Abstract
Background
The purpose of this study was to understand how the COVID-19 pandemic has affected health care patterns and outcomes for patients diagnosed with metastatic pancreatic ductal adenocarcinoma (mPDAC) in 2020 compared with those diagnosed with mPDAC in 2019.
Patients and Methods
We used the Flatiron Health database to identify adults diagnosed with mPDAC from March 1 to September 30, 2019 (pre-COVID-19 cohort) and March 1 to September 30, 2020 (post-COVID-19 cohort). Between-cohort comparisons included demographic and clinical characteristics and year-over-year data for diagnosis of mPDAC, newly treated patients, time to and types of first-line therapy, and adverse events (AEs) during first-line therapy. Overall survival (OS) and milestone survival rates were evaluated. Kaplan-Meier methods were used to assess OS.
Results
Pre-COVID-19 (n = 923) and post-COVID-19 (n = 796) cohorts had similar baseline demographic characteristics. A smaller proportion of patients in the pre-COVID-19 cohort were initially diagnosed with stage IV disease versus the post-COVID-19 cohort (62.2% vs 69.7%). Between 2019 and 2020, there was a 13.8% decrease in diagnosis of mPDAC and a 13.0% decrease in newly treated patients. Median (interquartile range) times to first-line treatment were similar (21 [13-40] and 19 [12-32] days). Median OS (months) was significantly longer in the pre-COVID-19 cohort (8·4 [95% CI: 7·5, 9·0]) versus the post-COVID-19 cohort (6·1 [95% CI: 5·4, 6·9]; P < .001). Survival rates were higher in the pre-COVID-19 versus post-COVID-19 cohorts.
Conclusions
During the pandemic, patients were initially diagnosed with PDAC at more advanced stages. While patients in both cohorts appeared to receive similar care, survival outcomes were adversely affected.
Keywords: COVID-19, metastatic pancreatic ductal adenocarcinomam, treatment patterns, overall survival
The negative impact of COVID-19 on patients at risk of or being treated for life-threatening diseases, including cancer, has been profound. This article focuses on the diagnosis of metastatic pancreatic ductal adenocarcinoma, reporting a relatively larger number of patients diagnosed with advanced-stage disease at initial presentation.
Implications for Practice.
During the COVID-19 pandemic, the diagnosis of metastatic pancreatic ductal adenocarcinoma (mPDAC) was affected, as a relatively larger number of patients were diagnosed with advanced-stage disease at initial presentation. These findings from a large contemporary database suggest while patients in the pre- and post-COVID-19 cohorts received similar levels of care, survival outcomes were adversely affected for the post-COVID-19 cohort. This study provides the first real-world evidence of treatment patterns and outcomes for patients with stage IV pancreatic cancer in the community oncology setting during the COVID-19 pandemic. Further research is warranted to characterize the impact of the COVID-19 pandemic on cancer care and outcomes.
Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It was first identified in December 2019 in Wuhan, China. In the US, the first case of COVID-19 was confirmed on January 21, 2020. The World Health Organization declared the outbreak as a pandemic on March 11, 2020. As of March 25, 2021, there have been ~30 million confirmed COVID-19 cases and ~540 000 COVID-19-related deaths.1 While the pandemic led to abrupt disruptions in the provision of health care for all patients across the country, the negative impact of COVID-19 on patients at risk of or being treated for other life-threatening diseases, including cancer, has been profound.
Recent research has shown cancer patients are at increased risk for COVID-19 infection,2 more frequently experience severe disease,3,4 and worse outcomes, including death.5 These increased risks are associated with institutional restrictions on hospital visits, stay-at-home orders for patients, and the reallocation of most of the health care workforce toward treating patients with COVID-19. In addition, there has been some patient hesitancy to schedule a screening or clinic visit owing to the associated risk of exposure to the virus.6 As a result, patients with cancer have not been receiving necessary treatments. Even cancer screening programs have been delayed, leading to cancer diagnoses at later stages of disease.7,8
Although pancreatic cancer accounts for ~3% of all cancer types in the US, it is the third leading cause of cancer-related deaths in the country after lung and colon cancer.9,10 In 2021, an estimated 60 430 Americans will be diagnosed with pancreatic cancer, and ~48 000 will die from the disease.9,10 To date, little is known about how the pandemic has affected the provision of health care to patients with de novo metastatic pancreatic ductal adenocarcinoma (mPDAC) and whether any of these changes also have affected treatment outcomes among these patients. The purpose of this study was to characterize the impact of the COVID-19 pandemic on health care utilization and outcomes among mPDAC patients in the US-based community oncology setting. The primary objective was to understand how COVID-19 has affected health care patterns and outcomes for patients newly diagnosed with mPDAC in 2020 compared with those diagnosed with mPDAC in 2019.
Materials and Methods
Study Design and Data Source
This was a retrospective cohort study of adult patients diagnosed with mPDAC between March 1, 2019 and November 30, 2020. We used the Flatiron Health database, a longitudinal, demographically and geographically diverse database derived from electronic health record (EHRs). The database contains information from >280 cancer clinics (~800 sites of care), representing >2.2 million active US-based patients with cancer available for analysis. Patient-level EHRs include structured (eg, laboratory values, medication prescriptions) and unstructured data (eg, physicians’ notes, biomarker reports), which were curated using technology-enabled abstraction. The majority of patients in the database originate from community-based oncology settings. Data provided to third parties are deidentified and provisions are in place to prevent re-identification to protect patient confidentiality. The study protocol received Institutional Review Board approval and included a waiver of informed consent. All authors had access to the study data and reviewed and approved the final manuscript.
Patient Population
The pre-COVID-19 cohort included those diagnosed with mPDAC between March 1 and September 30, 2019. The post-COVID-19 cohort included those diagnosed with mPDAC between March 1 and September 30, 2020. The identification periods were chosen to cover the same months in 2019 and 2020 and allow for follow-up of the post-COVID-19 cohort. For both cohorts, patients had to be aged ≥18 years on the date of the mPDAC diagnosis and had an mPDAC diagnosis date (index date) before their date of death (after assigning the 15th day of the month to derive the date of death).
Baseline Cohort Characteristics
The following demographic and clinical characteristics were compared between the pre- and post-COVID-19 cohorts: age at metastatic diagnosis, sex, race/ethnicity, geographic region, cancer stage at initial diagnosis, primary tumor location, Eastern Cooperative Oncology Group Performance Status (ECOG PS), previous treatment with first- and second-line therapies, number of clinic visits within 90 days of metastatic diagnosis, and time (days) to first clinic visit within 90 days of metastatic diagnosis.
Clinical Outcomes
Between-cohort comparisons for year-over-year (YoY) data included: number and percentage of patients with a diagnosis of de novo mPDAC, number and percentage of newly treated mPDAC patients, number of clinic visits per patient, time to first-line therapy among treated patients, types of first-line therapy regimens, mean number of days between first-line treatment administrations, and adverse event (AE) rates during first-line therapy. Adverse events were coded using the Common Terminology Criteria for Adverse Events version 4·03.11 Overall survival also was evaluated. For both cohorts, patients with a death event were assigned the 15th day of the month of death as the event date. Patients without a death recorded in the database were censored at their last recorded clinic visit or treatment administration. The same definitions were used to evaluate 60-, 90-, 120-, and 180-day milestone survival. A sensitivity analysis was conducted limiting the follow-up time to November of the year of interest to compare outcomes with equal follow-up time available for both cohorts.
Statistical Analysis
Results for continuous variables are summarized as means with SDs or medians and interquartile ranges (IQRs), depending on the nature of their distributions. Results for categorical variables are summarized using absolute (frequencies) and relative (percentages) terms. Kaplan-Meier methods were used to assess overall and 60-, 90-, 120-, and 180-day milestone survival. Associations between continuous variables were analyzed using the Student’s t test or Wilcoxon rank-sum test after testing for normality; categorical variables were analyzed using Chi-square tests. A P-value of < .05 was considered statistically significant. All statistical analyses were performed using SAS version 9·4 (SAS Institute, Cary, NC) or R package version 4·0·0 (R Core Team, Vienna, Austria).
Results
Patient Cohort Disposition and Characteristics
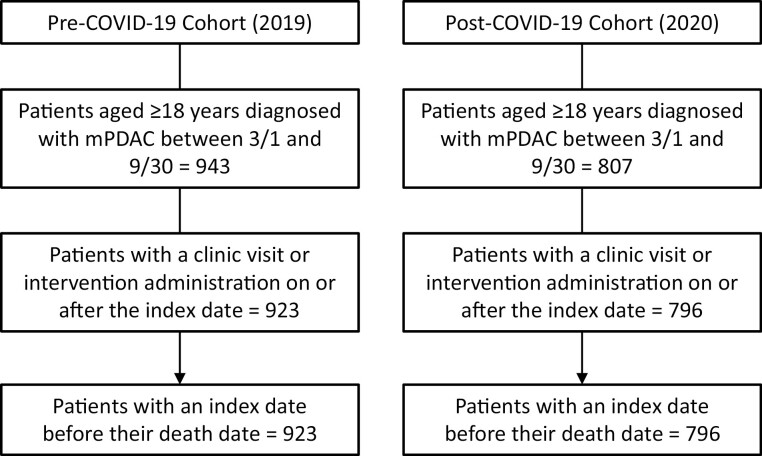
In total, 1750 patients were identified as having a diagnosis of mPDAC during the study periods; 1719 patients met all other study inclusion criteria. Of this total, the pre-COVID-19 cohort accounted for 53·7% (n = 923), and the post-COVID-19 cohort accounted for 46·3% (n = 796) (Fig. 1).
Figure 1.
Patient disposition.
The pre- and post-COVID-19 cohorts had similar baseline characteristics for age at metastatic diagnosis, sex, race, and geographic region (Table 1). While all patients were diagnosed with metastatic or stage IV disease in order to be included in our study cohort, a slightly smaller percentage of patients in the pre-COVID-19 cohort were initially diagnosed with de novo metastatic disease versus the post-COVID-19 cohort (62.2% [575/923] vs 69.7% [555/796]). Similar percentages of patients in the pre- and post-COVID-19 cohorts had ECOG PS of 0 or 1 (48·5% [448/923] and 47·9% [381/796]). Similar percentages of patients in the pre- and post-COVID-19 cohorts received first-line therapy (75·8% [700/923] and 76·5% [609/796]). In the pre- and post-COVID-19 cohorts, 94·5% (872/923) and 98·2% (782/796) had a clinic visit and/or treatment administration within 90 days of their mPDAC diagnosis; the median (IQR) number of days to the first clinic visit or treatment administration was 10 (12-13) in both groups.
Table 1.
Baseline demographic and clinical Characteristics.
| Characteristic | Pre-COVID-19 2019 cohort (N = 923) | Post-COVID-19 2020 cohort (N = 796) |
|---|---|---|
| Age, year | ||
| Mean (SD) | 69 (10) | 69 (10) |
| Median (IQR) | 70 (62,76) | 70 (62,76) |
| Gender, n (%) | ||
| Male | 482 (52.2) | 426 (53.5) |
| Female | 441 (47.8) | 370 (46.5) |
| Race, n (%) | ||
| White | 532 (57.6) | 419 (52.6) |
| Other | 139 (15.1) | 156 (19.6) |
| Black | 81 (8.8) | 66 (8.3) |
| Asian | 18 (2.0) | 19 (2.4) |
| Unknown | 101 (10.9) | 95 (11.9) |
| Hispanic or Latino ethnicity, n (%) | 52 (5.6) | 41 (5.2) |
| Region, n (%) | ||
| South | 393 (42.6) | 343 (43.1) |
| Northeast | 128 (13.9) | 105 (13.2) |
| West | 125 (13.5) | 125 (15.7) |
| Midwest | 93 (10.1) | 84 (10.6) |
| Unknown (academic center-blinded) | 184 (19.9) | 139 (17.5) |
| Stage at initial diagnosis, n (%) | ||
| Stage I | 53 (5.7) | 60 (7.5) |
| Stage II | 151 (16.4) | 79 (9.9) |
| Stage III | 79 (8.6) | 63 (7.9) |
| Stage IV | 575 (62.3) | 555 (69.7) |
| Unknown | 65 (7.0) | 39 (4.9) |
| Site of primary tumor, n (%) | ||
| Head | 476 (51.6) | 397 (49.9) |
| Tail | 168 (18.2) | 177 (22.2) |
| Body | 163 (17.7) | 130 (16.3) |
| Overlapping sites | 90 (9.8) | 68 (8.5) |
| Pancreas, NOS | 26 (2.8) | 24 (3.0) |
| ECOG PS score, n (%) | ||
| 0 | 191 (20.7) | 152 (19.1) |
| 1 | 257 (27.8) | 229 (28.8) |
| 2+ | 75 (8.1) | 87 (10.9) |
| Missing | 400 (43.3) | 328 (41.2) |
| Received first-line therapy, n (%) | 700 (75.8) | 609 (76.5) |
| Received second-line therapy, n (%) | 263 (28.5) | 109 (13.7) |
| Clinic visit within 90 days of mPDAC diagnosis, n (%) | 872 (94.5) | 782 (98.2) |
| Days to first clinic visit | ||
| Mean (SD) | 10 (12) | 10 (13) |
| Median (IQR) | 7 (2-14) | 7 (2-14) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; IQR, interquartile range; mPDAC, metastatic pancreatic ductal adenocarcinoma.
Outcomes
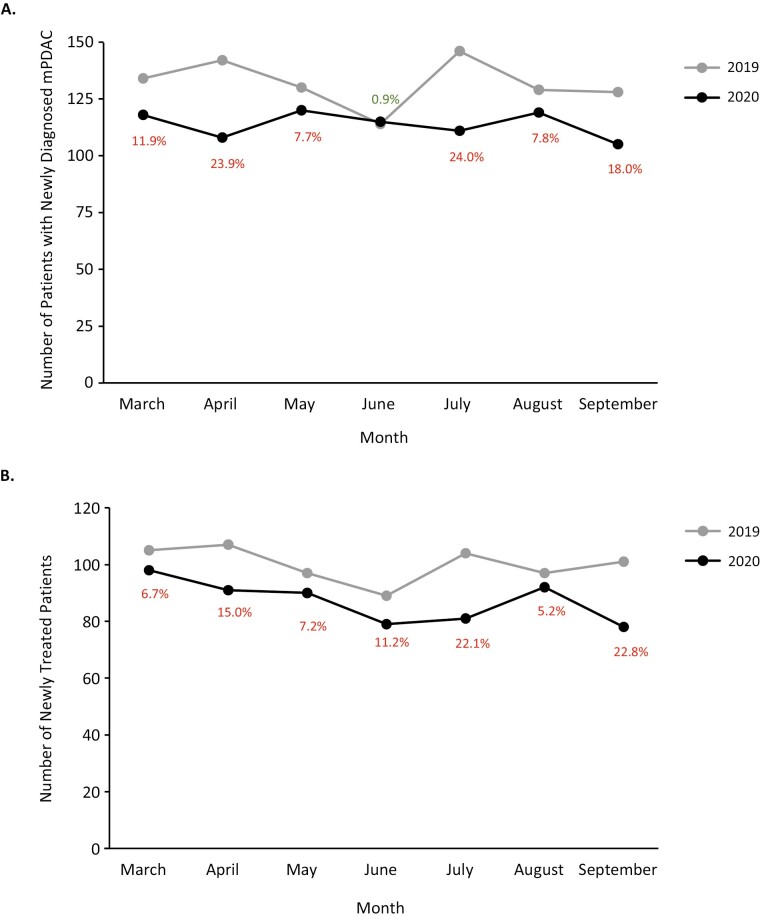
Overall, between 2019 and 2020, there was a 13·8% decrease in the diagnosis of mPDAC. The numbers of patients with newly diagnosed mPDAC by month (March to September) are shown in Fig. 2A. With the exception of the month of June, where the numbers of newly diagnosed patients in the two cohorts were comparable, the percentage reductions in the number of newly diagnosed cases ranged from 7.7%-24.0% for all other months evaluated. Similarly, overall, there was a 13.0% decrease in the number of newly treated patients with mPDAC between 2019 and 2020. The numbers of newly treated patients by month are shown in Fig. 2B. Between March and September, the percentage reductions in the number of newly treated patients ranged from 5.2% to 22.8%.
Figure 2.
Number of patients with newly diagnosed mPDAC (A) and number of newly treated patients with mPDAC (B).
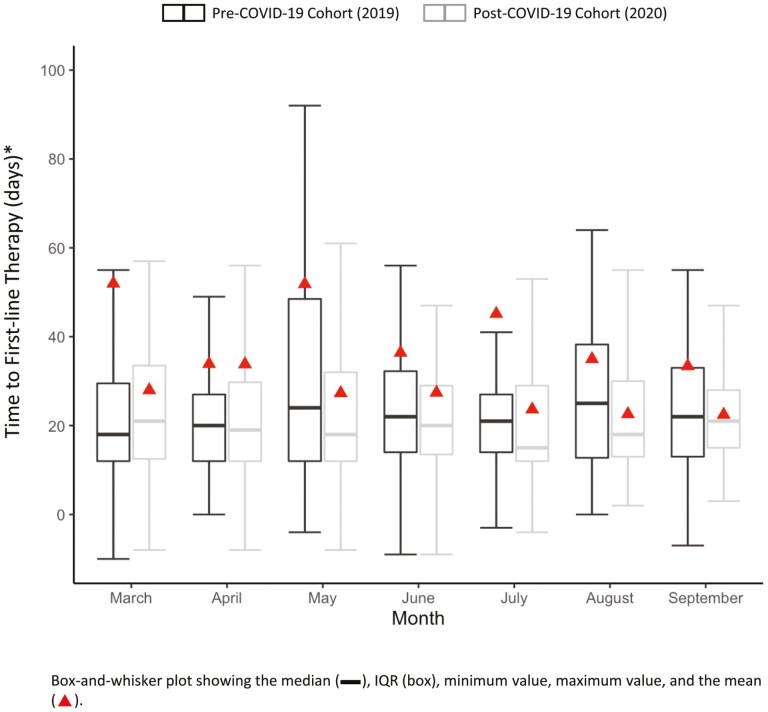
The most common first-line treatment regimen was combination therapy with gemcitabine plus nab-paclitaxel, followed by the combination treatment of leucovorin, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX), and gemcitabine monotherapy (Table 2). Overall, the median (IQR) time to first-line treatment was similar in the pre- versus post-COVID-19 cohorts (21 [13-40] and 19 [12-32] days). Among patients who received first-line therapy, 37.6% of patients in the pre-COVID-19 cohort and 17.9% of patients in the post-COVID-19 cohorts received second-line treatment. In the sensitivity analysis to control for the additional follow-up available to patients diagnosed pre-COVID-19, 16·9% of patients who received first-line therapy in 2019 went on to receive second-line therapy. Mean and median (IQR) times to first-line therapy by month are shown in Fig. 3. In the pre- and post-COVID-19 cohorts the median (IQR) number of visits recorded within 90 days of their mPDAC diagnosis was 8 (IQR: 3-14) and 9 (IQR: 4-14).
Table 2.
Most frequent first-line treatment regimens and time between administrations.
| Regimen | Pre-COVID-19 2019 cohort (n = 700) | Time between Aadministrations, days | Post-COVID-19 2020 cohort (n = 609) | Time between administrations, days |
|---|---|---|---|---|
| n (%) | mean (SD) median (IQR) | n (%) | Mean (SD) Median (IQR) | |
| Gemcitabine + nab-paclitaxel | 263 (37.6) | 12.6 (8.3) 14 (7.0, 14.0) |
249 (40.8) | 11.9 (5.5) 14.0 (7.0, 14.0) |
| FOLFIRINOX | 239 (34.1) | 17.0 (13.3) 14.0 (14.0, 20.0) |
206 (33.8) | 15.7 (4.7) 14.0 (14.0, 14.0) |
| Gemcitabine | 48 (6.8) | 11.4 (5.9) 7.0 (7.0, 14.0) |
36 (5.9) | 12.4 (8.8) 14.0 (7.0, 14.0) |
| FOLFOX | 25 (3.6) | 18.9 (18.2) 14.0 (7.0, 18.3) |
20 (3.3) | 11.5 (7.0) 14.0 (6.0, 14.0) |
| Clinical study drug | 23 (3.3) | 11.9 (13.0) 7.0 (7.0, 14.0) |
20 (3.3) | 13.9 (5.3) 14.0 (10.5, 14.5) |
| Capecitabine | 22 (3.1) | 50.0 (NA) 50.0 (50.0, 50.0) |
13 (2.1) | NA NA |
| Fluorouracil, Irinotecan Liposomal, Leucovorin | 15 (2.1) | 16.6 (9.9) 14.0 (14.0, 18.3) |
12 (2.0) | 18.2 (5.3) 14.0 (14.0, 21.0) |
| Other | 65 (9.3) | 12.2 (6.4) 12.0 (7.0, 14.0) |
53 (8.7) | 14.2 (8.5) 14.0 (7.0, 14.3) |
Abbreviations: FOLFIRINOX, leucovorin, fluorouracil, irinotecan, and oxaliplatin; FOLFOX, leucovorin, fluorouracil, oxaliplatin.
Figure 3.
Time to first-line therapy.
The most common grade 3 or 4 AEs that occurred during first-line therapy in the pre- and post-COVID-19 cohorts were neutropenia (21.3% and 16.7%), elevated liver enzymes (18.7% and 18.1%), and thrombocytopenia (14.6% and 10.7%).
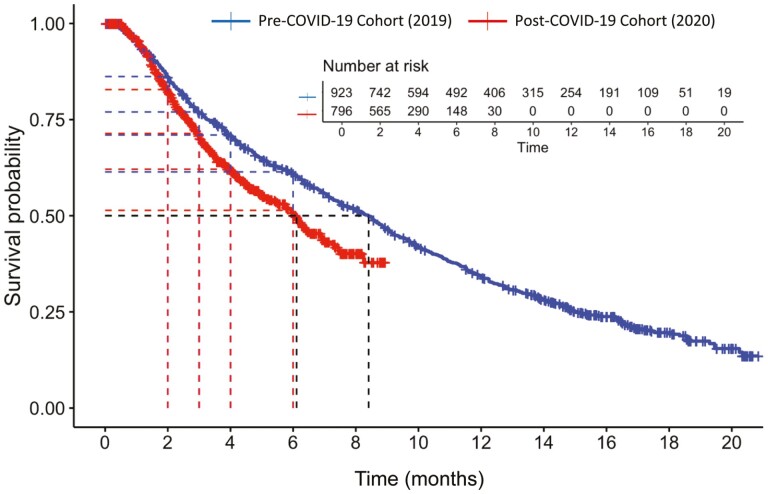
Median OS was significantly longer for patients in the pre-COVID-19 cohort (8·4 [95% CI: 7·5, 9·0] months versus the post-COVID-19 cohort (6.1 [95% CI: 5.4, 6.9]; P < .001). The results from the sensitivity analysis were similar: 8·2 (95% CI: 7·2, NA) versus 6·1 (95% CI: 5·4, 6·9) (P < .001). The YoY change from 2019 to 2020 was −27.4%. At all time points evaluated, survival rates were higher in the pre- versus post-COVID-19 cohorts: 60 days (86.2% vs 82.8%), 90 days (77.0% vs 71.4%), 120 days (71.0% vs 62.1%), and 180 days (61.4% vs 51.4%). The between-group difference in rates increased during this 2- to 6-month time period. Kaplan-Meier curves for OS and for 60-, 90-, 120-, and 180-day survival are presented in Fig. 4.
Figure 4.
Kaplan-Meier curves for OS and 60-, 90-, 120-, and 180-day milestone survival.
Discussion
In this retrospective analysis of data from the Flatiron Health database, we compared the diagnosis of de novo mPDAC and the provision of health care among patients in the pre-COVID-19 cohort (March to September, 2019) and the post-COVID-19 cohort (March to September, 2020). Overall, the YoY percentage decrease in mPDAC diagnoses from 2019 to 2020 was 13·8%, with month-to-month percentage decreases ranging 7.7% to 24.0%. Moreover, a larger percentage of patients in the post- versus pre-COVID-19 cohort was diagnosed with advanced-stage disease at presentation. Overall, the YoY percentage decrease in the number of newly treated patients was 13%, with month-to-month percentage decreases ranging from 5.2% to 22.8%. Overall survival was significantly shorter in the post- versus pre-COVID-19 cohort. After the patients’ first visit, the 2 cohorts appeared to receive similar levels of care, as assessed using the overall number of clinic visits and time to treatment. This was not unexpected, as mPDAC is a serious disease that requires in-person care. The benefit-to-risk ratio for not receiving care is highly skewed toward risk compared with other cancers, especially those detected and treated in early stages.
London et al used the TriNetX platform to analyze 20 health care institutions that have up-to-date patient encounter data and compared a pre-COVID cohort (January to April 2019) with a current cohort (January to April 2020). Interestingly, the group evaluated subgroups of patients, including those with new incidence malignant tumors. For the month of March, there was a 22% reduction in patient encounters for this subgroup from the pre-COVID to current cohorts; for the month of April, during the peak of the pandemic in 2020, patient encounters decreased by 65%.12 Unfortunately, while the study evaluated specific cancer types, pancreatic cancer was not included. We believe that this reduction in encounters may have contributed the reduced number of patients diagnosed with mPDAC during the post-COVID-19 period in our study.
During the past year, numerous studies have published similar findings demonstrating the enormous negative impact of the COVID-19 pandemic on screening, diagnosis, and management of patients with many types of cancer in the US. Using data from a large medical claims database, Patt et al reported significant decreases in cancer screening (56%-85%), health care visits (60%-74%), and treatments (26%-31%) when comparing the same 6-month period (March to July) in 2019 and 2020.13 Kaufman et al conducted a cross-sectional study to compare the number of patients with newly diagnosed cancers (breast, colorectal, esophageal, gastric, lung, pancreatic) during a baseline period (January 6, 2019 to February 29, 2020) and a COVID-19 period (March 1 to April 18, 2020). During the pandemic, the overall weekly number of newly diagnosed cancer patients decreased by 46%; the number of newly diagnosed pancreatic cancer patients decreased by 25%.14
Similar findings have been reported in countries worldwide. Analysis of data from the nationwide Netherlands Cancer registry from February to April 2020 showed a notable decrease in cancer diagnoses compared with data before the pandemic.15 In the UK, cancer screening programs were suspended in early 2020.16 Clark et al reported a significant reduction in the number of registrations for systemic anti-cancer treatments during the pandemic.17 Delays in cancer diagnoses have led to increased cancer-related mortality. Maringe et al conducted a population-based modeling study to estimate the impact of these delays on survival outcomes for breast, colorectal, esophageal, and lung cancer. Compared with pre-pandemic figures, estimated increases in cancer-related deaths ranged from 4.8%-5.3% (esophageal) to 15.3%-16.6% (colorectal).7 In another modeling study evaluating the effect of the 2-week-wait cancer referral pathway in the UK, Sud et al reported a ≤84% decrease in referrals during the COVID-19 lockdown and significant reductions in 10-year survival estimates owing to the backlogs from the referral delays.7 Of note, pancreatic, gastric, and liver cancers only contributed moderately to the estimates, as a large percentage of patients have stage IV disease at presentation.8
Our results should be interpreted in light of some study limitations. The data collected were retrospective and collected for routine clinical care and not for research purposes. Patients who received treatment were subject to non-random allocation. That is, the reason(s) for the patient- and/or physician-based decision to forgo treatment was not available in these data. The care received by patients in the pre-COVID cohort may not be reflective of the longer term patterns of care for patients with mPDAC prior to the COVID-19 pandemic. Finally, mortality-related data were incomplete, and cause of death information was not available; however, our sensitivity analyses were conducted to assess the source of uncertainty.
In conclusion, during the COVID-19 pandemic, the diagnosis of mPDAC appears to have been impacted with a relatively larger number of patients diagnosed with advanced-stage disease at initial presentation. These findings from a large contemporary database suggest while patients in the pre- and post-COVID-19 cohorts received comparable levels of care, their survival outcomes were adversely affected. Further research is warranted to further characterize the impact of the COVID-19 pandemic on cancer care and outcomes.
Acknowledgments
This study was funded by Ipsen. Linda A. Goldstein, PhD, CMPP, from The Write Source MSC, LLC, provided editorial and writing assistance funded by Ipsen in accordance with GPP3.
Contributor Information
Ravi Paluri, Wake Forest Baptist Health, Winston-Salem, NC, USA.
Ashley Laursen, Ipsen, Cambridge, MA, USA.
Joseph Gaeta, Ipsen, Cambridge, MA, USA.
Shu Wang, Genesis Research, Hoboken, NJ, USA.
Andy Surinach, Genesis Research, Hoboken, NJ, USA.
Paul Cockrum, Ipsen, Cambridge, MA, USA.
Conflict of Interest
Ravi Paluri: Ipsen, Exelixis (C/A); Ashley Laursen, Joseph Gaeta, Paul Cockrum: Ipsen (E, OI); Shu Wang, Andy Surinach: Genesis Research (E).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/design: all authors. Provision of study material/patients: P.C. Collection and/or assembly of data: P.C., A.S., S.W. Data analysis and interpretation: all authors. Manuscript writing: all authors. Final approval of manuscript: all authors
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. WHO Coronavirus (COVID-19) Dashboard. March 25, 2021. https://covid19.who.int/ (accessed March 25 2021).
- 2. Jyotsana N, King MR. The impact of COVID-19 on cancer risk and treatment. Cell Mol Bioeng. 2020;13(4):285-291. https://doi.org/10.1007/s12195-020-00630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25(6):e936-e45. https://doi.org/10.1634/theoncologist.2020-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ofori-Asenso R, Ogundipe O, Agyeman AA, et al. Cancer is associated with severe disease in COVID-19 patients: a systematic review and meta-analysis. Ecancermedicalscience. 2020;14:1047. https://doi.org/10.3332/ecancer.2020.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang H, Han H, He T, et al. Clinical characteristics and outcomes of COVID-19-infected cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2021;113(4):371-380. https://doi.org/10.1093/jnci/djaa168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nodora JN, Gupta S, Howard N, et al. The COVID-19 pandemic: identifying adaptive solutions for colorectal cancer screening in underserved communities. J Natl Cancer Inst. 2021;113(8):962-968. https://doi.org/10.1093/jnci/djaa117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023-1034. https://doi.org/10.1016/S1470-2045(20)30388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sud A, Torr B, Jones ME, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21(8):1035-1044. https://doi.org/10.1016/S1470-2045(20)30392-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Cancer Society. Pancreatic cancer. 2021. https://www.cancer.org/cancer/pancreatic-cancer.html (accessed March 5 2021).
- 10. National Cancer Institute. Pancreatic cancer. 2021. https://seer.cancer.gov/statfacts/html/pancreas.html (accessed March 5 2021).
- 11. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03, June 14, 2010. US Department of Health and Human Services. National Institutes of Health National Cancer Institute. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed November 11 2020). [Google Scholar]
- 12. London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform. 2020;4:657-665. https://doi.org/10.1200/CCI.20.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4:1059-1071. https://doi.org/10.1200/CCI.20.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) Pandemic. JAMA Netw Open. 2020;3(8):e2017267. https://doi.org/10.1001/jamanetworkopen.2020.17267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750-751. https://doi.org/10.1016/S1470-2045(20)30265-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones D, Neal RD, Duffy SRG, Scott SE, Whitaker KL, Brain K. Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol. 2020;21(6):748-750. https://doi.org/10.1016/S1470-2045(20)30242-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clark JJ, Dwyer D, Pinwill N, Clark P, Johnson P, Hackshaw A. The effect of clinical decision making for initiation of systemic anticancer treatments in response to the COVID-19 pandemic in England: a retrospective analysis. Lancet Oncol. 2021;22(1):66-73. https://doi.org/10.1016/S1470-2045(20)30619-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.