Abstract
Functional gastrointestinal disorders (FGIDs) are highly prevalent in medical students around the world. However, there is no specific data on FGIDs in Tunisia. The objectives of this study were to evaluate the prevalence of FGIDs in medical students according to the rome III criteria and to identify risk factors associated with these disorders. A self-administered questionnaire survey was carried out among the students from the first and the second year of medical studies. We studied the influence of socio-demographic characteristics, lifestyle, health care seeking, psychosomatic symptoms and hospital anxiety and depression scale on the prevalence of FGIDs among these students. Three hundred and forty-three students (20.3 ± 0.8years) were included in our study. The prevalence of FGIDs was 54.2%. The main FGIDs found were the unspecified functional bowel disorder (46.6%), functional constipation (11.6%), irritable bowel syndrome (7.6%) and functional dyspepsia (6.7%). In logistic regression, abnormal BMI (OR = 2.1, 95% CI= 1–4.3), living in school dormitory (OR = 3.7, 95% CI = 1.7–7.8), low water intake (OR = 2.2, 95% CI = 1.1–4.2), digestive medication use (OR = 3.4, 95% CI= 1.3–8.5), and probable or definite anxiety (OR = 2.5, 95% CI = 1.1–5.8) were the five risk factors associated with FGIDs. We demonstrate a high prevalence of FGIDs (54.2%) among our students. Risk factors for FGIDs were abnormal BMI, living in school dormitory, low water intake, digestive medication use and anxiety.
Keywords: Prevalence, Functional gastrointestinal disorders, Medical students, Rome III criteria, Tunisia
1. Introduction
Functional gastrointestinal disorders (FGIDs) are characterized by the presence of chronic and recurrent gastrointestinal symptoms whose origin is attributed to the digestive tract but for which no organic or metabolic abnormality can be demonstrated by the usual examinations [1]. FGIDare mainly represented by irritable bowel syndrome (IBS), functional constipation (FC) and functional dyspepsia (FD). The pathophysiology of FGIDs remains unclear; but it includes a bidirectional dysregulation of gut-brain interaction leading to visceral hypersensitivity, motility disturbance, alterations in gut microbiota, immune and mucosal function [2]. Psychological and social factors were also reported to contribute to the pathogenesis of FGIDs [3].
FGIDs are prevalent and account for up to 50% of diagnosed gastrointestinal disorders [4]. Although FGIDs are non- threatening, they do generally decrease the quality of life and are subject of a marked increase of health care seeking [5,6]. This, with the significant economic burden they put on health care system, qualify FGIDs as a serious public health problem [7].
Medical students face significant amounts of stress, not only due to their overloaded curriculum, but also due to other factors such as the socioeconomic and the psychological challenges they face when initiating such a curriculum [8,9]. This makes them more susceptible to FGIDs and particularly IBS. Several studies showed that FGIDs are highly prevalent in medical students and that their prevalence could achieve 68% in this population [10–13]. To the best of our knowledge no study has evaluated the prevalence of FGIDs in medical student in Tunisia (North Africa). Therefore the aims of our study were to evaluate the prevalence of FGIDs in medical students according to the rome III criteria and to identify risk factors associated with these disorders.
2. Materials and methods
2.1. Study population
This study was approved by the local ethics committee as well as the faculty dean (ethics committee approval number: 3408/151211). In a Cross-Sectional Design, all the students enrolled in the first And second year between the period of february and march 2015 in faculty of medicine of Monastir were solicited to participate in this study. In Tunisia, the studies for the national diploma of doctor of medicine last 8 years for family medicine and 11 years for the specialty. Medical studies are made up of 3 cycles: a first cycle which lasts 2 years with a training which is articulated around the teaching of fundamental sciences, general modules and internships of semiology in the hospital; a second cycle which lasts 4 years and dedicated to the learning of clinical sciences in a theoretical and practical way (hospital internships and night shift); a third cycle that leads either to the national diploma of doctor of medicine and the ability to practice family medicine after three years of training, or to the diploma of medical specialist after 4 to 5 years of training depending on the medical specialty chosen.
Subjects received a detailed explanation of the study aims by the investigators and were asked to provide a written informed consent before being given any study material. Subjects were excluded if they presented: symptoms or history of peptic ulcer disease, inflammatory bowel disease and abdominal surgery except appendectomy.
2.2. Study questionnaire
Two investigators (SG and AK in the list of authors) collected the data at the end of lectures and remotely from exams. Data were collected using a set of self-administered questionnaires assessing:
Sociodemographic characteristics, life habits, nutritional habits and digestive intolerance to certain foods;
History of digestive disorders: peptic ulcer disease, inflammatory bowel disease, abdominal surgery, digestive cancer;
History of medication during the last three months: type and mode of prescription (auto-medication or medical prescription). Each pharmacological agent was re-coded to its pharmacological class;
Health care seeking due to any gastrointestinal disorder: category of health care professional (general practitioner or specialist), number and reasons for consultations and re-consultations, additional tests performed and treatment prescribed;
Characteristics of bowel movements: change of bowel movements during the last Year, infection preceding change of bowel movements and number of stools per week. The stool consistency was determined by the validated Bristol stool scale [14];
Alarm signs that could indicate an organic digestive illness in the last three months were searched and included: gastrointestinal bleeding, persistent fever, painful swallowing, unintentional weight loss (≥ 6 Kg), anemia and a family history of digestive cancer or inflammatory bowel disease [15];
Digestive symptoms were explored using rome III questionnaire which diagnoses the different types of FGIDs [16];
Psychosomatic symptom checklist was used to assess psychosomatic symptoms. This checklist is a self-reported scale that measures the frequency and the intensity of 17 psychosomatic symptoms on a scale ranging from 0 (not a Pproblem both in the intensity and frequency scores) to 4 (occurs daily and extremely bothersome when occurs on the frequency and the intensity scales respectively) [17]. A symptom is considered frequent when occurs at least once a week (frequency score ≥ 2) and intense when is rated at least as moderately bothersome (intensity score ≥ 2);
A French version of the hospital anxiety and depression scale (HAD) was used to screen for anxiety and depressive symptoms in FGIDs. HAD is a 14-Items self-reported scale that was previously validated in french and is largely used to explore anxiety and depressive symptoms in FGIDs [18,19]. It contains two 7-item scales: one to screen for anxiety and one to screen for depressive symptoms. Subjects are required to rate each item using a four-point scale ranging from 0 to 3. The calculation of a total score by summing the individual scores of the two 7-item scales related to anxiety and depression is then required to distinguish between 3 different situations: score < 8: normal state, score between 8 and 10: probable anxious or depressive state and score > 10: confirmed anxious or depressive state.
After self-questionnaires completion, students who presented one or more alarming signs were instructed to consult a Physician.
2.3. Statistical analysi
Data were analyzed using statistical package for the social sciences (SPSS) (version 10.0, SPSS inc, USA). Distributions of continuous variables are expressed as means ± SD and qualitative data are presented as frequencies.
Whenever the assumptions were met, student t Test, Chi 2 test, and Mann-Whitney U test were used to compare these variables. To identify factors associated independently with FGIDs, a multivariate analysis by binary logistic regression step down hosmer and lemeshow was undertaken. The variables included in the initial model were retained following the univariate analysis with statistical significance p ≤ 0.2. This threshold was chosen to avoid potential confounding factors. A Spearman correlation study between these variables was performed in order to avoid the phenomenon of multicollinearity. The p value was considered significant if it is less than or equal to 0.05.
3. Results
Of the 489 students enrolled in the faculty, only 429 were solicited to participate. Three hundred and seventy five students accepted to participate of whom 54 declined after being given the study questionnaires. After checking for exclusion criteria a total of 343 students were considered for this study as outlined in Figure 1. Table 1 lists the characteristics of the students.
Figure 1.
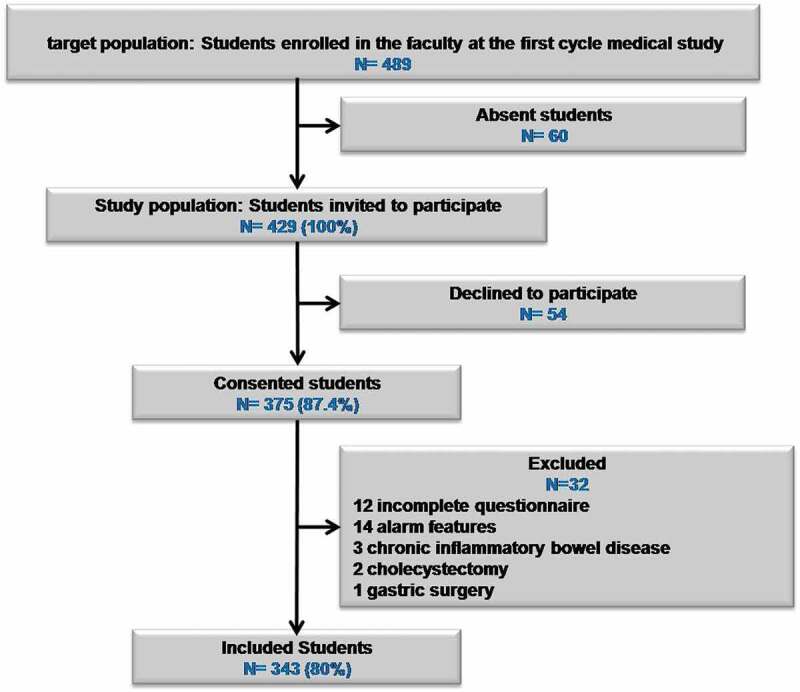
Flow chart of students inclusion in the study.
Table 1.
Sociodemographic characteristics of 1st and 2nd year students of a medical school in Tunisia
| Age (year) |
20.3 ± 0.8 |
|---|---|
| Female gender n(%) | 235 (68.5) |
| BMI n(%) | |
|
30 (8.7) 264 (77) 49 (14.3) |
| Residence n(%) | |
|
153 (44.6) 96 (28) 94 (27.4) |
| Regular physical activity (> 1 h/week) n(%) | 85 (24.9) |
| Material resources n(%) | |
|
37 (10.5) 307 (89.5) |
Diet n(%)
|
250 (72.9) 208 (60.6) 254 (74) 93 (27) 28 (8) 147 (42.8) |
Drug n(%)
|
162 (47) 157 (45.7) 42 (12.2) |
History of digestive problem n(%)
|
75 (21.8) 25 (7.2) 28 (8.1) 49 (14.2) |
Bowel movement frequency n(%)
|
306 (89.2) 37 (10.7) 0 (0) |
| At least one psychosomatic symptom n(%) | 260 (75.8) |
HAD n(%)
|
38 (11) 188 (54.8) 117 (34.2) |
3.1. Functional Gastrointestinal disorders prevalence
The overall prevalence of FGIDs was 54.2% (n = 186) of the total of our study population. Table 2 shows the prevalence of the different FGIDs according to the rome III criteria. Unspecified functional bowel disorder was the most prevalent FGID in our study population (46.6%, n = 160), followed by FC (11.6%, n = 40), IBS (7.6%, n = 26) and finally FD (6.7%, n = 23).
Table 2.
Functional gastrointestinal disorders prevalence in the 1st And 2nd Year students of a medical school in Tunisia
| Rome III disorders | Number of students | Percent among study population (n = 343) |
Percent among FGIDs (n = 186) |
|---|---|---|---|
| A. Functional esophageal disorders | |||
| A1. Functional heartburn | 11 | 3.2 | 5.9 |
| A2. Functional chest pain of presumed esophageal origin | 11 | 3.2 | 5.9 |
| A3. Functional dysphagia | 5 | 1.4 | 2.6 |
| A4. Globus | 4 | 1.1 | 2.1 |
| B. Functional gastroduodenal disorders | |||
| B1. Functional dyspepsia | 23 | 6.7 | 12.3 |
| B2. Belching disorders | 12 | 3.5 | 6.4 |
| B3. Nausea and vomiting disorders | 11 | 3.2 | 5.9 |
| B4. Rumination syndrome in adults | 5 | 1.4 | 2.6 |
| C. Functional bowel disorders | |||
| C1. Irritable bowel syndrome | 26 | 7.6 | 13.9 |
| C2. Functional bloating | 16 | 4.7 | 8.6 |
| C3. Functional constipation | 40 | 11.6 | 21.5 |
| C4. Functional diarrhea | 0 | 0 | 0 |
| C5. Unspecific functional bowel disorder | 160 | 46.6 | 86 |
| D. Functional abdominal pain syndrome | 0 | 0 | 0 |
| E. Functional gallbladder and Sphincter of oddi disorders |
0 | 0 | 0 |
| F. Functional anorectal disorders | |||
| F1. Functional fecal incontinence | 10 | 2.9 | 5.3 |
| F2. Functional anorectal pain | 17 | 4.9 | 9.1 |
| F3. Functional defecation disorders | 4 | 1.1 | 2.1 |
3.2. Risk factors of functional Gastrointestinal disorders
Table 3 summarizes the results of the multiple regression analysis of risk factors for FGIDs in medical students. Significant independent risk factors for FGIDs were abnormal BMI (OR= 2.1, 95% CI = 1–4.3), living in school dormitory (OR = 3.7, 95% CI = 1.7–7.8), low water intake (OR = 2.2, 95% CI= 1.1–4.2), digestive medication use (OR = 3.4, 95% CI= 1.3–8.5), and probable or definite anxiety (OR = 2.5, 95% CI= 1.1–5.8).
Table 3.
Multivariate analysis of risk factors for occurrence of functional gastrointestinal disorders in the 1st And 2nd year students of a medical school in Tunisia
| Parameters | Odd ratio(or) | Confidence interval (CI) 95% | p Value |
|---|---|---|---|
| Abnormal BMI | 2.1 | [1–4.3] | 0.046 |
| Living in school dormitory | 3.7 | [1.7–7.8] | 0.000 |
| Low water intake (< 1.5 l/day) | 2.2 | [1.1–4.2] | 0.016 |
| Digestive medication use | 3.4 | [1.3–8.5] | 0.009 |
| Probable or definite anxiety | 2.5 | [1.1–5.8] | 0.024 |
4. Discussion
To the best of authors’ knowledge, this is the first study that explored the epidemiology of FGIDs in a North African population and particularly in Tunisia. Overall, FGIDs were prevalent in our study population and accounted for 54.2%. Unspecified functional bowel disorder was the most prevalent (46.6%) followed by FC (11.6%), IBS (7.6%) and FD (6.7%).
Similar findings were reported by a chinese study using the rome III criteria in which the overall prevalence was 55.2% with unspecified functional bowel disorder as the most prevalent FGID [20]. A prevalence of 9.4%, 7.9% and 4% were respectively reported for fd, IBS and FC by another chinese study that also used rome III criteria [21].
The prevalence of FGIDs in students widely varies across countries and this could be explained by the difference in diagnostic criteria used. For example, using the rome iv criteria, an Indian study showed that FD and IBS were the most frequent FGIDs [2]. Medical students seem to be at increased risk of developing FGIDs compared to their non medical counterparts [20,23,24], this appears to be especially true as students progress through medical school [25,26]. This, however, remains subject of controversy; with some studies reporting increased prevalence of FGIDs with increased level of studies [25,26] while others showing that neither the level of studies, nor the medical curriculum compared to other non-medical curriculums do influence the prevalence of FGIDs [13,21,24,27].
Unspecified functional bowel disorder represent a set of symptoms that could originate in small intestine, colon or rectum but that do not meet the diagnostic criteria for IBS, FC, functional diarrhea or functional bloating [1]. In our study this disorder, as indicated above, was the most prevalent of FGIDs and accounted for 46.6% of the total of our study population. Unspecified functional bowel disorder was also reported to be the most frequent FGID by Chu et al [20].
The prevalence of FC was 11.6% in our study. The worldwide prevalence of FC among medical students varies between 2 to 34% [20–22,28]. This great variability in prevalence can be attributed to differences in food regimens across countries and different ethnic groups [29–32].
IBS is the most studied FGID in the literature. In our study the prevalence of this FGID was 7.6%. In medical students the prevalence of this disorder ranged from 9.2% to 31.8% and from 6.2% to 15.8% in studies using the rome III criteria [26,33] and rome IV criteria respectively [22,23]. It is important to highlight that the difference between the two criteria does not reside in the way they define IBS but instead in the frequency of its occurrence; from at least 3 days per month in the rome III criteria to at least 1 day per week in the rome IV criteria [34]. Therefore this wide heterogeneity between studies could not totally be attributed to non uniformity of diagnostic criteria. Many other factors like demographics, dietary habits, cultural and socioeconomic characteristics seem to play a role in mediating this heterogeneity [35–37]. Finally it is noteworthy to acknowledge that there is a huge lack of data regarding IBS in African students [38].
Finally the prevalence of FD was 6.7% which is within the range of that reported in the literature (4.5 à 15.2%) in medical students [20–22,39].
Analysis of risk factors in our study revealed that BMI is an independent risk factor for FGIDs and being a Student at both ends of abnormal weight spectrum (BMI < 18.5 Kg/m2 Or BMI ≥ 25 Kg/m2) increases up to four fold the risk of developing an FGID. In this regard, several studies showed that obesity increases the risk of developing esophageal burns, bloating, diarrhea and abdominal pain whereas early satiety was more associated with a low BMI [40–42]. In other studies, constipation was rather more frequent in obese subjects [43,44] and IBS was more frequent in underweight subjects [45,46]. Although our study was designed to investigate the effect of BMI on overall prevalence of FGIDs, our results confirmed previous findings by showing that an abnormal BMI irrespective of whether it favors overweight or underweight is a Strong risk factor for developing FGIDs.
Our study also confirmed previous findings that students living in a school dormitory had three times higher risk of developing an FGIDs[23,26,35,47]. The reasons behind this increased risk may involve multiple factors including the dormitory characteristic: i.e. the size of the rooms, the hygiene of the premises and the cohabitation of different students. Secondly, the change in lifestyle and particularly a change in eating habits and sleeping patterns could also contribute to this association. Finally, being away from parents, often for the first time in a student’s life, and facing new social challenges with new responsibilities could create a stressful environment that could in its turn contribute to this increased risk [37,48,49].
Poor hydration doubled the risk of having a FGID in our study and this would most likely be in favor of constipation. Indeed, previous research consistently showed increased risk of developing constipation with inadequate hydration [44,50,51]. It was demonstrated that poor hydration lowers stool frequency and modify their consistency [52,53]. Therefore according to the latest guidelines, it is recommended for individuals who suffer from constipation to appropriately hydrate to avoid constipation-related complications [54].
Our study showed a link between FGIDs and increased digestive medication intake in symptomatic individuals compared to their normal counterparts (18.5% vs 7%, p = 0.013). Increased gastrointestinal drug intake is largely reported in USA and could achieve 50% in some FGIDs [55]. Besides medication intake, the economic burden of FGIDs is also worsened by increased health care seeking and its related examination. In fact it was estimated that FGIDs cost us health care system up to 350 Million dollars and up to 8 Billion euros in Europe [55,56]. In studies conducted in medical students, Tan et al. [10] and Jafri el al. [57] reported respectively a 13% and 54% increase in medical visits of symptomatic students. This difference between studies could be explained by health care systems and the socio-economic characteristics of countries in which studies were conducted. Subject characteristics could in its turn contribute to this heterogeneity; it is known that medical training gives student certain autonomy of management and this is all the more true as their level of study is advanced.
Based on the HAD data, a confirmed anxious state was present in 21% of students showing symptoms of FGIDs compared to 6.4% in their non symptomatic counterparts. Depression did not, however, vary significantly between symptomatic and non symptomatic students. A probable or a confirmed anxious state more than doubled the risk of having an FGID (OR= 2.5; 95% CI: 1.1–5.8). FGIDs are defined as disorders of gut-brain interaction that integrate complex interaction between biological, psychological and social factors [2]. Abnormal brain areas activity was associated with visceral hypersensitivity, as well as anxiety and depression, in patients with FGIDs [58,59]. Considering the significant amount of stress imposed by the medical curriculum, an increased prevalence of these disorders is not an unexpected finding in this population and is indeed demonstrated by multiple studies [10–12,26,60,61]. However the cross-sectional nature of this study prevents the establishment of cause to effect relationship between FGIDs and their related comorbidities. In other words we cannot disentangle whether psychosocial factors such as anxiety caused these disorders or FGIDs influenced the levels of anxiety.
In our study, FGIDs tended to be more prevalent in females (69% vs 57.8%, p = 0.062). Whether sex influences the prevalence of FGIDs is currently subject of ongoing debate [62]. However most of the studies conducted on students report a sex ratio that favors women over men, particularly regarding IBS [10–12,21,22,33,57,63,64,65]. A recent meta-analyses conducted on 57 studies with a total of 426,362 from all over the globe reported an odds ratio of 1.46 (1.33–1.59), but this was variable according to what criteria were used (rome III or IV) and according to the geographical region [66].
The originality of this study stems from the fact that this is the first study of its kind investigating FGIDs in medical students in Tunisia. However like all studies, our study is not free of limits. The low sample size, the absence of a control group involving students from other curriculums and the limitation to one faculty for data collection constitutes major limitations of this study. Also of note that the cross-sectional design of this study constitutes one other limit that restricts the establishment of causality between FGIDs and their related co-morbidities. These results should, thus, be interpreted cautiously. Finally, the fact that we have not used the most recent criteria (rome IV) and we have not objectively explored for chronic gastrointestinal conditions constitute two other limits of this study. Yet, we have excluded from our analysis all the students who presented any alarm sign or a history for chronic condition.
In conclusion, this is the first study investigation on the prevalence of FGIDs in medical students in Tunisia according to rome III criteria. We demonstrate a high prevalence of FGIDs (54.2%) among our students. Risk factors for FGIDs were abnormal BMI, living in school dormitory, low water intake, digestive medication use and anxiety. Thus, the promotion of a healthy lifestyle and psychological support are recommended.
Acknowledgments
The authors would like to thank the medical students of the faculty of medicine of Monastir for their participation in this study.
Funding Statement
The author(s) reported there is no funding associated with the Work featured in this article.
Abbreviations
Functional constipation (FC); functional dyspepsia (FD); functional gastrointestinal disorders (FGIDs); irritable bowel syndrome (IBS).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Black Cj, Drossman Da, Talley Nj, et Al. Functional gastrointestinal disorders: advances in understanding and Management. Lancet. 2020;396(10263):1664–8. [DOI] [PubMed] [Google Scholar]
- [2].Longstreth GF, Thompson WG, Chey WD, et Al. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. [DOI] [PubMed] [Google Scholar]
- [3].Levy Rl, Olden, KW, Naliboff, BD.. Psychosocial aspects of the functional gastrointestinal Disorders. Gastroenterology. 2006;130(5):1447–1458. [DOI] [PubMed] [Google Scholar]
- [4].Talley Nj. Functional gastrointestinal disorders as a Public health Problem. Neurogastroenterol motil. 2008;20(1):121–129. [DOI] [PubMed] [Google Scholar]
- [5].Talley Nj, Boyce Pm, Jones M.. Predictors of health care seeking for irritable bowel syndrome: a Population based Study. Gut. 1997;41(3):394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chang L. Epidemiology and quality of life in functional gastrointestinal Disorders. Aliment pharmacol ther. 2004;20:31–39. [DOI] [PubMed] [Google Scholar]
- [7].Mohaghegh shalmani H, Soori, H, Khoshkrood Mansoori, B. Direct and indirect medical costs of functional constipation: a Population-Based Study. Int J Colorectal dis. 2011;26(4):515–522. [DOI] [PubMed] [Google Scholar]
- [8].Sansone Ra, Sansone La. Physician and medical student Stress. Psychiatry (edgmont). 2007;4(3):28–29. [PMC free article] [PubMed] [Google Scholar]
- [9].Lee J, Graham Av. Students’ perception of medical school stress and their evaluation of a Wellness elective. Med educ. 2001;35(7):652–659. [DOI] [PubMed] [Google Scholar]
- [10].Tan Ym, Goh Kl, Muhidayah R, et Al. Prevalence of irritable bowel syndrome in young adult malaysians: a Survey among medical Students. J Gastroenterol hepatol. 2003;18(12):1412–1416. [DOI] [PubMed] [Google Scholar]
- [11].Okami Y, Kato T, Nin G, et Al. Lifestyle and psychological factors related to irritable bowel syndrome in nursing and medical school Students. J Gastroenterol. 2011;46(12):1403–1410. [DOI] [PubMed] [Google Scholar]
- [12].Naeem SS, Siddiqui, EU, Kazi, AN, et Al. Prevalence and factors associated with irritable bowel syndrome among medical students of karachi, pakistan: a Cross-Sectional Study. Bmc res notes. 2012;5:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wells M, Roth, L, McWilliam, M, et Al. A Cross-Sectional study of the association between overnight call and irritable bowel syndrome in medical Students. Can j Gastroenterol. 2012;26(5):281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Riegler G, Esposito I. Bristol scale stool form. A Still valid help in medical practice and clinical Research. Tech coloproctol. 2001;5(3):163–164. [DOI] [PubMed] [Google Scholar]
- [15].Hammer J, Eslick, GD, Howell, SC, et Al. Diagnostic yield of alarm features in irritable bowel syndrome and functional Dyspepsia. Gut. 2004;53(5):666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Drossman Da. The functional gastrointestinal disorders and the rome III Process. Gastroenterology. 2006;130(5):1377–1390. [DOI] [PubMed] [Google Scholar]
- [17].Attanasio V, Andrasik, F, Blanchard, EB, et Al. Psychometric properties of the sunya revision of the psychosomatic symptom Checklist. J Behav med. 1984;7(2):247–257. [DOI] [PubMed] [Google Scholar]
- [18].Zigmond As, Snaith Rp. The hospital anxiety and depression Scale. Acta psychiatr scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- [19].Roberge P, Dore, I, Menear, M, et Al. A Psychometric evaluation of the french canadian version of the hospital anxiety and depression scale in a Large primary care Population. J Affect disord. 2012. [DOI] [PubMed] [Google Scholar]
- [20].Chu L, Zhou H, Lü B, et Al. [an epidemiological study of functional bowel disorders in zhejiang college students and its relationship with psychological Factors]. Zhonghua nei ke za zhi. 2012;51(6):429–432. [PubMed] [Google Scholar]
- [21].Dong YY, Chen , FX, Yu , YB, et Al. A School-Based study with rome iii criteria on the prevalence of functional gastrointestinal disorders in chinese college and university Students. Plos one. 2013;8(1):E54183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Goyal O, Nohria S, Dhaliwal As, et Al. Prevalence, overlap, and risk factors for rome iv functional gastrointestinal disorders among college students in northern India. Indian j Gastroenterol. 2020. Doi: 10.1007/s12664-020-01106-y. [DOI] [PubMed] [Google Scholar]
- [23].Albutaysh Of, Alquraini Aa, Almukhaitah Aa, et Al. Epidemiology of irritable bowel syndrome and its associated factors in saudi undergraduate Students. Saudi j Gastroenterol. 2020;26(2):89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wani FA, Almaeen, AH, Bandy, AH, et Al. Prevalence and risk factors of ibs among medical and nonmedical students in the jouf University. Niger j Clin pract. 2020;23(4):555–560. [DOI] [PubMed] [Google Scholar]
- [25].Vasquez-Rios G, Machicado , JD, Ticse, R, et Al. Stress and a Sedentary lifestyle are associated with irritable bowel syndrome in medical students from peru: a Cross-Sectional Study. Eur j Gastroenterol hepatol. 2019;31(11):1322–1327. [DOI] [PubMed] [Google Scholar]
- [26].Ibrahim NK, Battarjee WF, Almehmadi SA. Prevalence and predictors of irritable bowel syndrome among medical students and interns in king abdulaziz university Jeddah. Libyan j Med. 2013;8:21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wells M, Roth , L, McWilliam, M, et Al. A Cross-Sectional study of the association between overnight call and irritable bowel syndrome in medical Students. Can j Gastroenterol. 2012;26(5):281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Khatri Pk, Ali, AD, Alzadjali , N. et al, et Al. Frequency of functional constipation in 3 different populations and its causative Factors. J Pak med assoc. 2011;61(11):1149–1152. [PubMed] [Google Scholar]
- [29].Murakami K, Sasaki, S, Okubo , H, et Al. Food intake and functional constipation: a Cross-Sectional study of 3,835 japanese women aged 18-20 Years. J Nutr sci vitaminol (tokyo). 2007;53(1):30–36. [DOI] [PubMed] [Google Scholar]
- [30].Murakami K, Sasaki, S, Okubo , H, et Al. Association between dietary fiber, water and magnesium intake and functional constipation among young japanese Women. Eur j Clin nutr. 2007;61(5):616–622. [DOI] [PubMed] [Google Scholar]
- [31].Okubo H, Sasaki , S, Murakami K, K, et Al. Dietary patterns associated with functional constipation among japanese women aged 18 to 20 years: a Cross-Sectional Study. J Nutr sci vitaminol (tokyo). 2007;53(3):232–238. [DOI] [PubMed] [Google Scholar]
- [32].You Js, Park Jy, Chang Kj. A Case-Control study on the dietary taurine intake, nutrient status and life stress of functional constipation patients in korean male college Students. J Biomed sci. 2010;17 suppl 1:S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jung HJ, Park, MI, Moon, W. et al, et Al. Are food constituents relevant to the irritable bowel syndrome in young adults? - a Rome iii based prevalence study of the korean medical Students. J Neurogastroenterol motil. 2011;17(3):294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mearin F, et Al. Bowel Disorders. Gastroenterology; 2016. [DOI] [PubMed] [Google Scholar]
- [35].Kim Yj, Ban Dj. Prevalence of irritable bowel syndrome, influence of lifestyle factors and bowel habits in korean college Students. Int J Nurs stud. 2005;42(3):247–254. [DOI] [PubMed] [Google Scholar]
- [36].Shiotani A, Miyanishi T, Takahashi T. Sex differences in irritable bowel syndrome in japanese university Students. J Gastroenterol. 2006;41(6):562–568. [DOI] [PubMed] [Google Scholar]
- [37].El-Gilany Ah, Amr M, Hammad S. Perceived stress among male medical students in egypt and saudi arabia: effect of sociodemographic Factors. Ann saudi med. 2008;28(6):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Okeke EN, Agaba, EI, Gwamzhi, L. et al, et Al. Prevalence of irritable bowel syndrome in a Nigerian student Population. Afr j Med med sci. 2005;34(1):33–36. [PubMed] [Google Scholar]
- [39].Hori K, Matsumoto T, Miwa H. Analysis of the gastrointestinal symptoms of uninvestigated dyspepsia and irritable bowel Syndrome. Gut liver. 2009;3(3):192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Talley N, Quan, C, Jones, MP. et al, et Al. Association of upper and lower gastrointestinal tract symptoms with body mass index in an australian Cohort. Neurogastroenterol motil. 2004;16(4):413–419. [DOI] [PubMed] [Google Scholar]
- [41].Talley Nj, Howell S, Poulton R. Obesity and chronic gastrointestinal tract symptoms in young adults: a Birth cohort Study. Am j Gastroenterol. 2004;99(9):1807–1814. [DOI] [PubMed] [Google Scholar]
- [42].Ragins AI, Shan, J, Thom, DH, et Al. Effects of urinary incontinence, comorbidity and race on quality of life outcomes in Women. J Urol. 2008;179(2):651–655. Discussion 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pourhoseingholi MA, Kaboli, SA, Pourhoseingholi, A, et Al. Obesity and functional constipation; a communitybased study in Iran. J Gastrointestin liver dis. 2009;18(2):151–155. [PubMed] [Google Scholar]
- [44].Shen L, Huang, C, Lu, X, et Al. Lower dietary fibre intake, but not total water consumption, is associated with constipation: a Population-Based Analysis. J Hum nutr diet. 2019;32(4):422–431. [DOI] [PubMed] [Google Scholar]
- [45].Ebling B, et Al. Anthropological, demographic and socioeconomic characteristics of irritable bowel Syndrome. Coll antropol. 2011;35(2):513–521. [PubMed] [Google Scholar]
- [46].Kubo M, Fujiwara, Y, Shiba, M, et Al. Differences between risk factors among irritable bowel syndrome subtypes in japanese adults. Neurogastroenterol motil. 2011;23(3):249–254. [DOI] [PubMed] [Google Scholar]
- [47].Costanian C, Tamim H, Assaad S. Prevalence and factors associated with irritable bowel syndrome among university students in lebanon: findings from a Cross-Sectional Study. World j Gastroenterol. 2015;21(12):3628–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Supe An. A Study of stress in medical students at seth g.Smedical College. J Postgrad med. 1998;44(1):1–6. [PubMed] [Google Scholar]
- [49].Clouse RE, Shiba, EA, Aziz, Q, et Al. Functional abdominal pain Syndrome. Gastroenterology. 2006;130(5):1492–1497. [DOI] [PubMed] [Google Scholar]
- [50].Markland AD, Palsson, O, Goode, PSS , et Al. Association of low dietary intake of fiber and liquids with constipation: evidence from the National Health and Nutrition Examination Survey. Gastroenterology. 2011;140(5, supplement 1):S–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Arnaud Mj. Mild dehydration: a Risk factor of Constipation? Eur j Clin nutr. 2003;57(2):S88–95. [DOI] [PubMed] [Google Scholar]
- [52].Klauser Ag, et Al. Low fluid intake lowers stool output in healthy male Volunteers. Z Gastroenterol. 1990;28(11):606–609. [PubMed] [Google Scholar]
- [53].Young Rj, Beerman Le, Vanderhoof Ja. Increasing oral fluids in chronic constipation in Children. Gastroenterol nurs. 1998;21(4):156–161. [DOI] [PubMed] [Google Scholar]
- [54].Bharucha Ae, Lacy Be. Mechanisms, evaluation, and management of chronic Constipation. Gastroenterology. 2020;158(5):1232–1249 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ma C, Congly, SE, Novak, KL, et Al. Epidemiologic burden and treatment of chronic symptomatic functional bowel disorders in the usa: a Nationwide Analysis. Gastroenterology. 2021;160(1):88–98.E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Flacco ME, Manzoli, L, De Giorgio, RL, et Al. Costs of irritable bowel syndrome in european countries with universal healthcare coverage: a Meta-Analysis. Eur rev med pharmacol sci. 2019;23(7):2986–3000. [DOI] [PubMed] [Google Scholar]
- [57].Jafri W, Yakoob, J, Jafri, N, et Al. Frequency of irritable bowel syndrome in college Students. J Ayub med coll abbottabad. 2005;17(4): 9–11 [PubMed] [Google Scholar]
- [58].Mayer EA, Labus, J, Aziz, Q, et Al. Role of brain imaging in disorders of brain–gut interaction: a Rome working team report. Gut. 2019;68(9):1701–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lee IS, Wang, H, Chae, Y, et Al. Functional neuroimaging studies in functional dyspepsia patients: a Systematic Review. Neurogastroenterol motility. 2016;28(6):793–805. [DOI] [PubMed] [Google Scholar]
- [60].Hazlett-Stevens H, Craske, MG, Mayer, EA, et Al. Prevalence of irritable bowel syndrome among university students: the roles of worry, neuroticism, anxiety sensitivity and visceral Anxiety. J Psychosom res. 2003;55(6):501–505. [DOI] [PubMed] [Google Scholar]
- [61].Liu Y, Liu, L, Yang, Y, et Al. A School-Based study of irritable bowel syndrome in medical students in beijing, China: prevalence and some related factors. Gastroenterol res pract. 2014;2014:124261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chang L, Toner, BB, Fukudo, S, et Al. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130(5):1435–1446. [DOI] [PubMed] [Google Scholar]
- [63].Ibrahim Nk, Battarjee Wf, Almehmadi Sa. Prevalence and predictors of irritable bowel syndrome among medical students and interns in king abdulaziz university Jeddah. Libyan j Med. 2013;8(1):21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Dai N, Cong Y, Yuan H. Prevalence of irritable bowel syndrome among undergraduates in southeast China. Dig liver dis. 2008;40(6):418–424. [DOI] [PubMed] [Google Scholar]
- [65].Dong YY, Zuo, XL, Li, CK, et Al. Prevalence of irritable bowel syndrome in chinese college and university students assessed using rome III Criteria. World j Gastroenterol. 2010;16(33):4221–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Oka P, Parr, H, Barberio, B, et Al. Global prevalence of irritable bowel syndrome according to rome III or IV criteria: a systematic review and meta-analysis. Lancet gastroenterol hepatol. 2020;5(10):908–917. [DOI] [PubMed] [Google Scholar]


