Abstract
Reduced glutathione (GSH) levels and resistance to chlorine were measured for two isogenic Escherichia coli strains stressed by oxygenation and/or starvation. The E. coli mutant deficient in GSH was not more sensitive to the oxidant than its parent strain when the bacteria were cultured with a low oxygenation rate. Starvation or oxygenation increased the resistance of the parent strain to chlorine, while the resistance of the deficient strain remained unchanged.
All aerobic organisms cope with reactive oxygen species, such as superoxide anions, hydrogen peroxide, and hydroxyl radicals, which accumulate in cells as products of the incomplete reduction of molecular oxygen (10, 14, 15). Cells are equipped with several defense systems to protect them from the harmful effects of these reactive oxygen species (36). They include antioxidants, such as reduced glutathione (GSH; l-γ-glutamyl-l-cysteinylglycine) and enzymes (catalases and superoxide dismutases). GSH is usually the most abundant intracellular low-molecular-weight thiol in Escherichia coli. It is a major component of all the cell systems involved in protection against oxidants and free-radical-mediated cell injuries, since the redox status (E0′ = −240 mV) is mainly maintained by thiol-disulfide equilibrium inside the bacterial cell (2, 24, 32). GSH is also indirectly implicated in the regulation of the OxyR transcription factor (38), SoxR (7), and SOS (26) systems.
Recently, the question was raised of the benefit of such a protective system for microorganisms in specific environments, such as drinking water distribution networks, in which the bacteria grow in a low-nutrient environment (17, 33) that contains variable concentrations of oxidative species, such as chlorine used for postdisinfection (4). Water distribution networks are continuously colonized by autochthonous bacteria which are impossible to eradicate with the usual disinfectants. The limited transfer of oxidants through biofilms and bacterial envelopes (6, 25, 30, 31) and genetically controlled resistance are the two major explanations for this situation. Glutathione homeostasis and a high intracellular GSH level were recently proposed as a potential resistance pathway of E. coli cells to chlorine (5).
In this study we tested the validity of the hypothesis that oxygenation (oxygenation of the growth medium from 10% to saturation) and starvation (24 h of starvation in minimal medium) of cultures of E. coli lead to significant changes in glutathione homeostasis (concentration of GSH and its precursor, the γ-glutamylcysteine Glucys) and hence modify E. coli sensitivity to further chlorine disinfection.
Bacterial models.
Experiments were carried out with an isogenic pair of E. coli strains, since GSH was not detectable in indigenous biomass recovered from drinking water treatment plants. E. coli AB1157 and the GSH-deficient strain JTG10 (derived in Bruce Demple’s laboratory, Harvard University, Cambridge, Mass.), reported to be deficient in GSH synthetase (13), were provided by Danièle Touati (Institut Jacques Monod, Paris, France). E. coli AB1157 and JTG10 were grown in completely mixed 5-liter chemostats (dilution rate, 0.05 h−1; 30 ± 1°C; regulated oxygen) and continuously fed with Luria-Bertani (LB) medium (Difco), and 50 μg of kanamycin monosulfate ml−1 (final concentration) was added every 24 h to the JTG10 culture. The steady-state bacterial density in the reactor was ca. 2 × 109 ml−1 (determined by 4′,6-diamidino-2-phenylindole [DAPI] staining) (31), including about 50% culturable bacteria (counted as CFU after dilution in sterile 0.9% NaCl solution and incubation for 48 h at 30 ± 2°C in LB agar [Difco] containing 50 μg of kanamycin ml−1). The E. coli parental strain (AB1157) produced GSH (ca. 6 μmol/1012 cells, which is close to previously reported values) (13). A cellular GSH level was measurable in the mutant strain (JTG10), but the values were always low (5- to 35-fold lower than that of its parent strain) (Table 1). This fact could be explained by a mutation mechanism that was easily reversible even when the strain was cultured in the presence of kanamycin. Nevertheless, the difference between the GSH levels of the two strains was considered great enough to use them as an experimental model for the examination of the role of GSH in the response to stress factors.
TABLE 1.
Glucys, GSH, and GSHt concentrations in E. coli AB1157 and JTG10 cultured under different oxygenation conditionsa
| E. coli strain | Oxygenation rate (%) | Glucys (μmol/1012 cells) | GSH (μmol/1012 cells) | GSHt (μmol/1012 cells) | GSH/GSHt (%) |
|---|---|---|---|---|---|
| AB1157 | 10 (n = 1) | 0.040 | 5.02 | 5.06 | 99 |
| 50 (n = 3) | 0.20 ± 0.08 | 5.93 ± 0.59 | 6.51 ± 1.04 | 92 | |
| 95 (n = 6) | 0.19 ± 0.04 | 7.59 ± 1.90 | 8.37 ± 2.17 | 94 | |
| 100 (n = 3) | 0.03 ± 0.01 | 13.49 ± 0.62 | 16.92 ± 0.78 | 80 | |
| JTG10 | 10 (n = 1) | 0.04 | 0.95 | 1.29 | 74 |
| 50 (n = 3) | 0.06 ± 0.03 | 1.11 ± 0.85 | 1.27 ± 0.98 | 93 | |
| 95 (n = 6) | 0.07 ± 0.03 | 1.37 ± 0.72 | 1.40 ± 0.65 | 96 | |
| 100 (n = 3) | 0.02 ± 0.01 | 0.42 ± 0.13 | 0.49 ± 0.20 | 87 |
The data are reported with standard deviations.
Determination of thiol concentrations.
Suspensions of bacteria (180 ml) were centrifuged at 10,000 × g for 20 min at 20°C. The pellets were washed twice by resuspending them in 0.9% NaCl and centrifuging them at 10,000 × g for 5 min at 20°C. Thiols were extracted from the bacteria by immediately suspending each pellet in 10 ml of 3.3% HClO4 containing 2 mM disodium EDTA, with vigorous vortexing and sonication for 5 min in an ultrasound bath (Prolabo 670/H; power, 9) at 6°C. The suspensions were incubated for 10 min in an ice bath and centrifuged at 10,000 × g for 5 min at 4°C. The resulting supernatants were immediately frozen and stored in liquid nitrogen. The frozen samples were fast thawed in a water bath at 30°C and diluted with cold 0.1 M HCl containing 2 mM disodium EDTA (4°C), and a 50-μl aliquot was injected onto the column of the high-performance liquid chromatography system optimized for thiol measurement (18). This technique included reverse-phase separation, postcolumn derivatization with ortho-phthalaldehyde, and fluorimetric detection. The technique selectively measured the GSH and Glucys. Total GSH (GSHt; GSH plus oxidized forms [symmetrical and mixed disulfides]) was measured by the same high-performance liquid chromatography technique, but the samples were reduced with 1,4-d,l-dithiothreitol. GSHt was expressed as GSH equivalents. Thiol concentrations were expressed as micromoles (or micromolar concentrations) per 1012 total cells.
Chlorination assay.
The washed bacterial suspension (20 ml) to be tested was filtered through a 5-μm-pore-size filter (Millipore) to remove or break up the bacterial aggregates. The filtered suspension was aseptically placed in 500-ml brown flasks (previously cleaned of trace organic matter by heating them to 550°C for 4 h) containing 400 ml of sterile phosphate-buffered saline (PBS). Sodium hypochlorite (20 mg liter−1) was added to the diluted E. coli suspension (approximately 106 cells ml−1) to obtain final concentrations of from 2 × 10−3 to 0.1 mg of Cl2 liter−1 (one flask used as a control was not spiked with chlorine). Solutions of sodium hypochlorite (Sigma Chemical Co.) (20 mg liter−1) were prepared daily in sterile bacterium-free distilled water and adjusted to pH 7.0 with dilute HCl. The sodium hypochlorite concentration was determined by the N,N-diethyl-p-phenylene diamine method (1), and the results were expressed in milligrams of chlorine liter−1. The flasks were incubated for 30 min at 20°C in the dark with gentle shaking (160 rpm); then traces of residual chlorine were neutralized by adding sterile sodium thiosulfate (final concentration, 0.02%) to all flasks. The culturable bacteria were then counted, and the number of surviving bacteria was determined as CFU per milliliter. Plate counts (CFU/milliliter) were log10 transformed, and the means and standard deviations of the means were calculated. The calculated means for each assay corresponding to each chlorine concentration tested were compared by one-way analysis of variance (P = 0.05) (11). Differences in the chlorine sensitivities of the two strains and the influence of oxygenation rates or starvation were then analyzed. All data were evaluated by analysis of variance testing with StatView F.4.5. (Abacus Concepts, Inc., Berkeley, Calif.).
Effect of oxygenation on E. coli cultures.
The E. coli cultures were oxygenated with pure oxygen at 10, 50, 95, and 100% oxygen saturation of the medium (n = 1 for each value). One hundred milliliters of steady-state AB1157 and JTG10 cultures was washed twice by centrifugation at 10,000 × g for 5 min at 20°C and resuspended in 200 ml of sterile PBS (pH 6.5) (the washing protocol did not decrease the viable CFU [data not shown]). Samples (20 ml) were used for disinfection assays, and 180 ml was used to measure thiol concentrations. Changing the oxygenation rate of the parental E. coli growth cultures (AB1157) from 10 to 100% (Table 1) drastically increased its GSH content (threefold). There was a marked conversion of GSH to the oxidized form, as indicated by the decrease in the GSH/GSHt ratio from 99 to 80%. The concentration of the GSH precursor, Glucys, also increased when the oxygenation rate was increased from 10 to 50% and decreased when the oxygenation rate reached 100%. The E. coli mutant (JTG10) was unable to restore its internal redox potential by overproducing GSH, especially when the oxygenation rate increased. The GSH content of JTG10 remained stable (ca. 1 μmol/1012 cells) and dropped at the highest oxygenation rate (Table 1). The toxicity of molecular oxygen in the culture medium and its reactive species for the mutant strain was particularly dramatic, and the mutant was unable to adapt to this oxidative environment, as shown by a drop in CFU (2.5-fold) and in the total number of cells (1.6-fold) (data not shown).
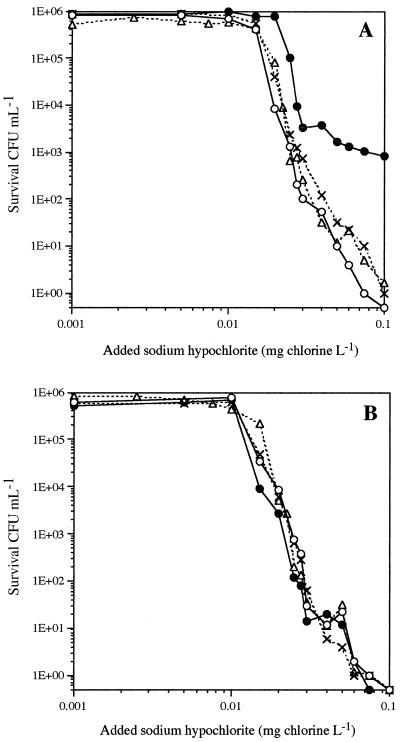
The sensitivities to chlorine of the two strains grown under low-oxygenation conditions (10%) were very similar, with 6-log-unit inactivation of the bacterial populations exposed to 0.1 mg of chlorine for 30 min at 30°C (Fig. 1). Increasing the oxygenation of the culture increased the resistance of the parental strain (P > 99.9%) to chlorine (Fig. 1A). The maximum oxygenation rate (100%) gave rise to a subpopulation of AB1157 with a high resistance to chlorine; they resisted 0.1 mg of chlorine for 30 min. The mutant strain showed no change in its sensitivity to chlorination (P > 95%), regardless of the previous oxygenation growth conditions (Fig. 1B).
FIG. 1.
Sensitivity of the E. coli parental strain (A) and GSH mutant (B) to exposure to chlorine for 30 min at 20°C. Cultures of parental (AB1157) and mutant (JTG10) strains were grown in a reactor with oxygen regulation at 10% (○), 50% (×), 95% (▵), or 100% (●) oxygen saturation of the medium (n = 1).
It was recently demonstrated that oxidative stress (sublethal doses of H2O2) (8) made E. coli suspensions more resistant to chlorine. We have also demonstrated that high oxygenation of the wild-type E. coli strain shifts glutathione homeostasis towards higher concentration of GSH and increases resistance to chlorine. These results assume that E. coli possesses a general response to oxidative stress that is independent of the nature of the oxidant and that GSH homeostasis plays a key role in stimulating this mechanism. GSH could protect bacteria against chlorination by acting as a scavenger, as previously shown (5), and also probably by triggering defense systems. The changes in the chlorine sensitivity of E. coli lacking gorA (coding for GSH reductase) after various stress factors could be used to test this method of chlorine defense regulation.
Effect of starvation on E. coli cultures.
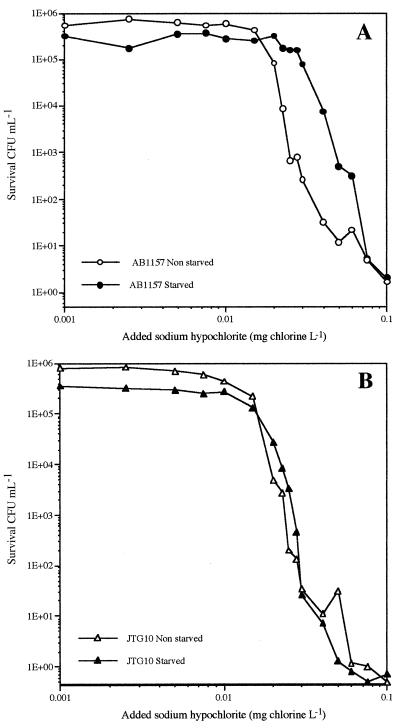
Cells were starved by incubating 200 ml of the washed AB1157 and JTG10 suspensions (obtained with a 95% oxygenation rate at 30°C) with gentle shaking (160 rpm) in 1-liter sterile flasks for 24 h. The starved cells were then washed by centrifugation at 10,000 × g for 5 min at 20°C and resuspended in 200 ml of PBS (pH 6.5), and the chlorination assays and thiol analysis were performed. All assays were carried out in triplicate. The populations of bacteria showed a slight decrease in the number of culturable bacteria (from 5.4 × 108 to 3.0 × 108 CFU per ml), while the total number of cells did not decrease (around 14.5 × 108 per ml). Lack of nutrients for a relatively long period led to a major reduction in GSH and to an increased fraction of its oxidized form and, hence, a decrease in the GSH/GSHt ratio (Table 2). The starved parental strain was more resistant to chlorine up to 0.05 mg/liter than the unstarved population (P > 99.9%), in spite of a threefold decrease in GSH (Fig. 2A). The sensitivity of the mutant strain to chlorine did not change after starvation (P < 95%) (Fig. 2B).
TABLE 2.
Glucys, GSH, and GSHt concentrations in E. coli AB1157 and JTG10 before and after 24 h of starvation in PBSa
| E. coli strain | Starvation | Glucys (μmol/1012 cells) | GSH (μmol/1012 cells) | GSHt (μmol/1012 cells) | GSH/GSHt (%) |
|---|---|---|---|---|---|
| AB1157 | Before | 0.014 ± 0.002 | 6.99 ± 0.38 | 7.39 ± 0.39 | 94.6 ± 0.3 |
| After | 0.07 ± 0.01 | 0.84 ± 0.06 | 2.48 ± 0.59 | 35.1 ± 8.6 | |
| JTG10 | Before | 0.005 ± 0.0003 | 1.27 ± 0.09 | 1.31 ± 0.10 | 97.0 ± 19.1 |
| After | 0.013 ± 0.003 | 0.12 ± 0.08 | 0.17 ± 0.08 | 66.6 ± 19.4 |
The data are reported with standard deviations; n = 3.
FIG. 2.
Sensitivity to chlorine of dilute suspensions of GSH-replete (A) and GSH-deficient (B) E. coli cells grown in LB medium (open symbols) and after 24 h (solid symbols) of starvation in phosphate buffer (pH 6.5) at 30°C. The number of surviving bacteria represent the average of three separate experiments.
Starvation is one of the most important factors that influence the sensitivity of bacteria to disinfectants (3, 19, 22, 23, 28, 35). However, the way in which bacteria develop resistance is poorly understood. The consensus is that the resistance of starved bacteria to chlorine is primarily due to limited chlorine transfer to intracellular target bacteria because of changes in the cell membrane permeability (19, 37), accumulation of exopolymers on the bacterial cell surface (29), and/or formation of aggregates (12, 27, 34, 35). We have demonstrated that only the wild-type GSH-containing E. coli strain increased its resistance to chlorine after 24 h of starvation, while the mutant strain without GSH did not develop such a resistance. These results suggest that GSH metabolism plays a role in the general starvation response, perhaps by activation of ςS, the major regulator of the general starvation response in E. coli produced by the rpoS gene (21). This potential action of GSH in the regulation of starvation has never been described, but it was demonstrated that GSH increased significantly during the transition from exponential to stationary phase (9, 20). More work is needed to understand the importance of GSH in the defense of starving cells against chlorine.
We conclude that starvation and oxidative stress cause E. coli to become resistant to chlorine in less than 24 h. GSH plays a key role in the cell defense against chlorine, acting as an oxidant scavenger and activator of defense systems. The same pathways involving GSH are probably also implicated in defense against other disinfectants, such as chloramines, H2O2, and ozone, which all deplete the intracellular GSH pool (5, 16). Tap water treatment processing includes the reduction of organic matter and the inactivation of bacteria by ozonation and chlorination. The resulting low nutrient level and the initial exposure of bacteria to oxidants could make the final chlorination less effective. Consequently, the ability of E. coli to remain physiologically active and develop resistance to chlorine after sublethal stresses has potential public health implications and may require further changes in water treatment practices, particularly where the disinfectant residual in the water is less than 0.1 mg of chlorine/liter.
Acknowledgments
This work was carried out as part of a larger research program (Biofilm IV) coordinated by the Centre International de l’Eau de Nancy (NANCIE-France) and funded by the Générale des Eaux (Paris, France), the Communauté Urbaine du Grand Nancy, the Syndicat des Eaux d’Ile de France (Paris, France), the Office National de l’Eau Potable (ONEP-Maroc), the Agence de l’Eau Seine-Normandie (Paris, France), Pont-à-Mousson S.A. (France), and NANCIE.
We thank D. J. Reasoner for his critical review of the manuscript and suggestions.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 19th ed. Washington, D.C.: American Public Health Association; 1995. [Google Scholar]
- 2.Aslund F, Berndt K D, Homgren A. Redox potentials of glutaredoxins and other thiol-disulfide oxidoreductases of the thioredoxin superfamily determined by direct protein-protein redox equilibria. J Biol Chem. 1997;272:30780–30786. doi: 10.1074/jbc.272.49.30780. [DOI] [PubMed] [Google Scholar]
- 3.Berg J D, Matin A, Roberts P V. Effects of antecedent growth conditions on sensitivity of Escherichia coli to chlorine dioxide. Appl Environ Microbiol. 1982;144:814–819. doi: 10.1128/aem.44.4.814-819.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block J C. Biofilms in drinking water distribution systems. In: Melo L F, Bott T R, Fletcher M, Capdeville B, editors. Biofilms—science and technology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 469–485. [Google Scholar]
- 5.Chesney J A, Eaton J W, Mahoney J R. Bacterial glutathione: a sacrificial defense against chlorine compounds. J Bacteriol. 1996;178:2131–2135. doi: 10.1128/jb.178.7.2131-2135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBeer D, Srinivasan R, Stewart P S. Direct measurement of chlorine penetration into biofilms during disinfection. Appl Environ Microbiol. 1994;60:4339–4344. doi: 10.1128/aem.60.12.4339-4344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding H, Demple B. Glutathione-mediated destabilization in vitro of [2Fe-2S] centers in the SoxR regulatory protein. Proc Natl Acad Sci USA. 1996;93:9449–9453. doi: 10.1073/pnas.93.18.9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dukan S, Touati D. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. J Bacteriol. 1996;178:6145–6150. doi: 10.1128/jb.178.21.6145-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahey R C, Brown W C, Adams W B, Worsham M B. Occurrence of glutathione on bacteria. J Bacteriol. 1978;133:1126–1129. doi: 10.1128/jb.133.3.1126-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 11.Fry J, editor. Biological data analysis: a practical approach. 1st ed. New York, N.Y: IRL Press; 1993. [Google Scholar]
- 12.Gauthier V, Redercher S, Block J C. Chlorine inactivation of Sphingomonas cells attached to goethite particles in drinking water. Appl Environ Microbiol. 1999;65:355–357. doi: 10.1128/aem.65.1.355-357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg J T, Demple B. Glutathione in Escherichia coli is indispensable for resistance to H2O2 and gamma radiation. J Bacteriol. 1986;124:140–148. doi: 10.1128/jb.168.2.1026-1029.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halliwell B, Gutteridge J M C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imlay J A, Fridovich I. Assay of metabolic superoxide production in Escherichia coli. J Biol Chem. 1991;266:6957–6965. [PubMed] [Google Scholar]
- 16.Komanapalli I R, Mudd J B, Benjamin H S L. Effect of ozone on metabolic activities of Escherichia coli K-12. Toxicol Lett. 1997;90:61–66. doi: 10.1016/s0378-4274(96)03830-1. [DOI] [PubMed] [Google Scholar]
- 17.LeChevallier M W, Cawthon C D, Lee R G. Factors promoting survival of bacteria in chlorinated water supplies. Appl Environ Microbiol. 1988;54:649–654. doi: 10.1128/aem.54.3.649-654.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leroy P, Nicolas A, Wellmann M, Michelet F, Oster T, Siest G. Evaluation of o-phthalaldehyde as bifunctional fluorogenic post-column reagent for glutathione in LC. Chromatographia. 1993;36:130–134. [Google Scholar]
- 19.Lisle J T, Broadaway S C, Prescott A M, Pyle B H, Fricker C, McFeters G A. Effects of starvation on physiological activity and chlorine disinfection resistance in Escherichia coli O157:H7. Appl Environ Microbiol. 1998;64:4658–4662. doi: 10.1128/aem.64.12.4658-4662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loewen P C. Levels of glutathione in Escherichia coli. Can J Biochem. 1979;57:107–111. doi: 10.1139/o79-013. [DOI] [PubMed] [Google Scholar]
- 21.Loewen P C, Hengge-Aronis R. The role of the sigma factor ςS (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 22.Matin A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol Microbiol. 1991;5:3–10. doi: 10.1111/j.1365-2958.1991.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 23.Matin A, Harakeh S. Effect of starvation on bacterial resistance to disinfectants. In: McFeters G A, editor. Drinking water microbiology. New York, N.Y: Springer-Verlag; 1990. pp. 88–103. [Google Scholar]
- 24.Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- 25.Morin P, Gauthier V, Saby S, Block J C. Bacterial resistance to chlorine through attachment to particles and pipe surfaces in drinking water distribution systems. In: Keevil C W, Godfree A F, Holt D M, Dow C S, editors. Biofilms in the aquatic environment. Cambridge, United Kingdom: Royal Society of Chemistry; 1999. pp. 171–190. [Google Scholar]
- 26.Müller J, Janz S. Modulation of the H2O2-induced SOS response in Escherichia coli PQ300 by amino acids, metal chelators, antioxidants, and scavengers of reactive oxygen species. Environ Mol Mutagen. 1993;22:157–163. doi: 10.1002/em.2850220308. [DOI] [PubMed] [Google Scholar]
- 27.Power K N, Schneider R P, Marshall K C. The effect of growth conditions on survival and recovery of Klebsiella oxytoca after exposure to chlorine. Water Res. 1997;31:135–139. [Google Scholar]
- 28.Preez M, Kfir R, Coubrough P. Investigation of injury of coliforms after chlorination. Water Sci Technol. 1995;31:115–118. [Google Scholar]
- 29.Rudd T, Sterritt R M, Lester J M. The use of extraction methods for the quantification of extracellular polymer production by Klebsiella aerogenes under varying cultural conditions. Eur J Appl Microbiol Biotechnol. 1982;16:23–27. [Google Scholar]
- 30.Russell A D, Chopra I, editors. Understanding antibacterial action and resistance. Chichester, United Kingdom: Ellis Horwood; 1990. pp. 96–146. [Google Scholar]
- 31.Saby S, Sibille I, Mathieu L, Paquin J L, Block J C. Influence of water chlorination on the counting of bacteria with DAPI (4′,6-diamidino-2-phenylindole) Appl Environ Microbiol. 1997;63:1564–1569. doi: 10.1128/aem.63.4.1564-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.She Z W, Mays D C, Sagone A L, Davis W B. Aminobenzoic acid compounds as HOCl traps for activated neutrophils. Free Radic Biol Med. 1997;22:989–998. doi: 10.1016/s0891-5849(96)00486-8. [DOI] [PubMed] [Google Scholar]
- 33.Sibille I, Mathieu L, Paquin J L, Gatel D, Block J C. Microbial characteristics of a distribution system fed with nanofiltered drinking water. Water Res. 1997;31:2318–2326. [Google Scholar]
- 34.Stewart M, Olson B. Impact of growth conditions on resistance of Klebsiella pneumoniae to chloramines. Appl Environ Microbiol. 1992;58:2649–2653. doi: 10.1128/aem.58.8.2649-2653.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart M, Olson B. Physiological studies of chloramine resistance developed by Klebsiella pneumoniae under low-nutrient growth conditions. Appl Environ Microbiol. 1992;58:2918–2927. doi: 10.1128/aem.58.9.2918-2927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storz G, Tartaglia L A, Farr S B, Ames B N. Bacterial defense against oxidative stress. Science. 1990;248:189–192. [Google Scholar]
- 37.Wang G, Doyle M P. Survival of enterohemorrhagic Escherichia coli O157:H7 in water. J Food Prot. 1998;61:662–667. doi: 10.4315/0362-028x-61.6.662. [DOI] [PubMed] [Google Scholar]
- 38.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]