01).
Figure 4.
GSH and GSSG levels in tumor homogenates in groups treated with combined regimen. PDT with TSPP decreased GSH levels in tumor tissue (a) while RES, RES+IR and RES+PDT increased GSH values compared to control group (p<0.001 and #p<0.01). GSSG levels in both combined regimens and in PDT associated with CUR did not increase. Data are depicted as mean ± SD. Statistical analysis was performed by a one-way ANOVA, with Tukey’s multiple comparisons posttest ***p<0.001 as compared to control group and ##p<0.01 as compared to PDT group).
There were no significant differences in the groups receiving CUR when compared to the PDT group, irrespective of the sequence of administration (Figure 4b). The same stayed true for GSSG levels in tumor tissue (Figure 4c and 4d) and in serum (Figure 5c and 5d) in the RES or CUR treated groups (p>0.05).
Figure 5.
GSH and GSSG levels in serum in groups treated with combined regimen. PDT with TSPP did not change significantly the GSH and GSSG levels in serum of animals treated with combined regimen. Data are depicted as mean ± SD. Statistical analysis was performed by a one-way ANOVA, with Tukey’s multiple comparisons posttest.
Evaluation of COX2 and NOS2 by immunohistochemistry
COX2
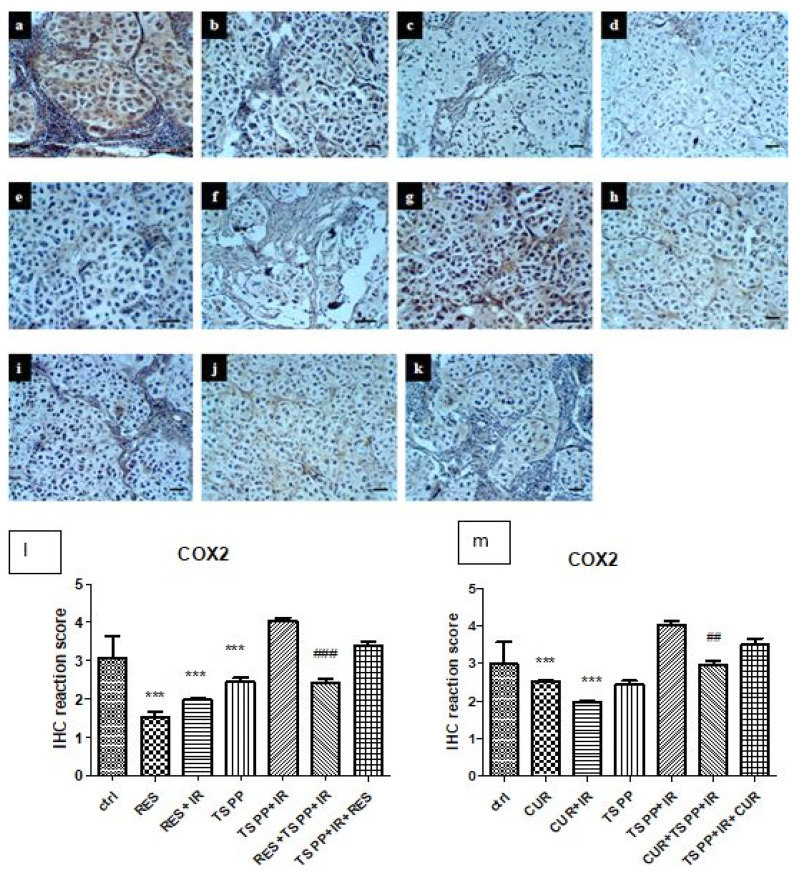
CUR and RES, both alone and in the irradiated groups (groups b–e), induced a significant decrease of COX2 expressions (p<0.001) (Figure 6 a–k and l–m). TSPP exposure followed by irradiation (group g) did not significantly change COX2 expression in experimental tumors. Nonetheless, the addition of CUR and RES to PDT significantly decrease COX2 compared to PDT group (groups h–k vs group g), especially when the antioxidant agent was administered before PDT (p<0.001).
Figure 6.
Immunohistochemistry for COX2 in control and experimental groups. a-Control, b-CUR, c-RES, d-CUR+IR, e-RES+IR, f-TSPP, g-TSPP+IR, h-CUR+TSPP+IR, i-RES+TSPP+IR, j-TSPP+IR+CUR, k-TSPP+IR+RES. ×200, scale bar = 20 μm. Data are depicted as mean ± SD. Statistical analysis was made with one-way ANOVA, using Tukey’s multiple comparisons posttest ***p<0.001 as compared to control group and ##p<0.01 and ##p<0.001 as compared to PDT group).
NOS2
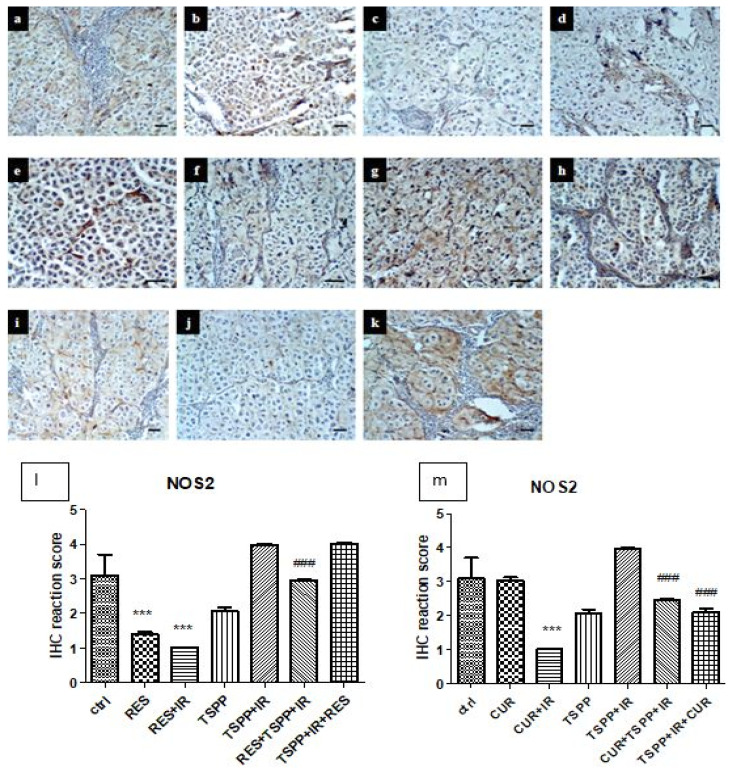
NOS2 expressions decreased significantly in the groups receiving RES, RES+IR and CUR+IR (groups c–e, Figure 7). A different expression pattern was observed after CUR administration and irradiation (group b). In this group, NOS2 decreased significantly compared to control group (p<0.001). The combined regimens (groups h–j) showed a significant decrease of NOS expression compared only to PDT (p<0.001). RES administered after PDT (group k), didn’t show significant changes in NOS2 immunoreactivity compared to PDT.
Figure 7.
Immunohistochemistry for NOS2 in control and experimental groups. a - Control, b-CUR, c-RES, d-CUR+IR, e-RES+IR, f-TSPP, g-TSPP+IR, h-CUR+TSPP+IR, i-RES+TSPP+IR, j-TSPP+IR+CUR, k-TSPP+IR+RES. ×200, scale bar = 20 μm. Data are depicted as mean ± SD. Statistical analysis was performed by a one-way ANOVA, with Tukey’s multiple comparisons posttest ***p<0.001 as compared to control group and ###p<0.001 as compared to PDT group).
Necrosis and apoptosis evaluation
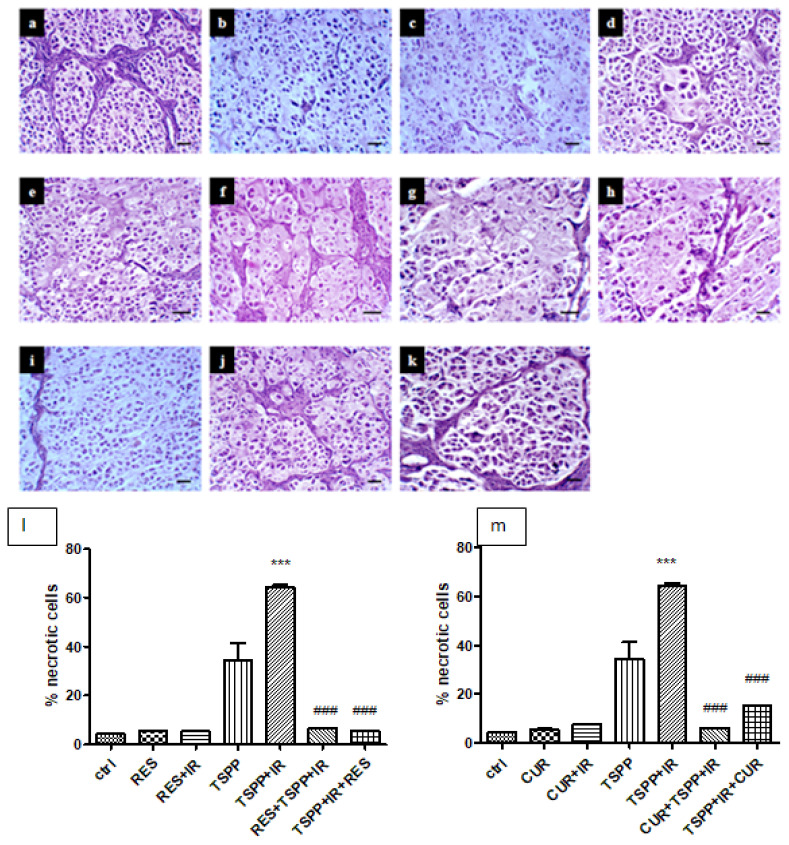
Histological evaluation revealed that CUR and RES (groups b and c) induced similar changes in tumor structure (Figure 8). Thus, karyolysis without inflammatory infiltrate, piknosys, spongious and hyperchromic nuclei and cytoplasm with vacuolar degeneration tendencies were noticed. CUR and irradiation (CUR+IR) (group d) induced focal karyorrhexis associated with hyperchromic nuclei whereas RES+IR (group e) induced slight changes such as hyperchromic nuclei. Pretreatment with CUR and RES before PDT (groups h and i), showed that the CUR had similar effect with RES on tumor necrosis.
Figure 8.
Histopathological examination of tumor sections for necrosis assessment. a-control, b-CUR c-RES, d-CUR+IR, e-RES+IR, f-TSPP, g-TSPP+IR, h-CUR+TSPP+IR, i-RES+TSPP+IR, j-TSPP+IR+CUR, k-TSPP+IR+RES, ×200, scale bar = 20 μm. Data are depicted as mean ± SD. Statistical analysis was made with one-way ANOVA, using Tukey’s multiple comparisons posttest ***p<0.001 as compared to control group and ###p<0.001 as compared to PDT group).
In the TSPP+IR+CUR group, histological exam revealed large areas of necrosis, diffuse cytoplasm, frequent karyolysis without inflammatory infiltrate whereas in the TSPP+IR+RES group, histological evaluation showed rare pyknotic nuclei, well condensed nucleoli and rare necrosis.
The same changes were observed when CUR or RES were administered before PDT (groups h and i). In tumors subjected to CUR+TSPP+IR, histological evaluation revealed vacuolated hyperchromic nuclei, clear cytoplasm, frequent necrosis and karyolysis, frequent cells in the post-mitotic phase marked by two nuclei in the same cell and nuclear dysplasia. The percentage of necrotic index calculated on 3 randomly chosen microscopic fields showed an important percentage in PDT group (64.27±1.75 %) compared to control group (4.23±0.12%, p<0.001). The administration of CUR after PDT increased the necrotic cells per mm2 (15.31±0.30 %) compared to control group (p<0.001) but the values were significantly lower than those obtained after PDT.
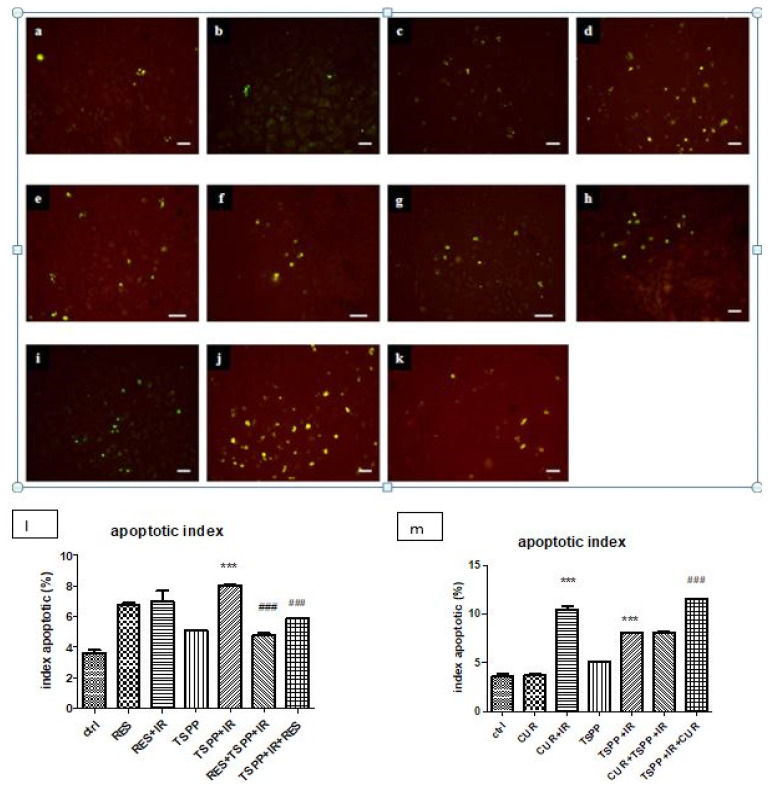
The TUNEL assay revealed a pro-apoptotic effect of both CUR and RES in association with irradiation (CUR+IR and RES+IR) (Figure 9, groups d and e) or in PDT (group g). CUR amplified the effect of PDT (apoptotic index >10% whereas in the control group the apoptotic index was 3.5 – 4±0.2% and in PDT group about 7.5 – 8±0.4%) similar with values obtained in CUR+IR (p<0.001).
Figure 9.
TUNEL assay and apoptotic index for programmed cell death assessment in control and experimental groups. a-control, b-CUR, c-RES, d-CUR+IR, e-RES+IR, f-TSPP, g-TSPP+IR, h-CUR+TSPP+IR, i-RES+TSPP+IR, j-TSPP+IR+CUR k-TSPP+IR+RES, ×200, scale bar = 20 μm. Data are depicted as mean ± SD. Statistical analysis was made with one-way ANOVA, using Tukey’s multiple comparisons posttest ***p<0.001 as compared to control group and ###p<0.001 as compared to PDT group).
Discussion
Photodynamic therapy made its way as a known weapon in the armamentarium against cancer, as did new conceptual approaches of tumors based on observations proposed by Hanahan and Weinberg in an update of their seminal paper [34]. They described new enabling and emerging hallmarks of cancer such as avoiding immune destruction, deregulating cellular energetics, and tumor-promoting inflammation. PDT can be synergistically used as a means of exploiting them in order to increase tumor response. It has been shown to downsize tumors, prevent growth of metastases and prolong survival in a mouse model subjected to PD-1/PD-L1 blockade [35], or help in establishing a DC - mediated immune response [36]. Some classes of drugs, such as nonsteroid anti-inflammatory drugs (NSAIDs) [37], have been shown to be a good match for photodynamic therapy, since COX2 expression offers tumor cells a growth advantage [38]. Clinical guidelines already indicate the use of this class of drugs for chemoprevention for colorectal cancer [39,40].
Lastly, deregulated cellular energetics, with shifted redox equilibrium, can modulate different cellular processes, including cell proliferation and survival [41]. In this context, the use of antioxidants, such as Curcumin [42] and Resveratrol [43] was proposed as a way of preventing cancer or be used as adjuvants to its treatment. PDT with TSPP increased lipid peroxides formation and induced apoptotic response of tumor cells as mechanisms of cell death. Both natural antioxidants diminished MDA generation, increased antioxidant capacity in tumor and reduced inflammation, especially when they were administered before PDT. The apoptotic index decreased in the combined regimen of PDT with RES, while CUR administered after PDT increased apoptosis, when compared to PDT.
Low levels of tumor and serum MDA, as a surrogate of ROS damage on membrane lipids in the groups subjected to the combined regimens (CUR and RES associated to PDT) might be interpreted in conjunction with an increase in antioxidant cell capacity, as shown by the GSH levels in the same groups. This was expected from two compounds well known for their purported antioxidant properties. In order to prevent oxidative damage, the GSSG reductase reduces GSSG to GSH. While in a normal cell there is an evident surplus of GSH at molar ratios greater than 100:1, different models of oxidative stress bring this ratio (i.e. GSH/GSSG) down to 1:1 [44]. Some studies tried to use this ratio as a surrogate for redox status and as a marker for oxidative stress in pediatric tumor patients [45].
On the other hand, added to PDT, irrespective of the sequence of administration, RES and CUR seem to have decreased tumor inflammation and down-regulated COX2 and NOS2 expressions. This was more obvious for NOS2 and for the groups treated with CUR, which raises the idea of CUR decreasing oxidative stress. Kumari et al. [46] brought further evidence in this regard. Thus, CUR decreased stress parameters (nitric oxide, MDA) and increased the antioxidant protection after intranasal administration for acute lung inflammation. Nitric oxide (NO), the end-product of NOS2 activity, seems to have a dual activity in tumors. On the one hand, it has an antitumoral effect due to its interaction with superoxide and peroxynitrite generation. On the other, NO-induced vasodilation sustains tumor nutrition, inhibits the expression of adhesion molecules and hinders the immune response [47].
A greater percentage of necrotic cells have been identified in the combined regimen groups when CUR was administered after PDT compared to control group. This, in conjunction with a downregulated NOS2, fairly supports the idea of the anti-tumor effect of NOS2 activity and NO generation, rather than its protective activity. This is further supported by a greater apoptotic index in the same regimen, compared to the effect of RES associated to PDT, administered before and after PDT. Moustapha et al described the existing crosstalk between autophagy and apoptosis induced by CUR [48]. Other studies stated the pro-apoptotic effect of CUR in cervical carcinoma cell lines by up-regulation of Bax and activation of caspases-3 and −9 [49]. In combination with light, CUR induced 95% phototoxicity of nasopharyngeal carcinoma cells [50] and diminished the cell viability to 5% when was used as photosensitizer in photodynamic therapy. The efficiency of PDT has increased due to higher internalization of Curcumin-nanoemulsion, both in cervical carcinoma cells and in HaCaT cells [51]. On pediatric epithelial liver tumor cells, PDT potentiated the antitumoral effect of Curcumin [17] and acted synergistically with it on Caco-2 cells and PC-3 lines [13]. In our study, CUR followed by irradiation induced a high rate of apoptosis in tumor tissue compared to PDT and administered after PDT maintained an elevated level of apoptosis.
Previous results obtained with combined regimen of PDT and Cornus mas fruits extract showed an increase of apoptotic and necrotic indices in tumor tissue, associated with a strong inflammatory reaction and DNA damage, particularly when the extract was administered before PDT [52]. Our results were in agreement with these and confirmed the potential antitumoral effect of Curcumin followed by irradiation or when was administered after PDT.
Data concerning the effect of combined regimen in PDT with antioxidants are rather contradictory and depend on the antioxidants used, dose and regimen of administration. In our study, a lower effect of RES on cell death can be explained in light of a new and spatial perspective on ROS [53]. It seems that the greater intrinsic tumor cell antioxidant capacity annihilates distant ROS damage, while at the same time it allows localized ROS signaling to promote cell proliferation and cell survival.
Another explanation for the results can be related to the small number of animals studied. Using small samples some of the statistical comparisons may not reach the threshold of statistical significance. However, the obtained results draw attention to these effects and motivate us to continue research with a larger study with greater power, in which to take into account all the factors that could be involved (heterogeneity of tumor, the variability of the amount of energy that reaches the cells, etc).
In this context, dietary antioxidants can increase the pool of antioxidants that will act distantly, whereas local tumorigenic ROS levels remain rather unperturbed. The same pattern was noticed when Silimarin was administered prior to PDT-it inhibited ROS generation and apoptosis and consequently reduced the efficacy of PDT on Walker carcinosarcoma in rats [54]. Unlike our results, other studies revealed that RES associated to ALA-PDT enhanced the effect of PDT on A431 cell proliferation and apoptosis. Moreover, the expressions of p-ERK, p-p53, p53 and caspase-3 increased after the combined therapy, suggesting an effect mediated by p38/MAPK pathway [55]. The extracts of Lumnitzera racemosa, Albizia procera and Cananga odorata photoactivated were cytotoxic against MCF-7 cells compared to the effects on non-tumor cells and induced apoptosis [56]. All these in vitro and in vivo results can be validated by confirmation in patient therapy. However, the translation to the clinic involves overcoming certain difficulties related to the low penetration of the light into the tissue. For this, a new class of up converting nanoparticles was designed, which can convert near-infrared light, more penetrable into the tissue, in visible red light in order to better activate the photosensitizers [57]. The side-effects of PDT in clinical practice, especially the damage on non-tumor cells, can be limited by using selective PDT drugs which are located mainly in the tumor cells or by using focused lasers as light source. PDT damage can be reduced by using two-photon excitation of photosensitizer [58] or by using metronomic PDT (mPDT), method in which the drug and light are delivered at very low dose over an extended period of time [59]. Probably when using natural extracts associated with PDT, an important step is the isolation of the active compounds from the extract and their purification in order to prepare specific drugs for PDT.
Conclusion
In conclusion, both RES and CUR reduced ROS generation and diminished the COX2 and NOS2 expressions, especially when the natural compounds were administered before PDT, thus increasing the antioxidant endogenous capacity. The combined regimen with CUR was associated with an improved necrotic rate and apoptotic index compared to control, particularly when CUR was administered after PDT. Conversely, RES enhanced the expression of NOS2, which might partially explain its inability to increase the apoptotic index when compared to the PDT or CUR-based PDT regimens. However, a new perspective on ROS might change the strategy of antioxidants use in PDT optimization by shedding a new light on their mechanisms and by emphasizing both their promises and perils.
Acknowledgements
We express our appreciation to the members of Oxidative Stress Laboratory and Physiology Department of “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, for their important support.
References
- 1.Bonnett R. Gordon and Breach Science, Amsterdam. London: CRC Press; 2000. Chemical Aspects of Photodynamic Therapy; p. 324. [Google Scholar]
- 2.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer WHO. Globocan. 2020. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf.
- 5.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 6.Gollnick SO. Photodynamic therapy and antitumor immunity. J Natl Compr Canc Netw. 2012;10(Suppl 2):S40–S43. doi: 10.6004/jnccn.2012.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016;473:347–364. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satrialdi, Munechika R, Biju V, Takano Y, Harashima H, Yamada Y. The optimization of cancer photodynamic therapy by utilization of a pi-extended porphyrin-type photosensitizer in combination with MITO-Porter. Chem Commun (Camb) 2020;56:1145–1148. doi: 10.1039/c9cc08563g. [DOI] [PubMed] [Google Scholar]
- 10.Araújo NC, Fontana CR, Gerbi ME, Bagnato VS. Overall-mouth disinfection by photodynamic therapy using curcumin. Photomed Laser Surg. 2012;30:96–101. doi: 10.1089/pho.2011.3053. [DOI] [PubMed] [Google Scholar]
- 11.Atsumi T, Fujisawa S, Tonosaki K. Relationship between intracellular ROS production and membrane mobility in curcumin- and tetrahydrocurcumin-treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Dis. 2005;11:236–242. doi: 10.1111/j.1601-0825.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 12.Khorsandi K, Chamani E, Hosseinzadeh G, Hosseinzadeh R. Comparative study of photodynamic activity of methylene blue in the presence of salicylic acid and curcumin phenolic compounds on human breast cancer. Lasers Med Sci. 2019;34:239–246. doi: 10.1007/s10103-018-2571-0. [DOI] [PubMed] [Google Scholar]
- 13.Şueki F, Ruhi MK, Gülsoy M. The effect of curcumin in antitumor photodynamic therapy: In vitro experiments with Caco-2 and PC-3 cancer lines. Photodiagnosis Photodyn Ther. 2019;27:95–99. doi: 10.1016/j.pdpdt.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, et al. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2018;8:1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vera-Ramirez L, Pérez-Lopez P, Varela-Lopez A, Ramirez-Tortosa M, Battino M, Quiles JL. Curcumin and liver disease. Biofactors. 2013;39:88–100. doi: 10.1002/biof.1057. [DOI] [PubMed] [Google Scholar]
- 16.Dujic J, Kippenberger S, Ramirez-Bosca A, Diaz-Alperi J, Bereiter-Hahn J, Kaufmann R, et al. Curcumin in combination with visible light inhibits tumor growth in a xenograft tumor model. Int J Cancer. 2009;124:1422–1428. doi: 10.1002/ijc.23997. [DOI] [PubMed] [Google Scholar]
- 17.Ellerkamp V, Bortel N, Schmid E, Kirchner B, Armeanu-Ebinger S, Fuchs J. Photodynamic Therapy Potentiates the Effects of Curcumin on Pediatric Epithelial Liver Tumor Cells. Anticancer Res. 2016;36:3363–3372. [PubMed] [Google Scholar]
- 18.Zhao Y, Chen B, Shen J, Wan L, Zhu Y, Yi T, et al. The Beneficial Effects of Quercetin, Curcumin, and Resveratrol in Obesity. Oxid Med Cell Longev. 2017;2017:1459497. doi: 10.1155/2017/1459497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Z, Chen K, Cheng L, Yan B, Qian W, Cao J, et al. Resveratrol and cancer treatment: updates. Ann N Y Acad Sci. 2017;1403:59–69. doi: 10.1111/nyas.13466. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinimehr SJ, Hosseini SA. Resveratrol sensitizes selectively thyroid cancer cell to 131-iodine toxicity. J Toxicol. 2014;2014:839597. doi: 10.1155/2014/839597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yousef M, Vlachogiannis IA, Tsiani E. Effects of Resveratrol against Lung Cancer: In Vitro and In Vivo Studies. Nutrients. 2017;9:1231. doi: 10.3390/nu9111231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferraz da Costa DC, Campos NPC, Santos RA, Guedes-da-Silva FH, Martins-Dinis MMDC, Zanphorlin L, et al. Resveratrol prevents p53 aggregation in vitro and in breast cancer cells. Oncotarget. 2018;9:29112–29122. doi: 10.18632/oncotarget.25631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai H, Deng HB, Wang YH, Guo JJ. Resveratrol inhibits the growth of gastric cancer via the Wnt/β-catenin pathway. Oncol Lett. 2018;16:1579–1583. doi: 10.3892/ol.2018.8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu XM, Jaskula-Sztul R, Ahmed K, Harrison AD, Kunnimalaiyaan M, Chen H. Resveratrol induces differentiation markers expression in anaplastic thyroid carcinoma via activation of Notch1 signaling and suppresses cell growth. Mol Cancer Ther. 2013;12:1276–1287. doi: 10.1158/1535-7163.MCT-12-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nenu I, Popescu T, Aldea MD, Craciun L, Olteanu D, Tatomir C, et al. Metformin associated with photodynamic therapy--a novel oncological direction. J Photochem Photobiol B. 2014;138:80–91. doi: 10.1016/j.jphotobiol.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 26.Filip A, Daicoviciu D, Clichici S, Bolfa P, Catoi C, Baldea I, et al. The effects of grape seeds polyphenols on SKH-1 mice skin irradiated with multiple doses of UV-B. J Photochem Photobiol B. 2011;105:133–142. doi: 10.1016/j.jphotobiol.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Noble JE, Bailey MJ. Quantitation of protein. Methods Enzymol. 2009;463:73–95. doi: 10.1016/S0076-6879(09)63008-1. [DOI] [PubMed] [Google Scholar]
- 28.Conti M, Morand PC, Levillain P, Lemonnier A. Improved fluorometric determination of malonaldehyde. Clin Chem. 1991;37:1273–1275. [PubMed] [Google Scholar]
- 29.Popescu T, Nenu I, Aldea MD, Olteanu D, Gheban D, Tatomir C, et al. The effect of TSPP-mediated photodynamic therapy and Parecoxib in experimental tumours. Life Sci. 2014;117:75–83. doi: 10.1016/j.lfs.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380–385. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 31.Toma VA, Farcas AD, Parvu M, Silaghi-Dumitrescu E, Roman I. CA3 hippocampal field: Cellular changes and its relation with blood nitro-oxidative stress reveal a balancing function of CA3 area in rats exposed to repetead restraint stress. Brain Res Bull. 2017;130:10–17. doi: 10.1016/j.brainresbull.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Grover J, Patel PN, Carnelio S, Chandrashekar C, Shergill AK, Solomon MC. Comparison of glycogen content, basement membrane integrity and mitotic index in stages of oral dysplasia progression to cancer and in oral lichen-lichenoid reactions: a histochemical study. J Adv Med. And Dental Sci. Res. 2015;393(3):3–8. [Google Scholar]
- 33.Bolfa P, Vidrighinescu R, Petruta A, Dezmirean D, Stan L, Vlase L, et al. Photoprotective effects of Romanian propolis on skin of mice exposed to UVB irradiation. Food Chem Toxicol. 2013;62:329–342. doi: 10.1016/j.fct.2013.08.078. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 35.O’Shaughnessy MJ, Murray KS, La Rosa SP, Budhu S, Merghoub T, Somma A, et al. Systemic Antitumor Immunity by PD-1/PD-L1 Inhibition Is Potentiated by Vascular-Targeted Photodynamic Therapy of Primary Tumors. Clin Cancer Res. 2018;24:592–599. doi: 10.1158/1078-0432.CCR-17-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ventura A, Vassall A, Robinson E, Filler R, Hanlon D, Meeth K, et al. Extracorporeal Photochemotherapy Drives Monocyte-to-Dendritic Cell Maturation to Induce Anticancer Immunity. Cancer Res. 2018;78:4045–4058. doi: 10.1158/0008-5472.CAN-18-0171. [DOI] [PubMed] [Google Scholar]
- 37.Makowski M, Grzela T, Niderla J, LAzarczyk M, Mróz P, Kopeé M, et al. Inhibition of cyclooxygenase-2 indirectly potentiates antitumor effects of photodynamic therapy in mice. Clin Cancer Res. 2003;9:5417–5422. [PubMed] [Google Scholar]
- 38.Gee J, Lee IL, Grossman HB, Sabichi AL. Forced COX-2 expression induces PGE(2) and invasion in immortalized urothelial cells. Urol Oncol. 2008;26:641–645. doi: 10.1016/j.urolonc.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 39.NCCN. Colon cancer, version 2. 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 40.NCCN. Rectal cancer, version 2. 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
- 41.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Memorial Sloan Kettering Cancer Center. Turmeric. Available from: https://www.mskcc.org/cancer-care/integrative-medicine/herbs/turmeric.
- 43.Memorial Sloan Kettering Cancer Center. Resveratrol. Available from: https://www.mskcc.org/cancer-care/integrative-medicine/herbs/resveratrol.
- 44.Chai YC, Ashraf SS, Rokutan K, Johnston RB, Jr, Thomas JA. S-thiolation of individual human neutrophil proteins including actin by stimulation of the respiratory burst: evidence against a role for glutathione disulfide. Arch Biochem Biophys. 1994;310:273–281. doi: 10.1006/abbi.1994.1167. [DOI] [PubMed] [Google Scholar]
- 45.Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, et al. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012;4:1247–1253. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumari A, Tyagi N, Dash D, Singh B. Intranasal curcumin ameliorates lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2015;38:1103–1112. doi: 10.1007/s10753-014-0076-y. [DOI] [PubMed] [Google Scholar]
- 47.Korbelik M, Parkins CS, Shibuya H, Cecic I, Startford MR, Chaplin DJ. Nitric oxide production by tumour tissue: impact on the response to photodynamic therapy. Br J Cancer. 2000;82:1835–1843. doi: 10.1054/bjoc.2000.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moustapha A, Pérétout PA, Rainey NE, Sureau F, Geze M, Petit JM, et al. Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discov. 2015;1:15017. doi: 10.1038/cddiscovery.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh M, Singh N. Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol Cell Biochem. 2009;325:107–119. doi: 10.1007/s11010-009-0025-5. [DOI] [PubMed] [Google Scholar]
- 50.Ahn JC, Kang JW, Shin JI, Chung PS. Combination treatment with photodynamic therapy and curcumin induces mitochondria-dependent apoptosis in AMC-HN3 cells. Int J Oncol. 2012;41:2184–2190. doi: 10.3892/ijo.2012.1661. [DOI] [PubMed] [Google Scholar]
- 51.de Matos RPA, Calmon MF, Amantino CF, Villa LL, Primo FL, Tedesco AC, et al. Effect of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Cervical Carcinoma Cell Lines. Biomed Res Int. 2018:4057959. doi: 10.1155/2018/4057959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laszló IP, Laszló MR, Toma V, Baldea I, Olteanu D, David L, et al. The in vivo modulatory effects of Cornus mas extract on photodynamic therapy in experimental tumors. Photodiagnosis Photodyn Ther. 2020;30:101656. doi: 10.1016/j.pdpdt.2020.101656. [DOI] [PubMed] [Google Scholar]
- 53.Chandel NS, Tuveson DA. The promise and perils of antioxidants for cancer patients. N Engl J Med. 2014;371:177–178. doi: 10.1056/NEJMcibr1405701. [DOI] [PubMed] [Google Scholar]
- 54.Dionisie V, Clichici S, Ion RM, Danila O, Moldovan R, Decea N, et al. In vivo silymarin’s antioxidant and anti-apoptotic effects on photodynamic therapy’s responsiveness. J. Porphyrins Phthalocyanines. 2017;21:1–9. [Google Scholar]
- 55.Zhang X, Liu X, Kang S, Liu C, Hao Y. Resveratrol enhances the effects of ALA-PDT on skin squamous cells A431 through p38/MAPK signaling pathway. Cancer Biomark. 2018;21:797–803. doi: 10.3233/CBM-170495. [DOI] [PubMed] [Google Scholar]
- 56.Villacorta BR, Roque KFJ, Tapang GA, Jacinto SD. Plant extracts as natural photosensitizers in photodynamic therapy: in vitro activity against human mammary adenocarcinoma MCF-7 cells. Asian Pac J Trop Biomed. 2017;7:358–366. [Google Scholar]
- 57.Punjabi A, Wu X, Tokatli-Apollon A, El-Rifai M, Lee H, Zhang Y, et al. Amplifying the red-emission of upconverting nanoparticles for biocompatible clinically used prodrug-induced photodynamic therapy. ACS Nano. 2014;8:10621–10630. doi: 10.1021/nn505051d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Probodh I, Cramb D. Two-photon excitation photodynamic therapy: working toward a new treatment for wet age-related macular degeneration. In: Ying GS, editor. The recent advances in basic research and clinical care. InTech; Shanghai: 2012. pp. 213–226. [Google Scholar]
- 59.Bisland SK, Lilge L, Lin A, Rusnov R, Wilson BC. Metronomic photodynamic therapy as a new paradigm for photodynamic therapy: rationale and preclinical evaluation of technical feasibility for treating malignant brain tumors. Photochem Photobiol. 2004;80:22–30. doi: 10.1562/2004-03-05-RA-100.1. [DOI] [PubMed] [Google Scholar]