SUMMARY
The reticulin stain is a critical diagnostic aide used to differentiate benign hepatocellular proliferations from well differentiated hepatocellular carcinoma. Rarely, however, hepatocellular carcinomas do not show definitive loss of reticulin in liver biopsy specimens. In order to study this group of tumors, 11 HCC with no reticulin loss in 10 patients were collected and studied. Analysis of demographics showed a typical enrichment for men with a typical age for HCC presentation of 69± 7 years for adults. The background livers showed advanced fibrosis or cirrhosis in 6/6 cases with available information. The tumors were all well differentiated. Cytological atypia was mild and consisted of very mild nuclear atypia (8 cases), mild increase in N:C ratio (3 cases), and pseudorosette formation (4 cases). The cytological/architectural atypia was insufficient in isolation to diagnose HCC. Additional studies, however, showed an increased Ki-67 proliferative rate (N= 10/10 stained cases). The Ki-67 proliferative rate was estimated to be between 5–10% in all tested cases and was clearly increased from adjacent liver at low power. Glypican 3 positivity (4 tumors) and alpha fetoprotein (1/8 stained cases) positivity also helped make the diagnosis of HCC. Morphologically, the HCC had conventional morphology with five showing steatosis / steatohepatitic features and one showing intratumoral fibrosis. A control group of macroregenerative/dysplastic nodules showed no increase in Ki-67 proliferation and no staining for glypican 3. These findings highlight an important diagnostic pitfall: rare HCC show no reticulin loss on biopsy. In these challenging cases, additional findings are useful to make a diagnosis of HCC: increased Ki-67, and positive staining for aberrant expression of proteins such as glypican 3 or alpha fetoprotein (AFP).
Keywords: Hepatocellular carcinoma, reticulin, glypican 3, beta-catenin, AFP, Ki-67
1. Introduction
Distinguishing well differentiated hepatocellular carcinoma from benign hepatocellular lesions can be challenging based on morphology alone because some well differentiated hepatocellular carcinomas have no or minimal cytological and architectural atypia. Based on morphology, the histological differential for these well differentiated tumors in non-cirrhotic livers is typically that of a well differentiated hepatocellular carcinoma versus hepatic adenoma or focal nodular hyperplasia, while in cirrhotic livers the differential is typically that of a well differentiated hepatocellular carcinoma versus macro-regenerative nodules or dysplastic nodules. In cases where the morphology alone does not distinguish benign from malignant lesions, the standard of care is to use special and immunohistochemical stains as diagnostic aides.
The most widely used histochemical stain to support a diagnosis of hepatocellular carcinoma is the reticulin stain, where loss of normal reticulin staining patterns indicates a diagnosis of hepatocellular carcinoma. Rarely, however, hepatocellular carcinomas show normal or near normal patterns of reticulin, with insufficient changes to reliably make a diagnosis based on reticulin abnormalities. Previously, a few case reports have documented well-differentiated hepatocellular carcinomas that showed no loss of reticulin staining [1, 2], but there have been no systematic studies to date. In our consult practice, we have noticed this to be a recurrent diagnostic challenge. In order to examine the clinical and pathological correlates, and to provide a diagnostic approach for these difficult cases, a cohort of these cases were collected and studied.
Other stains can be used to supplement the reticulin stain when evaluating for the possibility of hepatocellular carcinoma. For example, glypican 3 and alpha fetoprotein (AFP) are known to be negative in benign liver tissue, outside of focal glypican expression in markedly inflamed livers [3]. Beta-catenin nuclear staining and/or strong and diffuse glutamine synthetase are not seen in dysplastic nodules [4]. As a caveat, in a non-cirrhotic liver, beta catenin nuclear staining and diffuse glutamine synthetase staining can also be seen in hepatic adenomas [5], so do not distinguish HCC from hepatic adenoma. Ki-67 shows low proliferation in hepatic adenomas and in macro-regenerative and low grade dysplastic nodules [6, 7]. Thus, these immunostains were also examined for their utility in diagnosing well differentiated hepatocellular carcinoma when there is no definite reticulin loss.
2. Materials and Methods
After IRB approval, HCC cases were collected from the pathology files of Mayo Clinic Rochester. The majority of cases were consult cases (N=9) and they were supplemented with several in-house cases (N= 2). Clinical and imaging findings were collected when available. Macro-regenerative nodules (N=2) and low grade dysplastic (N=6) were selected from explanted livers to serve as controls. Macro-regenerative nodules and dysplastic nodules can be difficult to reliably distinguish from each other [8]. In this study, lesions were classified as macro-regenerative nodules when the cytology of the hepatocytes within the nodule was identical to that of the background liver. If there was mild cytological atypia, but more than the background liver, the lesions were classified as low-grade dysplastic nodules [9].
All cases were centrally reviewed and scored by two pathologists (SY, MST). Cytological atypia was assessed as none or mild (findings could be consistent with either benign or malignant proliferations) versus atypia that was moderate or greater (cytological atypia alone that would be strongly suggestive or diagnostic of malignancy). Glutamine synthetase staining was considered positive when it was strong and diffuse within the tumor cells (>90%). Positive beta-catenin staining required nuclear staining. Glypican 3 positivity was correlated with H&E morphology, to exclude nonspecific staining secondary to cross reaction with lipofuscin [10]. Ki-67 was evaluated in association with the background liver whenever possible and was estimated to the nearest 5%. This approach is in keeping with routine clinical practice, where Ki-67 proliferation is interpreted by comparison of the tumor to the adjacent liver, often best evaluated at low power, where a clear increase in the tumor over that of the non-tumor liver provides evidence for a malignant process. Reticulin loss was evaluated by looking for thickening of hepatic plates (>2 cells thick) and or foci of multiple adjacent tumor cells that were not touching reticulin on any of their surfaces. In order to determine the frequency of this rare finding, the number of cases of hepatocellular carcinoma with no reticulin loss was identified in 100 consecutive cases of well differentiated hepatocellular carcinoma in a consult practice (MST).
3. Results
3.1. Clinical findings
Eleven hepatocellular carcinomas with no reticulin loss were collected in 10 patients (8 biopsy specimens, 3 resection specimens). The clinicopathological features are summarized in Table 1. Nine tumors occurred in adults and 2 in a child. For the adults, the median tumor size based on imaging was 2.6 cm, range 1.7 to 5.6 cm. Demographic findings showed a typical enrichment for men (all were men) with an average age at presentation of 69± 7 years. The background livers showed advanced fibrosis or cirrhosis in 6/6 adult cases with available information. Underlying liver diseases were available in 5 adult cases: metabolic syndrome with obesity, hypertension, and type 2 diabetes mellitus (N=1), alcoholic hepatitis (N=1), chronic hepatitis C (N=1), chronic hepatitis C and HFE C282Y homozygosity (N=1), and concurrent chronic hepatitis C viral infection and steatohepatitis (N=1). The pediatric hepatocellular carcinoma occurred in the liver of a young boy with a history of cirrhosis from tyrosinemia.
Table 1.
Clinical and morphological findings of hepatocellular carcinoma
| Case | Specimen | Age | M/F | Radiology | Liver disease | Fibrosis | Size (cm) | HCC grade/morphology | Other findings | Immuno-histochemistry |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Liver explant | 2 | M | Numerous hypodense nonhyperenhancing lesions; sections of these showed macroregenerati ve nodules. The HCC were small and identified on gross examination only and not by imaging | tyrosinemia | cirrhosis | 0.5, 0.4 | Well |
HCC 1 Ki67 increased, 5% GP3 positive GS positive diffuse but weak AFP negative Retic focal equivocal loss HCC 2 Ki67 increased, 5% GP3 positive very focal GS negative AFP positive Retic focal equivocal loss |
|
| 2 | biopsy | 66 | M | Ill-defined area of diminished attenuation. Nonspecific | HCV SH |
Bridging fibrosis | 2.6 | Well Mild steatosis |
Pseudo-glands | GP3 positive AFP negative |
| 3 | biopsy | 84 | M | Not available | unavailable | Probable cirrhosis based on bx | unavailable | Well | Pseudo-glands | GP3 negative AFP negative Bcat negative GS negative Ki-67 increased, 10% |
| 4 | biopsy | 73 | M | Not available | unavailable | Insufficient background liver to evaluate | unavailable | Well | Focal necrosis Occasional mitoses | GP3 negative Ki-67 increased, 10% |
| 5 | biopsy | 63 | M | Indeterminate, mass suspicious for neoplasm | Unavailable But mild steatosis in background liver | Insufficient background liver to evaluate | 5.6 (biopsy target); 3.9 (not biopsied) | Well | GP3 negative AFP negative Bcat negative GS negative Ki-67 increased, ~5% |
|
| 6 | biopsy | 65 | M | Arterial enhancement, and other features diagnostic of HCC | unavailable | cirrhosis | 3.4 | Well Steatohepatitic |
Rare pseudo-glands Rare mitoses Possible portal tract invasion |
GP3 negative AFP negative Bcat negative GS negative Ki-67 increased, ~5% |
| 7 | biopsy | 61 | M | Two hyperenhancing lesions, suspicious for HCC | ETOH | cirrhosis | 2.3, also one smaller lesion, size not specified and not sampled | Well Mild steatosis |
GP3 negative AFP negative Bcat negative GS positive diffuse but weak Ki-67 increased, ~10% |
|
| 8 | Liver explant | 68 | M | Enhancement characteristics of HCC | HCV HFE |
cirrhosis | 0.9 (of viable tumor) | Well Status post treatment; 10% viable |
Mild | GP3 negative AFP negative Bcat positive, nuclear staining GS diffuse positive Ki-67 increased, ~5% |
| 9 | Biopsy | 63 | M | Not available | HCV | Cirrhosis | unavailable | Well Mild steatosis |
Rare Pseudo-glands | GP3 positive Ki-67 increased, ~10% |
| 10 | Biopsy | 74 | M | Radiology differential of HCC versus abscess | Metabolic syndrome | Insufficient background liver to evaluate | 5.2 | Well steatohepatitic |
Abundant intra-tumoral fibrosis | GP3 negative Ki-67 increased, ~10% |
GP3: Glypican 3
AFP: alpha fetoprotein
Bcat: beta catenin nuclear staining
GS: glutamine synthetase
ETOH: alcohol liver disease
SH: Steatohepatitis
HFE: Hereditary hemochromatosis
Imaging findings were available in 7 patients. Of those, 3 patients (case #1, #2, and #5) showed indeterminate and nonspecific findings. In 2 patients, radiology findings were consistent with hepatocellular carcinoma by imaging criteria, but clinical uncertainty led to a biopsy. Two patients had imaging features that were suspicious, but not diagnostic of hepatocellular carcinoma. Clinicopathological features of the control group of macro-regenerative/dysplastic nodules are shown in Table 2.
Table 2.
Clinical and histological findings of dysplastic and macroregenerative nodules
| Case | Specimen | Age | M/F | Liver disease | Fibrosis | Size (cm) | Diagnosis | Immuno-histochemistry |
|---|---|---|---|---|---|---|---|---|
| 1 | Liver explant | 58 | M | Cryptogenic | cirrhosis | 0.9 | Macro-regenerative nodule | Ki67, less than 1% GP3 negative GS negative AFP negative Retic intact Bcat negative |
| 2 | Liver explant | 20 | M | PSC-AIH overlap | Bridging fibrosis to cirrhosis | 0.5 | Dysplastic nodule, low grade | Ki67, 1% GP3 negative GS negative AFP negative Retic intact Bcat negative |
| 3 | Liver explant | 20 | M | PSC-AIH overlap | Bridging fibrosis to cirrhosis | 0.4 | Dysplastic nodule, low grade | Ki67, 1% GP3 negative GS negative AFP negative Retic intact Bcat negative |
| 4 | Liver explant | 20 | M | PSC-AIH overlap | Bridging fibrosis to cirrhosis | 0.5 | Dysplastic nodule, low grade | Ki67, 1% GP3 negative GS negative AFP negative Retic intact Bcat negative |
| 5 | Liver explant | 62 | M | Chronic hepatitis C and a focus of well diff HCC | Cirrhosis | 0.8 | Macro-regenerative nodule | Ki67, 1% GP3 negative GS negative AFP negative Retic intact Bcat negative |
| 6 | Liver explant | 64 | M | Steatohepatitis and multifocal HCC | cirrhosis | 0.9 | Dysplastic nodule, low grade | Ki67, 1% GP3 negative GS negative AFP negative Retic intact Bcat negative |
| 7 | Liver explant | 64 | M | Steatohepatitis and multifocal HCC | cirrhosis | 0.5 | Dysplastic nodule, low grade | Ki67, 1% GP3 negative GS negative AFP negative Retic focal equivocal loss Bcat negative |
| 8 | Liver explant | 64 | M | Steatohepatitis and multifocal HCC | cirrhosis | 0.6 | Dysplastic nodule, low grade | Ki67, 1% GP3 negative GS negative AFP negative Retic focal equivocal loss Bcat negative |
GP3: Glypican 3
AFP: alpha fetoprotein
Bcat: beta catenin nuclear staining
GS: glutamine synthetase
Retic: reticulin
PSC: Primary sclerosing cholangitis
AIH: Autoimmune hepatitis
HCC: Hepatocellular carcinoma
3.2. Histological findings
All of the tumors were well differentiated, with mild cytological atypia, manifesting primarily as mild nuclear atypia (N=8), subtly increased nuclear to cytoplasmic (N:C) ratios compared to adjacent non-neoplastic hepatocytes (N=3 cases) and pseudoglands (N=4 cases), (Figures 1, 2, 3). The mild cytological atypia was considered insufficient in isolation to diagnose hepatocellular carcinoma in all of the cases, by both the referring pathologists (in consult cases) and by the consultant pathologists. All of the tumors had conventional morphology with trabecular or solid growth patterns. Five cases showed intratumoral steatosis/steatohepatitic features and one showed intra-tumoral fibrosis. Rare mitotic figures were identified in two cases. A single case showed possible portal tract invasion.
Figure 1. Case 1 is illustrated.

Panel 1A. There is mild atypia; tumor is on the right side of the image and the non-neoplastic liver is on the left. Panel 1B. There is focal equivocal reticulin loss (circled); since reticulin loss was limited to this single, small focus, it was not considered diagnostic in isolation. Panel 1C. The Ki67 is definitely increased in the tumor compared to non-tumor (left side of image). Panel 1D. AFP is focally positive.
Figure 2. Case 2 is illustrated.

Panel 2A. There is mild atypia in the left side of the image. Mild fatty change is also present. Panel 2B. There is mild reticulin fragmentation in the area of fatty change, but no definite reticulin loss. Panel 2C. A higher magnification, confirming that there is no reticulin loss in areas without fat. Panel 2D. Glypican 3 is positive
Figure 3. Case 3 is illustrated.
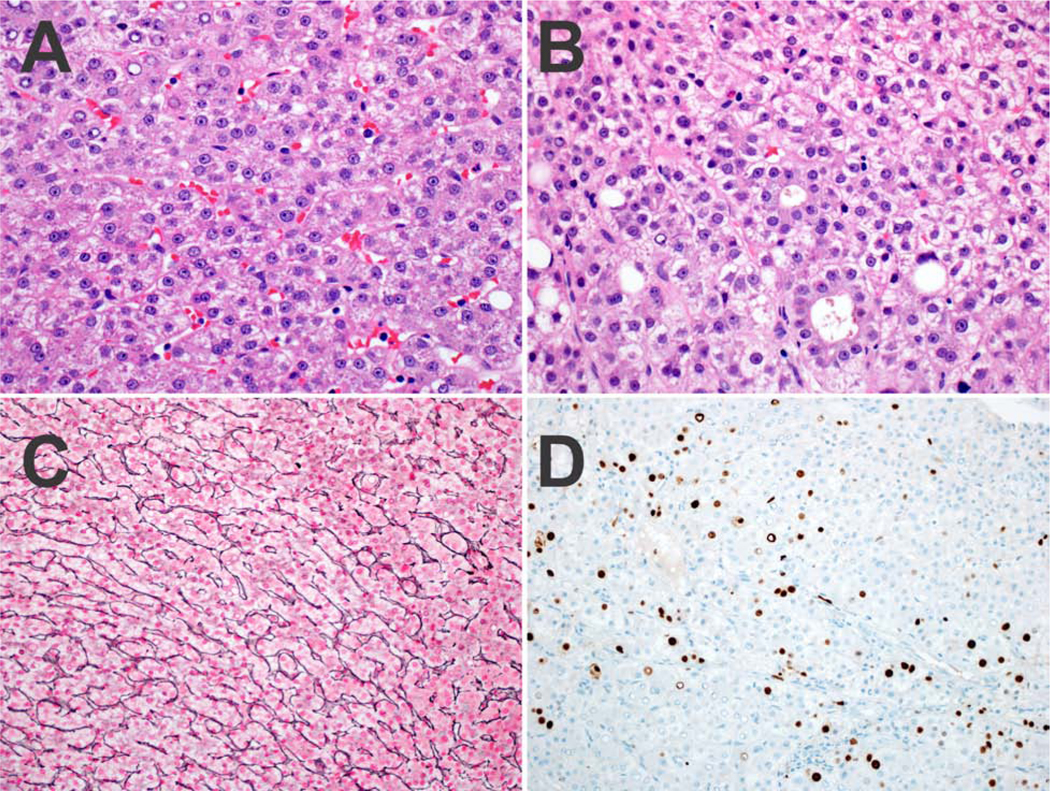
Panel 3A. There is no significant nuclear atypia, with an equivocal increase in the N:C ratio. Panel 3B. Focally, pseudoglands are present. Panel 3C. There is no reticulin loss. Panel 3D. A Ki-67 shows an increased proliferative rate.
3.3. Immunohistochemical findings
In nine cases, reticulin stains were essentially normal (Figures 2B, 3C), with no plate thickening and no loss of reticulin, while in two cases there was very focal and equivocal reduction in reticulin (Figure 1B). Ki-67 stain showed increased proliferation in all of 10 stained cases, with the Ki-67 proliferation rate estimated to be between 5–10% (Figures 1C, 3D). In all cases with adjacent non-tumor liver, the proliferation was clearly increased from adjacent liver at low power examination. AFP was positive in 1of 8 stained hepatocellular carcinomas (Figure 1D). Four hepatocellular carcinomas were also glypican 3 positive (Figure 2D); two separate nodules of HCC were identified in case #1 and both were glypican 3 positive. Beta catenin showed nuclear positive staining in 1 of the 5 stained cases. Glutamine synthetase showed diffuse positive staining in 3 of the 7 studied cases.
In the control group, there was one dysplastic nodule where focal equivocal disruption of reticulin was noted (case 8; Table 2), but the morphology and other immunostaining profile did not reach the level of hepatocellular carcinoma. All other dysplastic and macro-regenerative nodules in the control group showed intact reticulin meshwork. All dysplastic nodules in the control group showed a low Ki-67 staining that was indistinguishable from the background liver and were negative for glypican 3 and AFP.
3.4. Estimation of frequency
The frequency of hepatocellular carcinoma without reticulin loss was estimated by determining the number of hepatocellular carcinomas without reticulin loss in 100 consecutive hepatocellular carcinoma diagnoses made in a consult practice. Four cases were identified (4%), with the caveat that this approach likely overestimates the true frequency, because consult cases tend to be enriched for challenging specimens [11].
4. Discussion
The use of reticulin as an aide for diagnosing hepatocellular carcinoma represents a major advance in the diagnostic approach for hepatocellular carcinoma, as prior to this time the diagnosis was based exclusively on morphology, including cytological atypia, angiolymphatic invasion, and / or metastatic disease [12]. Reticulin loss in hepatocellular carcinoma was mentioned in a 1973 paper [13], but its potential was specifically highlighted in a 1977 study of hepatocellular carcinoma in cirrhotic livers [14]. Subsequently, this observation was extended to neoplasms in non-cirrhotic livers [15] and reticulin loss was fully incorporated into standardized criteria for diagnosing hepatocellular carcinoma [16], including fine needle aspiration specimens [15]. Since then, the use of reticulin for diagnosing hepatocellular carcinoma has been confirmed by decades of experience from centers across the world in all types of underlying liver disease.
Reticulin loss is defined essentially by two different approaches, both of which work well [9]. In the benign liver, hepatic cords are one to two cells in width, but the hepatic cords become consistently thickened (> 2 cells in thickness) in hepatocellular carcinoma. The other method of assessing the reticulin stain is based on the observation that each hepatocyte in the benign liver is touching reticulin on one of its borders, while with hepatocellular carcinoma, tumor cells are readily found that show no contact with reticulin. While the identification of reticulin loss is a very robust diagnostic tool, it needs to be interpreted in association with the morphology and with common sense. For example, focal equivocal reticulin loss is considered insufficient in isolation for a diagnosis of hepatocellular carcinoma [5, 9]. This point bears additional emphasis, being a diagnostic pitfall in its own right, where over interpretation of reticulin changes can lead to an incorrect diagnosis of malignancy. Known diagnostic pitfalls include poor quality stains and fatty liver disease, which can have physiological loss or fragmentation of the reticulin in areas of steatosis [17]. When evaluating tumors with fatty change, the best approach is to evaluate those areas of the tumor that have no or minimal steatosis. Rapidly regenerating benign liver can also show patchy mild thickening of the hepatic trabeculae [5]. Even outside these settings, the reticulin framework often has minor nonspecific changes that can be over interpreted as carcinoma. In one study of consult material [11], 15% of all submitted diagnoses of hepatocellular carcinoma were not confirmed on review, with the majority of diagnoses being changed from hepatocellular carcinoma to benign liver.
Previously, a few case reports have documented well-differentiated hepatocellular carcinomas that showed no loss of reticulin staining on biopsy specimens [1, 2], but this study is the first to systematically examine this important diagnostic pitfall. The cases in this study were all men, reflecting the general demographics of hepatocellular carcinoma. There are no reasons to suspect that the results do not extend to women, but the relatively small numbers and the lack of women are limitations of this study. Eight of the cases in this study were on biopsy material, so it seems likely that many or even all of these tumors may have reticulin loss in the resected specimen. Three of the cases were fully resected, but small, hepatocellular carcinomas found in cirrhotic livers, suggesting that early low grade hepatocellular carcinomas can sometimes show intact reticulin expression.
A rational, systematic approach is useful for these difficult biopsy specimens. The findings in this study support the following method for making a diagnosis of hepatocellular carcinoma when there is no definite reticulin loss: (1) at least mild cytological atypia should be present. In addition, there should be at least one of the following two features; both do not need to be present, although the diagnosis is strengthened when both are present: (2) Ki-67 proliferation that is clearly increased above the background liver; (3) strong expression of abnormal oncofetoproteins, such as Glypican 3 or AFP. These stains are helpful when positive, but are non-informative when negative. While not used in this study, EZH2 [18] and HSP70 [4, 19] are other useful immunostains to identify malignancy and could fulfill criteria number 3. In the current study, 1 of the 5 studied cases showed aberrant nuclear expression of beta-catenin and 3 of the 7 cases showed diffuse glutamine synthetase expression; these findings would also support a diagnosis of hepatocellular carcinoma when present in a nodule in a cirrhotic liver, fulfilling criteria 3. This approach will not solve every difficult case that has only mild cytological atypia, but will help with many of them. For cases that do not reach these criteria, then re-biopsy is likely the best course to establish a tissue diagnosis for a radiographically concerning lesion.
The reticulin stain is thought to identify mostly type III collagen [20], but also other extracellular proteins such as type IV collagen [21] and laminin [22]. Previous studies have shown a general correlation between the grade of hepatocellular carcinoma and reduced laminin and or type IV collagen staining by immunohistochemistry [23, 24]. The precise biological mechanism is unclear, however, for the reduced extracellular matrix accumulation. One possibility is the reduced reticulin results from increased extracellular matrix remodeling within the hepatocellular carcinoma, through the expression of various matrix metalloproteinases [25].
For biopsies showing neoplastic/lesional tissue with loss of portal tracts, the histological differential in non-cirrhotic livers is mostly that of hepatic adenomas and focal nodular hyperplasia. The diagnosis of focal nodular hyperplasia can sometimes be challenging [11], but the diagnosis is reliably established using the typical histological features of focal nodular hyperplasia along with glutamine synthetase stains. Hepatic adenomas should not have increased Ki-67 proliferation compared to the non-neoplastic livers and lack expression of glypican 3 and AFP. Other findings such as portal tract invasion and mitotic figures would also help exclude a hepatic adenoma. Immunostains used to subtype hepatic adenomas (LFABP, CRP, SAA, beta catenin, glutamine synthetase) are not useful to differentiate a hepatic adenoma from a well differentiated hepatocellular carcinoma [26].
In cirrhotic livers, the histological differential shifts to macro-regenerative nodules and dysplastic nodules. Here we studied 8 cases of macro-regenerative/dysplastic nodules. All cases showed intact reticulin, low Ki-67 staining (less than 1%), and negative glypican 3 and AFP. These findings are in keeping with that of others who have also found that macroregenerative/dysplastic nodules have a low proliferative rate and are negative for AFP and glypican 3 [27]. Therefore, the size of lesions (most macroregenerative nodules are <2 cm), the presence of portal tract invasion, increased proliferation by Ki-67 and the abnormal expression of glypican 3 and AFP can help establish a diagnosis of hepatocellular carcinoma.
In summary, rare hepatocellular carcinomas show no loss of reticulin or have only focal equivocal disruption of the reticulin meshwork, at least on biopsy specimens, and pose diagnostic challenges. These hepatocellular carcinomas occur in the same clinical setting of typical hepatocellular carcinoma, are well differentiated, and the diagnosis can be approached using a combination of histological and immunostain findings.
HIGHLIGHTS.
Reticulin is an important diagnostic stain used to make a diagnosis of well differentiated hepatocellular carcinoma.
Rare hepatocellular carcinomas show no definite reticulin loss or have focal equivocal reticulin disruption on liver biopsy specimens, which can be a diagnostic challenge.
In these challenging cases, the diagnosis of HCC can be made using a combination of histologic and immunohistochemical findings; increased Ki-67, and aberrant expression of proteins such as glypican 3 or alpha fetoprotein (AFP).
Acknowledgments
Grant support
P50 CA210964 (MT)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hong H, Patonay B, Finley J. Unusual reticulin staining pattern in well-differentiated hepatocellular carcinoma. Diagn Pathol 2011;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkens L, Becker T, Schlegelberger B, Kreipe HH, Flemming P. Preserved reticulin network in a case of hepatocellular carcinoma. Histopathology 2006;48:876–8. [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Al HM, Makhlouf HR, Wang G, Goodman ZD. Glypican-3 expression in benign liver tissue with active hepatitis C: implications for the diagnosis of hepatocellular carcinoma. Hum Pathol 2008;39:209–12. [DOI] [PubMed] [Google Scholar]
- 4.Di Tommaso L, Destro A, Seok JY, et al. The application of markers (HSP70 GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol 2009;50:746–54. [DOI] [PubMed] [Google Scholar]
- 5.Matsukuma KE, Yeh MM. Update on the pathology of liver neoplasms. Ann Diagn Pathol 2019;38:126–37. [DOI] [PubMed] [Google Scholar]
- 6.Yeh MM, Larson AM, Campbell JS, et al. The expression of transforming growth factor-alpha in cirrhosis, dysplastic nodules, and hepatocellular carcinoma: an immunohistochemical study of 70 cases. Am J Surg Pathol 2007;31:681–9. [DOI] [PubMed] [Google Scholar]
- 7.An HJ, Illei P, Diflo T, et al. Scirrhous changes in dysplastic nodules do not indicate high-grade status. J Gastroenterol Hepatol 2003;18:660–5. [DOI] [PubMed] [Google Scholar]
- 8.Wanless IR. International consensus on histologic diagnosis of early hepatocellular neoplasia. Hepatol Res 2007;37 Suppl 2:S139–41. [DOI] [PubMed] [Google Scholar]
- 9.Torbenson MS, Zen Y, Yeh MM, American Registry of Pathology. Tumors of the liver. Published by the American Registry of Pathology: Washington, DC, 2018. xv, 449 pages pp. [Google Scholar]
- 10.Mounajjed T, Yasir S, Aleff PA, Torbenson MS. Pigmented hepatocellular adenomas have a high risk of atypia and malignancy. Mod Pathol 2015;28:1265–74. [DOI] [PubMed] [Google Scholar]
- 11.Torbenson MS, Arnold CA, Graham RP, et al. Identification of key challenges in liver pathology: data from a multicenter study of extramural consults. Hum Pathol 2019;87:75–82. [DOI] [PubMed] [Google Scholar]
- 12.Torbenson M, Washington K. Pathology of liver disease: advances in the last 50years. Hum Pathol 2019. [DOI] [PubMed]
- 13.Anthony PP. Primary carcinoma of the liver in Uganda. J Pathol 1972;106:Piv-v. [PubMed] [Google Scholar]
- 14.Noorredam K. Primary carcinoma of the liver. A histological study of 27 cases of primary carcinoma of the liver from Malawi. Acta Pathol Microbiol Scand A 1977;85:461–9. [PubMed] [Google Scholar]
- 15.Koelma IA, Nap M, Huitema S, Krom RA, Houthoff HJ. Hepatocellular carcinoma, adenoma, and focal nodular hyperplasia. Comparative histopathologic study with immunohistochemical parameters. Arch Pathol Lab Med 1986;110:1035–40. [PubMed] [Google Scholar]
- 16.Ferrell LD, Crawford JM, Dhillon AP, Scheuer PJ, Nakanuma Y. Proposal for standardized criteria for the diagnosis of benign, borderline, and malignant hepatocellular lesions arising in chronic advanced liver disease. Am J Surg Pathol 1993;17:1113–23. [DOI] [PubMed] [Google Scholar]
- 17.Singhi AD, Jain D, Kakar S, et al. Reticulin loss in benign fatty liver: an important diagnostic pitfall when considering a diagnosis of hepatocellular carcinoma. Am J Surg Pathol 2012;36:710–5. [DOI] [PubMed] [Google Scholar]
- 18.Hajosi-Kalcakosz S, Dezso K, Bugyik E, et al. Enhancer of zeste homologue 2 (EZH2) is a reliable immunohistochemical marker to differentiate malignant and benign hepatic tumors. Diagn Pathol 2012;7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen TB, Roncalli M, Di Tommaso L, Kakar S. Combined use of heat-shock protein 70 and glutamine synthetase is useful in the distinction of typical hepatocellular adenoma from atypical hepatocellular neoplasms and well-differentiated hepatocellular carcinoma. Mod Pathol 2016;29:283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojkind M, Giambrone MA, Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology 1979;76:710–9. [PubMed] [Google Scholar]
- 21.Grimaud JA, Druguet M, Peyrol S, et al. Collagen immunotyping in human liver: light and electron microscope study. J Histochem Cytochem 1980;28:1145–56. [DOI] [PubMed] [Google Scholar]
- 22.Loreal O, Levavasseur F, Fromaget C, et al. Cooperation of Ito cells and hepatocytes in the deposition of an extracellular matrix in vitro. Am J Pathol 1993;143:538–44. [PMC free article] [PubMed] [Google Scholar]
- 23.Donato MF, Colombo M, Matarazzo M, Paronetto F. Distribution of basement membrane components in human hepatocellular carcinoma. Cancer 1989;63:272–9. [DOI] [PubMed] [Google Scholar]
- 24.Grigioni WF, Garbisa S, D’Errico A, et al. Evaluation of hepatocellular carcinoma aggressiveness by a panel of extracellular matrix antigens. Am J Pathol 1991;138:647–54. [PMC free article] [PubMed] [Google Scholar]
- 25.Theret N, Musso O, Turlin B, et al. Increased extracellular matrix remodeling is associated with tumor progression in human hepatocellular carcinomas. Hepatology 2001;34:82–8. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Shah SS, Naini BV, et al. Immunostains Used to Subtype Hepatic Adenomas Do Not Distinguish Hepatic Adenomas From Hepatocellular Carcinomas. Am J Surg Pathol 2016;40:1062–9. [DOI] [PubMed] [Google Scholar]
- 27.Libbrecht L, Severi T, Cassiman D, et al. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol 2006;30:1405–11. [DOI] [PubMed] [Google Scholar]


