Abstract
As of January 2022, at least 60 million individuals are estimated to develop post-acute sequelae of SARS-CoV-2 (PASC) after infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). While elevated levels of SARS-CoV-2-specific T cells have been observed in non-specific PASC, little is known about their impact on pulmonary function which is compromised in the majority of these individuals. This study compares frequencies of SARS-CoV-2-specific T cells and inflammatory markers with lung function in participants with pulmonary PASC and resolved COVID-19 (RC). Compared to RC, participants with respiratory PASC had between 6- and 105-fold higher frequencies of IFN-γ- and TNF-α-producing SARS-CoV-2-specific CD4+ and CD8+ T cells in peripheral blood, and elevated levels of plasma CRP and IL-6. Importantly, in PASC participants the frequency of TNF-α-producing SARS-CoV-2-specific CD4+ and CD8+ T cells, which exhibited the highest levels of Ki67 indicating they were activity dividing, correlated positively with plasma IL-6 and negatively with measures of lung function, including forced expiratory volume in one second (FEV1), while increased frequencies of IFN-γ-producing SARS-CoV-2-specific T cells associated with prolonged dyspnea. Statistical analyses stratified by age, number of comorbidities and hospitalization status demonstrated that none of these factors affect differences in the frequency of SARS-CoV-2 T cells and plasma IL-6 levels measured between PASC and RC cohorts. Taken together, these findings demonstrate elevated frequencies of SARS-CoV-2-specific T cells in individuals with pulmonary PASC are associated with increased systemic inflammation and decreased lung function, suggesting that SARS-CoV-2-specific T cells contribute to lingering pulmonary symptoms. These findings also provide mechanistic insight on the pathophysiology of PASC that can inform development of potential treatments to reduce symptom burden.
Author summary
Long COVID-19 or post-acute sequelae of SARS-CoV-2 (PASC) impacts 20–30% of those infected with SARS-CoV-2 and is characterized by COVID-19 symptoms exceeding 4 weeks from symptom onset. While those with PASC experience a wide variety of persistent symptoms including shortness of breath, cough, chest pain, irregular heartbeat, brain fog, fatigue and intermittent fever, lung-related conditions are the most common. Although, infection with SARS-CoV-2 is clearly the inciting factor for PASC, the mechanisms responsible for long-term lung dysfunction are unclear and current treatments are ineffective at resolving pulmonary symptoms. Generalized PASC has been associated with SARS-CoV-2-specific T cells, a component of adaptive immunity, suggesting that residual virus may persist. Here, we investigated the frequency and function of virus-specific T cells in the blood of individuals with pulmonary PASC and correlated their presence with systemic inflammation and lung function. Our findings demonstrated that T cells specific for SARS-CoV-2 are elevated in the blood of those with pulmonary PASC and are associated with increased IL-6, a cytokine strongly associated with COVID-19 severity, and decreased lung function. These findings provide mechanistic insight into the pathophysiology of pulmonary PASC needed for the development of new treatments to improve quality of life for those affected.
Introduction
After infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), 20–30% of survivors experience prolonged symptoms that can significantly impact quality of life [1]. “Long-COVID” or “Long-haul COVID” refers to individuals experiencing persistent symptoms that can involve multiple organ systems, including the lungs, heart and brain [2–4]. Officially named post-acute sequelae of SARS-CoV-2 (PASC), this syndrome is defined as new, continuing or recurring symptoms of COVID-19 that occur four or more weeks after initial infection [1]. Hallmark symptoms of PASC include persistent palpitations, neuropsychiatric conditions, anosmia and dysgeusia, with dyspnea and other respiratory ailments being the most common [5–7]. Reduced lung volume and exercise capacity are commonly observed in survivors of COVID-19 pneumonia [8], however, the appearance and persistence of PASC respiratory symptoms is not related to the severity of initial illness [9]. As new SARS-CoV-2 variants potentially increase infection rates and disease severity [10,11], mutations to viral surface proteins may also increase the prevalence of persistent symptoms [12]. Early reports show sustained frequencies of SARS-CoV-2-specific T cells and elevated inflammatory systemic markers have been observed in non-specific PASC [13,14], and understanding the immunologic mechanisms of pulmonary PASC is of vital importance for developing treatment options to reduce symptom burden.
The T cell adaptive immune response is well characterized in acute and convalescent cases of COVID-19 and contributes to virus clearance, protective immunity and inflammation [15]. The frequency of SARS-CoV-2-specific T cells positively correlates with both serum antibody levels and disease severity; however, virus-specific CD4+ and CD8+ T cell frequencies decline with a half-life of 3–5 months [16,17]. In mild/asymptomatic cases, SARS-CoV-2-specific T cells are polyfunctional and produce multiple cytokines [18]; conversely, during severe disease, polyfunctional virus-specific T cells are underrepresented and are skewed towards a cytotoxic phenotype [19]. Although SARS-CoV-2-specific T cells are protective in most cases, it has been shown they can contribute to the cytokine release syndrome seen in patients with severe COVID-19 [20]. Furthermore, CD8+ T cells in the lung during acute infection are associated with inflammation, fibrosis, biomarkers of vascular injury and poor outcomes [21,22]. Thus, while SARS-CoV-2-specific T cells likely play a role in PASC, the characteristics of these cells and their connections to systemic inflammation or pulmonary symptoms are currently unknown.
Here we determined the frequency and function of SARS-CoV-2-specific T cells in blood, and their relationship with the expression of plasma inflammatory markers and measures of lung function in individuals with pulmonary PASC. We found patients with pulmonary PASC had significantly elevated frequencies of IFN-γ- and TNF-α-producing SARS-CoV-2-specific T cells compared to participants with resolved COVID-19 (RC). These virus-specific T cells were strongly associated with increased markers of inflammation and decreased lung function in PASC. These findings indicate pulmonary PASC may be, in part, driven by the production of inflammatory cytokines by SARS-CoV-2-specific T cells.
Results
Cohort descriptions
Study participants were recruited between July 2020 and April 2021, prior to appearance of the B.1.617.2 or B.1.1.529 variants in Colorado [23]. Patients were confirmed SARS-CoV-2 PCR positive by nasopharyngeal swab during the acute phase of infection. Participants categorized as pulmonary PASC experienced prolonged pulmonary complications including tussis, dyspnea, fatigue, exercise intolerance and hypoxia (S1 Table). The pulmonary PASC cohort reported symptoms lasting for a median duration of six months from symptom onset or hospital discharge. All RC participants reported no symptoms at the time of sample collection, and if RC participants subsequently did experience relapse of symptoms, they were excluded from the study. All participants with chronic or active infections other than SARS-CoV-2, using medications targeting IL-6, or antibiotic use within one month of sample collection were also excluded.
Those with PASC are generally older and more likely to have pre-existing conditions compared to RC [1,9]. To control for these potential confounders, the cohorts were age matched and multiple clinical characteristics were compared and cataloged in Table 1. In the PASC cohort, 40% required hospitalization (duration 3–52 days: median = 11 (P = 0.01)) during acute COVID-19 infection (Table 1). Overall, no significant differences between PASC and RC cohorts in terms of pre-existing conditions were found (Table 1). Those with PASC experienced an average symptom duration of over 6 months while RC participants’ average symptom duration was 9 days (P<0.0001) (S1 Fig). PASC participants reported a median of 9 symptoms while RC participants reported 6 symptoms during initial infection (P = 0.002). The median number of prolonged symptoms reported by those with PASC was 5 while those with RC reported none (P<0.0001) (S1 Fig). There were no significant differences in the total duration of symptoms between PASC participants who were hospitalized (PASC-H) and those with PASC who were not hospitalized (PASC-NH) (P = 0.17). PASC-NH participants reported a greater variety of symptoms during both the acute (P = 0.03) and post-acute phases (P = 0.02) of disease when compared to symptoms reported by PASC-H participants (S1 Fig). The average time from symptom onset to blood collection was 212 days for the PASC and 197 days for the RC cohorts (Table 1). Statistical analyses stratified by number of comorbidities (S2 and S3 Figs), time to sample collection (S4 Fig), and hospitalization status demonstrated that none of these factors affected the differences in frequency of SARS-CoV-2 T cells and plasma IL-6 levels measured between PASC and RC cohorts.
Table 1. Demographics of Cohorts.
| Cohort | P Valuea | PASC Hospitalized | P Value | |||
|---|---|---|---|---|---|---|
| PASC | RC | PASC-RC | Yes | No | Yes-No | |
| Number of participantsb | 20 | 20 | 8Δ | 12 | ||
| Female | 10 (50) | 10 (50) | >0.99 | 2 (25) | 8 (75) | 0.17 |
| Male | 10 (50) | 10 (50) | >0.99 | 6 (75) | 4 (25) | 0.17 |
| Median time to samplec (Range) | 212 (64–396) | 197Δ (13–289) | 0.08 | 184.5 (31–383) | 183 (45–332) | 0.79 |
| Median Age (Range) | 53 (22–69) | 50 (27–73) | 0.18 | 60 (49–69) | 50 (22–62) | 0.01 |
| ICU Admission | 6 (32) | NAd | NA | 6 (75) | NA | NA |
| Race and Ethnicity | ||||||
| White | 17 (85) | 18 (90) | >0.99 | 6 (75) | 11 (92) | >0.99 |
| Black | 2 (10) | 2 (10) | >0.99 | 1 (13) | 1 (8) | >0.99 |
| American Indian/Alaska Native | 0 (0) | 2 (10) | 0.49 | 0 (0) | 0 (0) | >0.99 |
| Other | 1 (5) | 0 (0) | >0.99 | 1 (13) | 0 (0) | >0.99 |
| Hispanic or Latin Origin | 5 (25) | 3 (15) | 0.69 | 3 (38) | 2 (17) | 0.60 |
| Underlying Medical Condition | ||||||
| Any | 10 (50)Δ | 8 (40) | 0.75 | 4 (50) | 6 (50) | >0.99 |
| Hypertension | 7 (35) | 2 (10) | 0.13 | 2 (25) | 4 (33) | >0.99 |
| Pulmonary Disease | 2 (10) | 2 (10) | >0.99 | 0 (0) | 2 (17) | 0.50 |
| Cardiac Disease | 0 (0) | 1 (5) | >0.99 | 0 (0) | 0 (0) | >0.99 |
| Immune System Disease | 1 (5) | 1 (5) | >0.99 | 0 (0) | 1 (8) | >0.99 |
| Cancer | 1 (5) | 0 (0) | >0.99 | 0 (0) | 1 (8) | >0.99 |
| Kidney Disease | 1 (5) | 1 (5) | >0.99 | 0 (0) | 1 (8) | >0.99 |
| Metabolic Disease | 5 (25) | 1 (5) | 0.18 | 4 (50) | 1 (8) | 0.11 |
| Medications during Hospitalization | ||||||
| Convalescent Plasma | 3 (15) | NA | NA | 3 (38) | NA | NA |
| Hydroxychloroquine | 4 (20) | NA | NA | 4 (50) | NA | NA |
| Remdesivir | 4 (20) | NA | NA | 4 (50) | NA | NA |
| Dexamethasone | 4 (20) | NA | NA | 4 (50) | NA | NA |
| Tocilizumab | 0 (0) | NA | NA | 0 (0) | NA | NA |
aMann-Whitney tests were used to determine differences between groups where P<0.05 is considered significant.
bAll values are number of participants with the percentage of the cohort in parentheses unless otherwise specified.
cMedian number of days from first reported symptom to collection of blood.
dNA = Not applicable.
ΔIndicates stratification analyses were performed demonstrating these differences do not influence SARS-CoV-2-specific T cell frequencies and levels of plasma IL-6.
Elevated SARS-CoV-2-specific T cells in pulmonary PASC
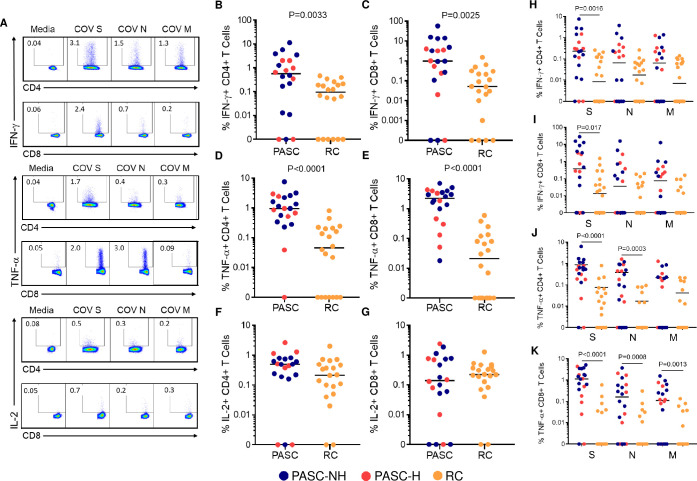
We measured the frequency of SARS-CoV-2-specific T cells in blood using intracellular cytokine (IFN-γ, TNF-α and IL-2) staining after stimulation with peptide pools of the SARS-CoV-2 spike (S), nucleocapsid (N) or membrane (M) surface-expressed proteins. Representative density plots of SARS-CoV-2-specific T cell populations are shown (Fig 1A). First, we analyzed the combined frequency of SARS-CoV-2-specific T cells for all three proteins. PASC participants had significantly increased frequencies of SARS-CoV-2-specific CD4+ and CD8+ T cells that produced IFN-γ or TNF-α compared to the RC cohort, while frequencies of IL-2-producing CD4+ SARS-CoV-2-specific T cells also trended higher (Fig 1B–1G). There was a 6- and 19.3-fold increased frequency of CD4+ and CD8+ SARS-CoV-2-specific T cells producing IFN-γ in PASC participants compared to RC participants (Fig 1B and 1C). The differences in the frequencies of TNF-α-producing CD4+ and CD8+ T cells in PASC participants compared to RC participants were even greater (20.9- and 105-fold, respectively) (Fig 1D and 1E). We chose to separate pulmonary PASC participants by prior hospitalization status to determine if this factor was associated with the frequency of virus-specific T cells. The same significant differences were observed when comparing only PASC-NH and RC participants and no significant differences were observed between PASC-NH and PASC-H groups (Fig 1B–1G). Stratification analyses for comorbidities and time of sample collection showed no significant differences within the RC or pulmonary PASC cohorts (S2 and S4 Figs).
Fig 1. Cumulative and S-, N- and M- specific SARS-CoV-2 specific T cell frequencies are elevated in PASC.
(A) Representative flow cytometry density plots showing the frequency of SARS-CoV-2 (S, N and M) IFN-γ-, TNF-α- and IL-2-specific T cells from a participant with PASC. The cummulative frequency of SARS-CoV-2 S, N and M IFN-γ-producing (B) CD4+ and (C) CD8+, TNF-α-producing (D) CD4+ and (E) CD8+ and IL-2-produing (F) CD4+ and (G) CD8+ T cells are shown. For B-G each point represents the sum of the combined frequencies of SARS-CoV-2 S, N and M-specific T cells of each participant. The frequency of individual SARS-CoV-2 S-, N- or M-specific IFN-γ-producing (H) CD4+ and (I) CD8+ and TNF-α-producing (J) CD4+ and (K) CD8+ T cells are shown. The horizontal bars depict median values for each cohort. Blue: PASC-NH (not hospitalized), red: PASC-Hospitalized and orange: RC participants. Mann-Whitney tests were used to determine statistical significance.
Next, we individually assessed IFN-γ- and TNF-α-producing SARS-CoV-2-specific T cell frequencies as these cytokines had the most significant differences overall. The frequency of IFN-γ-producing, S-specific CD4+ T cells was significantly higher in those with pulmonary PASC as compared to RC participants (median, range; PASC: 0.23%, 0–7.63%; RC: 0.0085%, 0–0.26%: P = 0.0016) (Fig 1H). No significant differences were noted in the frequency of SARS-CoV-2 N- and M-specific CD4+ T cells producing IFN-γ in PASC and RC participants (Fig 1H). Similar findings were seen for IFN-γ expression in CD8+ T cells between PASC and RC cohorts and again no difference was observed comparing the PASC-NH and PASC-H groups (Fig 1I). Again, there were no significant differences based on time of sample collection or comorbidities for IFN-γ-producing SARS-CoV-2 (S2 and S4 Figs).
PASC participants had significantly higher frequencies of TNF-α-producing SARS-CoV-2 S- and N-specific CD4+ T cells, (P<0.0001 and P = 0.0003, respectively) (Fig 1J) and significantly increased frequencies of TNF-α-producing CD8+ T cells in response to all three SARS-CoV-2 proteins (Fig 1K) compared to RC participants. Approximately 50% of CD4+ and CD8+ T cells from PASC participants produced TNF-α in response to all 3 SARS-CoV-2 proteins, whereas these percentages were 15% and 5%, respectively in RC participants. Only one PASC participant had no detectable CD4+ T cell cytokine response to any SARS-CoV-2 protein–this individual did have CD8+ T cell cytokine responses–while 7 RC participants had no detectable responses in either CD4+ or CD8+ T cells. Interestingly, female PASC participants had significantly higher S, N and M combined (P = 0.0015) and S-specific (P = 0.045) CD8+ T cell responses compared to male participants with PASC (S2 Fig). The frequencies of cytokine producing CD4+ and CD8+ T cells after stimulation were determined after subtracting the frequency of the unstimulated conditions. These unstimulated cells had low frequencies that were not significantly different between the PASC and RC group except for IFN-γ producing CD8+ T cells (PASC = 0.3% and RC = 0.16% (P = 0.025)) and TNF-α producing CD4+ T cells (PASC = 0.14 and RC = 0.08% (P = 0.032)). These data suggest that there may be more residual cytokine production directly ex vivo in PASC compared to RC participants, but it was minimal and had little effect on the frequency of SARS-CoV-2 T cells.
SARS-CoV-2-specific T cells in PASC are less polyfunctional than in RC and exhibit recent proliferation
Next, we compared the expression of cytokines and phenotypic markers on SARS-CoV-2-specific T cells in pulmonary PASC and RC participants. It has been established that T cell immunity to SARS-CoV-2 wanes rapidly after resolution of infection and symptoms [24]. As confirmation of waning immunity, we examined SARS-CoV-2-specific T cell frequencies in 2 RC participants who provided blood at 2- and 30-weeks post resolution of symptoms. As expected, their T cell responses decreased over time (S4 Fig). Based on these data, we collected blood from RC participants at early time points after resolution of infection to ensure that detectable frequencies of SARS-CoV-2-specific T cells were present. Thus, we were able to interrogate differences in T cell phenotype and function in both PASC and RC participants.
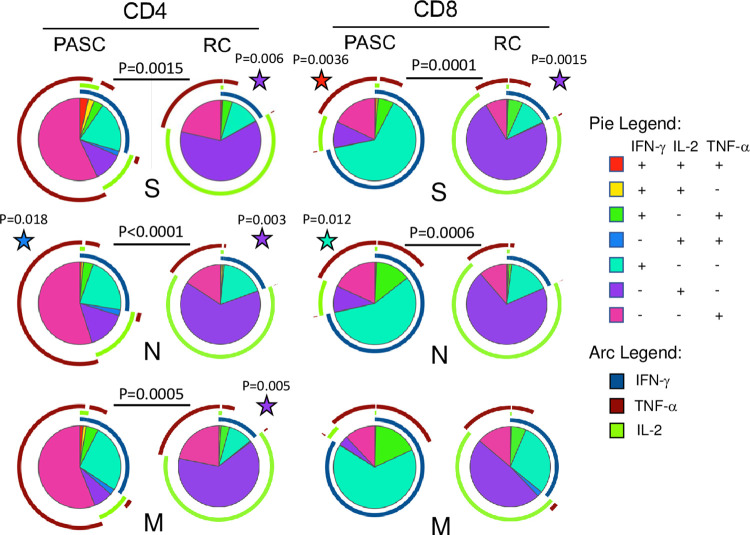
We assessed the cytokine production profiles of PASC and RC participants utilizing simplified presentation of incredibly complex evaluations (SPICE) [25]. The SPICE analysis revealed the majority of SARS-CoV-2-specific T cells for both cohorts only produce one of the three cytokines tested, with TNF-α dominating the virus-specific CD4+ T cell response and IFN-γ dominating the CD8+ T cell response in those with pulmonary PASC, while RC participants’ responses were IL-2 dominant. A deeper analysis revealed significant differences in the distribution of CD4+ T cell cytokine expression in response to S, N and M proteins when comparing PASC and RC participants (P = 0.0015, P<0.0001 and P = 0.0005, respectively) (Fig 2). The proportion of IL-2 producing CD4+ T cells was significantly higher in RC participants compared to PASC participants in response to S, N and M proteins (P = 0.006, P = 0.003 and P = 0.005, respectively), while the proportion of TNF-α- and IL-2-producing N-specific CD4+ T cells was higher in pulmonary PASC participants (P = 0.0036). The overall proportions of cytokine-producing CD8+ T cells were also significantly different between PASC and RC cohorts for S- and N-specific T cells (P = 0.0001 and P = 0.0006, respectively) and the proportion of SARS-CoV-2-specific T cells producing all three cytokines in response to S protein was higher in PASC (P = 0.0036) (Fig 2). Overall PASC participants had a greater proportion cytokine co-expression and a much lower proportion of IL-2-producing SARS-CoV-2-specific T cells.
Fig 2. Cytokine co-expression of SARS-CoV-2 specific T cells differs between PASC and RC.
Cytokine co-expression on SARS-CoV-2 specific T cells visualized using simplified presentation of incredibly complex evaluations (SPICE) analysis. Each pie chart represents the proportions of combinations of IFN-γ, TNF-α and IL-2 producing T cells in response to one SARS-CoV-2 protein. Arcs surrounding each pie chart depict the proportion of cells secreting each individual cytokine. Colors of pie charts and arcs represent different cytokines or combinations of cytokines and are listed in the corresponding legend. Stars denote significant differences determined by student t test corrected for multiple comparsons between PASC and RC cohorts for a particular combination of co-expressed cytokines matching as indicated by the color corresponding to the pie legend. Stars are positioned next to the cohort with the higher proportion. P values positioned between PASC and RC pie charts denote statistical significance of overall composition by permutation test with 10,000 iterations corrected for multiple comparsons.
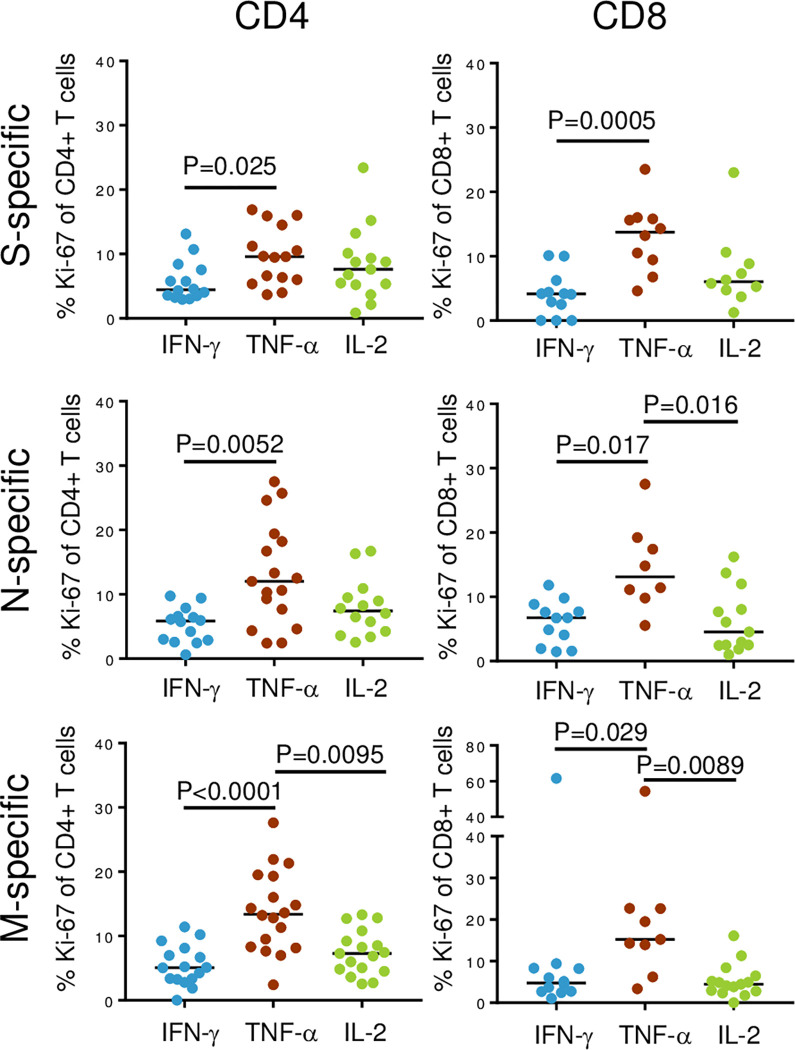
We then assessed markers of T cell maturation (CD27 and CD45RA), exhaustion (PD-1) and proliferative capacity (Ki-67) on total (S5 Fig) and virus-specific (Fig 3) T cells from PASC and RC participants. No differences were seen in the frequency of naïve, effector memory or terminally differentiated effector memory for total CD4+ or CD8+ T cells between the two groups (S5 Fig). Also, no significant differences in Ki-67 or PD-1 expression were seen. However, within the pulmonary PASC cohort, Ki-67 expression in SARS-CoV-2-specific TNF-α-producing CD4+ and CD8+ T cells was significantly higher than in cells expressing either IFN-γ or IL-2 (Fig 3). For example, in response to M protein, the number of TNF-α-producing cells expressing Ki-67 was 2.6-fold higher for CD4+ T cells (P<0.0001) and 3.2-fold higher for CD8+ T cells (P = 0.029) compared to IFN-γ-producing T cells (Fig 3). TNF-α-producing T cells exhibited significantly higher frequencies of Ki-67 than IFN-γ-producing T cells for both CD4+ and CD8+ T cell subsets, and Ki-67 was higher on TNF-α-producing T cells for all conditions, except for S-specific CD4+ T cells when compared to the frequency of Ki-67 on IL-2-producing T cells (Fig 3). Unlike the PASC cohort, no significant differences in Ki67 expression on IFN-γ-, TNF-α- and IL-2-producing CD4+ or CD8+ T cells were found in the RC cohort. Interestingly, there was no statistically significant difference in the frequencies of cytokine-producing CD4+ (IFN-γ, P = 0.27; IL-2, P = 0.21; TNF-α, P = 0.16) and CD8+ (IFN-γ, P = 0.51; IL-2, P = 0.12; TNF-α, P = 0.09) T cells after PMA/Ionomycin stimulation, indicating that T cells from RC and PASC participants had the same intrinsic ability to produce cytokines.
Fig 3. TNF-α-producing T cells have the highest proportion of Ki-67 expression.
The percentages of Ki67 expressing SARS-CoV-2-specific CD4+ (left panels) and CD8+ (right panels) T cells obtained from the blood of PASC participants are shown. SARS-CoV-2 S- (top panel), N- (middle panel) and M- (bottom panel) specific T cells are grouped by production of IFN-γ, TNF-α and IL-2. Note, data points from individual PASC participants were obtained for 1 or more of the cytokines assessed; however, in no instances are there multiple values obtained from the same participant for a particular cytokine. Blue represents IFN-γ+ T cells, brown represents TNF-α+ T cells and green represents IL-2+ T cells. Kruskal-Wallis tests with corrections for multiple comparisons were used to determine statistical significance.
Plasma IL-6 levels in pulmonary PASC correlated with the frequency of SARS-CoV-2-specific T cells
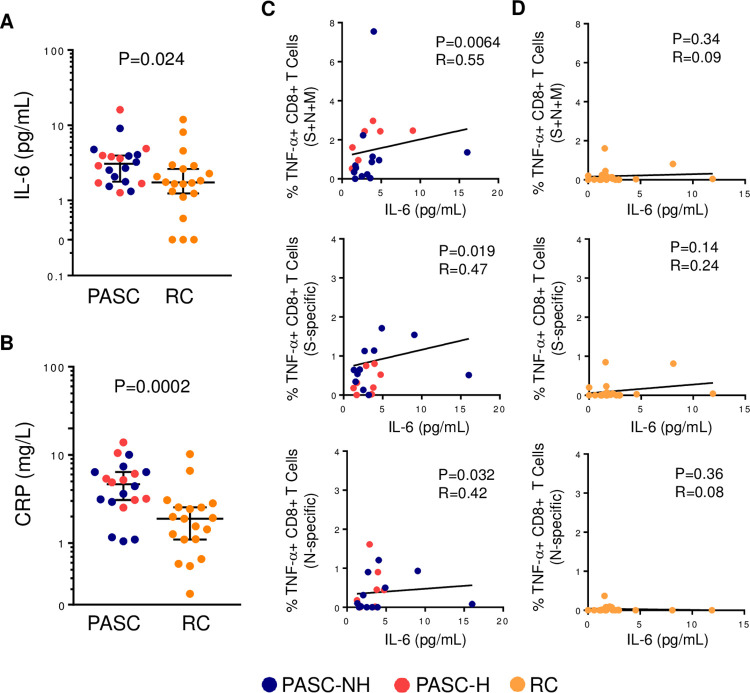
We measured plasma IL-6 and CRP in participants to characterize systemic inflammation in pulmonary PASC and correlate these markers with the frequency of virus-specific T cells. Assessed independently of hospitalization status, both IL-6 and CRP were significantly elevated compared to the RC cohort: IL-6 (PASC median = 3.1 pg/mL, RC median = 1.73 pg/mL, P = 0.024); CRP (PASC median = 4.64 mg/L, RC median = 1.89 mg/L, P = 0.0004) (Fig 4A and 4B). No significant correlations were found between plasma IL-6 or CRP levels and age (IL-6: P = 0.83, CRP: P = 0.15) or number of pre-existing conditions (IL-6: P = 0.35, CRP: P = 0.39) for all participants or each cohort separately. No significant difference in plasma IL-6 between PASC-H and PASC-NH (P = 0.62) were found and both were significantly elevated compared to RC. There was also no difference in IL-6 comparing female and male PASC participants (S3 Fig). Assessing CRP in PASC-NH participants, this group trended higher than RC participants (PASC-NH median = 3.10 mg/L, RC median = 1.76 mg/L, P = 0.074), whereas there was a significant difference between PASC-H and RC (PASC-H median = 5.74 mg/L, RC median = 1.76 mg/L, P = 0.004). Of note, PASC-H participants were significantly higher compared to PASC-NH (P = 0.025). Male PASC participants also had significantly higher plasma CRP compared to female PASC participants (P = 0.028) (S3 Fig). This observation suggests that elevated plasma CRP in pulmonary PASC is likely related to initial disease severity, known to be associated with male sex, while IL-6 elevation is specific to pulmonary PASC regardless of disease severity or gender. Stratification analyses show no significant differences within the PASC or RC cohorts based on pre-existing conditions, although CRP did trend higher in those with pre-existing conditions (S3 Fig). No correlations between duration of symptoms (IL-6: P = 0.42, CRP: P = 0.44) or time from onset to sample collection (IL-6: P = 0.46, CRP: P = 0.68) with IL-6 or CRP were found.
Fig 4. Plasma IL-6 in PASC is higher than RC and correlates with frequency of SARS-CoV-2-specific TNF-α producing CD8+ T cells.
(A) Plasma IL-6 levels (pg/mL) and (B) plasma CRP levels (mg/L) in PASC and RC. (C) Correlations between plasma IL-6 and frequency of SARS-CoV-2-specific TNF-α-producing CD8+ T cells in PASC participants. (D) Correlations between plasma IL-6 and frequency of SARS-CoV-2-specific TNF-α-producing CD8+ T cells in RC participants. For A-B, the horizontal bar represents median of cohort and error bars are 95% confidence index. Each point represents data from one participant. Blue: PASC-NH (not hospitalized), red: PASC-Hospitalized and orange: RC participants. For A-B Mann-Whitney tests were used to determine statistical significance. For C-D Spearman correlations were used to determine statistical significance.
We next explored the relationship between the frequencies of SARS-CoV-2-specific CD4+ and CD8+ T cells with IL-6 and CRP. We identified significant positive correlations between S, N and M combined, S-specific and N-specific frequencies of TNF-α-producing SARS-CoV-2-specific CD8+ (r = 0.55; P = 0.0064, r = 0.47; P = 0.019 and r = 0.42; P = 0.032, respectively) T cells and plasma IL-6 in PASC participants (Fig 4C). These correlations were not observed in the RC cohort (Fig 4D). No significant correlations between plasma CRP and the frequency of SARS-CoV-2-specific T cells in either PASC (CD8+ TNF-α+: r = 0.05, P = 0.42) or RC (CD8+ TNF-α+: r = 0.03, P = 0.46) cohorts were observed.
SARS-CoV-2-specific T cell frequencies correlate with decreased lung function
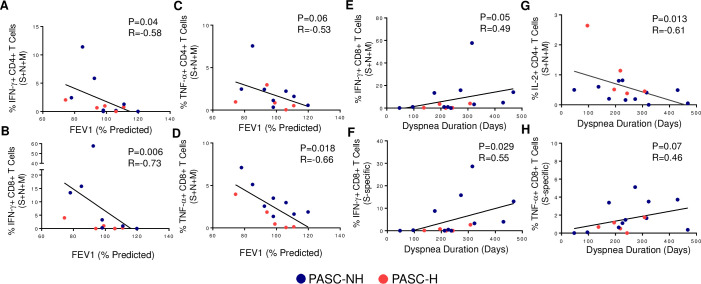
A subset of pulmonary PASC participants (n = 13) had pulmonary function tests (PFTs) performed during their period of prolonged respiratory symptoms as part of their standard of care. None of these participants reported pre-existing pulmonary conditions prior to infection with SARS-CoV-2. PFTs were performed between 45 and 315 days after symptom onset (median = 158 days). We correlated the frequencies of SARS-CoV-2-specific T cells with the following variables: percent predicted forced vital capacity (%FVC), absolute and percent predicted forced expiratory volume during the 1st second (FEV1 and %FEV1, respectively), FEV1/FVC, total lung capacity percent predicted (%TLC), single-breath diffusing capacity of the lung for CO percent predicted (%DLCO_SB), and diffusing capacity of the lung per alveolar volume percent predicted (%DLCO/VA). As shown in Fig 5A and 5B, the combined frequencies of IFN-γ-producing SARS-CoV-2-specific CD4+ and CD8+ T cells in response to S, N and M negatively correlated with %FEV1 (r = -0.58, P = 0.04; r = -0.73, P = 0.006, respectively). Similar findings were seen between TNF-α-producing CD4+ and CD8+ T cells and %FEV1 (Fig 5C and 5D). We then compared the frequency of SARS-CoV-2-specific T cells with the duration of prolonged dyspnea experienced by 80% (n = 16) of our pulmonary PASC participants. From this analysis, we identified positive correlations between dyspnea duration and frequencies of IFN-γ-producing SARS-CoV-2 S, N and M combined (r = 0.49, P = 0.02) and S-specific (r = 0.55, P = 0.015) CD8+ T cells (Fig 5E and 5F). There was also a positive correlation between TNF-α-producing SARS-CoV-2 S-specific (r = 0.45, P = 0.036) CD8+ T cells and dyspnea duration, and a negative correlation between S, N and M combined CD4+ IL-2-producing T cells and dyspnea duration (r = -0.61, P = 0.006) (Fig 5G and 5H). This was an exploratory analysis with a limited number of participants, so these associations were not corrected for multiple comparisons.
Fig 5. Correlations between SARS-CoV-2 specific T cells and FEV1 and symptoms in PASC.
Correlations between the percent predicted FEV1 and the combined SARS-CoV-2 S-, N- and M-specific frequencies of IFN-γ-producing (A) CD4+ and (B) CD8+ T cells and TNF-α-producing (C) CD4+ and (D) CD8+ T cells are shown. Correlations of between the duration of dyspnea in days and the frequencies of (E) combined SARS-CoV-2 S-, N- and M-specific CD8+ T cells, (F) S-specific IFN-γ-producing CD8+ T cells, (G) combined S-, N- and M-specific IL-2-producing CD4+ T cells and (H) S-specific TNF-α-producing CD8+ T cells are shown. Each point represents data from one PASC participant. Blue and red symbols represent PASC-NH (not hospitalized) and PASC-Hospitalized participants, respectively. Spearman correlations were used to determine statistical significance.
Discussion
As the number of SARS-CoV-2 infections accumulate worldwide, PASC is likely to remain a significant health concern for the foreseeable future. We examined SARS-CoV-2-specific immunity in convalescent COVID-19 patients recruited prior to the appearance of the B.1.617.2 “Delta” and B.1.1.529 “Omicron” variants [23]. Pulmonary PASC participants with a defined set of prolonged respiratory symptoms had dramatically higher frequencies of SARS-CoV-2-specific T cells in blood compared to participants who had recovered from infection without persistent COVID-19 symptoms. We also found that levels of key plasma inflammatory markers (IL-6 and CRP) were significantly elevated in individuals with ongoing pulmonary PASC and associated with the frequency of SARS-CoV-2-specific T cells. The frequency of SARS-CoV-2-specific T cells in pulmonary PASC participants correlated with reduced lung function and duration of dyspnea, linking the presence of these anti-viral T cells to lung dysfunction. Taken together, these data provide mechanistic insight into the immunopathogenesis of pulmonary PASC.
The most striking feature of PASC is the significantly elevated frequency of SARS-CoV-2-specific TNF-α-producing CD8+ T cells. This increased frequency could be detected in response to peptide pools of all the viral structural proteins in comparison to the RC cohort. Interestingly, these T cells were also significantly higher in female PASC participants compared to males, which may contribute to the higher prevalence of PASC in women [1]. TNF-α-producing CD8+ T cells also expressed the highest levels of Ki-67, indicating recent activation and proliferation. Because the half-life of SARS-CoV-2-specific T cells is between three and five months [24] and most of our PASC participants donated blood over 6 months from symptom onset, it suggests these cells are maintained by viral antigen. The presence of persistent viral reservoirs of SARS-CoV-2 has been proposed as a possible explanation of PASC pathophysiology [26]. Studies in macaques and humans demonstrated viral replication can persist months after initial infection in multiple organ systems [27–30] and viral presence in cerebrospinal fluid has been observed in neurological PASC [31]. Alternatively, damage resulting from severe disease during acute infection has also been proposed as a cause of PASC [26]. However, our results don’t support this idea since sixty percent of our pulmonary PASC cohort initially experienced mild disease [32], yet still developed PASC. Furthermore, there was no difference in the frequency of SARS-CoV-2-specific T cells when hospitalized and non-hospitalized PASC participants were compared. Thus, our findings that pulmonary PASC participants have elevated levels of SARS-CoV-2-specific T cells months after initial infection suggest ongoing viral replication that is maintaining the pool of inflammatory T cells.
The role of T cells in chronic inflammatory conditions is well documented and characterized by the production of TNF-α and other proinflammatory cytokines [33], so we examined the inflammatory markers CRP and IL-6. Both are closely associated with disease severity during acute SARS-CoV-2 infection [34], although previously, no differences in IL-6 levels were found in non-specific PASC when compared to those with resolved infection [35]. In contrast, we found that CRP and IL-6 were elevated in pulmonary PASC participants. Levels of CRP in hospitalized PASC participants were significantly higher compared to non-hospitalized PASC participants suggesting prolonged CRP elevation is more strongly associated with initial severity of disease than pulmonary PASC. Elevated IL-6, however, was not different between PASC-H and PASC-NH participants after controlling for sex, age or comorbidities. IL-6 is directly associated with inflammatory lung conditions [36] and targeting IL-6 pathways can effectively treat a variety of inflammatory conditions and decrease mortality in severe COVID-19 cases [37–40]. Interestingly, IL-6 levels strongly associated with the frequencies of SARS-CoV-2-specific CD8+ T cells which have been shown in other diseases to directly impact tissue-specific monocyte and macrophage production of IL-6 and TNF-α and contribute to feedback loops for innate immune cell recruitment and activation [41,42] which likely contributes to prolonged respiratory symptoms.
To further understand the role of SARS-CoV-2-specific T cells in pulmonary PASC, we evaluated their associations with lung function. TNF-α impacts asthma progression [43,44] and chronic obstructive pulmonary disease is associated with IFN-γ-producing T cells [45]. In severe COVID-19, decreased pulmonary function is connected to elevated levels of systemic IFN-γ and TNF-α, and analysis of immune cells isolated from bronchoalveolar lavage fluid suggests T cell dysfunction potentially exacerbates tissue damage in severe cases [46–48]. These studies indicate a strong connection between T cell cytokine production and lung function, particularly in SARS-CoV-2 infections. While initial studies have found associations between non-specific PASC and CD8+ T cells and that immunoglobulin signatures predicts risk of developing PASC, these associations have not been examined specifically in pulmonary PASC [49,50]. Here, we found that elevated frequencies of IFN-γ- and TNF-α-producing SARS-CoV-2-specific T cells were positively associated with decreased lung function in pulmonary PASC. We also found the duration of dyspnea correlated with increased frequencies of CD8+ IFN-γ- and TNF-α-producing SARS-CoV-2-specific T cells and decreased levels of CD4+ IL-2 producing T cells. These findings are further supported by recent studies that show elevated numbers of CD8+ T cells in the airways of individuals with ongoing respiratory symptoms after COVID-19 [51,52]. Similar to the effects of systemic cytokines and T cell expression of inflammatory cytokines in other pulmonary conditions, our findings suggest that the presence of persistently activated SARS-CoV-2-specific T cells in PASC likely contributes to lung dysfunction.
Collectively, our findings demonstrate that elevated frequencies of SARS-CoV-2-specific T cells are associated with systemic inflammation and decreased lung function in pulmonary PASC. We observed a striking difference in the frequency of activated and dividing T cells as well as correlations between SARS-CoV-2-specific T cell frequencies and levels of plasma IL-6. Most importantly, we found a strong association between the frequency of SARS-CoV-2-specific T cells and the duration of respiratory symptoms and lung function. While this study examines the responses after infection with one of the early strains of SARS-CoV-2, the characteristics of more recent variants may increase the prevalence of PASC via the same mechanisms supported by our findings [12]. Together, these findings suggest pulmonary PASC is in part driven by inflammatory cytokines produced by activated virus-specific T cells, which are likely maintained by persistent virus and contribute to systemic inflammation and prolonged disease morbidity.
Materials and methods
Ethics statement
This study was approved by the Colorado Multiple Institutional Review Board (COMIRB# 20–1219) at the University of Colorado Anschutz Medical Campus. All participants provided formal written informed consent prior to any study procedures.
Study participants and sample collection
Adult study participants were recruited from the Denver, Colorado metropolitan area via community flyers, and from the Anschutz Medical Campus Infectious Disease and Pulmonology PASC UCHealth outpatient clinics between July 2020 and April 2021, prior to detection of the Delta or Omicron variants in Colorado [23]. Information regarding symptom severity and duration was collected from all participants upon enrollment. 50 mL of blood was collected from study volunteers in sodium heparin tubes (BD, Vacutainer), and plasma and peripheral blood mononuclear cells (PBMCs) were isolated as described previously [53]. None of the participants in the study had received a SARS-CoV-2 vaccine at the time of enrollment. Participants for this study were included if they had a documented positive SARS-CoV-2 PCR nasal swab during acute infection or a replicated SARS-CoV-2 IgG antibody test, and were separated into PASC and RC cohorts based on the Center for Disease Control and Prevention definition of PASC [1,5]. The classification of pulmonary PASC was made by review of symptom questionnaire responses and chart review by a board-certified pulmonologist indicating all PASC participants experienced prolonged pulmonary complications after infection with SARS-CoV-2. Demographics of the study population are shown in Tables 1 and S1.
Cytokine and antigen ELISAs
Anti-SARS-CoV-2 Spike S1 IgG antigens, IL-6 and C-reactive protein (CRP) were assessed using the following ELISA kits per manufacturer protocols: (IgG; Euroimmun—EI2606-9601G, IL-6; Invitrogen—88–7066.22, CRP; Millipore Sigma—RAB0096-1KT). In brief, plasma and standards were diluted per manufacturer’s protocol in sample diluent and added to pre-coated microplate wells. Following incubation of the wells with biotinylated detection antibody, HRP conjugate, substrate reagent, and stop solution, the plates were read at 450nm.
T cell stimulation and immunofluorescent staining
The frequency of antigen-specific, cytokine-secreting T cells in blood was determined by intracellular cytokine staining, with minor modifications to our previously published protocol [54]. In brief, PBMCs (2–4 x 106 cells) were cultured 5 ml polypropylene tubes in RPMI medium containing 10% human serum and anti-CD28 and anti-CD49d mAbs (each at 1 μg/ml) (S2 Table). Cells were stimulated under the following conditions: peptide arrays of SARS-CoV-2 spike (S) glycoprotein, nucleocapsid (N) protein, membrane (M) protein (5 μg/ml final concentration of each peptide; BEI Resources from USA-WA1/2020 strain, NR-52402, NR-52404, NR-52403), combined phorbol 12-myristate 13-acetate (PMA) and ionomycin (25 μg/ml and 32.5 μg/ml, respectively; Sigma) or medium alone. S and N arrays were 17- or 13-mer peptides with 10 amino acid overlap, and the M array consisted of 17- or 12-mer peptides with 10 amino acid overlap. Cells were incubated for 6 hours at 37°C in a humidified 5% CO2 atmosphere and a 5-degree slant with 1 μg/ml Golgi Plug added after 4 hours. LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen L34957) was used per the manufacturer’s protocol after washing. Cells were surfaced stained with the following mAbs: anti-CD3, anti-CD4, anti-CD8, anti-CD27, anti-CD45RA and anti-PD-1 for 30 min at 4°C. Cells were washed and stored in a fix permeabilization buffer (eBioscience, 421403) overnight at 4°C. Cells were washed in permeabilization buffer and stained with anti-IFN-γ, anti-IL-2, anti-Ki-67 and anti-TNF-α mAbs for 120 min at 4°C, washed and fixed with 1% formaldehyde. Fluorescence−1 (FMO) controls were used in anti-PD-1, anti-CD27 and anti-CD45RA staining. Full information on staining fluorophores is provided in S2 Table.
Flow cytometry
Cells were analyzed using a LSRII flow cytometer (BD Immunocytometry Systems). At least 1 million events were collected for each tested condition. Antibody capture beads (BD Biosciences) were used to perform electronic compensation. Beads were stained separately with individual mAbs used in the test sample. Data were analyzed using Diva software (BD). A representation of the gating strategy is included in S6 Fig. Lymphocytes were gated by their forward and side scatter profile. Live and CD3+ cells were selected, and expression of CD4 was analyzed in a bivariate dot plot with CD8 to exclude CD4/CD8 double positive T cells. Bi-exponential scaling was used in all dot plots. The frequency of SARS-CoV-2-specific T cells was calculated by subtraction of the cytokine producing cells in the unstimulated condition from the SARS-CoV-2 peptide stimulated condition. Expression of CD27, CD45RA, PD-1 and Ki-67 was examined on cytokine-producing cells with at least 100 events to ensure an adequate number of events for analysis [55,56]. FMO controls were used to set gates for determining the percentage of PD-1- and cytokine-expressing T cells. To ensure accuracy and precision of the measurements taken from day-to-day, quality control was performed on the LSRII daily using the Cytometer Setup & Tracking (CS&T) feature of the BD FACSDiva software. This program uses standardized CS&T beads (BD Biosciences) to determine voltages, laser delays and area scaling to track these settings over time. A manual quality control (QC) using rainbow beads was also performed daily to verify the laser delay and area scaling determined by CS&T.
Statistics
Statistical analyses were performed using GraphPad-Prism (Graphpad, San Diego, CA). The Mann-Whitney U test or Wilcoxon’s matched pairs test were utilized to determine significance of differences between groups. Correlations were calculated using the nonparametric Spearman test, and these associations were not corrected for multiple comparisons. P values of <0.05 were considered statistically significant. To visualize and evaluate differences in expression of multiple cytokines between the PASC and RC cohorts, simplified presentation of incredibly complex evaluations (SPICE) analysis was utilized as well as permutation tests with 10,000 iterations and student T tests for statistical significance where P<0.05 were considered statistically significant. The frequency of Ki67 on SARS-CoV-2-specific T cells was analyzed by a Kruskal-Wallis 1-way ANOVA. Both all SPICE and Ki67 analysis were corrected for multiple comparisons [57].
Supporting information
Number of participants reporting each symptom at time of acute or prolonged disease. aPercentage of cohort.
(XLSX)
Monoclonal antibodies used in intracellular cytokine staining to determine frequencies and characteristics of SARS-CoV-2 specific T cells. aNA = Not applicable. bIndicates antibody was excluded for Fluorescence-3 staining control.
(XLSX)
(A) Symptom duration (days) reported in symptom questionnaires for PASC and RC participants. (B) Number of symptoms reported <4 weeks or >4 weeks from symptom onset for PASC and RC participants. For each graph, bars represent the median of each cohort and the error bar represents the upper 95% confidence interval. Blue represents PASC participants not hospitalized (PASC-NH, n = 12), red represents PASC-hospitalized (PASC-H, n = 8) and orange represents RC participants (n = 20). Mann-Whitney tests were used to determine statistical significance.
(TIF)
(A) Percent of combined S-, N- and M-specific frequencies of IFN-γ- or TNF-α-producing CD4+ or CD8+ T cells separated by number of pre-existing conditions. (B) Frequency of combined S-, N- and M-specific TNF-α-producing CD8+ T cells or (C) S-specific TNF-α-producing CD8+ T cells for PASC participants separated by sex. Each point represents data from one participant where blue represents PASC-NH (not hospitalized) and red represents PASC-hospitalized. Mann-Whitney tests were used to determine statistical significance.
(TIF)
(A) PASC plasma IL-6 (pg/mL) levels and (B) PASC plasma CRP levels (mg/L) in PASC separated by sex. (C) Serum IL-6 (pg/mL) and (D) CRP (mg/L) in PASC separated by pre-existing conditions. Each point represents data from one participant. Where applicable, bar represents median of cohort and error bars are 95% confidence index. Blue and red symbols represent PASC-NH (not hospitalized) and PASC-Hospitalized, respectively.
(TIF)
(A) Frequency of SARS-CoV-2 N- and M-specific TNF-α-producing T cells from samples taken 2 and 30 weeks from symptom onset. Each set of points connected by a line represents data from one participant. (B) Percent of cummulative S, N and M SARS-CoV-2-specific CD4+ or CD8+ T cells producing IFN-γ or TNF-α for the RC cohort split by time from symtom onset to sample collection. Orange symbols represent RC participants.
(TIF)
PBMCs were isolated from PASC and RC participants and stained with mAb to assess their ex vivo phenotype by flow cytometric analysis. Percentage of total CD4+ T cells expressing (A) Ki-67 and (B) PD-1 for PASC and RC participants. Percentage of total CD8+ T cells expressing (C) Ki-67 and (D) PD-1 for both cohorts. Shown are the frequencies of naïve (CD27+CD45RA+), central memory (CD27+CD45RA-), effector memory (CD27-CD45RA-) and terminally differentiated (TD)-effector memory (CD27-CD45RA+) on total (E) CD4+ and (F) CD8+ T cells. All data collected from flow cytometry in unstimulated conditions. For each box and whisker plot, the center line denotes the median value (50th percentile), and the box contains the 25th to 75th percentile values for each dataset. Whiskers mark the 5th and 95th percentiles, and values beyond these upper and lower bounds are not visualized. Dark grey plots represent the PASC cohort and light gray plots represent the RC cohort. Mann-Whitney tests were used to determine statistical significance.
(TIF)
An example of the gating strategy used to determine frequency of SARS-CoV-2-specific T cells. Each axis is labeled with the fluorochrome measured and corresponding marker if applicable and data is presented as pseudocolor dot plots (FlowJo). Arrows indicate the sequence of analysis and the number on each plot is the frequency of the gated population.
(TIF)
Acknowledgments
We would like to thank the individuals and organizations who generously shared their time, experience, and materials for the purposes of this project particularly those with PASC who made this possible.
Data Availability
Data cannot be shared publicly because of the inclusion of PHI subject to HIPAA regulations. Data are available from the University of Colorado Institutional Data Access Committee (contact Crao_Contracts@ucdenver.edu) for researchers who meet the criteria for access to confidential data.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nature Medicine. 2021;27(4):626–31. doi: 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins V, Sohaei D, Diamandis EP, Prassas I. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Critical Reviews in Clinical Laboratory Sciences. 2020:1–23. doi: 10.1080/10408363.2020.1860895 [DOI] [PubMed] [Google Scholar]
- 3.Yong SJ. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chemical Neuroscience. 2021;12(4):573–80. doi: 10.1021/acschemneuro.0c00793 [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Kream RM, Stefano GB. Long-Term Respiratory and Neurological Sequelae of COVID-19. Medical Science Monitor. 2020;26. doi: 10.12659/MSM.928996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfì A, Bernabei R, Landi F. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324(6):603. doi: 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenforde M, Investigators IN, Team CC-R. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network—United States, March–June 2020. CDC, 2020. Contract No.: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122. Epub 20210518. doi: 10.1016/j.lanepe.2021.100122 ; PubMed Central PMCID: PMC8129613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2021;27(4):328–37. Epub 20201125. doi: 10.1016/j.pulmoe.2020.10.013 ; PubMed Central PMCID: PMC7687368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend L, Dowds J, O’Brien K, Sheill G, Dyer AH, O’Kelly B, et al. Persistent Poor Health after COVID-19 Is Not Associated with Respiratory Complications or Initial Disease Severity. Annals of the American Thoracic Society. 2021;18(6):997–1003. doi: 10.1513/AnnalsATS.202009-1175OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butt AA, Dargham SR, Chemaitelly H, Al Khal A, Tang P, Hasan MR, et al. Severity of Illness in Persons Infected With the SARS-CoV-2 Delta Variant vs Beta Variant in Qatar. JAMA Internal Medicine. 2021. doi: 10.1001/jamainternmed.2021.7949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meo SA, Meo AS, Al-Jassir FF, Klonoff DC. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. 2021;25(24):8012–8. doi: 10.26355/eurrev_202112_27652 . [DOI] [PubMed] [Google Scholar]
- 12.Proal AD, VanElzakker MB. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front Microbiol. 2021;12:698169. Epub 20210623. doi: 10.3389/fmicb.2021.698169 ; PubMed Central PMCID: PMC8260991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doykov I, Hällqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. ‘The long tail of Covid-19’—The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Research. 2021;9:1349. doi: 10.12688/f1000research.27287.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Files JK, Sarkar S, Fram TR, Boppana S, Sterrett S, Qin K, et al. Duration of post-COVID-19 symptoms are associated with sustained SARS-CoV-2 specific immune responses. JCI Insight. 2021. doi: 10.1172/jci.insight.151544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–80. doi: 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rha MS, Jeong HW, Ko JH, Choi SJ, Seo IH, Lee JS, et al. PD-1-Expressing SARS-CoV-2-Specific CD8(+) T Cells Are Not Exhausted, but Functional in Patients with COVID-19. Immunity. 2021;54(1):44–52 e3. Epub 2020/12/19. doi: 10.1016/j.immuni.2020.12.002 ; PubMed Central PMCID: PMC7834198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demaret J, Lefèvre G, Vuotto F, Trauet J, Duhamel A, Labreuche J, et al. Severe SARS-CoV-2 patients develop a higher specific T-cell response. Clinical & Translational Immunology. 2020;9(12). doi: 10.1002/cti2.1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin J-B, Olsson A, et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020;183(1):158–68.e14. doi: 10.1016/j.cell.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meckiff BJ, Ramírez-Suástegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H, et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4+ T Cells in COVID-19. Cell. 2020;183(5):1340–53.e16. doi: 10.1016/j.cell.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arcanjo A, Pinto KG, Logullo J, Leite PEC, Menezes CCB, Freire-De-Lima L, et al. Critically ill COVID-19 patients exhibit hyperactive cytokine responses associated with effector exhausted senescent T cells in acute infection. The Journal of Infectious Diseases. 2021. doi: 10.1093/infdis/jiab425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneko N, Boucau J, Kuo H-H, Perugino C, Mahajan VS, Farmer JR, et al. Expansion of Cytotoxic CD4+ T cells in the lungs in severe COVID-19. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chioh FW, Fong S-W, Young BE, Wu K-X, Siau A, Krishnan S, et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. eLife. 2021;10. doi: 10.7554/eLife.64909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colorado_State_Tri-County_Health_Department. COVID-19 Variants: Tri-County Health Department 2020. [cited 2021 November 18]. Available from: https://www.tchd.org/896/COVID-19-Variants. [Google Scholar]
- 24.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roederer M, Nozzi JL, Nason MC. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry Part A. 2011;79A(2):167–74. doi: 10.1002/cyto.a.21015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nature Medicine. 2021;27(4):601–15. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Böszörményi KP, Stammes MA, Fagrouch ZC, Kiemenyi-Kayere G, Niphuis H, Mortier D, et al. The Post-Acute Phase of SARS-CoV-2 Infection in Two Macaque Species Is Associated with Signs of Ongoing Virus Replication and Pathology in Pulmonary and Extrapulmonary Tissues. Viruses. 2021;13(8):1673. doi: 10.3390/v13081673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. European Journal of Heart Failure. 2020;22(5):911–5. doi: 10.1002/ejhf.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nature Communications. 2021;12(1). doi: 10.1038/s41467-021-22781-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceulemans LJ, Khan M, Yoo S-J, Zapiec B, Van Gerven L, Van Slambrouck J, et al. Persistence of SARS-CoV-2 RNA in lung tissue after mild COVID-19. The Lancet Respiratory Medicine. 2021. doi: 10.1016/S2213-2600(21)00240-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viszlayová D, Sojka M, Dobrodenková S, Szabó S, Bilec O, Turzová M, et al. SARS-CoV-2 RNA in the Cerebrospinal Fluid of a Patient with Long COVID. Therapeutic Advances in Infectious Disease. 2021;8:204993612110485. doi: 10.1177/20499361211048572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization WHO R&D Blueprint: Novel Coronavirus COVID-19 Therapeutic Trial Synopsis 2020. [cited 2021 June 8]. Available from: https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis. [Google Scholar]
- 33.Cope AP. Studies of T-cell activation in chronic inflammation. Arthritis Research. 2002;4(Suppl 3):S197. doi: 10.1186/ar557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong J, Dong H, Xia Q-S, Huang Z-Y, Wang D-K, Zhao Y, et al. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19: a retrospective study. BMC Infectious Diseases. 2020;20(1). doi: 10.1186/s12879-020-05681-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peluso MJ, Deitchman AN, Torres L, Iyer NS, Munter SE, Nixon CC, et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Reports. 2021;36(6):109518. doi: 10.1016/j.celrep.2021.109518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rincon M, Irvin CG. Role of IL-6 in Asthma and Other Inflammatory Pulmonary Diseases. International Journal of Biological Sciences. 2012;8(9):1281–90. doi: 10.7150/ijbs.4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–5. Epub 2020/05/01. doi: 10.1073/pnas.2005615117 ; PubMed Central PMCID: PMC7245089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–29. Epub 2021/01/05. doi: 10.7150/thno.49713 ; PubMed Central PMCID: PMC7681075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parameswaran N, Patial S. Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20(2):87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10 ; PubMed Central PMCID: PMC3066460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting Interleukin-6 Signaling in Clinic. Immunity. 2019;50(4):1007–23. doi: 10.1016/j.immuni.2019.03.026 [DOI] [PubMed] [Google Scholar]
- 41.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature Medicine. 2009;15(8):914–20. doi: 10.1038/nm.1964 [DOI] [PubMed] [Google Scholar]
- 42.Moghaddami M, Cleland LG, Radisic G, Mayrhofer G. Recruitment of dendritic cells and macrophages during T cell-mediated synovial inflammation. Arthritis Research & Therapy. 2007;9(6):R120. doi: 10.1186/ar2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lykouras D, Sampsonas F, Kaparianos A, Karkoulias K, Spiropoulos K. Role and pharmacogenomics of TNF-alpha in asthma. Mini Rev Med Chem. 2008;8(9):934–42. Epub 2008/08/12. doi: 10.2174/138955708785132828 . [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Zhang X, Zheng J, Liu Y, Wang J, Wang G, et al. Depressive symptom-associated IL -1β and TNF -α release correlates with impaired bronchodilator response and neutrophilic airway inflammation in asthma. Clinical & Experimental Allergy. 2019;49(6):770–80. doi: 10.1111/cea.13346 [DOI] [PubMed] [Google Scholar]
- 45.Xu W, Li R, Sun Y. Increased IFN-γ-producing Th17/Th1 cells and their association with lung function and current smoking status in patients with chronic obstructive pulmonary disease. BMC Pulmonary Medicine. 2019;19(1). doi: 10.1186/s12890-019-0899-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell. 2021;184(1):149–68.e17. doi: 10.1016/j.cell.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerging Microbes & Infections. 2020;9(1):1123–30. doi: 10.1080/22221751.2020.1770129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wauters E, Van Mol P, Garg AD, Jansen S, Van Herck Y, Vanderbeke L, et al. Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalveolar lavages. Cell Research. 2021;31(3):272–90. doi: 10.1038/s41422-020-00455-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881–95.e20. doi: 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cervia C, Zurbuchen Y, Taeschler P, Ballouz T, Menges D, Hasler S, et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nature Communications. 2022;13(1). doi: 10.1038/s41467-021-27797-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheon IS, Li C, Son YM, Goplen NP, Wu Y, Cassmann T, Wang Z, Wei X, Tang J, Li Y, Marlow H, Hughes S, Hammel L, Cox TM, Goddery E, Ayasoufi K, Weiskopf D, Boonyaratanakornkit J, Dong H, Li H, Chakraborty R, Johnson AJ, Edell E, Taylor JJ, Kaplan MH, Sette A, Bartholmai BJ, Kern R, Vassallo R, Sun J. Immune signatures underlying post-acute COVID-19 lung sequelae. Science Immunology. 2021; 6(65):eabk1741. doi: 10.1126/sciimmunol.abk1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vijayakumar B, Boustani K, Ogger PP, Papadaki A, Tonkin J, Orton CM, et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity. 2022;55(3):542–56.e5. doi: 10.1016/j.immuni.2022.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neff CP, Krueger O, Xiong K, Arif S, Nusbacher N, Schneider JM, et al. Fecal Microbiota Composition Drives Immune Activation in HIV-infected Individuals. EBioMedicine. 2018;30:192–202. doi: 10.1016/j.ebiom.2018.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmer BE, Boritz E, Blyveis N, Wilson CC. Discordance between Frequency of Human Immunodeficiency Virus Type 1 (HIV-1)-Specific Gamma Interferon-Producing CD4 + T Cells and HIV-1-Specific Lymphoproliferation in HIV-1-Infected Subjects with Active Viral Replication. Journal of Virology. 2002;76(12):5925–36. doi: 10.1128/jvi.76.12.5925-5936.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, et al. Programmed Death 1 Expression on HIV-Specific CD4+T Cells Is Driven by Viral Replication and Associated with T Cell Dysfunction. The Journal of Immunology. 2007;179(3):1979–87. doi: 10.4049/jimmunol.179.3.1979 [DOI] [PubMed] [Google Scholar]
- 56.Kassu A, D’Souza M, O’Connor BP, Kelly-Mcknight E, Akkina R, Fontenot AP, et al. Decreased 4-1BB expression on HIV-specific CD4+ T cells is associated with sustained viral replication and reduced IL-2 production. Clinical Immunology. 2009;132(2):234–45. doi: 10.1016/j.clim.2009.03.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jafari M, Ansari-Pour N. Why, When and How to Adjust Your P Values? Cell J. 2019;20(4):604–7. Epub 2018/08/21. doi: 10.22074/cellj.2019.5992 ; PubMed Central PMCID: PMC6099145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of participants reporting each symptom at time of acute or prolonged disease. aPercentage of cohort.
(XLSX)
Monoclonal antibodies used in intracellular cytokine staining to determine frequencies and characteristics of SARS-CoV-2 specific T cells. aNA = Not applicable. bIndicates antibody was excluded for Fluorescence-3 staining control.
(XLSX)
(A) Symptom duration (days) reported in symptom questionnaires for PASC and RC participants. (B) Number of symptoms reported <4 weeks or >4 weeks from symptom onset for PASC and RC participants. For each graph, bars represent the median of each cohort and the error bar represents the upper 95% confidence interval. Blue represents PASC participants not hospitalized (PASC-NH, n = 12), red represents PASC-hospitalized (PASC-H, n = 8) and orange represents RC participants (n = 20). Mann-Whitney tests were used to determine statistical significance.
(TIF)
(A) Percent of combined S-, N- and M-specific frequencies of IFN-γ- or TNF-α-producing CD4+ or CD8+ T cells separated by number of pre-existing conditions. (B) Frequency of combined S-, N- and M-specific TNF-α-producing CD8+ T cells or (C) S-specific TNF-α-producing CD8+ T cells for PASC participants separated by sex. Each point represents data from one participant where blue represents PASC-NH (not hospitalized) and red represents PASC-hospitalized. Mann-Whitney tests were used to determine statistical significance.
(TIF)
(A) PASC plasma IL-6 (pg/mL) levels and (B) PASC plasma CRP levels (mg/L) in PASC separated by sex. (C) Serum IL-6 (pg/mL) and (D) CRP (mg/L) in PASC separated by pre-existing conditions. Each point represents data from one participant. Where applicable, bar represents median of cohort and error bars are 95% confidence index. Blue and red symbols represent PASC-NH (not hospitalized) and PASC-Hospitalized, respectively.
(TIF)
(A) Frequency of SARS-CoV-2 N- and M-specific TNF-α-producing T cells from samples taken 2 and 30 weeks from symptom onset. Each set of points connected by a line represents data from one participant. (B) Percent of cummulative S, N and M SARS-CoV-2-specific CD4+ or CD8+ T cells producing IFN-γ or TNF-α for the RC cohort split by time from symtom onset to sample collection. Orange symbols represent RC participants.
(TIF)
PBMCs were isolated from PASC and RC participants and stained with mAb to assess their ex vivo phenotype by flow cytometric analysis. Percentage of total CD4+ T cells expressing (A) Ki-67 and (B) PD-1 for PASC and RC participants. Percentage of total CD8+ T cells expressing (C) Ki-67 and (D) PD-1 for both cohorts. Shown are the frequencies of naïve (CD27+CD45RA+), central memory (CD27+CD45RA-), effector memory (CD27-CD45RA-) and terminally differentiated (TD)-effector memory (CD27-CD45RA+) on total (E) CD4+ and (F) CD8+ T cells. All data collected from flow cytometry in unstimulated conditions. For each box and whisker plot, the center line denotes the median value (50th percentile), and the box contains the 25th to 75th percentile values for each dataset. Whiskers mark the 5th and 95th percentiles, and values beyond these upper and lower bounds are not visualized. Dark grey plots represent the PASC cohort and light gray plots represent the RC cohort. Mann-Whitney tests were used to determine statistical significance.
(TIF)
An example of the gating strategy used to determine frequency of SARS-CoV-2-specific T cells. Each axis is labeled with the fluorochrome measured and corresponding marker if applicable and data is presented as pseudocolor dot plots (FlowJo). Arrows indicate the sequence of analysis and the number on each plot is the frequency of the gated population.
(TIF)
Data Availability Statement
Data cannot be shared publicly because of the inclusion of PHI subject to HIPAA regulations. Data are available from the University of Colorado Institutional Data Access Committee (contact Crao_Contracts@ucdenver.edu) for researchers who meet the criteria for access to confidential data.