Abstract
Three-dimensional (3D) virtual reconstruction (VR) in the medical sciences has emerged as a novel, exciting and effective tool, with promising results for patients, trainees, and even experienced surgeons. The purpose of this review is to summarize the information on the clinical value and applications of 3D VR in renal tumors published in the last ten years. A literary search of PubMed-MEDLINE databases was performed to identify studies reporting the clinical application and usefulness of 3D VR models in renal tumors. Thirty-seven studies were found to meet the selection criteria and were included in the analysis. Most studies have provided a quantitative assessment focused on the accuracy of 3D VR models in replication of anatomy and renal tumor, on measuring 3D tumor volume and on the clinical value and utility of 3D VR in pre-surgical planning and simulation of renal procedures, with significant reductions of intraoperative complications. Fourteen studies provided a qualitative assessment of the usefulness of 3D VR models, with results showing an improved patient understanding of renal anatomy and pathology, improved undergraduate and postgraduate urology education. Moreover, 3D printing technology is a novel technique, and we are currently in the dynamic era, expanding into new surgical nephron-sparing procedures and the development of printed kidneys for transplantation.
Keywords: renal tumor, virtual reconstruction (VR), three-dimensional (3D) renal models, application
Introduction
The application of three-dimensional (3D) virtual reconstruction (VR) in the medical sciences from a standard two-dimensional (2D) cross-sectional image processing has become increasingly used, as it has been shown it accurately reproduces anatomical structures and pathologies, providing more information which cannot always be supported only by conventional imaging visualizations [1,2]. In the urology field, the 3D VR has gained much attention especially in the development of models for planning or simulation complex surgical procedures, focusing mainly on renal cancer, kidney transplantation and nephrolithiasis [3,4].
There are two main imaging techniques that are used for the generation of 3D renal models, which are CT and MRI [5,6]. Computed tomography imaging (CT) has long been established as the gold standard imaging for renal tumors [7]. Magnetic resonance imaging (MRI) is an attractive alternative because it provides an excellent combination of soft-tissue detail and radiation-free imaging, but remains used only as a tool for characterizing indeterminate renal tumors on CT and in patients where CT is contraindicated due to impaired renal function [8].
To get a more comprehensive view of renal tumors, creating 3D VR models that show the anatomy of the kidney and tumor, it is possible to facilitate the surgeons’ better understanding of patients’ oncological conditions, better communication with patients and more advanced surgical training allowing the surgeon to manipulate the model before surgery, reducing the need for surgical experience and also a better understanding of the relation of the tumors with other structures such as vessels or excretory cavities [9]. As the field of urology moves away from open, radical nephrectomy to minimally invasive and laparoscopic and robot-assisted techniques, surgeons and trainees often do not get a tactile familiarity with the tumor until it is removed, and therefore a 3D model would be very useful for the practice [10,11].
The aim of this systematic review is to survey and analyze the current literature related to the clinical value and applications of 3D VR on renal tumors. Our main goal was threefold: first, to systematically review the accuracy of 3D VR in the delimitation of renal anatomy and renal tumor, second, to systematically review the clinical value and utility of 3D VR in pre-surgical planning and simulation of renal procedures, and third, to systematically review the usefulness of 3D virtual kidney models in patient or medical education and patient-doctor communication.
Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [12]. No ethics committee approval was necessary due to the nature of this study.
Search strategy
A comprehensive literature research was conducted to assess the current use of 3D VR in renal tumors. We have searched the PubMed-MEDLINE database for the last 10 years (last search: December, 2020) using keywords for the relevant literature to capture recent trends in clinical practice. The search strategy included the following terms in isolation or combination: “Three-dimensional”, “3D”, “virtual reconstruction”, “virtual models”, “renal cell carcinoma”, “renal tumor”, “renal cancer “, “three-dimensional printing”, and “3D printed models”. The results were limited to articles written in English.
Eligibility criteria
To be eligible for the analysis, studies had to meet the following criteria: (a) the accuracy of 3D VR models in delineating renal anatomy and renal tumors; (b) the usefulness of 3D VR models in pre-surgical planning and simulation of renal procedures; (c) the usefulness of 3D VR models in patient or medical education and in patient-doctor communication.
Data extraction
The initial screening for the identification of potentially eligible articles was performed by two independent readers (C.-G. M. and A.L.). The screening was made only based on the title and abstract of all independently identified references. Once all the relevant literature was collected, each study was analyzed and read in detail to identify and extract the discussions. This includes the accuracy of 3D VR to reproduce anatomical structures and renal tumors, usefulness and feasibility in medical education, simulation in surgical training and planning of renal procedures. The details of each publication were checked to avoid duplicates and any differences were resolved by consensus.
The following characteristics of the study were extracted: first author’s name, year of publication, imaging methods used for 3D imaging reconstruction, software used for image segmentation, study sample size/participants, study proposal and main key findings of the study.
Results
Literature search outcomes
The initial literature search retrieved a total of 112 potentially eligible articles. However, only 77 titles and abstracts were screened as most of them were found to be irrelevant for the topic. Of these 77 articles, 43 articles were excluded as they did not meet our eligibility criteria. Finally, 34 articles were included into the analysis. Three additional articles were added from a review of references/citations from other articles, leading to a total of 37 full-text articles included in final systematic review. Figure 1 presents the flow chart showing the research literature strategy to identify the eligible studies.
Figure 1.
Flow chart showing strategy to identify eligible studies.
Study characteristics
Out of the 37 studies analyzed, twenty-three [13–35] reported the usefulness and applications of virtual and printed 3D kidney models in “surgical planning” and “surgical training”. Fourteen [36–49] of these were cross-sectional studies, mainly surveying the impact of 3D models in understanding renal anatomy and renal tumor by young surgeons, medical students and patients. Table I is a summary of study characteristics of the use of 3D VR models in renal tumors.
Table I.
Summary of findings of the 37 studies reviewed.
| First Author and Year of Publication | Imaging Modalities Used for 3D Imaging Recon-struction | Software Used for Image Segmen-tation | Study Sample Size/Participants | Study Purpose | Key Findings |
|---|---|---|---|---|---|
| Knoedler et al. 2015 [45] | CT | Software N/A | 6 patients/28 participants | The usefulness of 3D-printed renal models with enhancing masses on junior medics’ understandings, localizations, and characterizations of renal tumors |
|
| Silberstein et al. 2014 [46] | CT | Software N/A | 5 patients | The usefulness of 3D-printed kidney models with enhancing lesions on education of trainees, patients, and surgeons for characterization and management of RCC |
|
| Belenchón et al. 2020 [13] | CT | MeshMixer® and Cura® | 7 patients | The efficacy and efficiency of surgery planning with 3D models of RCC with venous tumor thrombus extension compared to the standard images |
|
| Durso et al. 2014 [19] | CT | Aquarius iNtuition™ Edition (Terarecon, California) | 28 patients | The usefulness of 3D reconstruction volume in determining renal parenchymal volumes and renal tumor volumes |
|
| Fan et al. 2019 [40] | CT | Mimics 18.0 (Materialise, Leuven, Belgium) | 127 patients | The efficacy of 3D printing physical model-assisted laparoscopic partial nephrectomy in patients with renal tumors |
|
| Glybochko et al. 2018 [47] | CT | Amira, Version 5.4.4 (license ASTND. 44644) | 5 patients | The effectiveness of 3D printing application in urology for localized renal cancer treatment using 3D printed soft models |
|
| Golab et al. 2017 [30] | CT | 3D Slicer | 3 patients | To evaluate the clinical value of 3D-printed kidney models for training purposes in the context of laparoscopic partial nephrectomy surgery |
|
| Hyde et al. 2019 [38] | CT | 3D Slicer | 25 patients | To determine whether the interactive visualization of patient-specific virtual 3D models of the renal anatomy influences the pre-operative decision-making process of urological surgeons for complex renal cancer operations |
|
| Komai et al. 2015 [24] | CT | ZedView®, LEXI Co., Ltd., Tokyo, Japan | 10 patients | To report an experience with a novel style of 3D printed kidney, called “4D” surgical navigation in minimally invasive off-clamp partial nephrectomy |
|
| Komai et al. 2014 [17] | CT | Synapse Vincent | 28 patients | To report an experience with the novel 3D image analysis system Synapse Vincent in clamp less partial nephrectomy, describing its advantages regarding the short-term surgical outcomes and its usefulness as an informed consent tool |
|
| Kyung et al. 2019 [18] | CT | Asan Medical Center, Seoul, South Corea | 17 patients | To construct a 3D printed kidney models for patients scheduled to undergo partial nephrectomy to provide a better understanding of individual tumors and to predict surgical outcomes |
|
| Lasser et al. 2012 [25] | CT | Medical Modeling Inc, Golden, CO | 10 patients | To describe an experience with 3D preoperative virtual surgical planning and its utilization during robot-assisted laparoscopic partial nephrectomy |
|
| Lee et al. 2018 [48] | CT | Object 260 Connex 3 (Stratasys, Eden Prairie, MN, USA) | 10 patients | The clinical usefulness of 3D-printed renal model in performing partial nephrectomy and also in the education of medical students |
|
| Leslie et al. 2014 [26] | CT | Synapse 3D, Fujifilm | 200 patients | To develop the novel concept of tumor contact surface area, to capture this clinical observation using a radiologically measurable parameter |
|
| Maddox et al. 2017 [14] | CT | 3D Systems (Rock Hill, SC) | 7 patients | To develop a novel 3D printed kidney model using materials that closely approximated normal kidney |
|
| Michiels et al. 2019 [43] | CT | Synapse 3D (version 5.2, Fujifilm) | 16 patients | To assess the anatomic accuracy of the 3D model used for 3D model-guided robot-assisted partial nephrectomy |
|
| Porpiglia et al. 2019 [22] | CT | MEDICS (Turin, Italy) | 101 patients | To create 3D-based PADUA and RENAL nephrometry scores/categories for the reclassification of the surgical complexity of renal masses, and to compare the new 3D nephrometry score/category with the standard 2D-based nephrometry score/category |
|
| Porpiglia et al. 2018 [21] | CT | M3DISC | 10 patients | The usefulness of 3D printed models in pre-surgical planning, training and education |
|
| Wang et al. 2019 [27] | CT | IQQA; EDDA Technology, Princeton, NJ, USA | 49 patients | The role of 3D reconstruction technique in renal function protection and ipsilateral parenchymal mass preserved after laparoscopic partial nephrectomy in patients with complex renal tumor (R.E.N.A.L. score≥8) |
|
| Wang et al. 2017 [16] | CT | Yorktal, Inc., Shenzhen, Guangdong, China | 49 patients | The effectiveness and safety of individualized 3D visualization technology on surgical planning and perioperative outcomes in laparoscopic partial nephrectomy for renal cell carcinoma |
|
| Zhang et al. 2015 [41] | CT | Software N/A | 10 patients | The impact of 3D printing on the surgical planning, potential of training and patients’ comprehension of minimally invasive surgery for renal tumors |
|
| Wake et al. 2017 [37] | MRI | Mimics 16.0 (Mimics, Materialise, Leuven, BE) | 10 patients | To ability of patient-specific 3D-printed kidney models with tumors to enhance pre-surgical planning for complex cases of RCC |
|
| Dwivedi et al. 2018 [15] | MRI | 3D Slicer | 6 patients | The usefulness of patient-specific 3D-printed kidney molds for radiomics and radio genomic analyses |
|
| Alyaev et al. 2017 [34] | CT | Software N/A | 5 patients | The usefulness of soft 3D-printed kidney models for treatment and pre-operative planning for patients with localized kidney cancer |
|
| Bernhard et al. 2015 [49] | CT | Synapse 3D, Fujifilm, Tokyo, Japan | 7 patients | The ability of 3D-printed models of kidneys with renal tumors to facilitate patients’ understanding and education of their condition |
|
| Schmit et al. 2018 [36] | CT | Mimics, Materialize; Leuven, Belgium | 25 patients | To assess whether a 3D printed model improves patients’ understanding of renal cryoablation and the anatomy involved |
|
| Teishima et al. 2018 [29] | CT | Toyotsu Machinery co., Ltd. Nagoya, Japan | 29 patients | The usefulness of a personalized 3D printed model of the kidney for preoperative education among patients who underwent robot-assisted partial nephrectomy |
|
| Rai et al. 2018 [39] | CT | MathWorks; Natick, MA | 100 participants | To test a novel visuospatial testing platform, improve trainee ability to convert two-dimensional to three-dimensional space |
|
| Liu et al. 2020 [28] | CT | CSA, Hisense, Tsingtao, China | 135 patients | To develop a 3D scoring system which could improve radiological evaluation of renal tumors |
|
| Huang et al. 2020 [20] | CT and MRI | Software N/A | 134 patients | To develop a reproducible, quantifiable scoring system that describes the exact anatomic features and quantifies compression of the vessels and the collection system, thereby classifying the surgical complexity and assisting in treatment planning |
|
| Fan et al. 2018 [23] | CT | Software N/A | 5 patients | To evaluate that a 3D kidney models can be used not only for planning but also for navigating laparoscopic partial nephrectomy in patients with completely endophytic renal tumors |
|
| Wake et al. 2019 [42] | CT and MRI | Mimics 20.0 and 3-matic 12.0, Materialize, Leuven, Belgium | 20 patients | To quantify how surgeons translate 2D CT or MRI data to a 3D model and evaluate if 3D printed models improve tumor localization |
|
| Tapiero et al. 2020 [44] | CT | Software N/A | 11 patients/13 participants | The utility of a CT-based interactive virtual reality display to assist surgeons’ understanding of the precise location of the renal tumor |
|
| Meyer et al. 2015 [32] | CT | Software N/A | 158 patients | To analyze the relationship among various patient, operative and tumor characteristics to determine which factors correlate with renal parenchymal volume loss after nephron sparing surgery using a novel 3D volume assessment |
|
| Mitsui et al. 2018 [31] | CT | Software N/A | 114 patients | To investigate the relationship between postoperative renal function and resected cortex margin volume calculated by a 3D reconstruction technique based on the resected specimen, and to determine predictors of renal function after robot-assisted partial nephrectomy |
|
| Tobert et al. 2015 [33] | CT | Software N/A | 157 patients | To validate the findings of a prior single-surgeon series with a multi-institutional comparison of 3D imaging of volume preservation and surgeon assessment of volume preservation as predictors of renal function after partial nephrectomy |
|
| Porpiglia et al. 2018 [35] | CT | M3DICS | 52 patients | To present hyperaccuracy 3D reconstruction during robot-assisted partial nephrectomy and compare its efficacy in sponsoring successful selective clamping of renal arterial branches during robot-assisted partial nephrectomy. |
|
Legend: N/A - not applicable, RCC - renal cell carcinoma, 3D - three-dimensional, VR - virtual reconstruction.
CT was the most used imaging technique for segmentation of anatomical structures, with 34 studies using CT datasets as a source of imaging data for 3D VR, while two utilized MRI datasets [15,37], and other two utilized a combination of both imaging modalities [20,42]. 2D CT images alone may not be so accurate in describing anatomic structure for some complex tumors, such as renal hilar tumor and renal endophytic tumor [50]. The actual position between tumor peripheral vessels was ambiguous, which increased the risk of additional damage in surgery. In recent years, 3D CT reconstruction has been increasingly used to provide additional information, thus reducing accessory surgical injuries [51,52].
Discussion
Clinical applications of 3D imaging in renal tumors
The clinical applications of 3D printing in renal tumors can be summarized into five main areas, which include preoperative planning, presurgical simulation, intraoperative navigation mapping, medical education and patient-doctor communication.
Preoperative planning
In the recent decades, the technology of 3D VR models has gained much interest, particularly in making 3D models that can provide a topographical map of the renal surface and multiplane views of intrarenal anatomy facilitating an accurate preoperative planning for renal tumors [4,48,49,53].
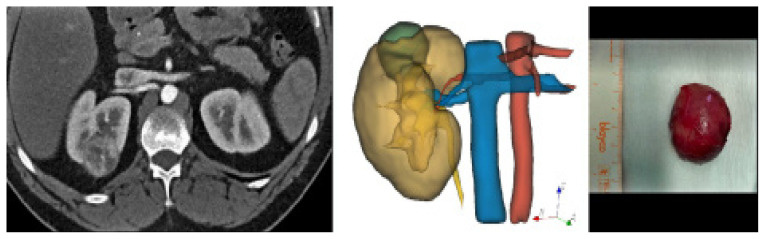
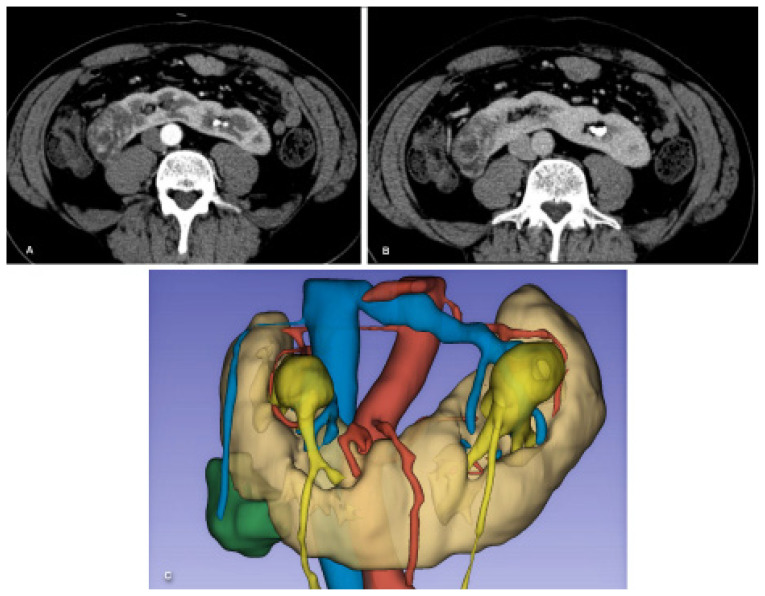
Two examples of 3D VR models are shown in figure 2 and figure 3. A thorough understanding of vascular and tumor anatomy in the kidneys is important to avoid damage to the renal parenchyma and major vessels and to achieve a complete excision of the tumor, which could be a challenging task in some cases, as is the case of endophytic tumors [50].
Figure 2.
3D VR model of a 54-year-old woman with a renal tumor at the upper pole of the right kidney. Comparative views of CT scan in the corticomedullary phase (axial planes), corresponding to the 3D VR model and surgical resection specimen.
Figure 3.
3D VR model of a 64-year-old male with a renal tumor at the lower pole of the right kidney (horseshoe kidneys). Comparative views of the CT scan in the corticomedullary and nephrographic phases (A, B, axial planes), and the corresponding 3D VR model (C).
Several steps are important to generate a patient-specific 3D VR model [54]. First, the accuracy of segmentation depends largely on image quality, including spatial resolution and contrast to noise ratio. All artefacts that occur within the 2D image will also be manifested in the 3D imaging [55]. The second and most important step is image segmentation. With the help of brain work and computer software, once segmented, a patient-specific 3D VR model is created, helping in a better anatomical understanding, with multiple benefits for both the patient and the surgeons [56]. Lately, more and more software packages are being developed and available for this purpose, which can be easily used for the same goal.
The studies included in this review found that 3D VR models technique had effective accuracy in reconstructing the anatomical structure, tiny vessels and tissues around the tumor, being an accurate technique compared to the original 2D datasets that helps surgeons remove the tumor with a minimal margin and a great postoperative renal function [13,19,21,22,42].
Moreover, printing 3D VR models with flexible materials that resemble life-like tissues could improve the understanding of renal masses, allowing surgeons and trainees to practice resection of individualized lesions before the actual surgery. Gill et al. [57] have shown the usefulness of 3D printing models in the pre-surgical planning of renal pedicle clamp management during partial nephrectomy, emphasizing the main role in 3D study, understanding of intrarenal vascular anatomy and its relationship to renal tumor.
Presurgical stimulation and intra-operative navigation mapping
Out of thirty-seven studies reviewed, twenty-three [13–35] reported the “surgical simulation” and “surgical training “as outcome of interest. The studies conducted a quantitative assessment of virtual and printed 3D kidney models on their usefulness and applications in areas including preoperative simulation and intraoperative navigation mapping.
Clear understanding of tumor anatomy including tumor location and size, the relationship of the renal tumor to normal parenchyma, collecting system, vascular structures, including divergence of tumor arterial feeders and veins around renal hilum is of paramount importance to optimize surgical outcome [25]. 3D imaging has improved the surgeon’s ability to better evaluate these features compared to the original 2D imaging datasets [32]. In addition, reconstructed 3D images allow 360° manipulation of all anatomic structures along all axes, removing structures from the screen for better visualization, and changing viewing windows from solid phase to the transparency phase, for a better evaluated intraparenchymal anatomy and tumor extension [31,34].
Lately, minimally invasive partial nephrectomy has been considered the procedure of choice for small renal masses, based on the functional advantages over traditional open surgery [26]. Most studies have focused on surgical planning for partial nephrectomy in order to predict surgical outcomes [15–18,21,23–25,27,34] and found that using 3D VR improves patient’s outcomes.
Bertolo et al. [58] published their experience with 3D reconstruction technologies in support of preoperative planning for highly complex renal tumors amenable to a conservative treatment. The authors demonstrated the reliability of 3D VR in reproducing anatomical structures visualized during robot-assisted partial nephrectomy, concluding that 3D virtual guidance technologies allowed more accurate preoperative planning of renal pedicle management compared to standard preoperative planning based on 2D imaging.
Over the past decade, the development of nephrometry scores, for example the PADUA and RENAL scores [22] have been developed as tools to predict objectively the surgical complexity of a renal tumor. Some studies [17,22,28,39,44,45] performed a quantitative analysis of 3D-based nephrometry scores (PADUA and RENAL) to reclassify the surgical complexity of renal masses (R.E.N.A.L. score ≥ 8). All studies found a high level of dimensional accuracy of the 3D scoring system compared to 2D-based scoring systems, especially in predicting major intra- and perioperative complications. On the other hand, Hwang et al. [59] proposed a new reporting system that combines and integrates advances in engineering technology with those from the medical sciences, the Multidimensional Interactive Radiology Report and Analysis (MIRRA) to standardize labeling, tracking, and quantifying of metrics for renal masses. The authors proposed a 3D atlas-based identification process for renal masses that includes a completely portable 3D model that will be able to better track lesions in patients with multiple masses and also an automatic process of the Tumor Node Metastasis (TNM) staging and the Response Evaluation Criteria in Solid Tumors (RECIST) linear measurements.
A recent paper [20] developed the ROADS scoring system which provides a standardized, quantitative, 3D anatomic classification, to stratify renal sinus tumors for surgical strategy. Postoperative renal function and chronic kidney disease remain a concern in the surgical management of renal masses, as they has been related to an increased risk of hospitalization, cardiovascular morbidity and death [33,58]. Few papers [31–33,60] have focused directly on the relationship between postoperative renal function and resected cortex margin volume, calculated by a 3D reconstruction technique based on the resected specimen, to determine the predictors of renal function after robot-assisted partial nephrectomy. Meyer et al. [32] found that using accurate 3D volumetric analysis, ischemia time, tumor size and endophytic/exophytic properties of a localized renal mass were the most important determiners of renal parenchymal volume loss. On the other hand, Mitsui et al. [31] reported that the healthy cortex margin volume calculated by the reconstruction technique was an independent risk factor for decreased post-operative renal function.
A recent study [61] showed that the using of 3D reconstructed images combined with 3D arteriography and 3D surface-rendered tumors coild facilitate maximal postoperative renal function preservation after partial nephrectomy.
Ukimura et al. [62] reported that 3D virtual models could provide improvements that facilitate the performance of zero-ischemia robotic and laparoscopic partial nephrectomies, especially in patients requiring nephron sparing surgery. Furthermore, Porpiglia et al. [21] have reported a high accuracy 3D reconstructive technique, succeeding in selectively clamping the branches of renal artery during long-time robot-assisted partial nephrectomy for complex renal masses, maximally decreasing the renal damages caused by ischemia.
Medical education and patient-doctor communication
Few papers [16,22,23,38,39,41,43–45,47,48] are focused on the value of 3D imaging models for educating medical students, urology residents and fellows. In their study, Rai et al. [39] demonstrated that medical students who are using the interactive 3D virtual reality simulator environment (dV-Trainer virtual) in addition to the standard 2D planar imaging, more accurately located renal masses in 3D physical models, compared to trainees who had access only to 2D planar images. Two studies [45,48] evaluated the ability of medical students to accurately characterize renal tumors, using the RENAL nephrometry scoring system compared to a reference standard generated by experienced surgeons. Both studies reported that the student group could locate the renal tumor more accurately using the 3D renal model, compared to standard 2D planar imaging alone, Knoedler et al. [45] finding that 3D printed physical models can increase medical students’ ability to correctly assign the R, N, and L components of the nephrometry score.
To perform a partial nephrectomy, it is of utmost importance to identify the exact location of the tumor to minimize both postoperative and major complications. Some studies [22,37,38,40,41,43,44,47] have investigated the usefulness of 3D imaging models in the learning capacity of urologists for presurgical planning of renal tumor and surgical training. Porpiglia et al. [22] reported that 3D imaging is a “hyper-accurate” method, capable to give the surgeon a more accurate understanding of the surgical complexity, being easier to understand a standard volume assessment obtained from 2D images, in a three-dimensional space.
Patient-doctor communication is very important for patient satisfaction. Since patients have significantly less experience looking at cross-sectional imaging than physicians, a patient-specific 3D model may be even capable to improve patients’ understanding of their disease status and the intended goals of any potential treatments. In aggregate, we found four studies [29,36,46,49] which reported the usefulness of 3D renal models to influence the patient’s understanding of renal anatomy and pathology. Schmit el al. [36] have shown that patients perceived the 3D cryoablation model to be “definitely recommended” (patient’s satisfaction score mean grade > 9 on a 0–10 point scale) for understanding their renal tumor anatomy. Moreover, they reported that the 3D printed models can improve patient-physician communication during the preprocedural consultation compared to review of the 2D imaging alone. In their study, Silberstein et al. [46] stated that patients and families verbally expressed their improved understanding of their condition and treatment options. Also, Bernhard et al. [49] showed that by using a personal 3D kidney model, patient’s understanding was significantly improved on basic kidney physiology by 16.7%, kidney anatomy by 50 %, tumor characteristics by 39.3 % and the planned surgical procedure by 44.6%, with an overall improvement of 37.6%. Teishima et al. [29] assessed the usefulness of a 3D model for the preoperative education among patients and their families, prior to partial nephrectomy, especially for elderly people. They reported that people of an older age group (65 years or older) had more difficulty understanding morphologic information about anatomy and tumors using just 2D CT scan imaging compared with those with lower age (younger patients). They also showed that the 3D model enabled them to overcome the difficulties of their understanding.
3D bioprinting technology is a new technology that has developed rapidly in recent years and has gained popularity in the creation of renal tumor models being used in preoperative surgical planning, resident education and patient information [63]. This type of technology is a step forward to meet the expectations of patients and surgeons but has some major disadvantages in terms of costs and production time [21,46,63].
There are some limitations to this review. The main limitation of this review is the inability to critically assess the strengths and biases of the chosen articles. Most of the studies included in this review evaluate the usefulness of 3D models from a quantitative perspective or mixed method analysis (a combination of qualitative and quantitative analysis). Second, this review shows that most studies did not provide information on the time spent on image processing and segmentation, most likely due to the use of various software tools for image-processing and segmentation by different research groups. The third type of limitation is the lack of technical standardization (file formats, software used and accessibility of the technology) and various cost estimates. Despite these limitations, 3D VR technology is widely accepted, some surgeons reporting a cognitive benefit that would be difficult to quantify.
Conclusion
In conclusion, this systemic review analyses thirty-seven studies reporting the clinical value and usefulness of 3D kidney models in renal tumor, with findings showing their feasibility and accuracy in delimiting both anatomical renal structures and renal tumors. Compared with the traditional 2D imaging, a 3D VR model is more intuitive, capable of 3D representation, and easy to understand. Successful integration of the 3D imaging into real-time operating technique can help urologists transfer the simulation of the technique into successful surgical performance, impacting patient outcomes and possibly reducing complications. This review also reports that 3D VR models are able to improve the patient’s understanding of their renal disease status and improve medical students’ and surgeons’ knowledge of the renal malignancy compared to viewing 2D images alone.
References
- 1.Timonen T, Iso-Mustajärvi M, Linder P, Lehtimäki A, Löppönen H, Elomaa AP, et al. Virtual reality improves the accuracy of simulated preoperative planning in temporal bones: a feasibility and validation study. Eur Arch Otorhinolaryngol. 2021 Aug;278(8):2795–2806. doi: 10.1007/s00405-020-06360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutherland J, Belec J, Sheikh A, Chepelev L, Althobaity W, Chow BJW, et al. Applying Modern Virtual and Augmented Reality Technologies to Medical Images and Models. J Digit Imaging. 2019;32:38–53. doi: 10.1007/s10278-018-0122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esperto F, Prata F, Autrán-Gómez AM, Rivas JG, Socarras M, Marchioni M, et al. New Technologies for Kidney Surgery Planning 3D, Impression, Augmented Reality 3D, Reconstruction: Current Realities and Expectations. Curr Urol Rep. 2021;22:35. doi: 10.1007/s11934-021-01052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lupulescu C, Sun Z. A Systematic Review of the Clinical Value and Applications of Three-Dimensional Printing in Renal Surgery. J Clin Med. 2019;8:990. doi: 10.3390/jcm8070990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Z. Insights into 3D printing in medical applications. Quant Imaging Med Surg. 2019;9:1–5. doi: 10.21037/qims.2019.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikken JJ, Krestin GP. MRI of the kidney-state of the art. Eur Radiol. 2007;17:2780–2793. doi: 10.1007/s00330-007-0701-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JH, Sun HY, Hwang J, Hong SS, Cho YJ, Doo SW, et al. Diagnostic accuracy of contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging of small renal masses in real practice: sensitivity and specificity according to subjective radiologic interpretation. World J Surg Oncol. 2016;14:260. doi: 10.1186/s12957-016-1017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edmund JM, Nyholm T. A review of substitute CT generation for MRI-only radiation therapy. Radiat Oncol. 2017;12:28. doi: 10.1186/s13014-016-0747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatar İ, Huri E, Selçuk İ, Moon YL, Paoluzzi A, Skolarikos A. Review of the effect of 3D medical printing and virtual reality on urology training with ‘MedTRain3DModsim’ Erasmus + European Union Project. Turk J Med Sci. 2019;49:1257–1270. doi: 10.3906/sag-1905-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potretzke AM, Weaver J, Benway BM. Review of robot-assisted partial nephrectomy in modern practice. J Kidney Cancer VHL. 2015;2:30–44. doi: 10.15586/jkcvhl.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagenaar S, Nederhoed JH, Hoksbergen AWJ, Jaap Bonjer HJ, Wisselink W, van Ramshorst GH. Minimally Invasive, Laparoscopic, and Robotic-assisted Techniques Versus Open Techniques for Kidney Transplant Recipients: A Systematic Review. Eur Urol. 2017;72:205–217. doi: 10.1016/j.eururo.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Mohr D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivero Belenchón I, Congregado Ruíz CB, Gómez Ciriza G, Gómez Dos Santos V, Rivas González JA, Gálvez García C, et al. How to obtain a 3D printed model of renal cell carcinoma (RCC) with venous tumor thrombus extension (VTE) for surgical simulation (phase I NCT03738488) Updates Surg. 2020;72:1237–1246. doi: 10.1007/s13304-020-00806-6. [DOI] [PubMed] [Google Scholar]
- 14.Maddox MM, Feibus A, Liu J, Wang J, Thomas R, Silberstein JL. 3D-printed soft-tissue physical models of renal malignancies for individualized surgical simulation: a feasibility study. J Robot Surg. 2018;12:27–33. doi: 10.1007/s11701-017-0680-6. [DOI] [PubMed] [Google Scholar]
- 15.Dwivedi DK, Chatzinoff Y, Zhang Y, Yuan Q, Fulkerson M, Chopra R, et al. Development of a Patient-specific Tumor Mold Using Magnetic Resonance Imaging and 3-Dimensional Printing Technology for Targeted Tissue Procurement and Radiomics Analysis of Renal Masses. Urology. 2018;112:209–214. doi: 10.1016/j.urology.2017.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Lu Y, Wu G, Wang T, Wang Y, Zhao H, et al. The role of three-dimensional reconstruction in laparoscopic partial nephrectomy for complex renal tumors. World J Surg Oncol. 2019;17:159. doi: 10.1186/s12957-019-1701-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komai Y, Sakai Y, Gotohda N, Kobayashi T, Kawakami S, Saito N. A novel 3-dimensional image analysis system for case-specific kidney anatomy and surgical simulation to facilitate clampless partial nephrectomy. Urology. 2014;83:500–506. doi: 10.1016/j.urology.2013.09.053. [DOI] [PubMed] [Google Scholar]
- 18.Kyung YS, Kim N, Jeong IG, Hong JH, Kim CS. Application of 3-D Printed Kidney Model in Partial Nephrectomy for Predicting Surgical Outcomes: A Feasibility Study. Clin Genitourin Cancer. 2019;17:e878–e884. doi: 10.1016/j.clgc.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Durso TA, Carnell J, Turk TT, Gupta GN. Three-dimensional reconstruction volume: a novel method for volume measurement in kidney cancer. J Endourol. 2014;28:745–750. doi: 10.1089/end.2013.0796. [DOI] [PubMed] [Google Scholar]
- 20.Huang Q, Gu L, Zhu J, Peng C, Du S, Liu Q, et al. A three-dimensional, anatomy-based nephrometry score to guide nephron-sparing surgery for renal sinus tumors. Cancer. 2020;126(Suppl 9):2062–2072. doi: 10.1002/cncr.32748. [DOI] [PubMed] [Google Scholar]
- 21.Porpiglia F, Amparore D, Checcucci E, Autorino R, Manfredi M, Iannizzi G, et al. Current Use of Three-dimensional Model Technology in Urology: A Road Map for Personalised Surgical Planning. Eur Urol Focus. 2018;4:652–656. doi: 10.1016/j.euf.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Porpiglia F, Amparore D, Checcucci E, Manfredi M, Stura I, Migliaretti G, et al. Three-dimensional virtual imaging of renal tumours: a new tool to improve the accuracy of nephrometry scores. BJU Int. 2019;124:945–954. doi: 10.1111/bju.14894. [DOI] [PubMed] [Google Scholar]
- 23.Fan G, Li J, Li M, Ye M, Pei X, Li F, et al. Three-Dimensional Physical Model-Assisted Planning and Navigation for Laparoscopic Partial Nephrectomy in Patients with Endophytic Renal Tumors. Sci Rep. 2018;12(8):582. doi: 10.1038/s41598-017-19056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komai Y, Sugimoto M, Gotohda N, Matsubara N, Kobayashi T, Sakai Y, et al. Patient-specific 3-dimensional Printed Kidney Designed for “4D” Surgical Navigation: A Novel Aid to Facilitate Minimally Invasive Off-clamp Partial Nephrectomy in Complex Tumor Cases. Urology. 2016;91:226–233. doi: 10.1016/j.urology.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 25.Lasser MS, Doscher M, Keehn A, Chernyak V, Garfein E, Ghavamian R. Virtual surgical planning: a novel aid to robot-assisted laparoscopic partial nephrectomy. J Endourol. 2012;26:1372–1379. doi: 10.1089/end.2012.0093. [DOI] [PubMed] [Google Scholar]
- 26.Leslie S, Gill IS, de Castro Abreu AL, Rahmanuddin S, Gill KS, Nguyen M, et al. Renal tumor contact surface area: a novel parameter for predicting complexity and outcomes of partial nephrectomy. Eur Urol. 2014;66:884–893. doi: 10.1016/j.eururo.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Qi L, Yuan P, Zu X, Chen W, Cao Z, et al. Application of Three-Dimensional Visualization Technology in Laparoscopic Partial Nephrectomy of Renal Tumor: A Comparative Study. J Laparoendosc Adv Surg Tech A. 2017;27:516–523. doi: 10.1089/lap.2016.0645. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Liu J, Wang S, Zhao H, Tian C, Shi B, et al. Three-dimensional nephrometry scoring system: a precise scoring system to evaluate complexity of renal tumors suitable for partial nephrectomy. PeerJ. 2020;8:e8637. doi: 10.7717/peerj.8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teishima J, Takayama Y, Iwaguro S, Hayashi T, Inoue S, Hieda K, et al. Usefulness of personalized three-dimensional printed model on the satisfaction of preoperative education for patients undergoing robot-assisted partial nephrectomy and their families. Int Urol Nephrol. 2018;50:1061–1066. doi: 10.1007/s11255-018-1881-2. [DOI] [PubMed] [Google Scholar]
- 30.Golab A, Smektala T, Kaczmarek K, Stamirowski R, Hrab M, Slojewski M. Laparoscopic Partial Nephrectomy Supported by Training Involving Personalized Silicone Replica Poured in Three-Dimensional Printed Casting Mold. J Laparoendosc Adv Surg Tech A. 2017;27:420–422. doi: 10.1089/lap.2016.0596. [DOI] [PubMed] [Google Scholar]
- 31.Mitsui Y, Sadahira T, Araki M, Maruyama Y, Nishimura S, Wada K, et al. The 3-D Volumetric Measurement Including Resected Specimen for Predicting Renal Function AfterRobot-assisted Partial Nephrectomy. Urology. 2019;125:104–110. doi: 10.1016/j.urology.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Meyer A, Woldu SL, Weinberg AC, Thoreson GR, Pierorazio P, Matulay JT, et al. Predicting Renal Parenchymal Loss after Nephron Sparing Surgery. J Urol. 2015;194:658–663. doi: 10.1016/j.juro.2015.03.098. [DOI] [PubMed] [Google Scholar]
- 33.Tobert CM, Takagi T, Liss MA, Lee H, Derweesh IH, Campbell SC, et al. Multicenter Validation of Surgeon Assessment of Renal Preservation in Comparison to Measurement With 3D Image Analysis. Urology. 2015;86:534–538. doi: 10.1016/j.urology.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Alyaev YG, Sirota ES, Bezrukov EA, Fiev DN, Bukatov MD, Letunovskii AV, et al. [Application of 3D soft print models of the kidney for treatment of patients with localized cancer of the kidney (a pilot study)]. Urologiia. 2017;(6):12–19. [PubMed] [Google Scholar]
- 35.Porpiglia F, Fiori C, Checcucci E, Amparore D, Bertolo R. Hyperaccuracy Three-dimensional Reconstruction Is Able to Maximize the Efficacy of Selective Clamping During Robot-assisted Partial Nephrectomy for Complex Renal Masses. Eur Urol. 2018;74:651–660. doi: 10.1016/j.eururo.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 36.Schmit C, Matsumoto J, Yost K, Alexander A, Ness L, Kurup AN, et al. Impact of a 3D printed model on patients’ understanding of renal cryoablation: a prospective pilot study. Abdom Radiol (NY) 2019;44:304–309. doi: 10.1007/s00261-018-1710-1. [DOI] [PubMed] [Google Scholar]
- 37.Wake N, Rude T, Kang SK, Stifelman MD, Borin JF, Sodickson DK, et al. 3D printed renal cancer models derived from MRI data: application in pre-surgical planning. Abdom Radiol (NY) 2017;42:1501–1509. doi: 10.1007/s00261-016-1022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyde ER, Berger LU, Ramachandran N, Hughes-Hallett A, Pavithran NP, Tran MGB, et al. Interactive virtual 3D models of renal cancer patient anatomies alter partial nephrectomy surgical planning decisions and increase surgeon confidence compared to volume-rendered images. Int J Comput Assist Radiol Surg. 2019;14:723–732. doi: 10.1007/s11548-019-01913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rai A, Scovell JM, Xu A, Balasubramanian A, Siller R, Kohn T, et al. Patient-specific Virtual Simulation-A State of the Art Approach to Teach Renal Tumor Localization. Urology. 2018;120:42–48. doi: 10.1016/j.urology.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 40.Fan G, Meng Y, Zhu S, Ye M, Li M, Li F, et al. Three-dimensional printing for laparoscopic partial nephrectomy in patients with renal tumors. J Int Med Res. 2019;47:4324–4332. doi: 10.1177/0300060519862058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Ge HW, Li NC, Yu CF, Guo HF, Jin SH, et al. Evaluation of three-dimensional printing for laparoscopic partial nephrectomy of renal tumors: a preliminary report. World J Urol. 2016;34:533–537. doi: 10.1007/s00345-015-1530-7. [DOI] [PubMed] [Google Scholar]
- 42.Wake N, Wysock JS, Bjurlin MA, Chandarana H, Huang WC. “Pin the Tumor on the Kidney:” An Evaluation of How Surgeons Translate CT and MRI Data to 3D Models. Urology. 2019;131:255–261. doi: 10.1016/j.urology.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michiels C, Jambon E, Bernhard JC. Measurement of the Accuracy of 3D-Printed Medical Models to Be Used for Robot-Assisted Partial Nephrectomy. AJR Am J Roentgenol. 2019;213:626–631. doi: 10.2214/AJR.18.21048. [DOI] [PubMed] [Google Scholar]
- 44.Tapiero S, Karani R, Limfueco L, Xie L, Jefferson FA, Reinwart C, et al. Evaluation of Interactive Virtual Reality as a Preoperative Aid in Localizing Renal Tumors. J Endourol. 2020;34:1180–1187. doi: 10.1089/end.2020.0234. [DOI] [PubMed] [Google Scholar]
- 45.Knoedler M, Feibus AH, Lange A, Maddox MM, Ledet E, Thomas R, et al. Individualized Physical 3-dimensional Kidney Tumor Models Constructed From 3-dimensional Printers Result in Improved Trainee Anatomic Understanding. Urology. 2015;85:1257–1261. doi: 10.1016/j.urology.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 46.Silberstein JL, Maddox MM, Dorsey P, Feibus A, Thomas R, Lee BR. Physical models of renal malignancies using standard cross-sectional imaging and 3-dimensional printers: a pilot study. Urology. 2014;84:268–272. doi: 10.1016/j.urology.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 47.Glybochko PV, Rapoport LM, Alyaev YG, Sirota ES, Bezrukov EA, Fiev DN, et al. Multiple application of three-dimensional soft kidney models with localized kidney cancer: A pilot study. Urologia. 2018;85:99–105. doi: 10.1177/0391560317749405. [DOI] [PubMed] [Google Scholar]
- 48.Lee H, Nguyen NH, Hwang SI, Lee HJ, Hong SK, Byun SS. Personalized 3D kidney model produced by rapid prototyping method and its usefulness in clinical applications. Int Braz J Urol. 2018;44:952–957. doi: 10.1590/S1677-5538.IBJU.2018.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernhard JC, Isotani S, Matsugasumi T, Duddalwar V, Hung AJ, et al. Personalized 3D printed model of kidney and tumor anatomy: a useful tool for patient education. World J Urol. 2016;34:337–345. doi: 10.1007/s00345-015-1632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park DS, Hong YK, Lee SR, Hwang JH, Kang MH, Oh JJ. Three-dimensional reconstructive kidney volume analyses according to the endophytic degree of tumors during open partial or radical nephrectomy. Int Braz J Urol. 2016;42:37–46. doi: 10.1590/S1677-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Oostenbrugge TJ, Fütterer JJ, Mulders PFA. Diagnostic Imaging for Solid Renal Tumors: A Pictorial Review. Kidney Cancer. 2018;2:79–93. doi: 10.3233/KCA-180028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otton JM, Birbara NS, Hussain T, Greil G, Foley TA, Pather N. 3D printing from cardiovascular CT: a practical guide and review. Cardiovasc Diagn Ther. 2017;7:507–526. doi: 10.21037/cdt.2017.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun Z, Liu D. A systematic review of clinical value of three-dimensional printing in renal disease. Quant Imaging Med Surg. 2018;8:311–325. doi: 10.21037/qims.2018.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wake N, Alexander AE, Christensen AM, Liacouras PC, Schickel M, Pietila T, et al. Creating patient-specific anatomical models for 3D printing and AR/VR: a supplement for the 2018 Radiological Society of North America (RSNA) hands-on course. 3D Print Med. 2019;5:17. doi: 10.1186/s41205-019-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z, Wang E, Zhu Y. Image segmentation evaluation: a survey of methods. Artif Intell Rev. 2020;53:5637–5674. [Google Scholar]
- 56.Virzì A, Muller CO, Marret JB, Mille E, Berteloot L, Grévent D, et al. Comprehensive Review of 3D Segmentation Software Tools for MRI Usable for Pelvic Surgery Planning. J Digit Imaging. 2020;33:99–110. doi: 10.1007/s10278-019-00239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gill IS, Eisenberg MS, Aron M, Berger A, Ukimura O, Patil MB, et al. “Zero ischemia” partial nephrectomy: novel laparoscopic and robotic technique. Eur Urol. 2011;59:128–134. doi: 10.1016/j.eururo.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Bertolo R, Autorino R, Fiori C, Amparore D, Checcucci E, Mottrie A, et al. Expanding the Indications of Robotic Partial Nephrectomy for Highly Complex Renal Tumors: Urologists’ Perception of the Impact of Hyperaccuracy Three-Dimensional Reconstruction. J Laparoendosc Adv Surg Tech A. 2019;29:233–239. doi: 10.1089/lap.2018.0486. [DOI] [PubMed] [Google Scholar]
- 59.Hwang DH, Ma K, Yepes F, Nadamuni M, Nayyar M, Liu B, et al. Multidimensional Interactive Radiology Report and Analysis: Standardization of workflow and reporting for renal mass tracking and quantification. Proc SPIE Int Soc Opt Eng. 2015;9681:96810C. doi: 10.1117/12.2211526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corradi R, Kabra A, Suarez M, Oppenheimer J, Okhunov Z, White H, et al. Validation of 3D volumetric-based renal function prediction calculator for nephron sparing surgery. Int Urol Nephrol. 2017;49:615–621. doi: 10.1007/s11255-017-1525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin WC, Chang CH, Chang YH, Lin CH. Three-dimensional Reconstruction of Renal Vascular Tumor Anatomy to facilitate accurate preoperative planning of partial nephrectomy. Biomedicine (Taipei) 2020;10:36–41. doi: 10.37796/2211-8039.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ukimura O, Nakamoto M, Gill IS. Three-dimensional reconstruction of renovascular-tumor anatomy to facilitate zero-ischemia partial nephrectomy. Eur Urol. 2012;61:211–217. doi: 10.1016/j.eururo.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 63.Ahmadi H, Liu JJ. 3-D Imaging and Simulation for Nephron Sparing Surgical Training. Curr Urol Rep. 2016;17:58. doi: 10.1007/s11934-016-0614-2. [DOI] [PubMed] [Google Scholar]