Abstract
The amount of polyphosphate in the intraradical and extraradical hyphae of Gigaspora margarita was estimated from successive extractions with trichloroacetic acid (TCA), EDTA, and phenol-chloroform (PC). In the intraradical hyphae, most of the polyphosphate was present in TCA- and EDTA-soluble (short-chain and long-chain) fractions, whereas most of the polyphosphate in the extraradical hyphae was present in EDTA- and PC-soluble (long-chain and granular) fractions.
The enhanced growth of plants colonized by arbuscular mycorrhizal (AM) fungi has been recognized to result from the supply of absorbed mineral nutrients, particularly phosphorus, by extraradical hyphae (22). It is now widely accepted that phosphate present in the soil is taken up into the extraradical hyphae by a phosphate transporter (12), subsequently condensed into polyphosphate (poly-P), and translocated by protoplasmic streaming into the intraradical hyphae (6–8). Thus, poly-P is considered to be a key phosphorus compound in the translocation processes. However, since the pioneering work in the 1980s, no quantification of poly-P in the hyphae of AM fungi has been performed. Because of the obligately symbiotic nature of AM fungi, the preparation of hyphae, particularly intraradical hyphae, is a major obstacle in studying their poly-P concentration. However, by a combination of enzyme digestion and Percoll centrifugation, a method which enables the investigation of the enzymes and the metabolic activities of intraradical hyphae in vitro (9, 10, 13, 23) was developed (20). In this study, therefore, we quantified poly-P in the hyphae of an AM fungus, Gigaspora margarita, and we employed a successive extraction procedure which does not hydrolyze long-chained poly-P to shorter chains (5).
Onion (Allium cepa L.) plants were inoculated with G. margarita MAFF 520054 and grown for 6 and 9 weeks after transplanting, as described previously (23). The intraradical hyphae were collected by a combination of enzyme digestion and Percoll centrifugation (20, 23). Extraradical hyphae were collected by the wet-sieving and decanting method (1). The fresh weight of hyphae was recorded after careful removal of excess moisture with small pieces of filter paper. Hyphal length was measured by the grid intersection method (23). Specific hyphal length was calculated from these measurements.
Different poly-P fractions in the hyphae were successively extracted with trichloroacetic acid (TCA), EDTA, and phenol-chloroform (PC), based upon the method of Clark et al. (5), except that the samples were first ground in ice-cold 2% aqueous TCA with zirconia beads by using a Bead-beater (Biospec Products, Bartlesville, Okla.). The extracted poly-P in aqueous solution was precipitated by adding Tris-HCl (1 M, pH 7.6) to a final concentration of 0.2 M and 2 volumes of acetone. The mixture was frozen at −80°C for more than 15 min, melted, and then centrifuged for 10 min. The residue was air dried, dissolved with water, and kept at −20°C until analysis. The poly-P content in the extracts was determined by measuring the metachromatic reaction of toluidine blue at 630 nm (11). The extracts were first treated with RNase A at 37°C for 45 min, and then the assay was performed by adding 10 μl of the poly-P sample to tubes containing 0.75 ml each of acetic acid (0.2 M) and toluidine blue (30 mg liter−1). The amount of poly-P was determined within 15 min of color development by comparison with a standard curve produced by using 1 to 5 μg of type 35 poly-P for the short-chain and type 65 for the long-chain poly-P fractions. Synthetic poly-P glasses of types 65, 35, and 15 were obtained from Sigma Chemical Co. Total P concentrations in hyphae were determined by digestion of the hyphae (ca. 20 to 40 mg [fresh weight]) in concentrated H2SO4 by using H2O2 as an oxidant for 1 h at approximately 200°C. Inorganic phosphate concentrations in the digests were colorimetrically determined (25).
Gel electrophoresis of RNase-treated samples was performed with Tris borate buffer and 10% polyacrylamide gels (1 by 120 by 150 mm). Ten microliters of the extract was loaded with glycerol, xylene cyanol, and bromophenol blue, and electrophoresis was performed at 120 V for about 75 min or until the sample dye, xylene cyanol, had migrated 2.5 cm and the reference marker dye, bromophenol blue, had migrated 5 cm. Poly-P on the gel was stained pinkish purple according to the method of Pepin and Wood (18).
Mycorrhizal colonization was 64% at 6 weeks and 73% at 9 weeks after transplanting. The extraradical hyphal biomass was almost half the intraradical hyphal biomass, because spore biomass was not included (Table 1). Specific hyphal length was within a range of 5.2 to 5.9 km g−1 (fresh weight). The differences in specific hyphal length between intraradical and extraradical hyphae or between sampling times were not large.
TABLE 1.
Hyphal biomass, length, and poly-P contents in G. margarita
| Wk | Type of hyphae | Biomassa (mg g−1 of colonized root) | Specific hyphala length (km of hyphae g−1) | Poly-P (μg of P g−1 [fresh wt])a
|
Poly-P/total P (%) | |||
|---|---|---|---|---|---|---|---|---|
| TCA solubleb | EDTA solubleb | PC solubleb | Total | |||||
| 6 | Intraradical | 26.2 ± 1.5 | 5.2 ± 0.1 | 137 ± 1.1 (40) | 175 ± 1.4 (52) | 28 ± 1.5 (8) | 340 ± 4 | 5.4 |
| Extraradical | 13.3 ± 0.6 | 5.3 ± 0.2 | 26 ± 2.0 (9) | 192 ± 3.6 (66) | 71 ± 4.3 (25) | 289 ± 10 | 8.4 | |
| 9 | Intraradical | 21.9 ± 0.8 | 5.9 ± 0.1 | 59 ± 0.9 (29) | 75 ± 1.4 (36) | 72 ± 0.6 (35) | 206 ± 3 | 7.7 |
| Extraradical | 7.2 ± 0.2 | 5.2 ± 0.2 | 15 ± 0.5 (8) | 102 ± 2.2 (56) | 65 ± 0.3 (36) | 182 ± 3 | 17.3 | |
Data are the means of triplicates ± standard deviations.
The percentage of each fraction in total poly-P is shown in parentheses.
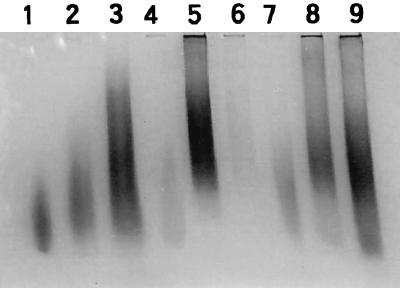
Poly-P was successively extracted into TCA-, EDTA-, and PC-soluble fractions. The recovery test, in which standard poly-P was added in each extraction step, revealed a >95% recovery rate. The enzyme digestion process of hyphae did not affect poly-P content or composition in any fraction. Poly-P in mycorrhizal hyphae showed a smear band which stained pinkish purple with toluidine blue (γ-metachromasy), indicating a range of poly-P molecules with different chain lengths in each extract (Fig. 1). Although the bands of poly-P in TCA-, EDTA-, and PC-soluble fractions overlapped each other in relative mobility, the poly-P band migrated with increasing speed in this order. Thus, we can assign short-chain, long-chain, and long-chain granular poly-P approximately to TCA-, EDTA-, and PC-soluble fractions, respectively, according to the system of Clark et al. (5). The band from the TCA-soluble fraction showed relatively faint staining compared to the others. This may have been due to the lower metachromasy of short-chain poly-P than that of long-chain poly-P (15).
FIG. 1.
Polyacrylamide gel of fractionated extracts of intraradical and extraradical hyphae collected at 6 weeks after transplanting for comparison with poly-P standards. Lanes 1 to 3, synthetic poly-P standards of average chain lengths (15, 35, and 65 Pi units, respectively); lanes 4 to 6, TCA-, EDTA-, and PC-soluble fractions, respectively of, poly-Ps from intraradical hyphae; lanes 7 to 9, TCA-, EDTA-, and PC-soluble fractions, respectively, of poly-Ps from extraradical hyphae. This figure is not a quantitative comparison between intraradical and extraradical hyphae because the hyphal biomass taken for extraction was different.
The quantification of poly-P in each fraction by the toluidine blue assay clearly demonstrated the differences in the composition of poly-P fractions between intraradical and extraradical hyphae. It was first observed that the proportion of short-chain poly-P to total poly-P was higher in the intraradical hyphae and that the proportion of long-chain poly-P was higher in the extraradical hyphae (Table 1). This suggests that poly-P synthesis to form long chains occurred in extraradical hyphae, possibly because long-chain granular molecules are more efficient in transporting phosphorus into the intraradical part of the fungi.
Poly-P was often determined as acid-hydrolyzable phosphate in the extracts (5). However, almost half of poly-P in EDTA- and PC-soluble fractions was not easily hydrolyzed with 1 N HCl, while poly-P in the TCA-soluble fraction was almost completely hydrolyzed (data not shown). Poly-P molecules in the hyphae of AM fungi have been considered to be mainly present in a granular form in vacuoles, based upon transmission electron microscopy (7, 19). However, it was reported that poly-P granules found in ectomycorrhizal fungal hyphae prepared for transmission electron microscopy were artifacts of the fixation processes (17), although this has been disputed recently (2). The present result suggests that, at least from the viewpoint of hydrolyzability, granular poly-P is present in the hyphae of AM fungi.
Poly-P concentrations in the hyphae of G. margarita ranged from 5.4 to 17.3% of total P content and were within the range reported earlier for the hyphae of mycorrhizal fungi, including Glomus mosse (4), some ericoid mycorrhizal fungi in culture (24), and some ectomycorrhizal fungi in culture (16).
Two possible mechanisms may account for the higher proportion of short-chain poly-P in intraradical hyphae. Firstly, the long-chain poly-P may be partly hydrolyzed into shorter chains with endopolyphosphatase (3, 14). Depolymerized short-chain poly-P may be further hydrolyzed with exopolyphosphatase to liberate inorganic phosphate. Secondly, long-chain poly-P may act as a phosphoryl donor for poly-P glucokinase so that only short-chain poly-P remains. Poly-P glucokinase of the anaerobic bacterium Propionibacterium shermanii uses long-chain poly-P preferentially, followed by the accumulation of short-chain poly-P (18). Poly-P glucokinase activity was previously found in the crude extract of mycorrhizal roots (4), and glucose is used by intraradical hyphae (21, 23). Glucose-6-phosphate produced from poly-P and glucose can serve as an intermediate both for liberation of inorganic phosphate and for glucose assimilation (9). Both mechanisms may operate in the hyphae of G. margarita.
In the present study, we have, for the first time, quantified the poly-P contents in both intraradical and extraradical hyphae of the AM fungus G. margarita, and we have also found that the poly-P chain was shorter in intraradical hyphae than in extraradical hyphae. The latter observation may be related to phosphate metabolism in arbuscular mycorrhizas. Investigation of the expression and regulation of the enzymes involved in poly-P metabolism may shed light on the storage, transport, and metabolism of phosphate in AM systems.
Acknowledgments
M.Z.S. is grateful to the Japan Science and Technology Corporation for providing a postdoctoral fellowship. This study was also partly supported by a grant-in-aid (Bio-media program) from the Ministry of Agriculture, Forestry and Fisheries, Japan.
REFERENCES
- 1.Bethlenfalvay G J, Ames R N. Comparison of two methods of quantifying extraradical mycelium of vesicular-arbuscular mycorrhizal fungi. Soil Sci Soc Am J. 1987;51:834–837. [Google Scholar]
- 2.Bucking H, Heyser W. Elemental composition and function of polyphosphates in ectomycorrhizal fungi—an X-ray microanalytical study. Mycol Res. 1999;103:31–39. [Google Scholar]
- 3.Callow J A, Capaccio L C M, Parish G, Tinker P B. Detection and estimation of polyphosphate in vesicular-arbuscular mycorrhizas. New Phytol. 1978;80:125–134. [Google Scholar]
- 4.Capaccio L C M, Callow J A. The enzymes of polyphosphate metabolism in vesicular-arbuscular mycorrhizas. New Phytol. 1982;91:81–91. [Google Scholar]
- 5.Clark J E, Beegen H, Wood H G. Isolation of intact chains of polyphosphate from “Propionibacterium shermanii” grown on glucose or lactate. J Bacteriol. 1986;168:1212–1219. doi: 10.1128/jb.168.3.1212-1219.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper K M, Tinker P B. Translocation and transfer of nutrients in vesicular-arbuscular mycorrhizas fungi. IV. Effect of environmental variables on movement of phosphorus. New Phytol. 1981;88:327–339. [Google Scholar]
- 7.Cox G C, Sanders F E, Tinker P B, Wild J A. Ultrastructural evidence relating to host-endophyte transfer in a vesicular-arbuscular mycorrhiza. In: Sanders F E, Mosse B, Tinker P B, editors. Endomycorrhizas. New York, N.Y: Academic Press; 1975. pp. 297–312. [Google Scholar]
- 8.Cox G C, Moran K J, Sanders F, Nockolds C, Tinker P B. Translocation and transfer of nutrients in vesicular-arbuscular mycorrhizas. III. Polyphosphate granules and phosphorus translocation. New Phytol. 1980;84:649–659. [Google Scholar]
- 9.Ezawa T, Kuwahara S, Yoshida T, Saito M. Specific inhibitor and substrate specificity of alkaline phosphatase expressed in the symbiotic phase of the arbuscular mycorrhizal fungus Glomus etunicatum. Mycologia. 1999;91:636–641. [Google Scholar]
- 10.Ezawa T, Saito M, Yoshida T. Comparison of phosphatase localization in the intraradical hyphae of arbuscular mycorrhizal fungi, Glomus spp. and Gigaspora spp. Plant Soil. 1995;176:57–63. [Google Scholar]
- 11.Griffin J B, Davidian N M, Penniall R. Studies of phosphorus metabolism by isolated nuclei. VII. Identification of polyphosphate as a product. J Biol Chem. 1965;240:4427–4434. [PubMed] [Google Scholar]
- 12.Harrison M J, van Buuren M L. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature. 1995;378:626–629. doi: 10.1038/378626a0. [DOI] [PubMed] [Google Scholar]
- 13.Kojima T, Hayatsu M, Saito M. Intraradical hyphae phosphatase of the arbuscular mycorrhizal fungus, Gigaspora margarita. Biol Fertil Soils. 1998;26:331–335. [Google Scholar]
- 14.Kumble K D, Kornberg A. Endopolyphosphatases for long-chain inorganic polyphosphate in yeast and mammals. J Biol Chem. 1996;271:27146–27151. doi: 10.1074/jbc.271.43.27146. [DOI] [PubMed] [Google Scholar]
- 15.Lorenz B, Münkner J, Oliveira M P, Leitãno J M, Müller W E G, Schröder H C. A novel method for determination of inorganic polyphosphates using the fluorescent dye fura-2. Anal Biochem. 1994;246:176–184. doi: 10.1006/abio.1996.9998. [DOI] [PubMed] [Google Scholar]
- 16.Martin F, Canet D, Rolin D, Marchal J-P, Larher F. Phosphorus-31 nuclear magnetic resonance study of polyphosphate metabolism in intact ectomycorrhizal fungi. Plant Soil. 1983;71:469–476. [Google Scholar]
- 17.Orlovich D A, Ashford A E. Polyphosphate granules are an artifact of specimen preparation in the ectomycorrhizal fungus, Pisolithus tinctorius. Protoplasma. 1993;178:66–80. [Google Scholar]
- 18.Pepin C A, Wood H G. Polyphosphate glucokinase from Propionibacterium shermanii. Kinetics and demonstration that the mechanism involves both processive and nonprocessive type reactions. J Biol Chem. 1986;261:4476–4480. [PubMed] [Google Scholar]
- 19.Peterson R L, Howarth M J. Morphometric and energy dispersive X-ray analysis of polyphosphate distribution in the VAM fungus Glomus versiforme associated with leek. Symbiosis. 1991;11:63–72. [Google Scholar]
- 20.Saito M. Enzyme activities of the internal hyphae and germinated spores of an arbuscular mycorrhizal fungus, Gigaspora margarita Becker & Hall. New Phytol. 1995;129:425–431. [Google Scholar]
- 21.Shachar-Hill Y, Pfeffer P E, Douds D, Osman S F, Doner L W, Ratcliffe R G. Partitioning of intermediary carbon metabolism in vesicular-arbuscular mycorrhizal leek. Plant Physiol (Rockville) 1995;108:7–15. doi: 10.1104/pp.108.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith S E, Read D J. Mycorrhizal symbiosis. 2nd ed. London, England: Academic Press; 1997. [Google Scholar]
- 23.Solaiman M Z, Saito M. Use of sugars by intraradical hyphae of arbuscular mycorrhizal fungi revealed by radiorespirometry. New Phytol. 1997;136:533–538. doi: 10.1046/j.1469-8137.1997.00757.x. [DOI] [PubMed] [Google Scholar]
- 24.Straker C J, Mitchell D T. The characterization and estimation of polyphosphates in endomycorrhizas of ericaceae. New Phytol. 1985;99:431–440. [Google Scholar]
- 25.Watanabe F S, Olsen S R. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci Soc Am Proc. 1965;29:677–678. [Google Scholar]