Abstract
In contrast to decreasing incidence rates of colorectal cancer (CRC) in older adults, incidence rates have nearly doubled in younger adults (age <50 years) in the U.S. since the early 1990s. Similar increases have occurred across the globe. Despite overall population trends in aging, by 2030, about 15% of CRCs will be diagnosed in younger adults. Mechanisms and factors contributing to early-onset CRC (EOCRC) remain puzzling, especially because most young adults diagnosed with CRC have no known risk factors or predisposing conditions, such as family history of CRC or polyps or a hereditary syndrome (e.g., Lynch syndrome, polyposis). In this up-to-date review, we discuss the current knowledge of EOCRC, including epidemiology, risk factors, clinical and molecular features, treatment and survival, and recognition and screening strategies.
Keywords: colorectal cancer, epidemiology, young adult, risk factors
Introduction
Colorectal cancer (CRC) is a major cause of cancer-related morbidity and mortality in the U.S. Incidence and mortality rates have decreased in adults older than age 50 years over the last three decades,1 likely due to increased uptake of screening and shifts in the distribution of risk factors (e.g., decreased cigarette use, increased aspirin use).2 By contrast, incidence rates have risen rapidly in younger adults (age <50 years), from 8.6 per 100,000 in 1992 to 12.9 per 100,000 in 2018,3 and as a result, 10–12% of all CRCs now occur in younger adults. Research on the mechanisms and factors contributing to early-onset CRC (EOCRC) has proliferated since increasing rates were first described in the mid-2000s.4, 5 In this up-to-date review, we discuss the current knowledge of EOCRC, including epidemiology, risk factors, clinical and molecular features, treatment and survival, and recognition and screening strategies.
Epidemiology of Early-Onset Colorectal Cancer
In contrast to dramatic decreases in older adults, incidence rates of CRC have nearly doubled in younger adults since the early 1990s. Specifically, incidence rates in the U.S. have risen rapidly among persons age 20–49 years, from 8.6 per 100,000 in 1992 to 12.9 per 100,000 in 2018, with the largest increases observed in those age 40–49 years (Figure 1).3, 6 Likewise, as mortality rates of CRC continue to decline in older adults2, 7 they remain stagnant at 2.8 per 100,000 in younger adults.8
Figure 1.
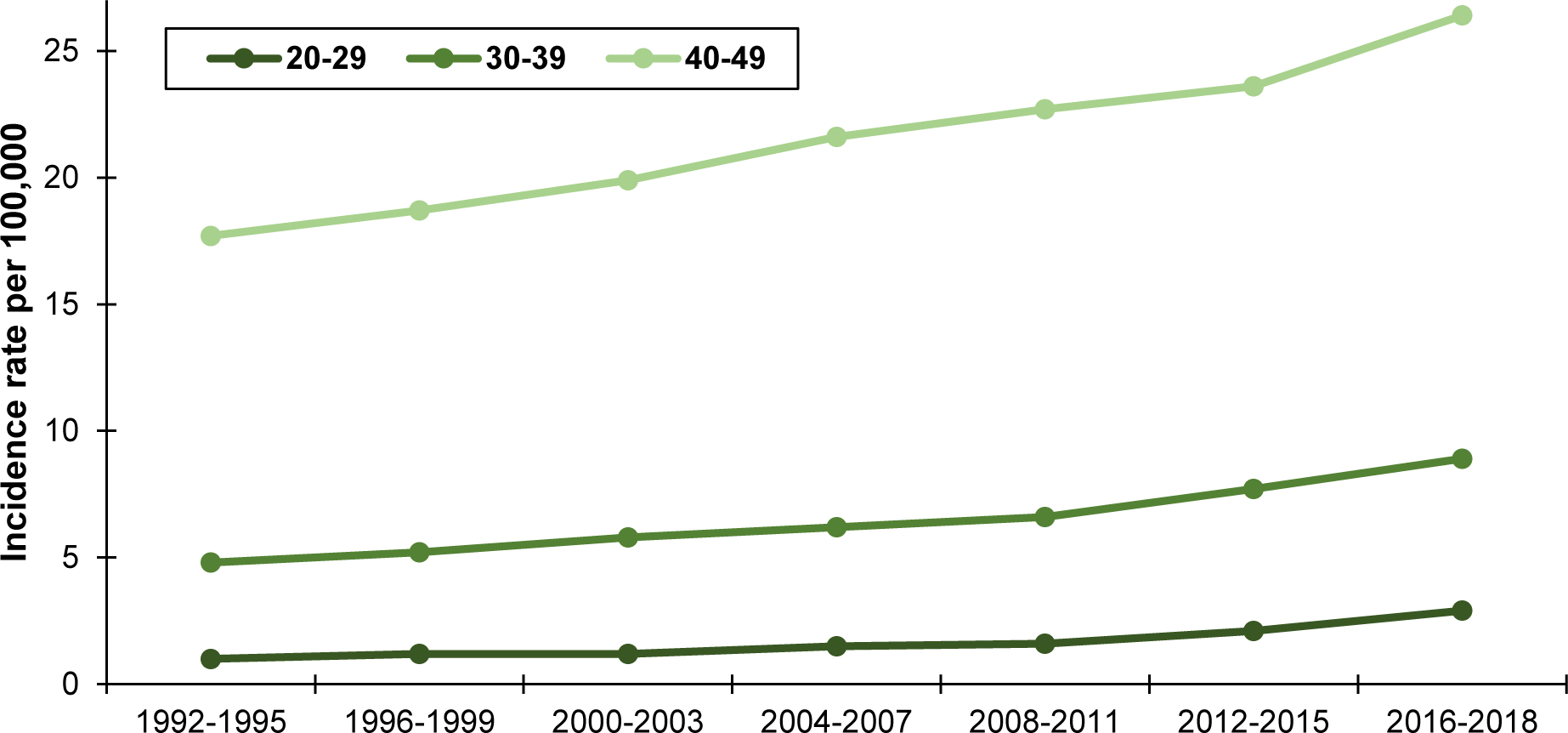
Incidence rates of colorectal cancer by 10-year age group, ages 20–49 years, SEER 13, 1992 – 2018
Global Trends
Incidence rates of EOCRC have increased across the globe, including many countries in western Europe, Australia, Brazil, Canada, China, Japan, Taiwan, Korea, and the United Kingdom.9–12 Incidence rates have decreased in only three countries—Austria, Italy, and Lithuania—by about 1% annually,12 although it is notable that screening programs in parts of Italy13 and in Austria include age ≥ 40 years.14
Geographic Trends in the United States
In the U.S., incidence rates of EOCRC vary widely by state. The lowest rates have been observed in western states (about 9.5 per 100,000), although rates in non-Hispanic Whites have recently begun to rise in this region.15 Rates remain highest in southern states, particularly in the Mississippi Delta Region and Appalachia (about 14.0 per 100,000). Specifically, Mississippi and Kentucky have the highest incidence rates of EOCRC, at 15.1 per 100,000 and 14.2 per 100,000, respectively. CRC mortality rates (across all age groups) are also high in these regions,16 as well as incidence rates of other gastrointestinal cancers, compared to other parts of the U.S.17 Reasons accounting for these geographic differences may include poor access to healthcare, high rates of poverty and unemployment, lifestyle factors (e.g., sedentary lifestyle, diet, obesity),18 occupational exposures (e.g., mineral dust, trace elements),19 and environmental exposures (e.g. industrial pollution, agricultural runoff).20
Birth Cohort Effects
Incidence rates of EOCRC have increased successively across generations.21, 22 For example, in the U.S., incidence rates are higher in 40-year-old persons born in 1970 (24.4 per 100,000) compared to 40-year-old persons born in 1950 (18.3 per 100,000).3 This pattern is most pronounced in younger generations, including Generation X (persons born in 1965–1980) and Millennials (persons born in 1981–1996). Increasing rates of EOCRC across generations have also been observed in Europe,9 Canada,23 Australia,24 and Asia.25, 26 The birth cohort effect points to exposures or factors in early life, such as infancy, childhood, and adolescence, that may increase risk of EOCRC.21, 27 Early life represents a window of susceptibility to exposures,28 which can translate into large effects on cancer risk in adulthood. This is consistent with literature showing events in utero and during infancy and childhood have important consequences for several adult cancers.29, 30
Differences Among Racial/Ethnic Groups
In the U.S., incidence rates of EOCRC are highest in non-Hispanic Blacks compared to other racial/ethnic groups. Although incidence rates remain highest in non-Hispanic Blacks, rates in this group have only modestly increased since the 1990s, from 12.7 to 15.0 per 100,000 persons, between 1992–1995 to 2016–2018.3 Conversely, incidence rates in non-Hispanic Whites and Hispanics have increased by about 85% over the same time period, from 7.7 to 14.4 per 100,000 persons and 6.1 to 11.3 per 100,000 persons, respectively.31 Rates have remained stable in Asians, increasing from 9.8 per 100,000 in 1992–1995 to 10.3 per 100,000 in 2016–2018. It is not yet clear why incidence rates have increased more rapidly in non-Hispanic Whites and Hispanics but not non-Hispanic Blacks or Asians, although some have suggested differences in the prevalence of risk factors across these groups may play a role.32
Differences by Sex
Incidence rates of EOCRC have increased in both men and women, although rates have been consistently higher in men. Specifically, incidence rates increased from 8.7 per 100,000 men in 1992–1995 to 13.9 per 100,000 men in 2016–2018; and from 7.7 per 100,000 women in 1992–1995 to 12.6 per 100,000 women in 2016–2018. Incidence rates between men and women are similar at ages 20–29 years and 30–39 years but begin to differentiate at age 40–49 years.
Genetic and Familial Risk
About 16–35% of EOCRC occurs in persons with hereditary cancer syndromes, which are more prevalent in younger age groups.33, 34 The most common syndrome is Lynch syndrome (10%) followed by polyposis syndromes, including familial adenomatous polyposis, MUTYH-associated polyposis, juvenile polyposis, and others (3%). Importantly, many patients diagnosed with EOCRC and a hereditary syndrome do not have phenotypes typically associated with these syndromes, and others have pathogenic mutations despite having no family history of CRC or cancer.34–36 Hereditary syndromes may also be underrecognized in persons of non-European ancestry due to the relative lack of knowledge of mutations in diverse groups.36 Given these findings, the National Comprehensive Cancer Network (NCCN) recommends all patients diagnosed with EOCRC receive germline genetic testing to evaluate for pathogenic variants in cancer susceptibility genes.37 Universal testing may also identify founder mutations or links between CRC and genes previously not believed to increase risk.
Across all ages, family history of CRC accounts for 10–30% of new diagnoses.38 Having a first-degree relative with a history of CRC diagnosed at age <50 years about triples risk (RR: 3.26; 95% CI, 2.82–3.77).39 For EOCRC, about 19% of all patients report a family history of CRC in a first degree relative, and 14% of patients with no hereditary syndrome report a family history.40 A few, albeit smaller, studies noted a higher proportion of patients diagnosed with EOCRC reported a family history of CRC in a second-degree relative compared to patients diagnosed with later-onset CRC (age ≥50 years).41, 42 Importantly, a recent case-control study found that one in four patients diagnosed with CRC at ages 40–49 years met criteria for earlier screening based on family history, and of these, nearly all (98.4%) should have undergone screening prior to their diagnosis.43 Therefore, identifying and screening based on family history of CRC remains an important factor in mitigating the rise of EOCRC.
Genome-wide association studies (GWAS) have not identified common or rare genetic events linked to EOCRC, including studies of high penetrance on whole-exome and whole-genome sequencing. However, studies have explored the pooled effects of common, low-penetrance genetic variants associated with EOCRC.44, 45 For example, a recent study developed a weighted polygenic risk score (derived from 95 single nucleotide polymorphisms), and the highest quartile of the risk score was associated with more than four-times the risk of EOCRC in persons without a family history in a first-degree relative.46 Polygenic risk scores – or the cumulative burden of CRC-associated genetic variants – along with lifestyle factors, environmental factors, and family history, may improve the accuracy of risk prediction models compared to using family history alone.44
Clinicopathologic Features
Tumor Location and Stage at Diagnosis
A higher proportion of young adults are diagnosed with tumors in the distal colon or rectum compared to older adults, among whom tumors in the proximal colon predominate.46 Multiple studies demonstrate left-sided tumors are more common in young adults (between 60–90%, including 30–50% with rectal tumors),47–55 and a recent meta-analysis suggests young adults are less likely to have right-sided tumors (pooled OR: 0.62; 95% CI: 0.56, 0.68).56 These findings are consistent with temporal trends in incidence rates. For example, from the early 1990s to about 2012, increasing incidence rates of EOCRC were largely driven by increasing rates of rectal cancer, particularly among non-Hispanic Whites.32 From 2012 to 2018, rates of EOCRC have increased similarly by tumor location, for proximal colon (3.2 to 4.2 per 100,000 persons), distal colon (3.0 to 3.4 per 100,000 persons), and rectum (3.5 to 3.9 per 100,000 persons).3
A higher proportion of young adults are also diagnosed with advanced stage CRC (stage III-IV) compared with older adults.41, 57, 58 A recent meta-analysis demonstrates younger adults are more likely to be diagnosed with regional (pooled OR: 1.27; 95% CI: 1.16, 1.40) or distant (pooled OR: 1.47; 95% CI: 1.30–1.67) stage disease compared to older adults.56 Reasons for this may include: 1) no routine screening, low awareness among patients and providers, and less attention to red flag symptoms of EOCRC, all of which likely contribute to delays in diagnosis;59 or 2) more aggressive disease, such as poor differentiation, mucinous or signet ring histology, and perineural or lymphovascular invasion.51, 60
Although not extensively studied, some studies suggest fewer differences in the proportion of left-sided tumors and advanced stage disease when comparing younger adults to unscreened older adults.42
Symptomatic Presentation
Because young adults do not undergo routine screening, they often present symptomatically. Common symptoms include hematochezia (even after adjusting for tumor sidedness50), anemia, and abdominal pain. For example, in an online survey conducted by the Colorectal Cancer Alliance, 81% of respondents reported at least three different symptoms prior to their diagnosis of EOCRC, and they often had symptoms for months or even years before undergoing an initial evaluation; 19% reported delays in diagnosis of >12 months from initial symptoms.61 Interestingly, younger adults diagnosed with advanced stage disease seem to have a shorter duration of symptoms41 compared to those diagnosed with early stage disease, suggesting differences in biology of more aggressive EOCRCs.
Microsatellite Instability
The aggressive pathologic features reported in EOCRCs, including poor differentiation, mucinous or signet-ring histology, and perineural or lymphovascular invasion,42, 48, 51 may be due to enriched microsatellite unstable (MSI) tumors in younger vs. older patients, related to a higher proportion of Lynch syndrome in younger adults.62 Consequently, studies have explored whether EOCRC has a distinct molecular and genomic profile. For example, in younger patients with MSI tumors, most tumors are attributable to Lynch syndrome, and epigenetic alterations in methylation of the CpG-rich region of the MLH1 promoter mediated by mutations in BRAF V600 (CpG-island methylator phenotype or CIMP) are less frequent.62, 63 Most sporadic EOCRCs are microsatellite stable (MSS) and likely develop through the chromosomal instability (CIN) pathway, which often results from somatic mutations in APC, although APC is less frequently mutated in younger patients. Instead, somatic mutations in TP53, CTTNB1, and POLE may be increased;62, 64 however, a recent study comparing MSS tumors between younger and older patients suggests no differences in tumor histology or somatic mutations between the two groups.
Tumors can be further characterized by consensus molecular subtypes (CMS),65 reflecting differences in gene expression. Willauer et al. examined prevalence of CMS in younger patients and found that the most common subtype in this age group (18–49 years) was CMS-1 (MSI-immune, immune infiltration and activation); CMS-2 was similar in younger and older patients, and CMS-3 and −4 were rare. This study included patients with Lynch syndrome, which may account for the higher prevalence of CMS-1 at younger ages.50, 63 Better characterizing tumors by CMS, particularly MSS tumors, may identify factors unique to the pathogenesis of sporadic (vs. hereditary) EOCRC.
Risk Factors
Some non-modifiable risk factors, including older age, male sex, and non-White race, are associated with EOCRC.6, 54, 66, 67 Because 80% of patients diagnosed with EOCRC have MSS tumors, implicating the adenoma-carcinoma sequence as in later-onset CRC, earlier exposures likely lead to an earlier sequence of carcinogenesis.68, 69 As detailed below, several modifiable lifestyle factors increase risk of EOCRC, including dietary patterns, obesity and metabolic syndrome, sedentary behavior, and alcohol and tobacco use. Exogenous exposures, such as factors related to intestinal dysbiosis, may also contribute to risk, and identifying these exposures may identify previously unknown carcinogens, relevant to both early- and later-onset CRC.70 Table 1 lists risk factors, describes the hypothesized mechanism by which they increase risk, and summarizes evidence related to EOCRC.
Table 1:
Risk factors of early-onset colorectal cancer
| Risk factor (direction of risk) | Hypothesized mechanism | Supporting evidence |
|---|---|---|
|
| ||
| Metabolic syndrome (+) | Increased risk in cohort, case-control studies, and meta-analyses | |
| Obesity (+) | Chronic inflammation mediated by adipocytokines (TNF-α, IL-6, CRP) trigger immune response; abnormal signaling pathways in insulin-like growth factors and sex hormones | Trend towards increased risk among cohort, case-control, and meta-analyses studies |
| Type 2 diabetes (+/−) | Impaired insulin receptor activation, high levels of insulin-like growth factors stimulate colonic mucosal cell growth and prevent apoptosis | Inconsistent results in few case-control studies |
| Dyslipidemia (?) | Triglycerides may increase fecal bile acid exposure or energy for neoplastic cells; cholesterol accumulates in membranes of cancer cells | Insufficient evidence |
| Hypertension (?) | Unclear, possibly prevents apoptosis | Insufficient evidence |
| Dietary patterns (+) | Increased risk in cohort, case-control studies, and meta-analyses | |
| Low fiber diet (+) | Decreased colonic motility causing increased exposure to fecal carcinogens; stimulates butyrogenic activity, which is anti-neoplastic | Increased risk in cohort, case-control studies, and meta-analyses |
| Sugar-sweetened beverages (+) | Induce insulin resistance; fructose may cause dysbiosis and increase gut permeability | Increased risk in few cohort studies |
| Red/processed meats (+) | Exposure to mutagenic compounds including N-nitroso compounds, heterocyclic amines, polycyclic aromatic hydrocarbons | Increased risk in cohort, case-control studies, and meta-analyses |
| High fat diet (+) | Increased bile acid metabolism with bile acid conversion to deoxycholic acid | Increased risk in cohort, case-control studies, and meta-analyses |
| Micronutrients | ||
| Calcium (?) | Prevents fatty acid/bile acid carcinogenic on intestinal mucosa; inhibits inflammation | Insufficient evidence |
| Vitamin D (−) | Inhibits proliferation and angiogenesis, promotes differentiation; vit D receptor binding inhibits Wnt/β-catenin pathway | Limited; decreased risk in cohort study |
| Alcohol use (+) | Direct and indirect genotoxic effects by metabolites | Increased risk in cohort, case-control studies, and meta-analyses |
| Smoking and tobacco (+/−) | Exposure to genotoxic compounds through circulatory system or direct ingestion leads to colorectal adenoma | Inconsistent results in cohort, case-control studies, and meta-analyses |
| Sedentary behavior (+/−) | Decreased colonic motility causing increased exposure to fecal carcinogens; impairment of glucose homeostasis; increased levels of pro-inflammatory factors, decreased levels of anti-inflammatory factors | Inconsistent results in few studies |
| Maternal weight gain/obesity (+) | Fetal programming leading to changes in adipose tissue and insulin sensitivity; epigenetic methylation of genes involved in energy metabolism | Limited; increased risk in cohort study |
| Birth weight (+) | Increased risk of obesity in adulthood | Limited; increased risk in cohort study |
| Dietary additives (?) | Variable | Insufficient evidence |
| Intestinal dysbiosis | Biofilm formation, increased inflammation, gut permeability | |
| Bacteria (+) | Potential oncogenic associations with Fusobacterium, B. fragilis, S. gallolytics, H. pylori | Meta-analysis with increased Fusobacterium in eoCRC patients |
| Antibiotic use (?) | Modifies intestinal flora | Insufficient evidence |
Dietary Patterns
Western diets, including high intake of processed foods,71 fatty foods,72–74 red meat,71, 75 and sugary beverages and desserts,55, 76, 77 and low intake of fiber72, 74, 78 and micronutrients (e.g., calcium, vitamin D79, beta-carotene, and vitamin E),55, 71 contribute to colorectal carcinogenesis via hyperinsulinemia, altered bile acid metabolism,72–74 chronic inflammation, and intestinal dysbiosis.55, 71, 80–83 For example, mice fed a Western diet had more colitis and tumorigenesis, mediated by increased interferon response and inflammation, changes in innate and adaptive immunity, and changes in antigen processing pathways; these effects were tempered when calcium and vitamin D were replaced in standard amounts.84 As an additional example, after a two-week food exchange, during which rural South Africans were provided a high-fat, low-fiber Western diet, and Black Americans were provided a low-fat, high-fiber South African diet, South Africans experienced alterations in colonic mucosal biomarkers, microbiota, and the metabolome linked to higher risk of CRC (across all ages). Meanwhile, Black Americans experienced beneficial changes.73
Although evidence on the independent effect of each of these dietary factors is mixed, the aggregate of a Western diet is consistently associated with EOCRC.55, 71, 85 For example, a recent analysis of the Nurses’ Health Study II demonstrated higher risk of early-onset, high-risk adenomas (defined as adenoma ≥1 cm, with villous features, or high-grade dysplasia) associated with a Western diet (adjusted OR: 1.67; 95% CI: 1.18, 2.37) compared to a prudent diet (aOR: 0.69; 95% CI: 0.48, 0.98), DASH (Dietary Approaches to Stop Hypertension) diet (aOR: 0.65; 95% CI: 0.45, 0.93), Mediterranean diet (aOR: 0.55; 95% CI: 0.38, 0.79), and the Alternative Healthy Eating Index-2010 (aOR: 0.71; 95% CI: 0.51, 1.01).85 A population-based case-control study in Ontario, Canada similarly found an increased risk of EOCRC associated with a Western diet (aOR: 1.92; 95% CI: 1.01, 3.66). Studies are ongoing to identify specific dietary factors (e.g., high fat, low fiber, excess sugar intake), but healthful dietary patterns should be encouraged as a means to prevent CRC in adults of all ages.
Obesity and Metabolic Syndrome
High body mass index (BMI), including overweight (BMI ≥25 kg/m2) and obese (BMI ≥30 kg/m2), increases risk of CRC across all age groups.86–89 Excess body fatness can lead to several tumorigenic mechanisms due to abnormalities in signaling pathways, insulin-like growth factors, sex hormones, and adipocytokines. Increasing incidence rates of EOCRC parallel the obesity epidemic in the U.S., and multiple studies have therefore examined the link between obesity and EOCRC. For example, Liu et al. demonstrated that obese women in the Nurses’ Health Study II had almost double the risk of EOCRC (adjusted RR: 1.93; 95% CI: 1.15, 3.25), even after adjusting for diabetes, alcohol and tobacco use, NSAID or aspirin use, and diet.90 This observation has been substantiated in other population-based studies91–94 and meta-analyses.95–97
A different study found an inverse relationship between BMI in participants’ early 20s (aOR: 0.43; 95% CI: 0.20, 0.90) and two years prior to the study (aOR: 0.59; 95% CI: 0.34, 1.01) and EOCRC.55 An inverse association between BMI and EOCRC was also observed in a study of U.S. veterans, although this may reflect weight loss due to symptoms of cancer.66 Other case-control and cohort studies54, 98 report no association between BMI and EOCRC. These conflicting findings suggest obesity – as measured by BMI alone – is not a risk factor but rather the metabolic dysregulation associated with higher BMI may matter most. Timing of obesity (i.e., during childhood or adolescence) may also contribute to risk of EOCRC later in life, although, to date, evidence has been mixed.99–103
Like obesity, metabolic syndrome, defined as the constellation of chronic conditions such as central obesity, hypertension, hyperglycemia or type 2 diabetes, and hyperlipidemia, has paralleled increasing incidence rates of EOCRC. Metabolic syndrome may be an especially important risk factor because insulin resistance104–107 and lipid metabolism108–110 appear to have independent effects on colorectal carcinogenesis. For example, in a large study of commercially-insured adults, metabolic syndrome was associated with early-onset colon cancer (aOR: 1.38; 95% CI: 1.18, 1.62) but not early-onset rectal cancer (aOR 1.04: 95% CI: 0.83, 1.32), and risk of early-onset colon cancer increased as the number of metabolic syndrome-defining conditions also increased.111 In a follow-up study, the same researchers identified an association between type 2 diabetes and early-onset colon cancer (proximal colon aOR: 1.35; 95% CI: 1.03,1.77 and distal colon aOR: 1.67; 95% CI: 1.30, 2.15).112 A case-control study conducted in a large, integrated healthcare system in Southern California examined type 2 diabetes, dyslipidemia, BMI, and hypertension as risk factors of early-onset adenocarcinoma but found that only obesity was associated with increased risk for colon but not rectal adenocarcinoma.93 Yet still, others have found no association between obesity, type 2 diabetes, and dyslipidemia and EOCRC.54 Ongoing and additional studies examining risks associated with metabolic syndrome may clarify these findings and provide insight into the protective effects of lipid- and glycemic-regulating therapies.
Sedentary Behavior
Sedentary behavior has been proposed as a risk factor for EOCRC because of its association with obesity, as well as its impact on colonic motility and intestinal stasis, especially in the rectum. Some studies demonstrate lower risk of EOCRC in persons with higher levels of occupational, physical, or leisure-time activity,94, 113–115 although definitions of activity differ across studies. For example, an analysis of the Nurses’ Health Study II used time watching television as a surrogate of sedentary behavior and found that more than 14 hours of television time per week increased risk of EOCRC (aRR: 1.69; 95% CI: 1.07, 2.67). This association was more pronounced for early-onset rectal vs. colon cancer.116 Conversely, in a pooled analysis of 13 population-based studies, there was no association between sedentary behavior and EOCRC (aOR: 1.13; 95% CI: 0.88, 1.44).78 Despite these mixed findings, most studies of physical activity and later-onset CRC demonstrate a protective effect,113–115, 117 and physical activity may continue to be encouraged as a prevention strategy, especially given the increase in sedentary time in work settings.118
Alcohol and Tobacco Use
Several meta-analyses demonstrate associations between regular alcohol consumption and CRC across all ages.119–122 This is consistent with recent meta-analyses and case-control studies suggesting alcohol increases risk of EOCRC, with some evidence of dose-response.55, 71, 78, 95, 97
Despite the well-established relationship between cigarette smoking and later-onset CRC, studies of EOCRC are less consistent.78, 94, 95, 97 Specifically, several case-control studies show no association between smoking and EOCRC.54, 55, 66 This may be due to: 1) long duration required for cigarette smoking to promote carcinogenesis; and 2) subsequent time needed to complete the adenoma to carcinoma sequence.68
Other Exogenous Exposures
Although dietary patterns, obesity and metabolic syndrome, sedentary behavior, and alcohol and tobacco use may be associated with increased risk of EOCRC, there may be other, as-yet-unknown, exogenous exposures that contribute to increasing incidence rates. Indeed, the recent “NIH Early-Onset CRC Think Tank” emphasized that obesity is an important risk factor but cannot fully explain trends.123 These other exogenous exposures may include in utero events, dietary additives, and intestinal dysbiosis, detailed below.
In Utero Events.
Increasing incidence rates of EOCRC across generations implicate exposures in early life – including in utero events – as risk factors. For example, in a large, population-based study linking pregnant mothers’ medical records to the California Cancer Registry, maternal obesity more than doubled the risk of CRC in adult offspring (adjusted HR: 2.51; 95% CI 1.05, 6.02). Almost half of offspring diagnosed with CRC in this study were diagnosed at age <50 years.124 Antibiotic exposure in utero or during childhood may also increase risk of EOCRC because of its effect on intestinal microbiota. Excess antibiotic exposure promotes colorectal tumorigenesis in mice125 but this association has not been demonstrated consistently outside of animal models.55
Dietary Additives.
Dietary additives, including nitrates and nitrites in processed meats, monosodium glutamate, titanium dioxide in confectionery, synthetic food coloring, and high-fructose corn syrup,126 are also possible risk factors.127 These additives are especially common in foods marketed to young children. In the Nurses’ Health Study II, Hur et al. demonstrated an increased risk of EOCRC in women consuming ≥2 servings of sugar-sweetened beverages per day during adolescence (age 13–18 years, aRR: 2.18; 95% CI: 1.10, 4.35) but not during adulthood. Each additional serving of sugar-sweetened beverages per day during adolescence was associated with a 32% increase in risk of EOCRC.76 Geographic differences in incidence rates may provide additional insight into the impact of dietary additives. For example, pollution from cigarette smoke, dust, and automobile engines contain similar carcinogenic compounds to those found in processed meats.20, 128
Intestinal Dysbiosis.
Several studies suggest dysbiosis and decreased diversity of intestinal flora, as well as specific bacteria, may increase risk of CRC across all ages.128 For example, Fusobacterium is an oral biofilm-forming gram-negative anaerobe that has been identified in colorectal tumors.129 Fusobacterium may contribute to carcinogenesis by promoting E-cadherin/β-catenin signaling through the FadA ligand or through downregulation of antitumor T-cell mediated immunity.130 Other bacteria that may be associated with CRC include enterotoxigenic Bacteroides fragilis, Streptococcus gallolyticus, and Helicobacter pylori.130 Many of the risk factors described above (e.g., in utero events, dietary patterns, obesity) may increase risk of EOCRC via their effects on intestinal dysbiosis. The gut microbiome begins developing at birth, and its diversity can be limited at multiple points in life, including by mode of delivery (vaginal versus Cesarean section), duration of breastfeeding, antibiotic treatment in childhood and adulthood,125, 131, 132 periodontal disease,133 obesity, and low-fiber and vegetable diets.130 Future studies will be critical to understand the gut microbiome’s role in EOCRC, the role of factors contributing to dysbiosis at different points in life, and the potential for therapies targeting dysbiosis in treatment and prevention.
Treatment and Survival
Treatment guidelines do not distinguish early-onset vs. later-onset CRC nor recommend different treatment strategies by age. However, several studies demonstrate that younger patients are more likely to be treated intensively, including more invasive surgery, multimodal chemotherapy, and radiation therapy, even if not clinically indicated.57, 134–137 Further, despite the fact that younger patients tend to have more aggressive tumor features (e.g., signet ring or mucinous histology, high grade, poor differentiation), there are generally no differences in stage-adjusted survival between younger vs. older patients, or those receiving vs. not receiving intensive treatment.47, 57, 135, 136, 138–140 It is possible that adjuvant chemotherapy confers no additional survival benefit for younger patients because a higher proportion have MSI-high tumors, and MSI-high tumors have a better prognosis for stage II and III141, 142 but are less sensitive to conventional chemotherapy.143 Recent guidelines recommend treating advanced, MSI or MMR-deficient tumors with immune-checkpoint inhibitors (e.g., PD-1 blockade).68, 143
Population-based studies suggest improvement in survival of patients with EOCRC over time. For example, an analysis of data from the Surveillance, Epidemiology, and End Results program of cancer registries shows five-year relative survival increased from 61.5% in the early 1990s to 67.7% after 2010.32 These improvements have not been consistent across racial/ethnic groups. Survival remains lower for non-Hispanic Black (62.8%) compared to non-Hispanic White (68.6%) and Hispanic (64.8%) patients, despite receiving near-equivalent treatment.32 144 Although racial/ethnic differences in survival may be due to differences in tumor characteristics among groups, other studies have demonstrated that, even after adjusting for these tumor characteristics, risk of all-cause- and cancer-specific mortality remains higher in non-Hispanic Black patients.145
Given the toxicities and costs associated with intensive treatment regimens, in addition to the well-documented challenges navigating cancer treatment (e.g., insurance, access), it will be important for future studies to address the risks and benefits of intensive treatment for younger patients and ensure equality in treatment and outcomes for underserved populations.
Surveillance and Survivorship
Life after cancer and cancer survivorship are important domains to address in patients diagnosed with EOCRC but have thus far not been rigorously studied. Across all ages, survivors may experience long-term and late effects, including neuropathy, cognitive dysfunction, change in bowel function, and higher risk of early-onset chronic conditions, recurrence, and second cancers.146–149 Many survivors also experience depression, anxiety, and changes in relationships with family members or friends, and these challenges may be especially difficult for younger survivors.70, 150, 151 For example, younger survivors may have unique struggles with intimacy, due to both the physical effects of treatment and overcoming the emotional challenges of re-experiencing intimacy with romantic partners previously serving as caregivers.61, 148, 150 Younger survivors are also concerned about the effects of treatment (e.g., pelvic radiation) on reproductive health and fertility;152, 153 yet fertility is rarely discussed by providers, and there may be additional cost and insurance barriers to fertility preservation.154, 155
The financial burden of surviving CRC also appears to be greater in younger vs. older adults. In a focus group of survivors of EOCRC, Blum-Barnett et al. identified four contributors of financial stress, including lost earnings, concern about health insurance coverage and benefits, decreased job performance, and a blunting of career trajectory.146 Araujo et al. also demonstrated challenges unique to the EOCRC experience: stress and frustration of being misdiagnosed, critical need for self-advocacy to undergo necessary workup, and frustration with the healthcare system.150 These challenges can create lasting mistrust of the medical team and healthcare system, as well as fear that future concerns will not be adequately addressed. Continued study of the needs of survivors of EOCRC will guide development of comprehensive survivorship care and help patients navigate the difficulties they experience during and after treatment.
Prevention and Early Recognition
In response to increasing incidence rates of EOCRC, the U.S. Preventive Services Task Force (USPSTF) updated in 2021156 its recommendation to start average-risk screening at age 45 (vs. 50) years. The American Cancer Society (ACS) made a similar recommendation in 2018.157 Although both of these recommendations are largely based on modeling studies, observational studies also suggest prevalence of advanced neoplasia is similar between 40- and 50-year-olds. For example, an analysis of the New Hampshire Colonoscopy Registry demonstrates that, despite a lower prevalence of any neoplasia in younger adults (17.5% vs 22.1%, p <0.01), prevalence of advanced neoplasia is similar between the two groups (3.3% vs 3.6%, p=0.50).158 A recent meta-analysis similarly reports a pooled prevalence of advanced neoplasia of 3.6% (95% CI, 1.9, 6.7) for age 45–49 years and 4.2% (95% CI, 3.2, 5.7) for age 50–54 years (p=0.69).159 Modeling studies suggest initiating screening at age 45 years is cost-effective,160 especially if using stool-based tests;161 given the recency of the USPSTF guidelines, it is not yet clear how these recommendations will impact clinical practice.
For adults younger than 45 years, it remains critical to identify those at higher risk and who may benefit from earlier screening, including those with hereditary cancer syndromes, a family history of CRC or advanced neoplasia, and predisposing conditions such as inflammatory bowel disease.43 Factors at multiple levels create barriers to adequately identify and screen higher-risk adults. At the patient-level, family history may be unknown, not discussed, or not disclosed,162 particularly for advanced neoplasia that has not progressed to cancer. At the provider-level, family history assessments may be underused or inadequately recorded.163 Providers may not uniformly refer patients to genetic counseling or testing, and efforts should be made to ensure equitable and culturally competent access to these services.36, 164
Finally, awareness and evaluation of symptoms (e.g., rectal bleeding or hematochezia, iron deficiency anemia) is important for both patients and providers, facilitating timely diagnosis and treatment.165, 166 For example, in a study of patients younger than age 40 years and referred for diagnostic colonoscopy due to rectal bleeding, 5% had advanced neoplasia or CRC.167 A large study of U.S. veterans found that those with iron deficiency anemia or hematochezia had 10-times the risk of EOCRC.168 EOCRC should also be considered as a rule-out diagnosis in young adults with unintentional or unexplained weight loss or abdominal pain without another identifiable cause.
Conclusion
In summary, incidence rates of EOCRC continue to increase across the globe, sounding an alarm to focus the scientific community’s efforts on understanding this phenomenon. In just the last few years, research has proliferated on the clinicopathologic features and risk factors of EOCRC. Although older studies initially suggested a difference in tumor biology between younger and older patients, these recent studies suggest more similarities between the two groups. We also have learned more about the role of dietary and lifestyle factors in EOCRC, which appear to confer a similar risk for younger adults as for later-onset CRC. In fact, as we identify additional, exogenous exposures associated with EOCRC, these may also be risk factors of later-onset CRC.
Several steps can enhance efforts to prevent and manage EOCRC. Universal genetic testing of all younger adults diagnosed with CRC will guide treatment and broaden the knowledge base of pathogenic variants relevant to this disease. Improved identification of family history of CRC can further risk stratify those who may benefit from earlier screening; risk prediction models incorporating family history, genetic risk, and environmental and lifestyle factors may identify others likely to benefit from earlier screening. Finally, trends in incidence and mortality rates over the next decade will provide empiric evidence of the impact of new guidelines to begin average-risk screening at age 45 years.
Grant support:
This work was supported by the National Cancer Institute at the National Institutes of Health under award number R01CA242558. The sponsor had no role in: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosures: CCM reports consulting for Freenome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy CC, Sandler RS, Sanoff HK, Yang YC, Lund JL, Baron JA. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. Jun 2017;15(6):903–909.e6. doi: 10.1016/j.cgh.2016.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. Feb 1 2010;116(3):544–73. doi: 10.1002/cncr.24760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SEER*Stat Database: Incidence-SEER Research Data, 13 Registries, Nov 2020 Sub (1992–2018) - Linked To County Attributes - Time Dependent (1990–2018) Income/Rurality, 1969–2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program.: Surveillance, Epidemiology, and End Results (SEER) Program; April 2021. [Google Scholar]
- 4.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. Jun 2009;18(6):1695–8. doi: 10.1158/1055-9965.epi-09-0186 [DOI] [PubMed] [Google Scholar]
- 5.O’Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Rates of colon and rectal cancers are increasing in young adults. The American surgeon. Oct 2003;69(10):866–72. [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA: a cancer journal for clinicians. May 2020;70(3):145–164. doi: 10.3322/caac.21601 [DOI] [PubMed] [Google Scholar]
- 7.Meester RG, Doubeni CA, Lansdorp-Vogelaar I, et al. Colorectal cancer deaths attributable to nonuse of screening in the United States. Ann Epidemiol. Mar 2015;25(3):208–213.e1. doi: 10.1016/j.annepidem.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Jemal A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970–2014. JAMA : the journal of the American Medical Association. Aug 8 2017;318(6):572–574. doi: 10.1001/jama.2017.7630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. October 2019;68(10):1820–1826. doi: 10.1136/gutjnl-2018-317592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lui RN, Tsoi KKF, Ho JMW, et al. Global Increasing Incidence of Young-Onset Colorectal Cancer Across 5 Continents: A Joinpoint Regression Analysis of 1,922,167 Cases. Cancer Epidemiol Biomarkers Prev. August 2019;28(8):1275–1282. doi: 10.1158/1055-9965.EPI-18-1111 [DOI] [PubMed] [Google Scholar]
- 11.Saad El Din K, Loree JM, Sayre EC, et al. Trends in the epidemiology of young-onset colorectal cancer: a worldwide systematic review. BMC Cancer. Apr 06 2020;20(1):288. doi: 10.1186/s12885-020-06766-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RL, Torre LA, Soerjomataram I, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut. December 2019;68(12):2179–2185. doi: 10.1136/gutjnl-2019-319511 [DOI] [PubMed] [Google Scholar]
- 13.Gini A, Jansen EEL, Zielonke N, et al. Impact of colorectal cancer screening on cancer-specific mortality in Europe: A systematic review. Eur J Cancer. March 2020;127:224–235. doi: 10.1016/j.ejca.2019.12.014 [DOI] [PubMed] [Google Scholar]
- 14.Jahn B, Sroczynski G, Bundo M, et al. Effectiveness, benefit harm and cost effectiveness of colorectal cancer screening in Austria. BMC Gastroenterol. Dec 05 2019;19(1):209. doi: 10.1186/s12876-019-1121-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel RL, Medhanie GA, Fedewa SA, Jemal A. State Variation in Early-Onset Colorectal Cancer in the United States, 1995–2015. Journal of the National Cancer Institute. Oct 1 2019;111(10):1104–1106. doi: 10.1093/jnci/djz098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel RL, Sahar L, Robbins A, Jemal A. Where can colorectal cancer screening interventions have the most impact? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. Aug 2015;24(8):1151–6. doi: 10.1158/1055-9965.Epi-15-0082 [DOI] [PubMed] [Google Scholar]
- 17.Whatley Z, Daram SR, Yousuf S, Tang SJ. Gastrointestinal cancers in Mississippi. Southern medical journal. Apr 2014;107(4):229–34. doi: 10.1097/smj.0000000000000093 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Zhang L, Penman A, May W. Using small-area estimation method to calculate county-level prevalence of obesity in Mississippi, 2007–2009. Preventing chronic disease. Jul 2011;8(4):A85. [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson N, Shelton BJ, Hopenhayn C, et al. Concentrations of arsenic, chromium, and nickel in toenail samples from Appalachian Kentucky residents. Journal of environmental pathology, toxicology and oncology : official organ of the International Society for Environmental Toxicology and Cancer. 2011;30(3):213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James W, Jia C, Kedia S. Uneven magnitude of disparities in cancer risks from air toxics. International journal of environmental research and public health. Dec 2012;9(12):4365–85. doi: 10.3390/ijerph9124365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. Journal of the National Cancer Institute. Aug 1 2017;109(8)doi: 10.1093/jnci/djw322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy CC, Singal AG, Baron JA, Sandler RS. Decrease in Incidence of Young-Onset Colorectal Cancer Before Recent Increase. Gastroenterology. December 2018;155(6):1716–1719.e4. doi: 10.1053/j.gastro.2018.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenner DR, Ruan Y, Shaw E, De P, Heitman SJ, Hilsden RJ. Increasing colorectal cancer incidence trends among younger adults in Canada. Preventive medicine. Dec 2017;105:345–349. doi: 10.1016/j.ypmed.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 24.Feletto E, Yu XQ, Lew JB, et al. Trends in Colon and Rectal Cancer Incidence in Australia from 1982 to 2014: Analysis of Data on Over 375,000 Cases. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. Jan 2019;28(1):83–90. doi: 10.1158/1055-9965.Epi-18-0523 [DOI] [PubMed] [Google Scholar]
- 25.Chung RY, Tsoi KKF, Kyaw MH, Lui AR, Lai FTT, Sung JJ. A population-based age-period-cohort study of colorectal cancer incidence comparing Asia against the West. Cancer epidemiology. Jan 16 2019;59:29–36. doi: 10.1016/j.canep.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 26.Sung JJY, Chiu HM, Jung KW, et al. Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Am J Gastroenterol. February 2019;114(2):322–329. doi: 10.14309/ajg.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 27.Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology. Jan 2020;158(2):341–353. doi: 10.1053/j.gastro.2019.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahabir S, Aagaard K, Anderson LM, et al. Challenges and opportunities in research on early-life events/exposures and cancer development later in life. Cancer causes & control : CCC. Jun 2012;23(6):983–90. doi: 10.1007/s10552-012-9962-5 [DOI] [PubMed] [Google Scholar]
- 29.Wild CP. How much of a contribution do exposures experienced between conception and adolescence make to the burden of cancer in adults? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. Apr 2011;20(4):580–1. doi: 10.1158/1055-9965.Epi-11-0187 [DOI] [PubMed] [Google Scholar]
- 30.Perera FP. Cancer: the big questions to address in coming years. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. Apr 2011;20(4):571–3. doi: 10.1158/1055-9965.Epi-11-0184 [DOI] [PubMed] [Google Scholar]
- 31.Chang SH, Patel N, Du M, Liang PS. Trends in Early-onset vs Late-onset Colorectal Cancer Incidence by Race/Ethnicity in the United States Cancer Statistics Database. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. Jul 26 2021;doi: 10.1016/j.cgh.2021.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy CC, Wallace K, Sandler RS, Baron JA. Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology. March 2019;156(4):958–965. doi: 10.1053/j.gastro.2018.11.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. Apr 2017;3(4):464–471. doi: 10.1001/jamaoncol.2016.5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoffel EM, Koeppe E, Everett J, et al. Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology. Mar 2018;154(4):897–905.e1. doi: 10.1053/j.gastro.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yurgelun MB, Kulke MH, Fuchs CS, et al. Cancer Susceptibility Gene Mutations in Individuals With Colorectal Cancer. J Clin Oncol. Apr 1 2017;35(10):1086–1095. doi: 10.1200/jco.2016.71.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dharwadkar P, Greenan G, Stoffel EM, et al. Racial and Ethnic Disparities in Germline Genetic Testing of Patients With Young-Onset Colorectal Cancer. Clin Gastroenterol Hepatol. Dec 2020;doi: 10.1016/j.cgh.2020.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta S, Provenzale D, Llor X, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 2.2019. J Natl Compr Canc Netw September 01 2019;17(9):1032–1041. doi: 10.6004/jnccn.2019.0044 [DOI] [PubMed] [Google Scholar]
- 38.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. Jun 2010;138(6):2044–58. doi: 10.1053/j.gastro.2010.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roos VH, Mangas-Sanjuan C, Rodriguez-Girondo M, et al. Effects of Family History on Relative and Absolute Risks for Colorectal Cancer: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. December 2019;17(13):2657–2667.e9. doi: 10.1016/j.cgh.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 40.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA oncology. Apr 1 2017;3(4):464–471. doi: 10.1001/jamaoncol.2016.5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen FW, Sundaram V, Chew TA, Ladabaum U. Advanced-Stage Colorectal Cancer in Persons Younger Than 50 Years Not Associated With Longer Duration of Symptoms or Time to Diagnosis. Clin Gastroenterol Hepatol. May 2017;15(5):728–737.e3. doi: 10.1016/j.cgh.2016.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dharwadkar P, Greenan G, Singal AG, Murphy CC. Is Colorectal Cancer in Patients Younger Than 50 Years of Age the Same Disease as in Older Patients? Clin Gastroenterol Hepatol. January 2021;19(1):192–194.e3. doi: 10.1016/j.cgh.2019.10.028 [DOI] [PubMed] [Google Scholar]
- 43.Gupta S, Bharti B, Ahnen DJ, et al. Potential impact of family history-based screening guidelines on the detection of early-onset colorectal cancer. Cancer. July 01 2020;126(13):3013–3020. doi: 10.1002/cncr.32851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeon J, Du M, Schoen RE, et al. Determining Risk of Colorectal Cancer and Starting Age of Screening Based on Lifestyle, Environmental, and Genetic Factors. Gastroenterology. June 2018;154(8):2152–2164.e19. doi: 10.1053/j.gastro.2018.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu L, Jeon J, Brenner H, et al. A model to determine colorectal cancer risk using common genetic susceptibility loci. Gastroenterology. Jun 2015;148(7):1330–9.e14. doi: 10.1053/j.gastro.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Archambault AN, Su YR, Jeon J, et al. Cumulative Burden of Colorectal Cancer-Associated Genetic Variants Is More Strongly Associated With Early-Onset vs Late-Onset Cancer. Gastroenterology. April 2020;158(5):1274–1286.e12. doi: 10.1053/j.gastro.2019.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdelsattar ZM, Wong SL, Regenbogen SE, Jomaa DM, Hardiman KM, Hendren S. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer. Mar 15 2016;122(6):929–34. doi: 10.1002/cncr.29716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rho YS, Gilabert M, Polom K, et al. Comparing Clinical Characteristics and Outcomes of Young-onset and Late-onset Colorectal Cancer: An International Collaborative Study. Clin Colorectal Cancer. December 2017;16(4):334–342. doi: 10.1016/j.clcc.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 49.Patel SG, Ahnen DJ. Colorectal Cancer in the Young. Current gastroenterology reports. Mar 28 2018;20(4):15. doi: 10.1007/s11894-018-0618-9 [DOI] [PubMed] [Google Scholar]
- 50.Cercek A, Chatila WK, Yaeger R, et al. A Comprehensive Comparison of Early-Onset and Average-Onset Colorectal Cancers. J Natl Cancer Inst. Aug 18 2021;doi: 10.1093/jnci/djab124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. Aug 2012;25(8):1128–39. doi: 10.1038/modpathol.2012.61 [DOI] [PubMed] [Google Scholar]
- 52.Murphy CC, Sanoff HK, Stitzenberg KB, Baron JA, Lund JL, Sandler RS. Patterns of Sociodemographic and Clinicopathologic Characteristics of Stages II and III Colorectal Cancer Patients by Age: Examining Potential Mechanisms of Young-Onset Disease. Journal of cancer epidemiology. 2017;2017:4024580. doi: 10.1155/2017/4024580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Leo M, Zuppardo RA, Puzzono M, et al. Risk factors and clinical characteristics of early-onset colorectal cancer vs. late-onset colorectal cancer: a case-case study. Eur J Gastroenterol Hepatol. Nov 17 2020;doi: 10.1097/MEG.0000000000002000 [DOI] [PubMed] [Google Scholar]
- 54.Gausman V, Dornblaser D, Anand S, et al. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin Gastroenterol Hepatol. November 2020;18(12):2752–2759.e2. doi: 10.1016/j.cgh.2019.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang VC, Cotterchio M, De P, Tinmouth J. Risk factors for early-onset colorectal cancer: a population-based case-control study in Ontario, Canada. Cancer Causes Control. Jun 13 2021;doi: 10.1007/s10552-021-01456-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffiths CD, McKechnie T, Lee Y, et al. Presentation and survival among patients with colorectal cancer before the age of screening: a systematic review and meta-analysis. Can J Surg. February 18 2021;64(1):E91–E100. doi: 10.1503/cjs.013019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kneuertz PJ, Chang GJ, Hu CY, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA surgery. May 2015;150(5):402–9. doi: 10.1001/jamasurg.2014.3572 [DOI] [PubMed] [Google Scholar]
- 58.Myers EA, Feingold DL, Forde KA, Arnell T, Jang JH, Whelan RL. Colorectal cancer in patients under 50 years of age: a retrospective analysis of two institutions’ experience. World J Gastroenterol. Sep 14 2013;19(34):5651–7. doi: 10.3748/wjg.v19.i34.5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siegel RL, Jakubowski CD, Fedewa SA, Davis A, Azad NS. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am Soc Clin Oncol Educ Book. Mar 2020;40:1–14. doi: 10.1200/EDBK_279901 [DOI] [PubMed] [Google Scholar]
- 60.Yantiss RK, Goodarzi M, Zhou XK, et al. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. The American journal of surgical pathology. Apr 2009;33(4):572–82. doi: 10.1097/PAS.0b013e31818afd6b [DOI] [PubMed] [Google Scholar]
- 61.Colorectal Cancer Alliance - Never Too Young Survey Report 2020. 2021.
- 62.Kirzin S, Marisa L, Guimbaud R, et al. Sporadic early-onset colorectal cancer is a specific sub-type of cancer: a morphological, molecular and genetics study. PLoS One. 2014;9(8):e103159. doi: 10.1371/journal.pone.0103159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. Jun 15 2019;125(12):2002–2010. doi: 10.1002/cncr.31994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lieu CH, Golemis EA, Serebriiskii IG, et al. Comprehensive Genomic Landscapes in Early and Later Onset Colorectal Cancer. Clin Cancer Res. October 01 2019;25(19):5852–5858. doi: 10.1158/1078-0432.CCR-19-0899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med Nov 2015;21(11):1350–6. doi: 10.1038/nm.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Low EE, Demb J, Liu L, et al. Risk Factors for Early-Onset Colorectal Cancer. Gastroenterology. Aug 2020;159(2):492–501.e7. doi: 10.1053/j.gastro.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahman R, Schmaltz C, Jackson CS, Simoes EJ, Jackson-Thompson J, Ibdah JA. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer Med. Dec 2015;4(12):1863–70. doi: 10.1002/cam4.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cavestro GM, Mannucci A, Zuppardo RA, Di Leo M, Stoffel E, Tonon G. Early onset sporadic colorectal cancer: Worrisome trends and oncogenic features. Dig Liver Dis. Jun 2018;50(6):521–532. doi: 10.1016/j.dld.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 69.Ballester V, Rashtak S, Boardman L. Clinical and molecular features of young-onset colorectal cancer. World J Gastroenterol. Feb 07 2016;22(5):1736–44. doi: 10.3748/wjg.v22.i5.1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siegel RL. Early-onset colorectal cancer: when hoof beats are zebras. Colorectal Cancer. 2020;9(3)doi: 10.2217/crc-2020-0013 [DOI] [Google Scholar]
- 71.Rosato V, Bosetti C, Levi F, et al. Risk factors for young-onset colorectal cancer. Cancer causes & control : CCC. Feb 2013;24(2):335–41. doi: 10.1007/s10552-012-0119-3 [DOI] [PubMed] [Google Scholar]
- 72.Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O’Keefe SJD. Fiber, Fat, and Colorectal Cancer: New Insight into Modifiable Dietary Risk Factors. Curr Gastroenterol Rep. Dec 02 2019;21(11):62. doi: 10.1007/s11894-019-0725-2 [DOI] [PubMed] [Google Scholar]
- 73.O’Keefe SJ, Li JV, Lahti L, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. Apr 28 2015;6:6342. doi: 10.1038/ncomms7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. Dec 2016;13(12):691–706. doi: 10.1038/nrgastro.2016.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carr PR, Walter V, Brenner H, Hoffmeister M. Meat subtypes and their association with colorectal cancer: Systematic review and meta-analysis. Int J Cancer. Jan 15 2016;138(2):293–302. doi: 10.1002/ijc.29423 [DOI] [PubMed] [Google Scholar]
- 76.Hur J, Otegbeye E, Joh HK, et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut May 06 2021;doi: 10.1136/gutjnl-2020-323450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joh HK, Lee DH, Hur J, et al. Simple Sugar and Sugar-Sweetened Beverage Intake During Adolescence and Risk of Colorectal Cancer Precursors. Gastroenterology. Jul 2021;161(1):128–142.e20. doi: 10.1053/j.gastro.2021.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Archambault AN, Lin Y, Jeon J, et al. Nongenetic Determinants of Risk for Early-Onset Colorectal Cancer. JNCI Cancer Spectr. Jun 2021;5(3):pkab029. doi: 10.1093/jncics/pkab029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim H, Lipsyc-Sharf M, Zong X, et al. Total Vitamin D Intake and Risks of Early-Onset Colorectal Cancer and Precursors. Gastroenterology. Jul 07 2021;doi: 10.1053/j.gastro.2021.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Veettil SK, Wong TY, Loo YS, et al. Role of Diet in Colorectal Cancer Incidence: Umbrella Review of Meta-analyses of Prospective Observational Studies. JAMA Netw Open. February 01 2021;4(2):e2037341. doi: 10.1001/jamanetworkopen.2020.37341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park Y, Lee J, Oh JH, Shin A, Kim J. Dietary patterns and colorectal cancer risk in a Korean population: A case-control study. Medicine (Baltimore). Jun 2016;95(25):e3759. doi: 10.1097/MD.0000000000003759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mehta RS, Song M, Nishihara R, et al. Dietary Patterns and Risk of Colorectal Cancer: Analysis by Tumor Location and Molecular Subtypes. Gastroenterology. June 2017;152(8):1944–1953.e1. doi: 10.1053/j.gastro.2017.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy N, Moreno V, Hughes DJ, et al. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol Aspects Med October 2019;69:2–9. doi: 10.1016/j.mam.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 84.Benninghoff AD, Hintze KJ, Monsanto SP, et al. Consumption of the Total Western Diet Promotes Colitis and Inflammation-Associated Colorectal Cancer in Mice. Nutrients. Feb 20 2020;12(2)doi: 10.3390/nu12020544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng X, Hur J, Nguyen LH, et al. Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer. J Natl Cancer Inst. May 04 2021;113(5):543–552. doi: 10.1093/jnci/djaa164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. Jun 2013;62(6):933–47. doi: 10.1136/gutjnl-2013-304701 [DOI] [PubMed] [Google Scholar]
- 87.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PloS one. 2013;8(1):e53916. doi: 10.1371/journal.pone.0053916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harriss DJ, Atkinson G, George K, et al. Lifestyle factors and colorectal cancer risk (1): systematic review and meta-analysis of associations with body mass index. Colorectal Dis. Jul 2009;11(6):547–63. doi: 10.1111/j.1463-1318.2009.01766.x [DOI] [PubMed] [Google Scholar]
- 89.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. Aug 21 2007;13(31):4199–206. doi: 10.3748/wjg.v13.i31.4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu PH, Wu K, Ng K, et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA oncology. Jan 1 2019;5(1):37–44. doi: 10.1001/jamaoncol.2018.4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Syed AR, Thakkar P, Horne ZD, et al. Old. World J Gastrointest Oncol Nov 15 2019;11(11):1011–1020. doi: 10.4251/wjgo.v11.i11.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glover M, Mansoor E, Panhwar M, Parasa S, Cooper GS. Epidemiology of Colorectal Cancer in Average Risk Adults 20–39 Years of Age: A Population-Based National Study. Dig Dis Sci. December 2019;64(12):3602–3609. doi: 10.1007/s10620-019-05690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schumacher AJ, Chen Q, Attaluri V, McLemore EC, Chao CR. Metabolic Risk Factors Associated with Early Onset Colorectal Adenocarcinoma: A Case-Control Study at Kaiser Permanente Southern California. Cancer Epidemiol Biomarkers Prev. Jul 22 2021;doi: 10.1158/1055-9965.EPI-20-1127 [DOI] [PubMed] [Google Scholar]
- 94.Kim NH, Jung YS, Yang HJ, et al. Prevalence of and Risk Factors for Colorectal Neoplasia in Asymptomatic Young Adults (20–39 Years Old). Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. Jan 2019;17(1):115–122. doi: 10.1016/j.cgh.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 95.Breau G, Ellis U. Risk Factors Associated With Young-Onset Colorectal Adenomas and Cancer: A Systematic Review and Meta-Analysis of Observational Research. Cancer Control. 2020 Jan-Dec 2020;27(1):1073274820976670. doi: 10.1177/1073274820976670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li H, Boakye D, Chen X, Hoffmeister M, Brenner H. Association of Body Mass Index With Risk of Early-Onset Colorectal Cancer: Systematic Review and Meta-Analysis. Am J Gastroenterol. Jul 23 2021;doi: 10.14309/ajg.0000000000001393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Sullivan DE, Sutherland RL, Town S, et al. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. Jan 29 2021;doi: 10.1016/j.cgh.2021.01.037 [DOI] [PubMed] [Google Scholar]
- 98.Dash C, Yu J, Nomura S, et al. Obesity is an initiator of colon adenomas but not a promoter of colorectal cancer in the Black Women’s Health Study. Cancer Causes Control. Apr 2020;31(4):291–302. doi: 10.1007/s10552-020-01283-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hidayat K, Yang CM, Shi BM. Body fatness at an early age and risk of colorectal cancer. Int J Cancer. February 15 2018;142(4):729–740. doi: 10.1002/ijc.31100 [DOI] [PubMed] [Google Scholar]
- 100.Nimptsch K, Wu K. Is Timing Important? The Role of Diet and Lifestyle during Early Life on Colorectal Neoplasia. Curr Colorectal Cancer Rep. Feb 2018;14(1):1–11. doi: 10.1007/s11888-018-0396-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X, Wu K, Giovannucci EL, et al. Early life body fatness and risk of colorectal cancer in u.s. Women and men-results from two large cohort studies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. Apr 2015;24(4):690–7. doi: 10.1158/1055-9965.Epi-14-0909-t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bjørge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. Jul 01 2008;168(1):30–7. doi: 10.1093/aje/kwn096 [DOI] [PubMed] [Google Scholar]
- 103.Célind J, Ohlsson C, Bygdell M, Nethander M, Kindblom JM. Childhood Body Mass Index Is Associated with Risk of Adult Colon Cancer in Men: An Association Modulated by Pubertal Change in Body Mass Index. Cancer Epidemiol Biomarkers Prev. May 2019;28(5):974–979. doi: 10.1158/1055-9965.EPI-18-1077 [DOI] [PubMed] [Google Scholar]
- 104.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. March 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 105.Davies M, Gupta S, Goldspink G, Winslet M. The insulin-like growth factor system and colorectal cancer: clinical and experimental evidence. Int J Colorectal Dis. Apr 2006;21(3):201–8. doi: 10.1007/s00384-005-0776-8 [DOI] [PubMed] [Google Scholar]
- 106.Insulin Giovannucci E., insulin-like growth factors and colon cancer: a review of the evidence. The Journal of nutrition. Nov 2001;131(11 Suppl):3109s–20s. doi: 10.1093/jn/131.11.3109S [DOI] [PubMed] [Google Scholar]
- 107.Komninou D, Ayonote A, Richie JP, Rigas B. Insulin resistance and its contribution to colon carcinogenesis. Exp Biol Med (Maywood). Apr 2003;228(4):396–405. doi: 10.1177/153537020322800410 [DOI] [PubMed] [Google Scholar]
- 108.Pakiet A, Kobiela J, Stepnowski P, Sledzinski T, Mika A. Changes in lipids composition and metabolism in colorectal cancer: a review. Lipids Health Dis. Jan 26 2019;18(1):29. doi: 10.1186/s12944-019-0977-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yan G, Li L, Zhu B, Li Y. Lipidome in colorectal cancer. Oncotarget. May 31 2016;7(22):33429–39. doi: 10.18632/oncotarget.7960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zaytseva Y Lipid Metabolism as a Targetable Metabolic Vulnerability in Colorectal Cancer. Cancers (Basel). Jan 15 2021;13(2)doi: 10.3390/cancers13020301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen H, Zheng X, Zong X, et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut. Jun 2021;70(6):1147–1154. doi: 10.1136/gutjnl-2020-321661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Z, Chen H, Fritz CDL, et al. Type 2 Diabetes and Risk of Early-Onset Colorectal Cancer. medRxiv preprint. 2021;doi: 10.1101/2021.06.02.21257972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst. Oct 17 2012;104(20):1548–61. doi: 10.1093/jnci/djs354 [DOI] [PubMed] [Google Scholar]
- 114.Carr PR, Weigl K, Jansen L, et al. Healthy Lifestyle Factors Associated With Lower Risk of Colorectal Cancer Irrespective of Genetic Risk. Gastroenterology. December 2018;155(6):1805–1815.e5. doi: 10.1053/j.gastro.2018.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Diet, nutrition, physical activity, and colorectal cancer. World Cancer Research Fund; 2017.
- 116.Nguyen LH, Liu PH, Zheng X, et al. Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr. Nov 2018;2(4):pky073. doi: 10.1093/jncics/pky073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morris JS, Bradbury KE, Cross AJ, Gunter MJ, Murphy N. Physical activity, sedentary behaviour and colorectal cancer risk in the UK Biobank. Br J Cancer. March 20 2018;118(6):920–929. doi: 10.1038/bjc.2017.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Parry S, Straker L. The contribution of office work to sedentary behaviour associated risk. BMC Public Health. 2013;13:296. doi: 10.1186/1471-2458-13-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. Sep 2011;22(9):1958–1972. doi: 10.1093/annonc/mdq653 [DOI] [PubMed] [Google Scholar]
- 120.Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. Feb 03 2015;112(3):580–93. doi: 10.1038/bjc.2014.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rumgay H, Shield K, Charvat H, et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol. August 2021;22(8):1071–1080. doi: 10.1016/S1470-2045(21)00279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McNabb S, Harrison TA, Albanes D, et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int J Cancer. February 01 2020;146(3):861–873. doi: 10.1002/ijc.32377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brockway-Lunardi L, Nelson S, Pandiri AR, et al. Early-onset colorectal cancer research: gaps and opportunities. Colorectal Cancer. 2020;9(3)doi: 10.2217/crc-2020-0028 [DOI] [Google Scholar]
- 124.Murphy CC, Cirillo PM, Krigbaum NY, et al. Maternal obesity, pregnancy weight gain, and birth weight and risk of colorectal cancer. Gut. Aug 24 2021;doi: 10.1136/gutjnl-2021-325001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaur K, Saxena A, Debnath I, et al. Antibiotic-mediated bacteriome depletion in Apc. Cancer Med. May 2018;7(5):2003–2012. doi: 10.1002/cam4.1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goncalves MD, Lu C, Tutnauer J, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science. March 22 2019;363(6433):1345–1349. doi: 10.1126/science.aat8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hofseth LJ, Hebert JR, Chanda A, et al. Early-onset colorectal cancer: initial clues and current views. Nature reviews Gastroenterology & hepatology. Jun 2020;17(6):352–364. doi: 10.1038/s41575-019-0253-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rattray NJW, Charkoftaki G, Rattray Z, Hansen JE, Vasiliou V, Johnson CH. Environmental influences in the etiology of colorectal cancer: the premise of metabolomics. Curr Pharmacol Rep. Jun 2017;3(3):114–125. doi: 10.1007/s40495-017-0088-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. Feb 2012;22(2):299–306. doi: 10.1101/gr.126516.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mukherji R, Weinberg BA. The gut microbiome and potential implications for early-onset colorectal cancer. Colorectal Cancer. 2020;9(3)doi: 10.2217/crc-2020-0007 [DOI] [Google Scholar]
- 131.Zhang J, Haines C, Watson AJM, et al. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989–2012: a matched case-control study. Gut. November 2019;68(11):1971–1978. doi: 10.1136/gutjnl-2019-318593 [DOI] [PubMed] [Google Scholar]
- 132.Song M, Nguyen LH, Emilsson L, Chan AT, Ludvigsson JF. Antibiotic Use Associated With Risk of Colorectal Polyps in a Nationwide Study. Clin Gastroenterol Hepatol. July 2021;19(7):1426–1435.e6. doi: 10.1016/j.cgh.2020.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Momen-Heravi F, Babic A, Tworoger SS, et al. Periodontal disease, tooth loss and colorectal cancer risk: Results from the Nurses’ Health Study. Int J Cancer. Feb 01 2017;140(3):646–652. doi: 10.1002/ijc.30486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Quah HM, Joseph R, Schrag D, et al. Young age influences treatment but not outcome of colon cancer. Annals of surgical oncology. Oct 2007;14(10):2759–65. doi: 10.1245/s10434-007-9465-x [DOI] [PubMed] [Google Scholar]
- 135.Manjelievskaia J, Brown D, McGlynn KA, Anderson W, Shriver CD, Zhu K. Chemotherapy Use and Survival Among Young and Middle-Aged Patients With Colon Cancer. JAMA Surg. May 01 2017;152(5):452–459. doi: 10.1001/jamasurg.2016.5050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kolarich A, George TJ, Hughes SJ, et al. Rectal cancer patients younger than 50 years lack a survival benefit from NCCN guideline-directed treatment for stage II and III disease. Cancer. September 01 2018;124(17):3510–3519. doi: 10.1002/cncr.31527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chagpar R, Xing Y, Chiang YJ, et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol. Mar 20 2012;30(9):972–9. doi: 10.1200/JCO.2011.39.6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burnett-Hartman AN, Powers JD, Chubak J, et al. Treatment patterns and survival differ between early-onset and late-onset colorectal cancer patients: the patient outcomes to advance learning network. Cancer causes & control : CCC. Jul 2019;30(7):747–755. doi: 10.1007/s10552-019-01181-3 [DOI] [PubMed] [Google Scholar]
- 139.Cheng E, Blackburn HN, Ng K, et al. Analysis of Survival Among Adults With Early-Onset Colorectal Cancer in the National Cancer Database. JAMA Netw Open. Jun 01 2021;4(6):e2112539. doi: 10.1001/jamanetworkopen.2021.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jin Z, Dixon JG, Fiskum JM, et al. Clinicopathological and Molecular Characteristics of Early-Onset Stage III Colon Adenocarcinoma: An Analysis of the ACCENT Database. J Natl Cancer Inst. Aug 18 2021;doi: 10.1093/jnci/djab123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. Jan 20 2005;23(3):609–18. doi: 10.1200/JCO.2005.01.086 [DOI] [PubMed] [Google Scholar]
- 142.Stigliano V, Assisi D, Cosimelli M, et al. Survival of hereditary non-polyposis colorectal cancer patients compared with sporadic colorectal cancer patients. J Exp Clin Cancer Res. Sep 19 2008;27:39. doi: 10.1186/1756-9966-27-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.André T, Shiu KK, Kim TW, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. December 03 2020;383(23):2207–2218. doi: 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 144.Alese OB, Jiang R, Zakka KM, et al. Analysis of racial disparities in the treatment and outcomes of colorectal cancer in young adults. Cancer Epidemiol December 2019;63:101618. doi: 10.1016/j.canep.2019.101618 [DOI] [PubMed] [Google Scholar]
- 145.Holowatyj AN, Ruterbusch JJ, Rozek LS, Cote ML, Stoffel EM. Racial/Ethnic Disparities in Survival Among Patients With Young-Onset Colorectal Cancer. J Clin Oncol. Jun 20 2016;34(18):2148–56. doi: 10.1200/jco.2015.65.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Blum-Barnett E, Madrid S, Burnett-Hartman A, et al. Financial burden and quality of life among early-onset colorectal cancer survivors: A qualitative analysis. Health Expect October 2019;22(5):1050–1057. doi: 10.1111/hex.12919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.El-Shami K, Oeffinger KC, Erb NL, et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin. 2015 Nov-Dec 2015;65(6):428–55. doi: 10.3322/caac.21286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hydeman JA, Uwazurike OC, Adeyemi EI, Beaupin LK. Survivorship needs of adolescent and young adult cancer survivors: a concept mapping analysis. J Cancer Surviv February 2019;13(1):34–42. doi: 10.1007/s11764-018-0725-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McMullen C, Bulkley J, Corley DA, et al. Health care improvement and survivorship priorities of colorectal cancer survivors: findings from the PORTAL colorectal cancer cohort survey. Support Care Cancer. Jan 2019;27(1):147–156. doi: 10.1007/s00520-018-4299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Araujo L, Breau G, George M, et al. Shared experiences of diagnosis and treatment of young-onset colorectal cancer: a patient-oriented qualitative study. Journal of Psychosocial Oncology Research and Practice. 2020;2(1) [Google Scholar]
- 151.Bailey CE, Tran Cao HS, Hu CY, et al. Functional deficits and symptoms of long-term survivors of colorectal cancer treated by multimodality therapy differ by age at diagnosis. J Gastrointest Surg. Jan 2015;19(1):180–8; discussio 188. doi: 10.1007/s11605-014-2645-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Oktay K, Harvey BE, Partridge AH, et al. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. July 01 2018;36(19):1994–2001. doi: 10.1200/JCO.2018.78.1914 [DOI] [PubMed] [Google Scholar]
- 153.Shandley LM, McKenzie LJ. Recent Advances in Fertility Preservation and Counseling for Reproductive-Aged Women with Colorectal Cancer: A Systematic Review. Dis Colon Rectum. June 2019;62(6):762–771. doi: 10.1097/DCR.0000000000001351 [DOI] [PubMed] [Google Scholar]
- 154.Mobley EM, Ryan GL, Sparks AE, Monga V, Terry WW. Factors Impacting Fertility Preservation in Adolescents and Young Adults with Cancer: A Retrospective Study. J Adolesc Young Adult Oncol. April 2020;9(2):208–221. doi: 10.1089/jayao.2019.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Waimey KE, Smith BM, Confino R, Jeruss JS, Pavone ME. Understanding Fertility in Young Female Cancer Patients. J Womens Health (Larchmt). Oct 2015;24(10):812–8. doi: 10.1089/jwh.2015.5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Colorectal Cancer: Screening. US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening#fullrecommendationstart
- 157.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. July 2018;68(4):250–281. doi: 10.3322/caac.21457 [DOI] [PubMed] [Google Scholar]
- 158.Butterly LF, Siegel RL, Fedewa S, Robinson CM, Jemal A, Anderson JC. Colonoscopy Outcomes in Average-Risk Screening Equivalent Young Adults: Data From the New Hampshire Colonoscopy Registry. The American journal of gastroenterology. Jan 1 2021;116(1):171–179. doi: 10.14309/ajg.0000000000000820 [DOI] [PubMed] [Google Scholar]
- 159.Kolb JM, Hu J, DeSanto K, et al. Early-Age Onset Colorectal Neoplasia in Average-Risk Individuals Undergoing Screening Colonoscopy: A Systematic Review and Meta-Analysis. Gastroenterology. Jun 10 2021;doi: 10.1053/j.gastro.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ladabaum U, Mannalithara A, Meester RGS, Gupta S, Schoen RE. Cost-Effectiveness and National Effects of Initiating Colorectal Cancer Screening for Average-Risk Persons at Age 45 Years Instead of 50 Years. Gastroenterology. July 2019;157(1):137–148. doi: 10.1053/j.gastro.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen CH, Tsai MK, Wen CP. Extending Colorectal Cancer Screening to Persons Aged 40 to 49 Years With Immunochemical Fecal Occult Blood Test: A Prospective Cohort Study of 513,283 Individuals. J Clin Gastroenterol. October 2016;50(9):761–8. doi: 10.1097/MCG.0000000000000495 [DOI] [PubMed] [Google Scholar]
- 162.Mitchell RJ, Brewster D, Campbell H, et al. Accuracy of reporting of family history of colorectal cancer. Gut. Feb 2004;53(2):291–5. doi: 10.1136/gut.2003.027896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Murff HJ, Greevy RA, Syngal S. The comprehensiveness of family cancer history assessments in primary care. Community Genet. 2007;10(3):174–80. doi: 10.1159/000101759 [DOI] [PubMed] [Google Scholar]
- 164.Muller C, Lee SM, Barge W, et al. Low Referral Rate for Genetic Testing in Racially and Ethnically Diverse Patients Despite Universal Colorectal Cancer Screening. Clin Gastroenterol Hepatol. December 2018;16(12):1911–1918.e2. doi: 10.1016/j.cgh.2018.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ko CW, Siddique SM, Patel A, et al. AGA Clinical Practice Guidelines on the Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology. September 2020;159(3):1085–1094. doi: 10.1053/j.gastro.2020.06.046 [DOI] [PubMed] [Google Scholar]
- 166.Pasha SF, Shergill A, Acosta RD, et al. The role of endoscopy in the patient with lower GI bleeding. Gastrointest Endosc. Jun 2014;79(6):875–85. doi: 10.1016/j.gie.2013.10.039 [DOI] [PubMed] [Google Scholar]
- 167.Lu R, Kassim T, Dave D, et al. Diagnostic Yield of Colonoscopy in Young Adults with Lower Gastrointestinal Symptoms in a Multicenter Midwest Cohort. Dig Dis. 2020;38(6):484–489. doi: 10.1159/000506073 [DOI] [PubMed] [Google Scholar]
- 168.Demb J, Liu L, Murphy CC, Doubeni CA, Martínez ME, Gupta S. Young-onset colorectal cancer risk among individuals with iron-deficiency anaemia and haematochezia. Gut. Dec 18 2020;doi: 10.1136/gutjnl-2020-321849 [DOI] [PMC free article] [PubMed] [Google Scholar]


