Abstract
Background
ADXS31-142 is an attenuated Listeria monocytogenes-based immunotherapy targeting prostate-specific antigen (PSA), being evaluated as monotherapy and combined with pembrolizumab for metastatic castration-resistant prostate cancer (mCRPC).
Patients and Methods
The 2-part phase I/II KEYNOTE-046 study enrolled men with mCRPC who have progressed after 2 or fewer prior systemic treatment regimens in the metastatic setting. In Part A, intravenous ADXS31-142 monotherapy was given every 3 weeks (q3w) to 3 dose-escalation cohorts. In Part B, ADXS31-142 (1 × 109 colony-forming units) plus pembrolizumab (200 mg) was administered intravenously q3w for 3 doses with a fourth pembrolizumab dose 3 weeks later (12-week cycles) for up to 24 months or until progression/toxicity. Endpoints included safety, overall response rate, progression-free survival (PFS), overall survival (OS), and immunogenicity.
Results
Fifty patients received ADXS31-142 alone (n = 13) or with pembrolizumab (n = 37). Among the 37 RECIST-evaluable patients (n = 8 Part A; n = 29 Part B), there were no objective responses. Median PFS was 2.2 months (95% CI: 0.8-7.4) with monotherapy and 5.4 months (95% CI: 2.3-7.9) with the combination; median OS was 7.8 months (95% CI: 4.4-18.5) and 33.7 months (95% CI: 15.4–not evaluable), respectively. Promising OS benefit was observed in combination-treated patients who had received prior docetaxel (16.0 months, 95% CI: 6.4-34.6; n = 20) and those with visceral metastasis (16.4 months, 95% CI 4.0-not evaluable; n = 11). All patients had ≥1 treatment-related adverse event, mostly grade 1/2 manageable events. No additive toxicity was observed with combination treatment.
Conclusions
Combining ADXS31-142 with pembrolizumab was safe and well tolerated. The observed OS in mCRPC warrants further testing of this combination.
Clinical Trial registration
Keywords: immunotherapy, metastatic castration-resistant prostate cancer (mCRPC), Lm vectors, pembrolizumab, prostate-specific antigen (PSA)
The phase I/II KEYNOTE-046 trial evaluated the antitumor activity and safety of ADXS31-142 alone or in combination with pembrolizumab in patients with metastatic castration-resistant prostate cancer.
Implications for Practice.
Patients with metastatic prostate cancer initially respond well to androgen-deprivation therapy; however, castration resistance inevitably develops. There are multiple treatment options that confer survival benefits for patients with metastatic castration-resistant prostate cancer (mCRPC); however, these treatments are not curative and are sometimes associated with poor tolerability. Immunotherapy with checkpoint inhibitors (CPIs) alone has been evaluated but has limited activity in mCRPC. Hence, there is a need to evaluate novel therapies that may enhance the activity of CPIs. Preliminary results from this study suggest that the combination of a PSA-specific immunotherapy (ADXS31-142) with the CPI pembrolizumab may improve clinical outcomes in patients with mCRPC and warrants further testing.
Introduction
Prostate cancer is the second most common non-cutaneous malignancy in men worldwide.1 According to estimates by GLOBOCAN, in 2020 there were approximately 1 414 259 new cases of prostate cancer and 375 304 deaths from the disease.1 An estimated 20% of men in the United States diagnosed with prostate cancer have regional or metastatic disease at presentation.2 Furthermore, patients with metastatic disease at diagnosis who are treated with androgen-deprivation therapy often develop a resistant phenotype within 1-3 years of therapy,3 a condition known as metastatic castration-resistant prostate cancer (mCRPC). There are multiple therapeutic options for mCRPC that confer a survival benefit including chemotherapy (eg, docetaxel, cabazitaxel), next-generation hormonal agents (NGHAs; eg, abiraterone, enzalutamide, darolutamide), immunotherapy with sipuleucel-T, and the bone-specific radionuclide radium 223. The humanized anti-programmed cell death-1 (PD-1) antibody pembrolizumab is also approved in a subset of patients with microsatellite instability (MSI) high (MSI-H) or deficient mismatch repair (dMMR) including mCRPC patients. Beyond these, the outlook for patients with mCRPC remains poor, with median overall survival (OS) of 2 years.4 Immunotherapy with checkpoint inhibitors alone has had limited or no activity in mCRPC due to the well-characterized immune-suppressive tumor microenvironment, particularly in those patients with microsatellite stable disease, prior use of chemotherapy and NGHAs, and the presence of visceral metastases, among other factors.5,6 In the KEYNOTE-199 trial, pembrolizumab was evaluated in mCRPC patients who had received one or more NGHAs and 1-2 chemotherapy regimens, one of which was docetaxel. Overall response rates were limited (3%-5%) but there were encouraging survival estimates in patients with measurable disease and in those with bone-predominant mCRPC.5 Median OS was 9.5 months in patients with programmed cell death ligand-1 (PD-L1)- positive disease (n = 133) and 7.9 months in those with PD-L1-negative (n = 66) disease. In bone-predominant disease (n = 59), the mOS was 14.1 months, regardless of PD-L1 expression.
The limited effectiveness of PD-1 treatment in these patients can be attributed to T-cell anergy, low CD8+ T-cell infiltration in the tumor, inability to generate tumor-specific cytotoxic T lymphocytes (CTLs) that do not already exist, and/or the inability for PD-1 blockade to have any impact on regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) that reside within and protect the tumor microenvironment.7 We hypothesized that one strategy to improve efficacy would be to combine PD-1 blockade with a treatment that both ensures tumor-reactive T cells are generated and present and also reduces or eliminates the effects of central tolerance mediated by Tregs and MDSCs in the tumor microenvironment.
ADXS31-142 is a live attenuated Listeria monocytogenes (Lm) immunotherapy bioengineered to secrete an antigen adjuvant fusion protein (tLLO-PSA) consisting of a truncated fragment of the listeriolysin toxin (tLLO) fused to human prostate-specific antigen (PSA). Upon intravenous (IV) administration, ADXS31-142 is rapidly taken up by antigen-presenting cells, stimulating adaptive immunity and resulting in the generation of a new population of tumor antigen-specific CTLs. In addition, ADXS31-142 has been shown to alter the tumor microenvironment, facilitate T-cell infiltration, and reduce immune suppression mediated by Tregs and MDSCs.8 Furthermore, synergistic activity of the combination of Lm-based immunotherapies with PD-1-blocking antibodies has been shown in animal models and in clinical evaluations.9-11
The phase I/II KEYNOTE-046 trial evaluated the antitumor activity and safety of ADXS31142 monotherapy or in combination with pembrolizumab in patients with mCRPC.
Methods
Study Design and Treatment
KEYNOTE-046 (NCT02325557) was a phase I/II, open-label, multicenter (8 sites), 2-part, dose-determining, safety and tolerability study of ADXS31142 administered as monotherapy or in combination with pembrolizumab. An expansion cohort further evaluated the safety and antitumor activity of combination treatment. Eligible patients were men aged ≥18 years with histologically confirmed mCRPC on androgen-deprivation therapy that had progressed or become resistant to ≤2 prior systemic treatment regimens comprising chemotherapy, hormonal (including NGHAs, abiraterone and/or enzalutamide), radiopharmaceuticals, or immunotherapy in the metastatic setting. Patients were enrolled regardless of the PD-L1 expression status of their tumors.
Patients in Part A were administered ADXS31142 IV at doses of 1 × 109 colony-forming units (CFUs), 5 × 109 CFUs, or 1 × 1010 CFUs every 3 weeks in a 12-week cycle for up to 24 months or until disease progression or discontinuation. Part A was designed to select a recommended phase II dose (RP2D) of ADXS31142 for use in Part B. Patients in Part B were administered ADXS31142 at the RP2D (1 × 109 CFUs) in combination with pembrolizumab 200 mg IV every 3 weeks for 3 doses with a fourth pembrolizumab dose given alone 3 weeks later (in 12-week cycles), for up to 24 months or until disease progression or discontinuation.
The study protocol and all amendments were approved by institutional review boards or ethics committees of all participating sites, and the study was conducted in accordance with the International Council for Harmonisation for Good Clinical Practice guidelines and the Declaration of Helsinki. All enrolled patients provided written informed consent.
Procedures
Tumor response was assessed through computed tomography or magnetic resonance imaging of the abdomen/pelvis and bone scan. Scans were obtained every 10 weeks (±1 week) during the first cycle and every 12 weeks (±1 week) thereafter during treatment. Serum levels of PSA were measured at Week 1 of Cycle 1 and then every 4 weeks. During Cycle 1, blood samples for T-cell testing were collected before ADXS31-142 infusion and 11-14 days (2 weeks) after every ADXS31-142 infusion (Weeks 1, 4, and 7). In addition, retrospective testing was performed to determine the MSI status. Adverse events (AEs) were monitored throughout the study and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0).
Immune Correlative Assays
Adaptive’s proprietary T-cell receptor (TCR) beta-chain sequencing was performed to evaluate changes in clonality and diversity of T cells.12 Sequencing analysis included identification and quantification of all clones in a given sample, including: (1) number of unique clones and frequency of each clone; (2) identification and comparison of common clones in all samples (between patients, and between pre-treatment and post-treatment samples for the same patient); (3) comparison of expansion of clones; and (4) computation of the diversity and clonality of all samples.
ELISpot assays were performed on cryopreserved peripheral blood mononuclear cells (PBMCs) isolated at Week 1 (baseline), Week 3, Week 6, and Week 9 from patients with mCRPC who had received 3 doses of ADXS-PSA monotherapy. Cryopreserved unfractionated PBMCs were thawed and rested overnight at 37 ºC prior to ex vivo stimulation. The ELISpot assay protocol followed the protocol outlined by Janetzki et al for assay harmonization.13 The reactivity of peripheral CD4+ and CD8+ T cells was assayed to peptides derived from PSA, the target antigen of ADXS-PSA, as well as to peptides derived from prostatic acid phosphatase (PAP), prostate-specific membrane antigen (PSMA), prostate stem cell antigen (PSCA), and prostein to determine the extent of antigen spreading after ADXS-PSA treatment. All peptide mixes contained peptides that were 15 amino acids in length with 11 amino acid overlap. Secretion of IFNγ, TNFα, and the cytolytic granule granzyme B was measured using the 3-color fluoroSpot kit (Cellular Technology Limited, Cleveland, OH).
MSI status was evaluated via PlasmaSELECT R64 by PGDx along with identification of potential somatic and genomic mutations.
Outcomes
The primary objective of this trial was to characterize the safety and tolerability of ADXS31-142 alone (Part A) and in combination with pembrolizumab (Part B) and select the RP2D. Safety endpoints included the incidence of AEs, SAEs, physical examinations, vital signs, concomitant medications, and laboratory safety test results.
Secondary objectives were to evaluate antitumor activity and survival signals of ADXS31-142 alone and in combination with pembrolizumab. Key efficacy endpoints were overall response rate, progression-free survival (PFS), OS, disease control rate (DCR), duration of response, and serum PSA levels. Overall response rate and DCR were based on best tumor response on treatment until disease progression/recurrence, utilizing RECIST (version 1.1) and Prostate Cancer Working Group 2 (PCWG2) criteria. DCR was defined as the proportion of subjects with complete response (CR), partial response (PR) or stable disease (SD) per RECIST 1.1 criteria for a minimum of 12 weeks following the first day of treatment. Duration of response was measured from the date of first documented CR/PR to the date of first documented disease progression or death. PFS was defined as the length of time from the start of treatment to the date of first documented disease progression or death. OS was defined as the time from the start of treatment to the date of death.
Exploratory objectives included evaluating correlative immunologic profiles of PBMCs for ADXS-PSA monotherapy and ADXS-PSA + pembrolizumab combination therapy, as well as direct ELISpot assessment of T-cell responses to PSA and antigen spreading to other prostate cancer–associated antigens.
Details of the methods used have been reported elsewhere.10,11
Statistical Analysis
Statistical analyses were performed using SAS software (version 9.2 or later; SAS Institute, Cary, NC, USA). All patients who received at least one dose of ADXS31-142 or pembrolizumab were included in the safety analysis; those patients who had at least one post-baseline radiologic tumor-response assessment were included in the efficacy analysis. Data were presented by treatment group (ADXS31-142 alone and ADXS31-142 and pembrolizumab combination). Descriptive statistics were used to summarize the continuous variables. Summary statistics for categorical variables were presented in terms of frequencies and percentages. Time to event data were summarized using the Kaplan-Meier method. AEs were graded in severity according to the guidelines outlined in the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version (v) 4.03. AEs were coded using the Medical Dictionary for Regulatory Activities (version 20.1).
Results
Patient Baseline Characteristics and Treatment
A total of 13 patients received ADXS31-142 monotherapy in Part A at doses of 1 × 109 CFU (n = 10), 5 × 109 CFU (n = 1), and 1 × 1010 CFU (n = 2). Reversible dose-limiting toxicities were documented in the first cycle of ADXS31-142 at 1 × 1010 CFU in 2 patients from Part A: one patient with grade 4 cytokine-release syndrome and grade 4 transaminases increased and one with grade 3 hypertension. The RP2D was defined in Part A and established for Part B as 1 × 109 CFU ADXS31-142 + 200 mg pembrolizumab. A total of 37 patients received combination therapy with ADXS31-142 (1 × 109 CFU) and pembrolizumab (200 mg) in Part B. Patients in the monotherapy and combination therapy arms had comparable demographic characteristics (Table 1). Patients in the combination therapy arm had higher median baseline PSA compared with patients in the monotherapy arm (42 ng/mL vs 19 ng/mL, respectively). Of the 13 patients in the monotherapy arm, 4 (31%) had received prior chemotherapy, 6 (46%) had received prior immunotherapy, and 11 (85%) had received hormonal therapy as their primary treatment. Of the 37 patients in the combination therapy arm, 21 (57%) had received prior chemotherapy, 7 (19%) had received immunotherapy, and 34 (92%) had received first-generation hormonal therapy. Patients in the combination therapy arm reported higher use of prior enzalutamide than those in the monotherapy arm (32% vs 15%, respectively). The primary reason for premature discontinuation was disease progression in both treatment arms (31% with monotherapy and 68% with combination therapy).
Table 1.
Summary of patient demographics and baseline characteristics.
| Category | ADXS31-142 monotherapy (n = 13) |
ADXS31-142 + pembrolizumab (n = 37) |
|---|---|---|
| Age, median (range), years | 69.0 (57-80) | 68.0 (45-92) |
| Weight, mean ± SD (range), kg | 87.7 ± 16.98 (56-117) | 93.7 ± 21.91 (55-157) |
| BMI, mean ± SD (range), kg/m2 | 28.5 ± 5.24 (19-37) | 29.8 ± 6.03 (21-50) |
| ECOG PS 0/1, % | 53.8/46.2 | 48.6/51.4 |
| PSA level, median (range), ng/mL | 19.0 (4.2-2456.0) | 41.5 (0.1-426.3) |
| Time from initial diagnosis to first dose of ADXS31-142, mean ± SD (range), months | 90.2 ± 70.30 (17-247) | 72.3 ± 55.57 (11-215) |
| Prior therapy, n (%) | ||
| Chemotherapy | 4 (30.8) | 21 (56.8) |
| Docetaxel | 4 (30.8) | 20 (54.1) |
| Immunotherapy | 6 (46.2) | 7 (18.9) |
| Hormonal | 11 (84.6) | 34 (91.9) |
| Next-generation hormonal agents, n (%) | ||
| Abiraterone only | 3 (23.1) | 6 (16.2) |
| Enzalutamide only | 2 (15.4) | 12 (32.4) |
| Abiraterone and enzalutamide | 1 (7.7) | 12 (32.4) |
| Presence of visceral metastases, n (%) | ||
| Yes | 2 (15.4) | 11 (29.7) |
| No | 11 (84.6) | 26 (70.3) |
Abbreviations: BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; PS, performance status; PSA, prostate-specific antigen; SD, standard deviation.
Safety
All 50 patients who received at least one dose of the study drug experienced at least one treatment-related AE (Table 2). Chills and pyrexia were the most commonly reported AEs in both treatment arms (Table 3). The severity of AEs was similar between the 2 treatment arms with grade ≥3 AEs reported in 8 patients (61.5%) in the monotherapy arm and in 24 patients (65%) in the combination therapy arm. Treatment-related grade ≥3 AEs were reported in 5 patients (38%) in the monotherapy arm and 11 patients (30%) in the combination therapy arm.
Table 2.
Summary of AEs.
| AE | No. of patients (%) | |
|---|---|---|
| ADXS31-142 monotherapy (n = 13) |
ADXS31-142 + pembrolizumab (n = 37) |
|
| Any AE | 13 (100.0) | 37 (100.0) |
| Treatment-related AE | 13 (100.0) | 37 (100.0) |
| Grade ≥3 AE | 8 (61.5) | 24 (64.9) |
| Treatment-related grade ≥3 AE | 5 (38.5) | 11 (29.7) |
| SAE | 3 (23.1) | 20 (54.1) |
| Treatment-related SAE | 2 (15.4) | 8 (21.6) |
| Deatha | 2 (15.4) | 0 |
After the first dose and within 30 days of the last dose.
Abbreviations: AE, adverse event; SAE, serious adverse event.
Table 3.
ADXS31-142 treatment-related AEs reported in >2 patients in any treatment arm.
| Preferred term | No. of patients (%) | |
|---|---|---|
| ADXS31-142 monotherapy (n = 13) |
ADXS31-142 + pembrolizumab (n = 37) |
|
| Patients with at least 1 AE | 13 (100.0) | 37 (100.0) |
| Chills | 10 (76.9) | 33 (89.2) |
| Pyrexia | 8 (61.5) | 20 (54.1) |
| Hypotension | 6 (46.2) | 8 (21.6) |
| Nausea | 5 (38.5) | 17 (45.9) |
| Fatigue | 5 (38.5) | 13 (35.1) |
| Hypertension | 2 (15.4) | 9 (24.3) |
| Vomiting | 2 (15.4) | 5 (13.5) |
| Tachycardia | 2 (15.4) | 4 (10.8) |
| Decreased appetite | 1 (7.7) | 6 (16.2) |
| Anemia | 1 (7.7) | 5 (13.5) |
| Headache | 1 (7.7) | 5 (13.5) |
| Diarrhea | 1 (7.7) | 4 (10.8) |
| Pain | 1 (7.7) | 4 (10.8) |
| Hypothyroidism | 0 | 7 (18.9) |
| Thrombocytopenia | 0 | 3 (8.1) |
| Back pain | 0 | 3 (8.1) |
| Hypoxia | 0 | 3 (8.1) |
| Rash | 0 | 3 (8.1) |
Abbreviation: AE, adverse event.
Serious AEs were reported in 3 patients (23%) in the monotherapy arm and 20 patients (54%) in the combination therapy arm. 2 patients (15%) in the monotherapy arm had 3 SAEs that were considered treatment-related, including cytokine-release syndrome in one patient and septic shock and acute kidney injury in one patient. Eight patients (22%) in the combination therapy arm had 10 SAEs that were treatment-related, including hypertension and hypotension in one patient, hypotension in, hypertension in one patient, thrombocytopenia in one patient, fatigue and dehydration in, pyrexia in one patient, infusion-related reaction in one patient, and pneumonitis in one patient.
Two deaths were reported within 30 days of the last dose of study medication: one was due to progressive disease unrelated to treatment and the other was due to septic shock which was considered possibly related; both deaths were reported in the monotherapy arm.
Efficacy
Thirty-seven patients had the measurable disease by RECIST 1.1, comprising 8 patients who received ADXS31-142 monotherapy and 29 patients who received combination treatment with ADXS31-142 and pembrolizumab. There were no objective responses in the 37 RECIST-evaluable patients. Four patients (31%) in the monotherapy arm and 21 (57%) patients in the combination therapy arm had SD as the best response. Thus, the DCR was 31% with monotherapy and 56.8% with the combination.
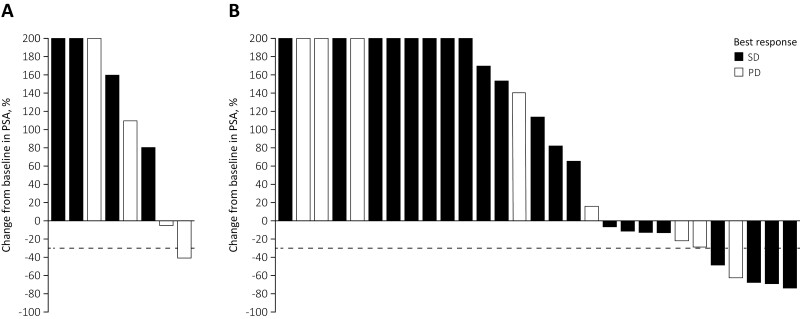
Of the 37 evaluable patients, 2 (25%) of the 8 evaluable patients in the monotherapy arm (Fig. 1A) and 11 (38%) of the 29 evaluable patients in the combination therapy arm had decreased PSA levels versus baseline at any time during treatment (Fig. 1B). Of these, one patient (13%) in the monotherapy arm and 5 patients (17%) in the combination therapy arm achieved a PSA reduction ≥50% from baseline.
Figure 1.
Maximal change in PSA at any time since treatment initiation. (A) ADXS31-142 monotherapy and (B) ADXS31-142 plus pembrolizumab combination therapy. PSA changes of >200% are truncated at 200% for clarity. Abbreviations: MK, pembrolizumab; ADXS, ADXS31-142.
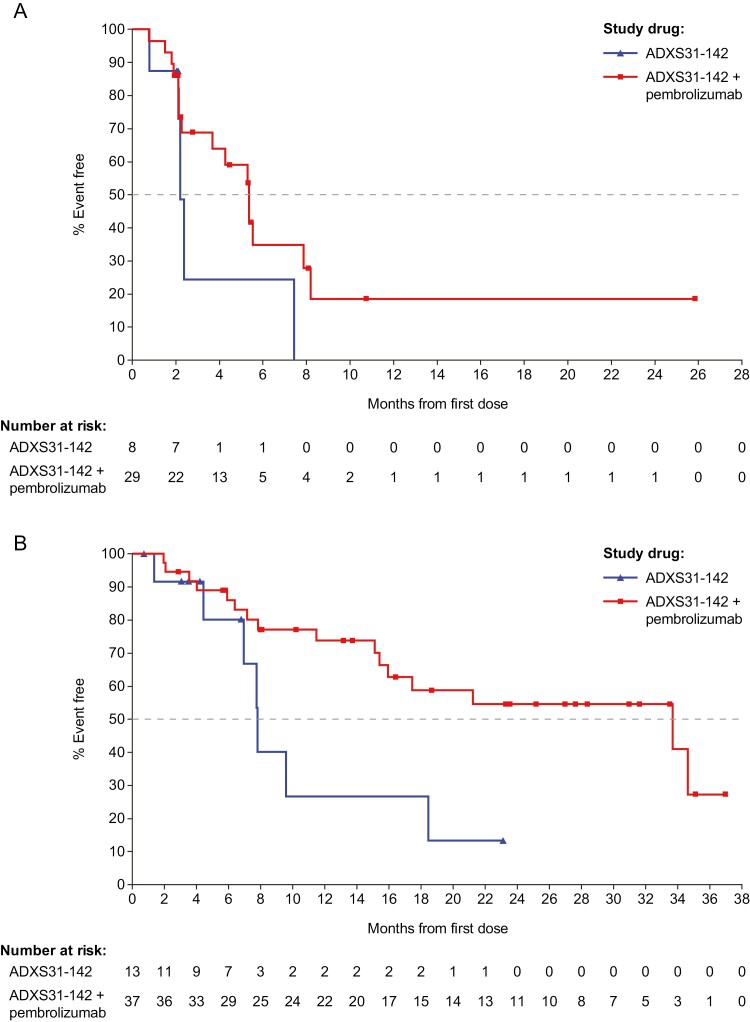
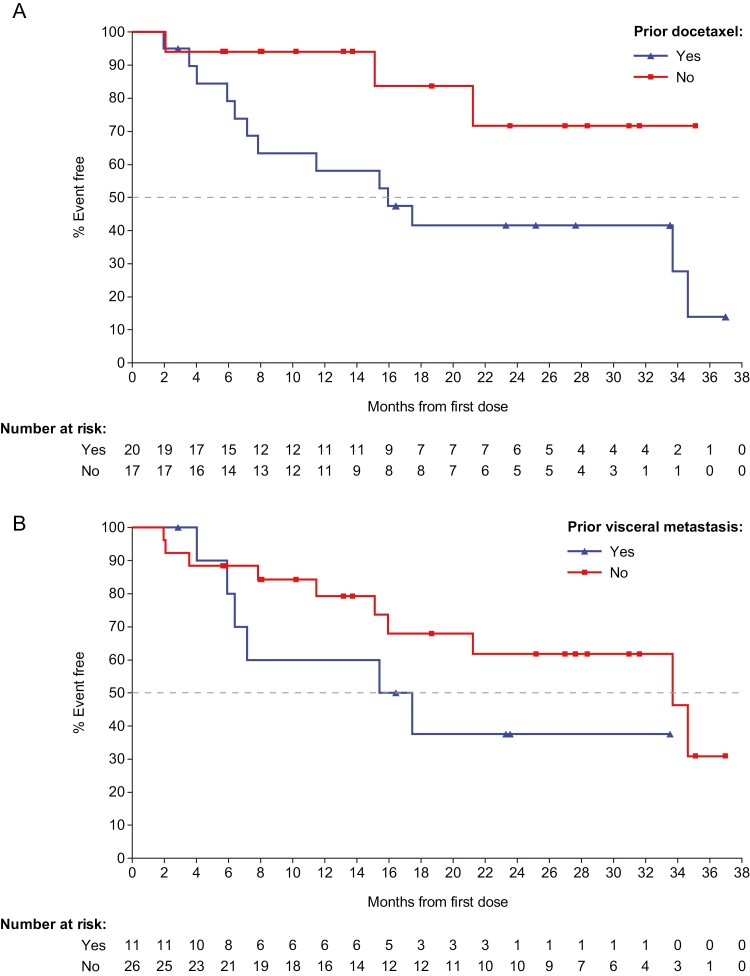
Median PFS was 2.2 months (95% CI: 0.8-7.4) in the monotherapy arm and 5.4 months (95% CI: 2.3-7.9) in the combination therapy arm (Fig. 2A). Median OS was 7.5 months (95% CI: 4.4-18.5) in the monotherapy arm and 33.7 months (95% CI: 15.4-not evaluable [NE]) in the combination therapy arm (Fig. 2B). Prolonged survival was observed in patients in the combination therapy arm regardless of prior therapy with docetaxel or presence of visceral metastasis (Fig. 3). In patients who had not received prior docetaxel (n = 17), median OS was not reached (95% CI: 15.1-NE) and in patients who had received prior docetaxel (n = 20), median OS was 16.0 months (95% CI: 6.4-34.6). Seventeen of 20 patients (85%) who had received prior docetaxel had also received one or 2 prior NGHA therapies. Similarly, in patients who had prior visceral metastasis (n=11), median OS was 16.4 months (95% CI: 4.0-NE) and in patients with no visceral metastasis (n = 26), median OS was 33.7 months (95% CI: 15.1-NE). Ten of the 11 patients with prior visceral metastasis (91%) had received prior docetaxel and 9 (82%) had received one or 2 prior NGHA therapy.
Figure 2.
Kaplan-Meier curve for (A) progression-free survival and (B) overall survival after ADXS31-142 monotherapy or ADXS31-142 plus pembrolizumab combination therapy.
Abbreviations: MK, pembrolizumab; ADXS, ADXS31-142.
Figure 3.
Kaplan-Meier curve for overall survival after ADXS31-142 plus pembrolizumab combination therapy in patients based on (A) prior docetaxel or (B) presence of visceral metastases. Abbreviations: MK, pembrolizumab; ADXS, ADXS31-142.
One patient with SD continued to receive the combination of pembrolizumab and ADXS31-142 for 21 months beyond the 2-year limit of this protocol on a separate Investigational New Drug Application.
Immunogenicity
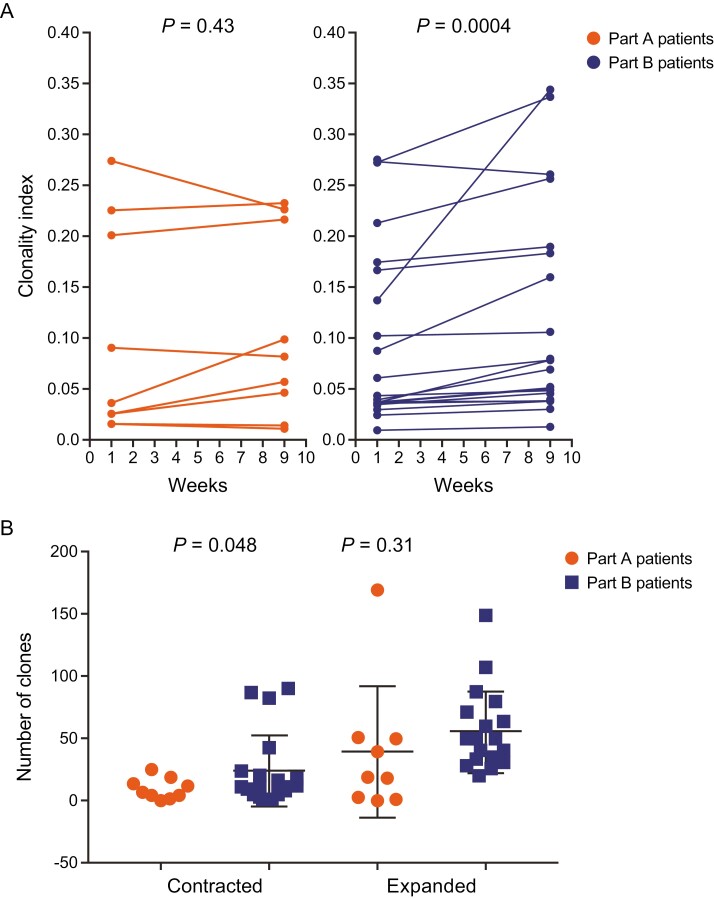
Immunogenicity data were available for 9 patients who received ADXS31-142 monotherapy and 17 patients who received combination treatment with ADXS31-142 and pembrolizumab. It is known that after activation by an antigen, T cells undergo a clonal expansion and differentiation followed by a contraction phase once the antigen/carrier has been cleared. As shown in Fig. 4A, combination treatment with ADXS31-142 plus pembrolizumab extended T-cell expansion at Week 9 compared with ADXS31-142 monotherapy, suggesting broader immune stimulation. Increased contraction of T-cell clones was observed in the combination group, which suggests that lower-avidity T-cell clones to PSA (or other prostate-related antigens) were reduced in favor of high-avidity T cells under PD-1 blockade (Fig. 4B). The frequency of functional PSA-specific T cells in the peripheral blood increased from baseline in 5 patients (56%) in the monotherapy arm and 11 patients (65%) in the combination therapy arm. Antigen spreading, or the expansion of T-cell responses to prostate cancer antigens that are not expressed by ADXS31-142, was seen in all 9 patients tested (100%) in the monotherapy arm and in 14 patients (82%) in the combination arm (plot not shown). Antigen-specific T-cell responses were documented against PAP, PSMA, PSCA, and prostein.10,11
Figure 4.
T-cell receptor beta-chain sequencing in peripheral blood mononuclear cells in Part A with ADXS31-142 monotherapy and in Part B with ADXS31-142 plus pembrolizumab combination therapy. (A) Clonal expansions at Week 9. (B) Differences in the numbers of contracted and expanded clones at Week 9. The Simpson Clonality index is one of the metrics used to understand and interpret the diversity of T cells and to quantify how focused the immune repertoire is on a particular set of antigens.14
Thirty-six of 37 patients in the combination therapy arm were tested for the MSI status of their disease and all had negative results, including the patient going beyond the 2 years of study treatment.10
Discussion
Data from the open-label, phase I/II KEYNOTE-046 study suggest that combination treatment with ADXS31-142 plus pembrolizumab may improve OS across different mCRPC populations, particularly in the presence of visceral metastases or prior therapy with docetaxel. In Part A of this study, ADXS31-142 monotherapy was evaluated in patients with mCRPC to characterize the safety, tolerability, RP2D, and immunogenicity of the drug. Part B evaluated the safety and efficacy of the combination of ADXS31-142 with pembrolizumab in a more refractory mCRPC population where the majority of the patients had bone-predominant disease (70%), had received prior docetaxel (54%) and/or one or 2 NGHA (82%) therapies, regardless of PD-L1 expression status.
ADXS31-142 was generally well tolerated as monotherapy and in combination with pembrolizumab. The most common treatment-related AEs in both treatment arms were chills and pyrexia, which were transient and manageable. These were usually grade 1-2 “flu-like” symptoms that lasted 2-4 h after treatment infusion and were consistent with immune cell activation, induced by ADXS31-142 and other vectors based on Lm technology. These symptoms usually resolved without specific intervention or responded rapidly to limited symptomatic treatment. No additive toxicity was observed with combination treatment compared with monotherapy.
There were no objective responses in RECIST-evaluable patients in Part B, but SD was shown in 72.4% of patients. PFS was 5.4 months with combination therapy, as expected for this population. However, median OS was promising in the overall population (33.7 months) as well as in patients who had received prior therapy with docetaxel (16.0 months) and even in those who had visceral metastasis (16.4 months). The latter 2 groups of patients are of particular interest because their therapeutic options are currently limited to chemotherapy such as cabazitaxel, pembrolizumab if disease is MSI-H or dMMR, radium-223 dichloride, lutetium 177, best supportive care, or receiving investigational treatment as part of a clinical trial.
Comparing the results of this study with those of other studies is challenging as the baseline characteristics and prior therapies of patients widely vary at this late stage of the disease. Also, the potential survival benefit of adding on ADXS31-142 to pembrolizumab for the entire group (mainly for patients with bone-predominant disease), as well as for those with prior docetaxel therapy and/or visceral metastasis remains to be further explored. Visceral metastases are found in approximately 22%-30% of patients with mCRPC and are associated with unfavorable outcomes.15 The median OS in patients with mCRPC with bone-predominant disease (ie, ≤ 25% patients with visceral metastasis) who have also progressed on docetaxel and NGHAs has been reported as 8.2-13.6 months with cabazitaxel14,16,17 and 14.1 months with pembrolizumab.5 In the current study, median OS for patients with bone-predominant disease was 33.7 months, with 38% of patients with prior docetaxel and 80% with one or 2 prior NGHAs. Data are sparse on median OS in patients with visceral/measurable disease who have already received prior docetaxel and NGHA. For example, median OS in patients with predominant visceral metastasis with prior docetaxel and no prior NGHA was 9.5 months with best supportive care and 13 months with enzalutamide.18 In contrast, almost all patients with visceral metastasis in this study had received prior docetaxel and NGHA therapies and their median OS was 16.4 months.
Within the limitations of the sample size of this study and bearing in mind the caveats of cross-study comparisons, it is possible that there may be a signal of OS benefit (ie, median 16.4 months) with pembrolizumab plus ADXS31-142 in patients with measurable disease. Our results compare favorably with those from patients with similar characteristics treated with pembrolizumab alone in the KN-199 study (Cohort 3), which reported a median OS of 9.5 months (95% CI: 6.4-11.9) and 7.9 months (95% CI: 5.9-10.2) in patients with PD-L1-positive and PD-L1-negative disease, respectively.5
There was a broader immune stimulation in the combination arm (which included B-cell activation) compared with the ADXS13-142 monotherapy arm. Correlative immune analyses showed T-cell responses against PSA (65%) and antigen spreading (85%) in patients in the combination arm. These results could potentially explain the mechanism of action by which ADXS31-142 could potentiate pembrolizumab activity in mCRPC. The contribution of the potential reduction of Tregs and MDSCs by ADXS31-142 in the tumor microenvironment remains to be defined in future studies.
Conclusion
The combination of ADXS31-142 with pembrolizumab in patients with mCRPC was safe and deserves a further evaluation of the improvement in OS, particularly in patients with visceral metastasis.
Acknowledgments
We would like to express sincere thanks to all participating patients and their families and the staff at the various clinical sites who were involved in the trial. Medical editing/writing assistance was provided by Neha Arora, PhD, Veristat LLC, Miller Medical Communications Ltd (both funded by Advaxis, Inc.), and Ariana Parsi, Advaxis Inc. The authors are fully responsible for all content and editorial decisions for this manuscript.
Contributor Information
Mark N Stein, Columbia University Medical Center, New York, NY, USA.
Lawrence Fong, University of California, San Francisco, CA, USA.
Ronald Tutrone, Chesapeake Urology Research Associates, Towson, MD, USA.
Anthony Mega, Lifespan Oncology Clinical Research, Rhode Island Hospital, Providence, RI, USA.
Elaine T Lam, University of Colorado Cancer Center, University of Colorado Anschutz Medical Center, Aurora, CO, USA.
Megan Parsi, Advaxis, Monmouth Junction, NJ, USA.
Surya Vangala, Advaxis, Monmouth Junction, NJ, USA.
Andres A Gutierrez, Advaxis, Monmouth Junction, NJ, USA.
Naomi B Haas, Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA, USA.
Funding
This work was supported by Advaxis, Inc. and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. No grant number is applicable.
Disclaimer
ADXS31-142 and pembrolizumab are being developed by Advaxis, Inc. and Merck & Co., Inc., Kenilworth, NJ, USA, respectively.
Conflict of Interest
Lawrence Fong: Abbvie, Amgen, Bavarian Nordic, BMS, Dendreon, Janssen, Merck, Roche-Genentech (RF); Anthony Mega: Astellas, Bristol Myers Squibb (C/A); Elaine T. Lam: Merck, Advaxis, Amgen, Harpoon, Astellas (RF—inst.); Megan Parsi, Surya Vangala, and Andres A. Gutierrez: Advaxis, Inc (E, OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/Design: M.N.S., N.B.H., L.F., A.M., R.T., E.T.L., A.A.G., M.P., S.V. Provision of study material/patients: M.S., N.H., L.F., A.M., R.T., E.T.L. Collection and/or assembly of data: M.S., N.H., L.F., A.M., R.T., E.T.L., A.A.G., M.P., S.V. Data analysis and interpretation: A.A.G., M.P., S.V. Manuscript writing: A.A.G., M.P. Final approval of manuscript: All authors.
Data Availability
The data underlying this article were provided by Advaxis, Inc. under licence/ by permission. Data will be shared on request to the corresponding author with the permission of Advaxis, Inc.
References
- 1. Global Cancer Statistics, GLOBOCAN. 2020. https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf. Accessed January 26, 2022.
- 2. National Cancer Institute. Surveillance, epidemiology, and end results program.2018. http://seer.cancer.gov/statfacts/html/prost.html. Accessed January 26, 2022.
- 3. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: prostate cancer, version 3. 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 26, 2022.
- 4. Mehtälä J, Zong J, Vassilev Z, et al. Overall survival and second primary malignancies in men with metastatic prostate cancer. PLoS One. 2020;15(2):e0227552. https://doi.org/10.1371/journal.pone.0227552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38(5):395-405. https://doi.org/10.1200/JCO.19.01638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powers E, Karachaliou GS, Kao C, et al. Novel therapies are changing treatment paradigms in metastatic prostate cancer. J Hematol Oncol. 2020;13(144): 1-13. https://doi.org/10.1186/s13045-020-00978-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Venkatachalam S, McFarland TR, Agarwal N, et al. Immune checkpoint inhibitors in prostate cancer. Cancers. 2021;13(2187):1-23. https://doi.org/10.3390/cancers13092187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wood LM, Paterson Y.. Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Front Cell Infect Microbiol. 2014;4(51):1-22. https://doi.org/10.3389/fcimb.2014.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bongiorno EK, Baybutt T, Portocarrero C, et al. Abstract B15: combination immunotherapy of murine prostate cancer using a Listeria-based PSA vaccine: immune correlates of efficacy and resistance development. Cancer Immunol Res. 2018;6(9 Suppl):B15. [Google Scholar]
- 10. Stein M, Fong L, Tutrone R, et al. Abstract CT098: KEYNOTE-046: effects of ADXS-PSA with or without pembrolizumab on survival and antigen spreading in metastatic, castration-resistant prostate cancer patients. Cancer Res. 2019;79(13 Suppl):CT098-CT098. [Google Scholar]
- 11. Hayes S, Lobo M, Petit R, et al. Magnitude of anti-PSA T cell response is associated with antigen spreading and slowing in PSA and PAP velocity in ADXS-PSA-treated mCRPC patients. Keystone Symposia Conference on Cancer Vaccines. Vancouver, BC, Canada. 2019. [Google Scholar]
- 12. Technical Note. immunoSEQ analyzer: Understanding clonality. Available at https://www.adaptivebiotech.com/wp-content/uploads/2020/06/immunoSEQ_Analyzer-Tech-Note_Clonality_WEB_MRK-00355.pdf. Accessed January 26, 2022.
- 13. Janetzki S, Price L, Schroeder H, et al. Guidelines for the automated evaluation of Elispot assays. Nat Protocol. 2015;10(7):1098-1115. https://doi.org/10.1038/nprot.2015.068 [DOI] [PubMed] [Google Scholar]
- 14. de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506-2518. https://doi.org/10.1056/NEJMoa1911206 [DOI] [PubMed] [Google Scholar]
- 15. Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32(7):671-677. https://doi.org/10.1200/JCO.2013.52.3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sella A, Sella T, Peer A, et al. Activity of cabazitaxel after docetaxel and abiraterone acetate therapy in patients with castration-resistant prostate cancer. Clin Genitourin Cancer. 2014;12(6):428-432. https://doi.org/10.1016/j.clgc.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 17. Al Nakouzi N, Le Moulec S, Albigès L, et al. Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway targeted therapies. Eur Urol. 2015;68(2):228-235. https://doi.org/10.1016/j.eururo.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 18. Loriot Y, Fizazi K, de Bono JS, et al. Enzalutamide in castration-resistant prostate cancer patients with visceral disease in the liver and/or lung: outcomes from the randomized controlled phase 3 AFFIRM trial. Cancer. 2017;123(2):253-262. https://doi.org/10.1002/cncr.30336 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article were provided by Advaxis, Inc. under licence/ by permission. Data will be shared on request to the corresponding author with the permission of Advaxis, Inc.