SUMMARY
The mammalian placenta, which is responsible for bonding between the mother and the fetus, is one of the first organs to develop. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection has caused a great threat to public health and affected almost all the organs including the placenta. Owing to limited available data on vertical transmission and pathological changes in the placenta of SARS-CoV-2 positive patients, we aim to review and summarize histopathological and ultrastructural changes in the placental tissue following SARS-CoV-2 infection. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines were used for review writing. Multiple studies have reported significant pathological changes in the placental tissue of SARS-CoV-2 positive mothers. On the other hand, some studies have demonstrated either no or very little involvement of the placental tissue. The most common pathological changes reported are fetal and maternal vascular malformation, villitis of unknown etiology, thrombus formation in the intervillous space and sub-chorionic space, and chorangiosis. Reports on vertical transmission are less in number. The observations of this review present a strong base for the pathological involvement of the placenta in SARS-CoV-2 infected mothers. However, a smaller number of original studies have been done until now, and most of them have small sample sizes and lack matched control groups, which are the big limitations for drawing an effective conclusion at this stage. Antenatal care can be improved by a better understanding of the correlation between maternal SARS-CoV-2 infection and placental pathology in COVID-19.
Keywords: COVID-19, histopathology, placenta, SARS-CoV-2, trophoblast, ultrastructure
INTRODUCTION
Coronavirus disease-2019 (COVID-19), caused by a positive single-stranded enveloped RNA virus has led to the deadly pandemic of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection around the world which has become a serious threat to public health [1]. The World Health Organization (WHO) labeled it a global pandemic on March 11, 2020, because of its rapid spread worldwide [2]. SARS-CoV-2 enters human cells through surface receptor protein Angiotensin-Converting Enzyme-2 (ACE2), which is ubiquitously distributed in the various human organs including the placenta [3]. Viral spike protein priming, which is generated by trans-membrane serine protease 2 (TMPRSS2) expressed in host tissues, aids cell entrance even further [3]. However, coronavirus particles have also been detected in placental tissue of infected mothers in some studies [4]. The placenta is one of the first organs to develop in a mammalian embryo and takes an important role in the transfer of nutrients from mother to fetus besides imparting protection against various hazards [5].
The placenta plays an important role in protecting the fetus from maternal infections and any chemical toxins. SARS-CoV-2, a newly emerged virus has been established in some of the studies to cross the placenta and infect the developing fetus [6]. The entry point of this virus in the feto-maternal circulation is mediated by the presence of ACE2 receptor on the cell membrane via its surface spike (S) proteins as observed in various other organs such as lungs, kidneys, testes, and gastrointestinal tract. Concerning the feto-maternal interface which consists of the placental trophoblast and the maternal decidua, the ACE2 is widely expressed in the female genital tract and fetal and maternal components of the placental tissue [7].
Although various modes of transmission of SARS-CoV-2 have been described from human to human the vertical transmission of infection from mother to fetus remains a pertinent and unresolved question. This review is an effort toward collecting and summarizing all the data available on the pathological changes in the placentas, and incidences of vertical transmission of the virus to the fetus in COVID-19 positive women reported until now.
MATERIALS AND METHODS
Objectives
Several studies have been done on intrapartum clinical features of COVID-19 positive women during pregnancy and their newborns, but studies on placental tissue of infected mothers are still limited to date. Due to the scarcity of data on the morphology and microscopic changes of the placental tissue of SARS-CoV-2 infected pregnant women, we aimed to review and summarize the reported data concerning the various changes in the placental tissue of COVID-19 positive pregnant women.
Literature search and information sources
The authors performed an extensive literature search, from 31st Dec 2019 to 5th August 2021. The search engines included PubMed, Medline (EBSCO & Ovid), the Cochrane Library, Science Direct, Scopus, Google Scholar, Medrxiv, and BioMedical. Keywords and MeSH terms used for searching the articles were ‘COVID-19 and placenta’, ‘Histopathology and COVID-19’, ‘Pregnancy and COVID-19’, SARS-CoV-2’, ‘Coronavirus disease’, ‘SARS-CoV-2 and placenta’. Only the English language was used in the search and selection criteria. Data extraction was performed by all the authors independently and reviewed with inclusion and exclusion criteria.
Protocol followed
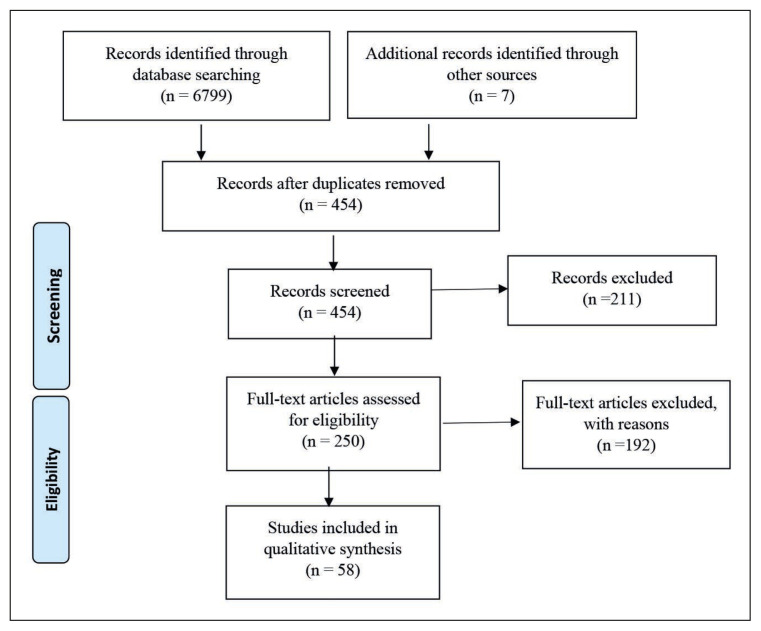
Systematic review writing was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines (Figure 1).
Figure 1.
Flow Diagram for Selection of Studies using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Quality Assessment
Quality assessment of the present systematic review was done by two investigators independently using the National Institutes of Health (NIH) tool for quality assessment of systematic review and meta-analysis (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools).
Inclusion criteria
We included articles (original, case series, and case reports both published and preprints) related to morphological, histopathological, immunohistochemical as well as ultrastructural findings of placental tissues of COVID-19 positive pregnant women irrespective of the trimester. We also reviewed the references of the articles that were used in our systematic study. Exclusive articles on SARS and MERS related to the histopathological study of placenta and pregnancy and very few studies on animal models related to the topic were also included. The last literature search was till 5th August 2021.
Exclusion criteria
We excluded articles in which a confirmed diagnosis of COVID-19 using RT-PCR testing was not made and studies in which histopathological examination of the placenta was not done. Editorials, letters to the editor, newsletters, commentaries, reviews, and conference abstracts were excluded from the study. Articles with missing or incomplete data were also eliminated.
RESULTS
For the initial screening of the articles, 454 articles were identified from various sources as mentioned in the methodology, out of which 211 articles were excluded on the basis of the title, details in the abstract, and relevance of the research question. Two hundred and fifty (250) articles were chosen for full-text review, out of which 192 were excluded for various reasons including research design, ineligible population, failure to indicate outcomes of interest, or inability to locate text. There were 58 studies that were found eligible after full-text screening (Figure 1). We created two tables to better understand the histopathological observations of various cases: one depicting details of only case reports (single case studies), in which we included 22 case reports [Supplementary table 1], and the other depicting details of case series or case studies, in which we included 36 studies according to inclusion and exclusion criteria.
On the basis of existing literature on the effect of COVID-19 on the placenta, we observed two categories broadly. According to one, significant histopathological changes are observed in the placentas of SARS-CoV-2 positive mothers. Whereas no differences in placental histopathology were observed as per another category. The most common finding across the studies we noted was fetal and maternal vascular malperfusion (FVM and MVM). Few studies reported inflammatory changes in the chorionic villi, intervillous thrombi formation, and decidual arteriopathy. Viral particles in the endothelial cells of chorionic villi vessels and Syncytiotrophoblast (STB) were demonstrated in some studies.
DISCUSSION
Though the placental barrier protects the fetus from many intra-uterine infections, several viruses including rubella virus, Cytomegalovirus (CMV), and Herpes simplex virus (HSV) have been reported to cause congenital infections or syndromes and affect the health of new born babies [8]. Transmission of some virulent, polytrophic strains of mouse hepatitis virus (which is a coronavirus) have been detected in placental tissue and are reported to affect the fetus as well [9]. Literature suggests vertical transmission of SARS in humans and fetal vascular malperfusion (FVM) in the form of thrombotic vasculopathy have also been reported (Figure 2 A and B) [10]. Whereas some earlier studies do not support the vertical transmission of SARS-CoV-2 in humans, however, many new studies are coming up which are suggestive of some intrauterine transmission [11–13]. Thus, congenital infection by the SARS-CoV-2 virus is still a controversial topic, as related data available to the date is limited and the best way to confirm this infection is by identifying viral particles in the placental tissue. Few reports are suggestive of complications, such as preterm birth or premature rupture of membranes (PROM) associated with COVID-19 in pregnancy [14].
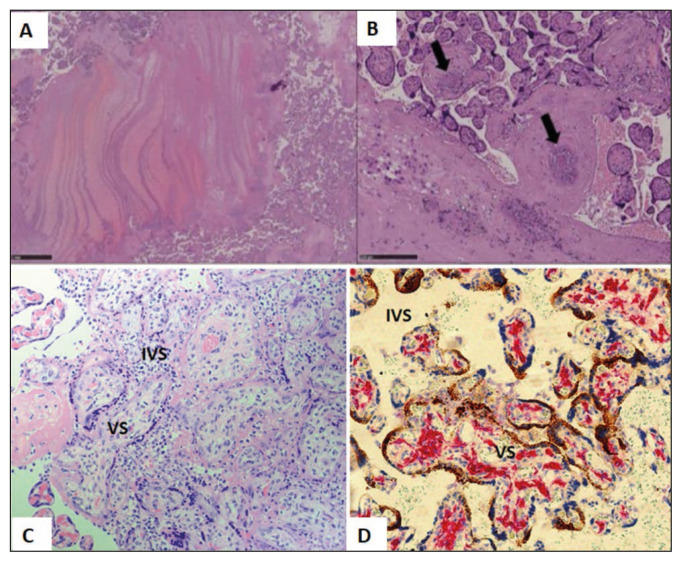
Figure 2.
Histopathological findings of placenta from SARS-CoV-2 positive mother. (A) showing Thrombo-hemorrhagic areas with fibrin laminar deposition (H&E, 20X). (B): Showing thrombosis of blood vessels (arrows) (B: HE, 100X). (Courtesy: Bertero L, Borella F, Botta G et al. Placenta in SARS-CoV-2 infection: a new target for inflammatory and thrombotic events; 202). (C): Placental tissue showing Chronic Histiocytic Intervillositis (CHI) with number of mononuclear macrophages in the Intervillous Space (IVS) (H&E 40X). (D): Placental tissue with double staining with antibody to CD163 and SARS-CoV-2 RNA Scope (x20) showing increased number of Hofbauer cells (red) in the villous stroma (VS) of placental villi (Hofbauer cell hyperplasia). SARS-CoV-2 staining (brown) can be seen only in Syncytiotrophoblast (STB). (Courtesy: Morotti D, Cadamuro M, Rigoli E, et al. Molecular Pathology Analysis of SARS-CoV-2 in Syncytiotrophoblast and Hofbauer Cells in Placenta from a Pregnant Woman and Fetus with COVID-19. Pathogens. 2021 Apr 15; 10(4), 479).
In some studies, on placental tissues of COVID-19 positive mothers, viral particles were not identified which indicates that there might be a low risk of vertical transmission of SARS-CoV-2 [15–17]. On the other hand, electron microscopic identification of SARS-CoV-2 virus in the STB of the chorionic villi and in the cell processes of fibroblasts of COVID-19 positive mothers, represents a risk of vertical transmission of coronavirus [18–20]. Khong et al studied 20 placentas of COVID-19 positive mothers and categorized its lesion using Amsterdam criteria [21]. In a histopathological study with Hematoxylin and Eosin (H&E) staining, the most common findings observed were malperfusion of fetal vasculature and intramural fibrin deposition (Figure 2A). In two cases, the focal presence of villous stromal-vascular karyorrhexis was seen. Other changes observed in a few placentas were intramural nonocclusive thrombi, meconium, macrophages, lesions of MVM, and perivillous fibrin deposition. In one case with high-grade infection (pneumonia and acute hypoxia), placental tissue depicted features of acute chorioamnionitis and acute funisitis. While the other four cases had chronic villitis with obliterative vasculopathy in one case. Increased incidence of fibrin deposition, microcalcifications, syncytial knots, small fibrotic villi, and villous agglutination was observed in a case-control study on the placenta of SARS-CoV-2 positive mothers [22]. Microcalcification and thrombus formation are suggestive of an underlying hypercoagulable state induced by COVID-19 infection or could be due to excessive STB injury.
Bertero et al. observed placental histopathological changes of chronic villitis and decidual infiltration of CD8-positive T cells along with thrombo-hemorrhagic areas with fibrin laminar deposition within intervillous space in 66.67% (4 out of 6) cases. In 2 cases they demonstrated infiltration of histiocytes in the intervillous space and thrombotic changes in blood vessels (Figure 2B) [23]. Shanes and colleagues in their study of 16 placentas from SARS-CoV-2 positive mothers, observed one intrauterine death (IUD) in the second trimester and 15 live births in the third trimester. Microscopic changes were suggestive of MVM like intervillous thrombi formation and decidual arteriopathy were the characteristic findings. Additional changes in a few placentas were fibrinoid necrosis, villous infarctions, villous agglutination, and accelerated villous maturation. Edematous villi and a retroplacental hematoma were found in the placenta of the mother of the fetal IUD [24].
In a case series of 5 placentas from COVID-19 positive mothers, histopathological findings revealed thrombin formation in the large blood vessels in all the cases. In one placenta, they found, fibrin in blood vessels of villous stroma while focal avascular villi were observed in another placenta. There was no abnormal complement deposition in the blood vessels of the placentas and staining for viral spike protein and viral RNA was also negative for all 5 placentas. Observations of this study reflect the systemic nature of SARS-CoV-2 infection leading to thrombosis irrespective of systemic complement activation [25]. Whereas, Zhang P et al observed vasculopathy, villitis, and thrombosis in most and chorioamnionitis in a few of the positive placentas. Increased expression of CD68 and CD42b (maternal macrophages and platelet aggregates) around the infarcts was reported. The atrophic endometrial glandular epithelium and subchorionic plate both had SARS-CoV-2 viral RNA signals. There were no viral signals seen in any other maternal, fetal, or placental cell types [26].
Smithgall et al in their case-control study of 51 placentas of mothers positive for SARS-CoV-2 infection and 25 control groups, demonstrated microscopic changes of MVM and FVM which included, thrombus formation in the intervillous spaces and subchorionic spaces with associated chorangiosis. The most remarkable histopathological findings observed were thrombosis in fetal vessels and villous agglutination. However, no viral particle was observed in the placental tissues either by immunohistochemistry (IHC) or in-situ hybridization (ISH), as an indicator of vertical transmission [27]. Similar findings were reported by some authors on the placenta of third trimester SARS-CoV-2 positive mothers including trophoblast necrosis and debris accumulation in the intervillous space (IVS) [28–30].
One of the studies done by Patane et al., 2020 reported two cases of COVID-19 vertical transmission with some placental pathological findings such as vascular malperfusion with multiple thromboses. Electron microscopic studies were also done on these placental tissues of COVID-positive pregnant mothers which identified the localization of the virions in the cytoplasm of the STB (Figure 3 and 4) [31]. Hecht et al did not find any specific gross or histopathologic changes in the 19 placentas from COVID-19 confirmed mothers. SARS-CoV-2 RNA was detected focally in the placenta of only in 10.5% cases (2/19). They also studied viral protein expression using Immunohistochemistry (IHC) and In-Situ Hybridization (ISH), where they observed expression of ACE2 in the STB of the chorionic villi on its stromal side, and in a few cases, it was even expressed in the cytotrophoblast (CTB) and extra-villous trophoblast (EVT) [32]. Weak expression of TMPRSS2 was seen in villous endothelium a part of STB.
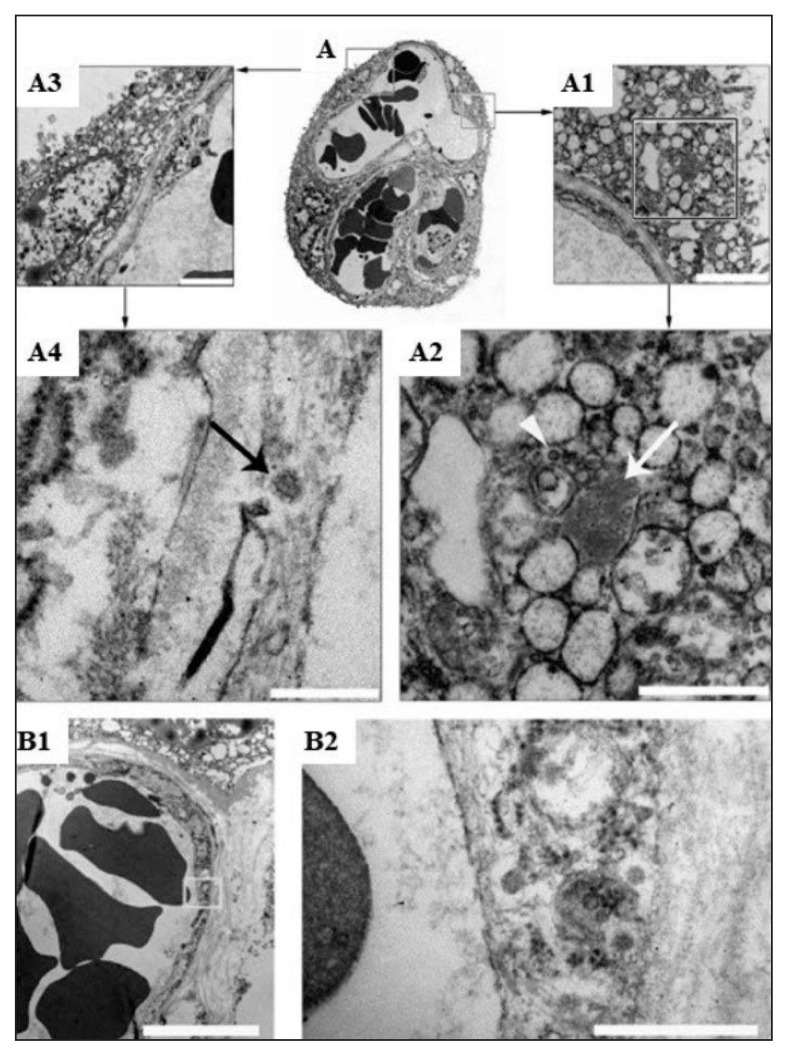
Figure 3.
Electron microscopic (EM) study of COVID-19 positive placenta.
(A): Showing thin section of placental villous, (A3, A1): Part of fetal capillary and syncytiotrophoblast (STB) (A4): the arrow is pointing to a particle with the typical morphological features of coronavirus within the endothelium. (A2): SARS-Cov-2 nucleocapsid inclusion aggregates (arrow) and particle that looks like a mature coronavirus (arrow head). (A1, bar 250 nm; A2, bar 2 μm). (B1, B2): Showing fetal capillary endothelium (B1, bar 5 μm), particles similar to the corona virus seen near to the villus (B2, high magnification of the area shown in A bar 500 nm). (Courtesy: Facchetti F, Bugatti M, Drera E, et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine. 2020 Sep; 59, 102951. doi: 10.1016/j.ebiom.2020.102951. Epub 2020 Aug 17. PMID: 32818801; PMCID: PMC7430280).
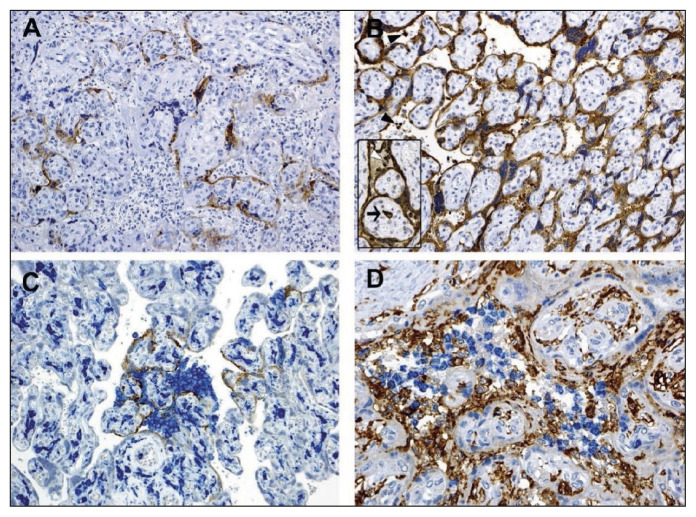
Figure 4.
Immunohistochemical study of SARS-CoV-2 placental tissue.
(A): Showing staining for SARS-CoV-2 spike protein. (B): Immunostaining for nucleocapsid proteins, showing stronger and more positivity than spike protein. Inset with arrow showing Hofbauer cell. (C): Showing double staining for spike protein (brown) and CD14 (blue), mainly at the sites where viral protein is expressed more intensely. (D): Showing staining for CD66b (blue) for neutrophils and CD14 (brown) expression for monocyte macrophages in the intevillous space (IVS) with presence of CD14 Hofbauer cells in the villi. (Courtesy: Facchetti F, Bugatti M., et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine. 2020 Sep; 59, 102951).
A recent study by Facchetti et al. on 15 placentas of COVID-19 infected mothers revealed prominent expression of SARS-CoV-2 S and N proteins in one of the placentas, and the newborn of the same patient was also tested positive for coronavirus and postnatally suffered from pneumonia. SARS-CoV-2 viral particles were demonstrated in the cytoplasm of STB, fibroblasts, endothelial cells of fetal capillaries, maternal macrophages, and Hofbauer cells. Viral particles were also found in the circulating mononuclear cells of the fetus, which is suggestive of vertical transmission of infection. In a few placentas, they observed infiltration of neutrophils and macrophages confirmed by immunostaining of CD66b (blue) for neutrophils and CD14 (brown) expression for monocyte macrophages (Figure 4) in the intervillous space and demonstrated scattered neutrophil extracellular traps (NETs) under immunofluorescence microscope [33]. A recent report about the presence of aberrant NETs with predominant neutrophil infiltration in autopsied COVID-19 positive lung tissue suggests some role of NETs in the development of complications following SARS-CoV-2 infection [34].
A study on 5 placentas of COVID-19 positive pregnant women demonstrated histopathological signs of MVM showing fibrin deposition in the intervillous spaces and vascular changes in all the cases. Signs of FVM were observed in only 2 cases in the form of vascular thrombus formation in the fetus and delayed villous maturation. The placenta from another mother with moderate respiratory symptoms had lympho-histiocytic villitis and intervillositis, where the most common cell population observed were CD8-positive T-cells, followed by a few CD68-positive macrophages, CD4-positive T-cells, and a scarce amount of plasma cells, with no increase in neutrophils. In the same placenta, the authors demonstrated SARS-CoV-2 viral particles in the decidua and the umbilical cord by use of ISH [35]. A similar finding of chronic villitis was observed by other authors, which they named as Villitis of Unknown etiology (VUE) [36–38]. Bouachba A et al in their study on 5 placental tissues showed massive perivillous fibrin deposition (MPFD) and numerous large intervillous thrombi (LIT). Severe chronic histiocytic intervillositis (CHI) (CD68+ macrophages along with lymphocytes T CD3+, CD8+) (Figure 2C) was also observed in all cases except one (case 5). SARS-CoV-2 RT-PCR from placenta samples was positive for cases 1, 2, and 3 and was negative for case 4 [39]. Acute chorioamnionitis, villous chorangiosis are other common observations of some studies (16,30). CHI and MPFD along with SARS-CoV-2 spike glycoprotein, mainly in the trophoblast layer were also observed in some case reports [40–43]. In a COVID positive 13-week twin pregnancy, SARS-CoV-2 N-protein and RNA as well as viral replication was detected in fetal lungs and kidneys, apart from that found in the placentas. Placental tissue showed diffuse infarction, CHI, and subchorial inflammation.CD163 positivity in the villous stroma and IVS [44].
In a retrospective study on 7 placentas of SARS-CoV-2 positive mothers, authors observed a triad of histopathological changes that included histiocytic intervillositis, perivillous fibrin deposition, and trophoblast necrosis which they defined as placentitis These characteristics necessitate confirmation of SARS-CoV-2 infection via RNA in ISH or anti–SARS-CoV-2 IHC, if available. They mentioned that complement activation is most likely responsible for the damage caused by SARS-CoV-2 placentitis [45].
A case report on the placenta of a symptomatic COVID-19 positive second-trimester pregnant mother with associated pre-eclampsia and placental abruption demonstrated the presence of high levels of SARS-CoV-2 in the STB of chorionic villi at the maternal-fetal interface under electron microscopy along with massive mononuclear cell infiltration (intervillositis) and fibrin deposits in the intervillous spaces. Immunohistochemical observations revealed CD68 and CD3 positive T lymphocytes [46]. The authors were the opinion that pre-eclampsia and coagulopathy were due to placental infection by SARS-CoV-2 (4). Inflammatory changes demonstrated by them in placental tissue were like the changes observed in lung tissue in COVID-19 positive patients with pneumonia, which suggests a similar mechanism of action [47]. In another case report with similar histopathological findings, authors also identified SARS-CoV-2 nucleocapsid and spike protein in STB [48].
While some authors did not find any remarkable difference between placentas of COVID-19 positive mothers and the uninfected ones, a case-control study on 77 placentas of COVID-19 positive mothers, reported the presence of thrombus formation in the chorionic plate and stem villi vessels, suggestive of FVM and VUE [13]. These findings were seen in most of the placental tissues of SARS-CoV-2 positive patients irrespective of their symptoms [30]. While FVM (thrombi of blood vessels) was a major change observed by Prabhu et al in their study in a large number of samples, in a case report on a single third-trimester placenta of a SARS-CoV-2 positive mother, the authors observed changes of MVM but no evidence of FVM [29]. They demonstrated viral particles in the endothelial cells of chorionic villi vessels but rarely in the trophoblast (Figure 3) [19]. Another case reported was of fetal demise in a SARS-CoV-2 positive mother with no significant preexisting conditions or complications related to the pregnancy, with placental changes of FVM and presence of infarction of placenta which lead to damage to a good number of chorionic villi [49]. Similar findings including microglial hyperplasia, and lymphocytic infiltration in the pla centa and fetal tissue were reported in another case study [50]. Another study by Rebutini PZ et al. did not observe significant differences in the histomorphometry of placenta of matched case and control study except an increase in villous fibrin deposition which was more in the cases [51].
As some of the studies suggest that inflammatory condition is not a prominent feature in the placenta of the mother infected with SARS-CoV-2, this might be due to the immune response of the placenta where cytokines may play a key role In other tissue SARS-CoV-2 has been reported to induce pyroptosis in the cells by activating inflammasomes, which are found in severe COVID-19 positive cases [53]. Pyroptosis is a critical inflammatory pathway reported to occur in trophoblast cells in pre-eclampsia, responsible for poor pregnancy outcomes. But SARS-CoV-2 has not been reported to induce severe inflammation in the placenta, for which a possible hypothesis suggested is that virus can replicate in these cells but might not be released. Pyroptosis leads to the release of lactate dehydrogenase (LDH) which is an enzyme present in the cytoplasm of the cells and released to the extracellular environment after rupturing of the cell membrane, hence pyroptosis is monitored by LDH level. This fact can be used in COVID-19 infected mothers to evaluate the severity [54].
A prospective histopathological study on 27 placentas of asymptomatic or mildly symptomatic SARS-CoV-2 positive mothers observed features of MVM which included retroplacental hematomas (RPH), accelerated villous maturation (AVM), distal villous hyperplasia (DVH), atherosis, fibrinoid necrosis, mural hypertrophy of membrane arterioles (MHMA), vessel ectasia and persistence of intramural endovascular trophoblast (PIEVT). Features of FVM reported in the placenta of cases showed chorangiosis, thrombosis of the fetal chorionic plate (TFCP), intramural fibrin deposition (IMFD), and vascular ectasia along with, perivillous fibrin deposition [55]. A study done on 34 African American COVID-19 positive mothers and their placentas did not report any specific placental histopathological changes. According to this study, there is no increased susceptibility or worse outcomes of COVID-19 infection in pregnancy and no evidence of vertical transmission observed in the neonates [56]. Similar findings were observed by Cribiù et al. in the placentas of 37 SARS-CoV-2 positive mothers. Only in one patient, did they observe adverse neonatal outcomes with neurological symptoms with obvious placental injury on pathological examination. The findings of both studies suggest that the placenta acts as an effective barrier even against COVID-19 infection [57].
A clinico-pathological study was done by Debelenko et al on 75 placentas of SARS-CoV-2 positive mothers, where they observed features of injury of the cell as dusky nuclei with homogenized chromatin, pyknosis, karyorrhexis, loss of nuclear basophilia, and clearing of cytoplasm with the formation of ghost cells eventually. Other changes demonstrated included perivillous fibrin deposition and inflammatory changes in the IVS. Inflammatory infiltrates predominantly consisted of monocytes/macrophages, neutrophils, and a few lymphocytes that showed positive staining, respectively with CD68, CD15, and CD3. Villous stroma showed reactive vascular proliferation and activated fibroblasts (myofibroblasts) [58].
Reports of vertical transmission of SARS-CoV-2 infection from mother to fetus have been very rare. The authors observed significant pathological changes in the placenta of COVID positive mother; more than 50% of placental tissue was found involved with massive fibrin deposition in the IVS covering along with infiltration of neutrophilic granulocytes (predominantly myeloperoxidase positive) and CD68 positive macrophages [59]. Viral protein was detected in the cytoplasm and nucleus of villous CTB and STB at the sites of intervillositis by IHC (Figure 4). [59, 60]. The fetus was delivered by the caesarian section as was necessitated by reduced fetal movements and baseline variability of fetal heart rate. The neonate was detected positive for the viral RNA in the nasopharyngeal swab tested 48 hours after delivery. An external transmission post-birth was ruled out as the neonate had no contact with any family member, including the mother, during the first 60 hours of life, neither skin-to-skin nor any other contact with the mother occurred during this period. A matching of the viral sequences from the mother and neonate strongly indicated intra-uterine transfer of the infection. Neonate suffered from transient asphyxia, which was possibly due to dysfunction of the infected placenta which lead to intrauterine hypoxia [20]. Another case series on eight COVID-19 confirmed pregnant females, presented at 33–40 weeks of gestation where 4 out of 8 cases were associated with major complications like anemia, hypertension, pericardial effusion, thrombocytopenia. Histopathology of placental tissue showed increased syncytial knots (MVM) and an increase in focal perivillous fibrin deposition in all the cases [61, 62].
CONCLUSION
This review suggests that SARS-CoV-2 infection in pregnancy can lead to significant histopathological changes in the placental tissue, most common being fetal and maternal vascular malformation and villitis of unknown etiology. The relationship between these microscopic changes and the final obstetric outcome is to be studied in detail, which could help clinicians for better obstetric care of their patients during the COVID-19 pandemic. Increasing the sample size and involving matched control groups, and earlier identification of COVID-19 positive mothers would increase the reliability of the study findings. Hence, we suggest for large multicenter studies to be conducted with routine, blinded histopathologic evaluation of placentas. These studies must plan a prior sensitivity analysis based on whether or not the pregnant women were symptomatic, gestational age at infection, and whether or not the pregnancy was affected by other comorbidities.
Supplementary Information
Footnotes
Statement of Ethics
As the study is a systematic review, based exclusively on published literature, and ethics statement is not applicable. As human subjects were not directly involved, informed consent was also not applicable as we limited our study to published information. All the authors participated in the process of data collection and qualitative analysis, and errors or disagreements among researchers if any were resolved by discussion. Any further statistical analysis and quantitative data analysis were not done for this review.
Authors contribution
RM: Writing of the first draft of the manuscript including tables and image collection. VD: reference management, AK, CK, KR, VD and HK: Review and editing of the final manuscript. All authors consented to the submission of the final manuscript.
Funding
The author(s) received no substantial financial support for this review study.
Declaration of conflicting interests
The authors declare no potential conflicts of interest.
Data availability statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus (COVID-19) Dashboard [Internet] [cited 2022 May 6]. Available from: https://covid19.who.int.
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–80e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosier H, Farhadian SF, Morotti RA, et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130(9):4947–53. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins JR, Bakardjiev AI. Pathogens and the placental fortress. Curr Opin Microbiol. 2012;15(1):36–43. doi: 10.1016/j.mib.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X, Jiang Y, Zhao Y, et al. Analysis of the susceptibility to COVID-19 in pregnancy and recommendations on potential drug screening. Eur J Clin Microbiol Infect Dis. 2020;39(7):1209–20. doi: 10.1007/s10096-020-03897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jing Y, Run-Qian L, Hao-Ran W, et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod. 2020;26(6):367–73. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aplin JD, Myers JE, Timms K, et al. Tracking placental development in health and disease. Nat Rev Endocrinol. 2020;16(9):479–94. doi: 10.1038/s41574-020-0372-6. [DOI] [PubMed] [Google Scholar]
- 9.Barthold SW, Beck DS, Smith AL. Mouse hepatitis virus and host determinants of vertical transmission and maternally-derived passive immunity in mice. Arch Virol. 1988;100(3–4):171–83. doi: 10.1007/BF01487681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng WF, Wong SF, Lam A, et al. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology (Phila) 2006;38(3):210–8. doi: 10.1080/00313020600696280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. The Lancet. 2020;395(10226):809–15. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi-Zarchi M, Neamatzadeh H, Dastgheib SA, et al. Vertical Transmission of Coronavirus Disease 19 (COVID-19) from Infected Pregnant Mothers to Neonates: A Review. Fetal Pediatr Pathol. 2020;39(3):246–50. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He M, Skaria P, Kreutz K, et al. Histopathology of Third Trimester Placenta from SARS-CoV-2-Positive Women. Fetal Pediatr Pathol. 2020;12:1–10. doi: 10.1080/15513815.2020.1828517. [DOI] [PubMed] [Google Scholar]
- 14.Mullins E, Evans D, Viner RM, et al. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. doi: 10.1002/uog.22014. [DOI] [PubMed] [Google Scholar]
- 15.Fan C, Lei D, Fang C, et al. Perinatal Transmission of 2019 Coronavirus Disease–Associated Severe Acute Respiratory Syndrome Coronavirus 2: Should We Worry? Clin Infect Dis. 2021;72(5):862–4. doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasco Santana L, Miraval Wong E, Álvarez-Troncoso J, et al. Maternal and perinatal outcomes and placental pathologic examination of 29 SARS-CoV-2 infected patients in the third trimester of gestation. J Obstet Gynaecol Res. 2021;47(6):2131–9. doi: 10.1111/jog.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lokken EM, Walker CL, Delaney S, et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2020;223(6):911e1–911.e14. doi: 10.1016/j.ajog.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Algarroba GN, Rekawek P, Vahanian SA, et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223(2):275–8. doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu AL, Guan M, Johannesen E, et al. Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease. J Med Virol. 2021;93(2):1038–44. doi: 10.1002/jmv.26386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaigham M, Holmberg A, Karlberg M, et al. Intrauterine vertical SARS-CoV-2 infection: a case confirming transplacental transmission followed by divergence of the viral genome. BJOG Int J Obstet Gynaecol. 128(8):1388–94. doi: 10.1111/1471-0528.16682. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khong TY, Mooney EE, Ariel I, et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016;140(7):698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 22.Baergen RN, Heller DS. Placental Pathology in Covid-19 Positive Mothers: Preliminary Findings. Pediatr Dev Pathol. 2020;23(3):177–80. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertero L, Borella F, Botta G, et al. Placenta in SARS-CoV-2 infection: a new target for inflammatory and thrombotic event. In Review. 2020. May, [cited 2022 May 6]. Available from: https://www.researchsquare.com/article/rs-30412/v1.
- 24.Shanes ED, Mithal LB, Otero S, et al. Placental Pathology in COVID-19. Am J Clin Pathol. 2020;154(1):23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulvey JJ, Magro CM, Ma LX, et al. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann Diagn Pathol. 2020;46:151530. doi: 10.1016/j.anndiagpath.2020.151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang P, Salafia C, Heyman T, et al. Detection of severe acute respiratory syndrome coronavirus 2 in placentas with pathology and vertical transmission. Am J Obstet Gynecol MFM. 2020;2(4):100197. doi: 10.1016/j.ajogmf.2020.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smithgall MC, Liu-Jarin X, Hamele-Bena D, et al. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology. 2020;77(6):994–9. doi: 10.1111/his.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linehan L, O’Donoghue K, Dineen S, et al. SARS-CoV-2 placentitis: An uncommon complication of maternal COVID-19. Placenta. 2021;104:261–6. doi: 10.1016/j.placenta.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prabhu M, Cagino K, Matthews K, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG Int J Obstet Gynaecol. 2020;127(12):1548–56. doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patberg ET, Adams T, Rekawek P, et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol. 2021;224(4):382e1–382.e18. doi: 10.1016/j.ajog.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patanè L, Morotti D, Giunta MR, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019–positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2(3):100145. doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020;33(11):2092–103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Facchetti F, Bugatti M, Drera E, et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. eBioMedicine. 2020;59:102951. doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6):e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menter T, Mertz KD, Jiang S, et al. Placental Pathology Findings during and after SARS-CoV-2 Infection: Features of Villitis and Malperfusion. Pathobiology. 2021;88(1):69–77. doi: 10.1159/000511324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marinho PS, da Cunha AJLA, Chimelli L, et al. Case Report: SARS-CoV-2 Mother-to-child transmission and fetal death associated with severe placental thromboembolism. Front Med. 2021;16(8):677001. doi: 10.3389/fmed.2021.677001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozer E, Cagliyan E, Yuzuguldu RI, et al. Villitis of unknown etiology in the placenta of a pregnancy complicated by Covid-19. Turk J Pathol. 2021;37(2):167–71. doi: 10.5146/tjpath.2020.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts J, Cheng JD, Moore E, et al. Extensive perivillous fibrin and intervillous histiocytosis in a SARS-CoV-2 infected placenta from an uninfected newborn: a case report including immunohistochemical profiling. Pediatr Dev Pathol. 2021;24(6):581–4. doi: 10.1177/10935266211025122. [DOI] [PubMed] [Google Scholar]
- 39.Bouachba A, Allias F, Nadaud B, et al. Placental lesions and SARS-Cov-2 infection: Diffuse placenta damage associated to poor fetal outcome. Placenta. 2021;112:97–104. doi: 10.1016/j.placenta.2021.07.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marton T, Hargitai B, Hunter K, et al. Massive Perivillous Fibrin Deposition and Chronic Histiocytic Intervillositis a Complication of SARS-CoV-2 Infection. Pediatr Dev Pathol. 2021;24(5):450–4. doi: 10.1177/10935266211020723. [DOI] [PubMed] [Google Scholar]
- 41.Pulinx B, Kieffer D, Michiels I, et al. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur J Clin Microbiol Infect Dis. 2020;39(12):2441–5. doi: 10.1007/s10096-020-03964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morotti D, Cadamuro M, Rigoli E, et al. Molecular Pathology Analysis of SARS-CoV-2 in Syncytiotrophoblast and Hofbauer Cells in Placenta from a Pregnant Woman and Fetus with COVID-19. Pathogens. 2021;10(4):479. doi: 10.3390/pathogens10040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanna N, Lin X, Thomas K, et al. Underestimation of SARS-CoV-2 infection in placental samples. Am J Obstet Gynecol. 2021;225(5):572–575. doi: 10.1016/j.ajog.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valdespino-Vázquez MY, Helguera-Repetto CA, León-Juárez M, et al. Fetal and placental infection with SARS-CoV-2 in early pregnancy. J Med Virol. 2021;93(7):4480–7. doi: 10.1002/jmv.26965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watkins JC, Torous VF, Roberts DJ. Defining Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Placentitis. Arch Pathol Lab Med. 2021;145(11):1341–9. doi: 10.5858/arpa.2021-0246-SA. [DOI] [PubMed] [Google Scholar]
- 46.Rosner-Tenerowicz A, Fuchs T, Zimmer-Stelmach A, et al. Placental pathology in a pregnant woman with severe COVID-19 and successful ECMO treatment: a case report. BMC Pregnancy Childbirth. 2021;21(1):760. doi: 10.1186/s12884-021-04228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sallenave JM, Guillot L. Innate immune signaling and proteolytic pathways in the resolution or exacerbation of SARS-CoV-2 in Covid-19: Key Therapeutic Targets? Front Immunol. 2020;11:1229. doi: 10.3389/fimmu.2020.01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babal P, Krivosikova L, Sarvaicova L, et al. Intrauterine Fetal Demise After Uncomplicated COVID-19: What Can We Learn from the Case? Viruses. 2021;13(12):2545. doi: 10.3390/v13122545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poisson TM, Pierone G. Placental pathology and fetal demise at 35 weeks of gestation in a woman with SARS-CoV-2 infection: A case report. Case Rep Womens Health. 2021;30:e00289. doi: 10.1016/j.crwh.2021.e00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stonoga ETS, de Almeida Lanzoni L, Rebutini PZ, et al. Intrauterine Transmission of SARS-CoV-2. Emerg Infect Dis. 2021;27(2):638–41. doi: 10.3201/eid2702.203824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rebutini PZ, Zanchettin AC, Stonoga ETS, et al. Association Between COVID-19 Pregnant Women Symptoms Severity and Placental Morphologic Features. Front Immunol. 2021;12:685919. doi: 10.3389/fimmu.2021.685919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Komine-Aizawa S, Takada K, Hayakawa S. Placental barrier against COVID-19. Placenta. 2020;99:45–9. doi: 10.1016/j.placenta.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han Y, Zhang H, Mu S, et al. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: a retrospective and observational study. Aging. 2020;12(12):11245–58. doi: 10.18632/aging.103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rayamajhi M, Zhang Y, Miao EA. Detection of Pyroptosis by Measuring Released Lactate Dehydrogenase Activity. Methods in Molecular Biology. 2013;1040:85–90. doi: 10.1007/978-1-62703-523-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaiswal N, Puri M, Agarwal K, et al. COVID-19 as an independent risk factor for subclinical placental dysfunction. Eur J Obstet Gynecol Reprod Biol. 2021;259:7–11. doi: 10.1016/j.ejogrb.2021.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jani S, Jacques SM, Qureshi F, et al. Clinical Characteristics of Mother-Infant Dyad and Placental Pathology in COVID-19 Cases in Predominantly African American Population. Am J Perinatol Rep. 2021;11(01):e15–20. doi: 10.1055/s-0040-1721673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cribiù FM, Erra R, Pugni L, et al. Severe SARS-CoV-2 placenta infection can impact neonatal outcome in the absence of vertical transmission. J Clin Invest. 2021;131(6):e145427. doi: 10.1172/JCI145427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Debelenko L, Katsyv I, Chong AM, et al. Trophoblast damage with acute and chronic intervillositis: disruption of the placental barrier by severe acute respiratory syndrome coronavirus 2. Hum Pathol. 2021;109:69–79. doi: 10.1016/j.humpath.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Resta L, Vimercati A, Sablone S, et al. Is the first of the two born saved? A rare and dramatic case of double placental damage from SARS-CoV-2. Viruses. 2021;13(6):995. doi: 10.3390/v13060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schoenmakers S, Snijder P, Verdijk RM, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Placental Infection and Inflammation Leading to Fetal Distress and Neonatal Multi-Organ Failure in an Asymptomatic Woman. J Pediatr Infect Dis Soc. 2021;10(5):556–61. doi: 10.1093/jpids/piaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He JR, Xiao YH, Ding W, et al. Maternal, placental and neonatal outcomes after asymptomatic SARS-CoV-2 infection in the first trimester of pregnancy: A case report. Case Rep Womens Health. 2021;31:e00321. doi: 10.1016/j.crwh.2021.e00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao L, Ren J, Xu L, et al. Placental pathology of the third trimester pregnant wmen from COVID-19. Diagn Pathol. 2021;16(1):8. doi: 10.1186/s13000-021-01067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.