Abstract
Background:
A line of evidence has shown that childhood trauma and patterns of H-shaped sulci in the orbitofrontal cortex (OFC) are associated with cognitive deficits in patients with schizophrenia. Studies have also suggested that childhood trauma is associated with OFC volumetrics. This study investigated the interrelationship between childhood trauma, OFC H-shaped sulci volume and cognitive function in patients with first-episode schizophrenia. We hypothesized that OFC H-shaped sulci volume would mediate the relationship between childhood trauma and cognitive function in patients with first-episode schizophrenia.
Methods:
We recruited patients with first-episode schizophrenia (n = 63) and healthy controls (n = 48), and quantified OFC H-shaped sulci volumes with 3.0 T high-resolution MRI. We assessed cognitive function and childhood trauma experiences using the MATRICS Consensus Cognitive Battery (MCCB) and the Childhood Trauma Questionnaire (CTQ).
Results:
Patients with first-episode schizophrenia had smaller left OFC H-shaped sulci volumes, more severe childhood trauma experiences and worse cognitive function than healthy controls. CTQ total score and emotional and physical neglect subscores were negatively correlated with left OFC H-shaped sulci volume. CTQ total score and emotional neglect and sexual abuse subscores were negatively correlated with cognitive function in patients with first-episode schizophrenia. Interestingly, the CTQ total score and physical neglect subscore were positively correlated with cognitive function in healthy controls. Left OFC H-shaped sulci volume played a mediating role in CTQ emotional neglect subscore, CTQ total score and MCCB composite score.
Limitations:
The small sample size and retrospective design need to be considered.
Conclusion:
Childhood trauma might contribute to cognitive deficits in patients with first-episode schizophrenia by affecting left OFC H-shaped sulci volume. This finding can help in the design of strategies to improve cognitive function in patients with first-episode schizophrenia.
Introduction
The orbitofrontal cortex (OFC), located in the ventral prefrontal cortex, plays a critical role in the regulation of human emotion, decision-making, motivation and social behaviour.1 Studies have shown that damage to the OFC can lead to emotional instability, apathy and social withdrawal, similar to the negative symptoms of schizophrenia.2 Moreover, recent studies have indicated that changes in the surface morphology of the OFC are associated with schizophrenia and ultra-high risk for psychosis.3,4
Previous studies have focused mainly on variations in H-shaped sulci patterns and reduced numbers of orbital (including posterior and intermediate) sulci in the OFC.5 Patients with schizophrenia show type III H-shaped sulci patterns more frequently than controls, and type I patterns less frequently than controls.3,6 The patterns of the OFC H-shaped sulci have also been associated with psychiatric symptoms and cognitive function. Patients with OFC type III sulci patterns demonstrated higher scores on the Positive and Negative Syndrome Scale, with more severe positive symptoms, social withdrawal and poorer cognitive function.7,8 Other studies have shown diminished total and subregional OFC volumes (middle orbital gyrus) in patients with chronic and first-episode schizophrenia.6,9
The OFC H-shaped sulcus is a configuration of multiple component patterns of the medial, lateral and transverse sulcus of the OFC.10 It is formed in middle and late gestation (16–44 wk) and is relatively stable after formation.11 However, synaptic pruning and cortical thinning during puberty may alter OFC volume.12 In addition, the pattern of the OFC is independent of changes in OFC volume.9
Childhood trauma not only represents a risk factor for the development of schizophrenia, but also has substantial impact on cognitive function.13 Patients with schizophrenia who have a history of childhood trauma have lower IQ scores (especially in verbal intelligence) and poorer performance with respect to delayed memory, attention, executive function, visual learning and social cognition.14–16 Other studies have investigated the effects of subtypes of childhood trauma on cognitive function and presented contrasting findings. For instance, one study reported that childhood sexual abuse was specifically associated with deficits in working memory, executive function and processing speed.17 Another showed effects of emotional neglect on verbal memory, visual learning and social cognition, but no effects of other subtypes of childhood trauma.13
Importantly, childhood trauma has been associated with morphometric features in the OFC. Using tensor-based morphometry, Hanson and colleagues18 found that children who had experienced physical abuse had reduced OFC grey matter volume compared to children who were not abused. De Brito and colleagues19 used voxel-based morphometry to study community-recruited children aged 10 to 14 years with a history of childhood maltreatment and found that those children had reduced grey matter volume in the medial OFC. A prospective study with 20 years of follow-up showed that OFC and hippocampal volumes were both reduced in women with chronic stress.20
Given the relationship between childhood trauma and morphometrics in the OFC, as well as findings of distinct OFC morphometric features associated with psychiatric and cognitive symptoms in schizophrenia, we hypothesized that childhood trauma would be associated with a smaller OFC H-shaped sulci volume and poorer cognitive function in patients with first-episode schizophrenia. We also hypothesized that lower OFC H-shaped sulci volume would serve as a mediator between childhood trauma and cognitive impairment in patients with first-episode schizophrenia.
Methods
Participants and study procedures
We recruited 63 patients with first-episode schizophrenia who were admitted to Beijing Huilongguan Hospital. Inclusion criteria were as follows: patients who met DSM-IV criteria for a diagnosis of first-episode schizophrenia, a disease duration of less than 3 years, taking antipsychotic medications for less than 2 weeks, and age 18 to 45 years. Exclusion criteria were as follows: any other DSM-IV Axis I disorder; any organic brain or other serious physical illness; drug or alcohol dependence, or substance abuse; intellectual disability; and pregnancy or lactation.
Using advertisements, we recruited 48 healthy control participants from the community who matched patients’ sex, age and education level. The educational level of participants in our study ranged from 6 to 20 years (mean ± standard deviation 13.4 ± 3.1 yr), and we found no difference between patients with first-episode schizophrenia and healthy controls (13.2 ± 3.3 yr v. 13.6 ± 2.8 yr; t = −0.96, p = 0.34).
This study was approved by the ethics committee of Beijing Huilongguan Hospital. All participants provided written informed consent before participating in the study.
Clinical assessment and imaging data
We used the Childhood Trauma Questionnaire (CTQ) to assess childhood trauma experiences in patients and healthy controls. The CTQ includes 5 subscales: emotional abuse, physical abuse, sexual abuse, emotional neglect and physical neglect. Each subscale is scored from 5 to 25; a higher score indicates more severe abuse. The CTQ has good reliability and validity in patients with first-episode schizophrenia and healthy controls.21,22
We used the Chinese version of the MATRICS Consensus Cognitive Battery (MCCB) to evaluate cognitive function in patients and healthy controls. The MCCB includes 7 psychological dimensions and 10 subtests: processing speed (including a connection test, symbol coding and semantic fluency); attention or vigilance (continuous operation test); working memory (including number sequence and spatial span); verbal memory; visual memory; reasoning and problem-solving ability (maze test); and social cognition (emotion management test). Studies have shown the MCCB to have good reliability and validity in Chinese patients with first-episode schizophrenia.23 All patients completed the MCCB; 4 healthy controls declined to complete it.
We obtained imaging data using a 3.0 T MRI system ( Siemens) at Beijing Huilongguan Hospital, using standard cranial coils positioned in the median sagittal plane. Scanning parameters were as follows: echo time 2.98 ms, repetition time 2530 ms, flip angle 7°, matrix size 256 × 224, field of view 256 × 224 mm, inversion time 1100 ms, thickness/gap 1/0 mm. After we had collected the required images, we collected imaging data.
We used Freesurfer version 5.3.0 for automatic and effective segmentation (surfer.nmr.mgh.harvard/freesurfer/). We extracted volume measurements using standard procedures, including motion correction, brain tissue mixed watershed or surface deformation procedures and automatic Talairach transformation. Then, we segmented the OFC H-shaped groove as the region of interest and performed intensity normalization and automatic topological correction. We also manually checked image quality and ensured motion correction.24
Statistical analyses
We used Student t and χ2 tests for group comparison of continuous and categorical variables, respectively.
We used multivariate analysis with a generalized linear model to compare group differences between left and right OFC H-shaped sulci volume, as well as differences in MCCB composite and subtest scores, with age, sex, education level and total intracranial volume as covariates.
We used the Mann–Whitney U test from the nonparametric test to compare CTQ total and subscale scores between groups. We input left and right OFC H-shaped sulci volumes, MCCB dimension scores and composite score into the model as dependent variables, and added the 5 CTQ subscale scores as independent variables to conduct multivariate linear regression using sex, age, education level and total intracranial volume as covariates.
We used general linear regression to analyze the relationship between CTQ total score and left and right OFC H-shaped sulci volume, MCCB dimension scores and MCCB composite score, using age, sex, educational level and total intracranial volume as covariates.
We considered p values < 0.05 (2-sided) to be significant. We corrected for the results of multiple comparisons using false discovery rate (FDR) correction. We used SPSS version 20.0 (IBM) software for all analyses.
Mediation analyses
Based on the results of the linear regression, we used the PROCESS version 3.4 macro in SPSS to conduct mediation effect analysis. In the mediation effect model, we used CTQ total score as an independent variable. Emotional neglect was also nominally correlated with visual learning and MCCB composite score, so we included it as an independent variable in the mediation analysis. We included MCCB visual learning and composite scores as dependent variables. We used left OFC H-shaped sulci volume as a mediator.
Pearson correlation analysis showed that sex and total intracranial volume were not correlated with CTQ emotional neglect subscore or total score, left OFC H-shaped sulci volume, or MCCB visual learning or composite score. Age was negatively correlated with left OFC H-shaped sulci volume (r = −0.263, p = 0.037), and education level was positively correlated with MCCB visual learning score and composite scores (r = 0.481, p < 0.001; r = 0.304, p = 0.016), so we included age and education level as covariates. We used a bootstrap method of 5000 samples to test the significance of the mediating effect. A significant mediating effect was indicated by a 95 % confidence interval for the indirect effect that did not include zero.
Results
As shown in Table 1, patients with first-episode schizophrenia and healthy controls were matched by age, sex and education level.
Table 1.
Participant characteristics
| Characteristica | Patients with first-episode schizophrenia n = 63 |
Healthy controls n = 48 |
Statistic | p value |
|---|---|---|---|---|
| Male/female | 30/33 | 28/20 | χ2 = 1.25 | 0.26 |
| Age, yr | 27.5 ± 6.8 | 30.5 ± 7.8 | t = −2.18 | 0.31 |
| Education, yr | 13.2 ± 3.3 | 13.8 ± 2.6 | t = −0.96 | 0.34 |
| Age at onset, yr | 26.8 ± 7.5 | NA | NA | NA |
| Illness duration, mo | 11.6 ± 12.2 | NA | NA | NA |
| CTQ | ||||
| Emotional abuse subscore | 7.09 ± 2.31 | 6.00 ± 1.46 | z = −2.89 | 0.004 |
| Physical abuse subscore | 5.68 ± 1.58 | 5.40 ± 1.25 | z = −1.53 | 0.13 |
| Sexual abuse subscore | 6.05 ± 2.83 | 5.17 ± 0.37 | z = −2.31 | 0.020 |
| Emotional neglect subscore | 9.32 ± 4.02 | 7.67 ± 2.94 | z = −2.17 | 0.030 |
| Physical neglect subscore | 11.14 ± 2.23 | 10.10 ± 1.36 | z = −2.42 | 0.016 |
| Total score | 39.29 ± 7.91 | 34.33 ± 5.12 | z = −3.76 | < 0.001 |
| MCCB | ||||
| Speed of processing subscore | 49.65 ± 11.15 | 56.72 ± 7.55 | F = 44.81 | < 0.001 |
| Attention or vigilance subscore | 42.33 ± 8.72 | 57.98 ± 7.01 | F = 87.14 | < 0.001 |
| Working memory subscore | 48.35 ± 9.80 | 58.89 ± 5.45 | F = 36.17 | 0.002 |
| Verbal learning subscore | 49.65 ± 11.15 | 56.73 ± 7.55 | F = 10.18 < | 0.001 |
| Visual learning subscore | 47.35 ± 9.56 | 55.82 ± 6.04 | F = 10.18 < | 0.001 |
| Reason and problem-solving subscore | 49.10 ± 9.02 | 57.05 ± 6.16 | F = 32.34 | < 0.001 |
| Social cognition subscore | 46.56 ± 8.50 | 54.20 ± 10.26 | F = 12.58 | 0.001 |
| Composite score | 46.00 ± 8.32 | 58.89 ± 5.80 | F = 78.95 | < 0.001 |
| Right OFC H-shaped sulci volume, cm3 | 2.49 ± 0.26 | 2.52 ± 0.37 | F = 0.06 | 0.81 |
| Left OFC H-shaped sulci volume, cm3 | 2.56 ± 0.31 | 2.69 ± 0.36 | F = 6.25 | 0.014 |
CTQ = Childhood Trauma Questionnaire; MCCB = MATRICS Consensus Cognitive Battery; NA = not applicable; OFC = orbitofrontal cortex.
Adjusting for sex, age, education level and total intracranial volume as covariates in the general linear model. Values are mean ± standard deviation unless otherwise indicated.
In patients with first-episode schizophrenia, the mean age at illness onset (± standard deviation) was 26.8 ± 7.5 years, and the mean illness duration was 11.6 ± 12.2 months. Compared to healthy controls, patients with first-episode schizophrenia showed higher CTQ emotional abuse, sexual abuse, emotional and physical neglect subscale scores and a higher CTQ total score (all p < 0.05). Patients with first-episode schizophrenia also showed significantly lower MCCB dimension and composite scores (all p < 0.05).
Compared to healthy controls, patients with first-episode schizophrenia had smaller left OFC H-shaped sulci volumes. We found no between-group difference in right OFC H-shaped sulci volume.
Childhood trauma and OFC H-shaped sulci volume
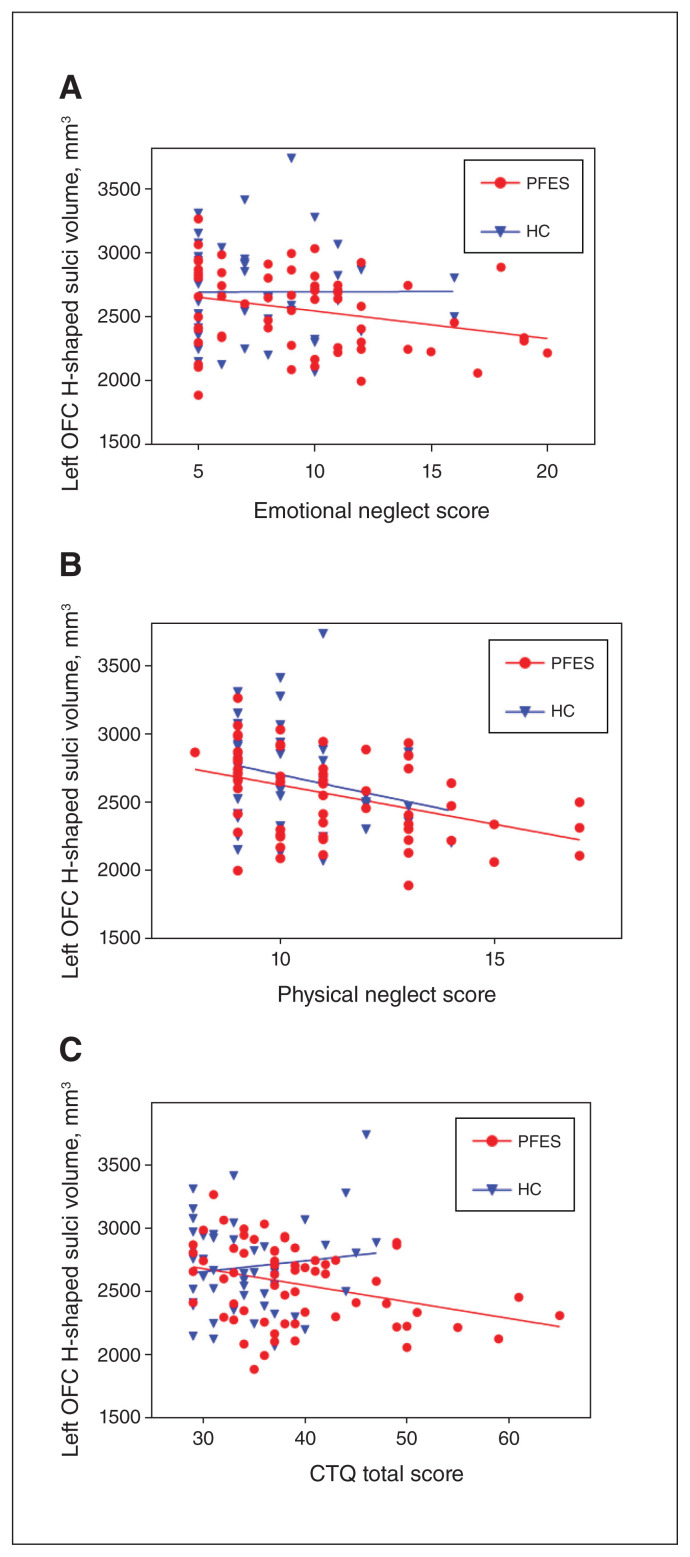
In patients with first-episode schizophrenia, the results of linear regression analysis (with sex, age, education level and total intracranial volume as covariates) showed that CTQ emotional and physical neglect subscale scores and CTQ total score were negatively correlated with left OFC H-shaped sulci volume (t = −2.16, p = 0.036; t = −2.26, p = 0.028; and t = −2.53, p = 0.014, respectively). Only the CTQ physical abuse subscale score was positively associated with right OFC H-shaped sulci volume (t = 2.05, p = 0.046). We found no correlations with other CTQ subscale scores, or for right OFC H-shaped sulci volume and CTQ total score.
In healthy controls, we found no correlation between CTQ subscale or total scores and left or right OFC H-shaped sulci volumes (Figure 1 and Appendix 1, Tables S1 and S2, available at www.jpn.ca/lookup/doi/10.1503/jpn.210178/tab-related-content).
Figure 1.
Correlation between childhood trauma and left OFC H-shaped sulci volume in patients with first-episode schizophrenia and healthy controls: (A) emotional neglect subscore; B) physical neglect subscore; (C) CTQ total score. Associations were significant at p < 0.05 after adjusting for age, sex, education level and total intracranial volume for PFES. CTQ = Childhood Trauma Questionnaire; HC = healthy controls; OFC = orbitofrontal cortex; PFES = patients with first-episode schizophrenia.
Childhood trauma and cognitive function
In patients with first-episode schizophrenia, the results of multivariate linear regression showed that CTQ sexual abuse subscale score was negatively correlated with MCCB working memory dimension score (t = −2.23, p = 0.030). We also found negative correlations between CTQ emotional neglect subscale score and MCCB visual learning and composite scores (t = −2.23, p = 0.030; and t = −2.49, p = 0.016, respectively). However, after FDR correction, none of these correlations was significant.
In healthy controls, CTQ emotional neglect subscale score was negatively correlated with MCCB reasoning and problem-solving ability score (t = −2.68, p = 0.011). CTQ physical neglect subscale score was positively correlated with MCCB working memory, visual learning and composite scores (t = 2.31, p = 0.027; t = 4.49, p < 0.001; t = 2.59, p = 0.014, respectively). However, after FDR correction, we found only a nominal correlation between CTQ physical neglect subscale and MCCB working memory dimension scores.
We also found that in patients with first-episode schizophrenia, CTQ total score was negatively correlated with MCCB dimension scores for attention or vigilance, working memory, visual memory, and reasoning and problem-solving ability, as well as the MCCB composite score, (t = −2.13, p = 0.038; t = −2.56, p = 0.013; t = −2.60, p = 0.012; t = −2.53, p = 0.013; and t = −2.44, p = 0.018, respectively). However, the correlation between CTQ total score and MCCB attention or vigilance dimension score was not significant after FDR correction (Appendix 1, Tables S3 and S4).
OFC H-shaped sulci volume and cognitive function
In patients with first-episode schizophrenia, the results of general linear regression analysis (with age, sex, educational level and total intracranial volume as covariates) showed a positive correlation between left OFC H-shaped sulci volume and MCCB speed of processing, working memory and visual learning dimension scores, as well as MCCB composite scores (t = 2.54, p = 0.014; t = 2.45, p = 0.017; t = 2.29, p = 0.026; t = 3.09, p = 0.003, respectively). However, the correlation between left OFC H-shaped sulci volume and MCCB visual learning dimension score was not significant after FDR correction. We found no correlation between right OFC H-shaped sulci volume and cognitive function in patients with first-episode schizophrenia (Appendix 1, Tables S5 and S6).
In healthy controls, neither left nor right OFC H-shaped sulci volume was associated with MCCB scores.
Mediation analysis
In the analyses above, we found that CTQ emotional neglect subscale and total scores showed nominal or significant negative correlations with left OFC H-shaped sulci volume and with MCCB visual learning dimension and composite scores; we also found that left OFC H-shaped sulci volume showed nominal or significant positive correlations with MCCB visual learning dimension and composite scores. Because of these findings, we employed mediation analyses to investigate the interrelationship between CTQ emotional neglect subscale and total scores, and left OFC H-shaped sulci volume.
In mediating effect models, we used CTQ measures as predictors and MCCB visual learning dimension and composite scores as outcome measures. We used left OFC H-shaped sulci volume as the mediator in all models. We focused on the relationships among CTQ emotional neglect subscale score, CTQ total score, left OFC H-shaped sulci volume and MCCB composite score in models 1 and 2 (Figure 2 and Figure 3). We also focused on the relationships among CTQ emotional neglect subscale score, CTQ total score, left OFC H-shaped sulci volume and MCCB visual learning dimension score in models 3 and 4 (Appendix 1, Figures S1 and S2).
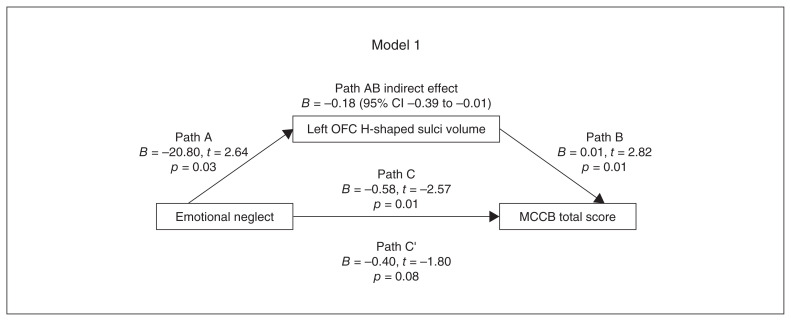
Figure 2.
Model 1, path diagram of the mediation model (x = emotional neglect; y = MCCB total score; left OFC H-shaped sulci volume as mediator). Paths C’ and C represent the direct and total effects between emotional neglect and left OFC H-shaped sulci volume. Path AB represents the mediation effect and was significant at p < 0.05. CI = confidence interval; MCCB = MATRICS Consensus Cognitive Battery; OFC = orbitofrontal cortex.
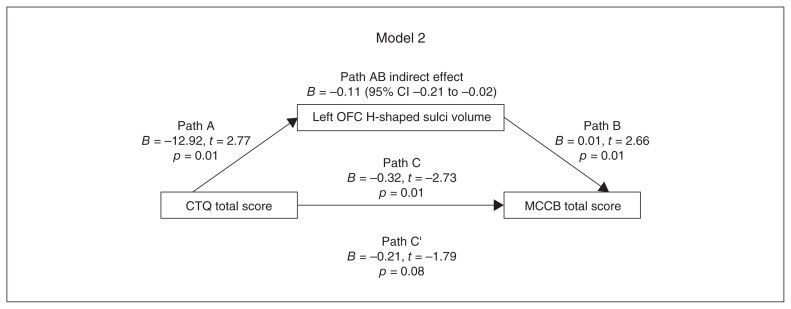
Figure 3.
Model 2, path diagram of the mediation model (x = CTQ total score; y = MCCB total score; left OFC H-shaped sulci volume as mediator). Paths C’ and C represent the direct and total effects between CTQ total score and left OFC H-shaped sulci volume. Path AB represents the mediation effect and is significant at p < 0.05. CI = confidence interval; CTQ = Childhood Trauma Questionnaire; MCCB = MATRICS Consensus Cognitive Battery; OFC = orbitofrontal cortex.
In models 1 and 2, the indirect effect was significant, but the direct effect was not significant, suggesting a full mediating effect. In models 3 and 4, the indirect effect was not significant, suggesting that left OFC H-shaped sulci volume did not play a mediating role between CTQ emotional neglect subscale score, MCCB composite score and MCCB visual learning dimension score.
Discussion
Our findings showed that patients with first-episode schizophrenia had experienced more severe childhood trauma than healthy controls, and that childhood trauma was associated with poorer cognitive function and a smaller left OFC H-shaped sulci volume in patients with first-episode schizophrenia. Most importantly, we found that left OFC H-shaped sulci volume mediated the relationship between childhood trauma and cognitive function in patients with first-episode schizophrenia.
Consistent with previous studies,25,26 our findings suggest that childhood trauma as indexed by CTQ total and subscale scores (except for physical abuse) was higher in patients with first-episode schizophrenia than in healthy controls. Our lack of significant findings related to the CTQ physical abuse subscore in patients with first-episode schizophrenia and healthy controls may have had something to do with the traditional Chinese belief of “no beating, no success”; many Chinese parents think “stick education” is reasonable.27 Sampling error caused by our small sample size is also an important factor to consider. Our findings from a larger previous study (patients with first-episode schizophrenia [n = 192] and healthy controls [n = 136]) showed that CTQ physical abuse scores were significantly higher among patients with first-episode schizophrenia compared to healthy controls.28
Cognitive dysfunction is a core symptom of schizophrenia.29 We found that the 7 MCCB domain scores and composite score were impaired in patients with first-episode schizophrenia, consistent with the results of previous studies.30 Moreover, CTQ total score was significantly associated with cognitive deficits in patients with first-episode schizophrenia, especially the MCCB visual learning and reasoning and problem-solving ability dimensions. The CTQ total score represents an accumulation of multiple complex traumatic experiences. A British birth cohort study of 2322 children found that participants with complex traumatic experiences had poorer spatial working memory and repetitive visual information processing than participants who had not experienced childhood trauma or a single childhood traumatic experience.31 Another study of 23 807 people older than 45 years found that childhood trauma was significantly associated with impaired cognitive function, particularly episodic memory.32 In addition, studies have shown that childhood trauma can predict worse cognitive function in people with depressive disorders and bipolar disorder.33
Although previous studies have found that sexual abuse is associated with poorer cognitive function in schizophrenia, anxiety disorders and depressive disorders, our study found only a nominal association; this relationship deserves further investigation.17,34,35 We found that emotional neglect also had an effect on cognitive function, but this finding did not survive FDR correction. The effect of emotional neglect on cognitive function may be related to changes in the volume of hippocampus and amygdala.36,37
In healthy controls, CTQ physical neglect subscale score was associated with better MCCB visual learning and composite scores, and CTQ total score was associated with a better MCCB visual learning score. This finding might be related to the fact that healthy controls had more external protective factors when exposed to physical neglect than patients with schizophrenia. Previous studies have suggested that healthy controls had better social support, self-esteem and positive coping skills than patients with schizophrenia, and these protective factors were associated with a more acute cortisol awakening response.38 Increased cortisol arousal response is associated with better episodic memory, processing speed, spatial working memory, executive function and attention or vigilance in healthy adults.39,40
Patients with first-episode schizophrenia had a smaller left OFC H-shaped sulci volumes than the healthy controls. In patients with early-onset schizophrenia, bilateral OFC grey matter volume was decreased, especially in the left lateral OFC.41 Another study found that increased functional connectivity in the left OFC and anterior cingulate gyrus was associated with violent behaviour in schizophrenia.42 These findings all suggest a closer relationship between the left OFC and schizophrenia, but to our knowledge, no study has explored the relationship between H-shaped sulci volume and schizophrenia.
Childhood is a critical period for brain development, and numerous studies have shown that childhood trauma can lead to changes in brain structure and function in patients with schizophrenia.43,44 In a study of grey matter volume in the prefrontal corticolimbic system, childhood trauma had the most significant effect on volume reduction in the OFC, insula and left thalamus in patients with schizophrenia, followed by patients with bipolar disorder and healthy controls.45 We also found that CTQ emotional neglect, physical neglect and total scores were associated with reduced left OFC H-shaped sulci volume. The reasons for this are unclear, but increased inflammatory states, abnormal brain-derived neurotrophic factor levels and interactions between genes and the environment caused by childhood trauma are factors worthy of investigation.46,47 Animal experiments showed that dendrite refinement in the OFC was increased and the morphology of OFC dendritic spines was changed in rats undergoing chronic stress.48 Several studies have shown that the OFC is associated with race, low socioeconomic status, stress and other factors associated with childhood trauma.49
The present study demonstrated that only smaller left OFC H-shaped sulci volumes were significantly associated with poorer cognitive function, especially MCCB speed of processing, working memory and composite scores. The OFC is a cortical structure with important roles in cognition, memory, decision-making and learning. Individuals with smaller OFC volumes may exhibit poor learning and memory abilities.50 Structural and functional changes in the OFC are also found in diseases associated with severe cognitive impairment, such as dementia and Alzheimer disease.51,52 Imaging studies have shown that the OFC is closely related to brain regions associated with cognitive function, such as the hippocampus, amygdala and striatum.53,54 Studies have also shown that left OFC volume is related to cognitive function in depressive disorder, especially visual learning and instantaneous memory, which seems to suggest that the left OFC is more closely related to cognitive function.55 Future neuroimaging studies could extend such volumetric findings to better understand how the OFC contributes to the interrelationship between childhood trauma and cognitive dysfunction in schizophrenia and other clinical populations.
Our study indicated that reduced left OFC H-shaped sulci volume mediates the relationship between CTQ total score, CTQ emotional neglect subscore and cognitive function in patients with first-episode schizophrenia. Although previous studies have suggested that childhood trauma might lead to cognitive decline in schizophrenia, the underlying mechanism was unclear. Previous studies in participants with major depressive disorder have found that exposure to more severe early-life stress is associated with slower processing speed, poorer working memory and smaller OFC volume.56 In the present study, we found that reduced left H-shaped sulci volume was associated with childhood trauma (especially emotional neglect), and this in turn was a risk factor for cognitive deficits in people with first-episode schizophrenia.
Limitations
The following limitations should be considered. Our sample size was relatively small, which led to an increase in type II errors. As well, this was a retrospective study of childhood trauma with a recall bias. Still, some studies have shown that recall bias was less than 1 % compared to prospective studies.57 In addition, the CTQ was assessed retrospectively and might have been subject to memory bias. Negative events might be easier to remember for patients with first-episode schizophrenia compared to healthy controls, who might be thinking more positively.58 Future studies should take negative memory bias into account when exploring the effects of childhood trauma on brain structure and cognitive function.
The CTQ does not investigate the age at which childhood trauma occurred, or its duration, and these factors may influence the clinical manifestations of schizophrenia.59 Future studies should use larger sample sizes and prospective designs to explore the effects of these factors on schizophrenia.
Conclusion
The present study suggested that childhood trauma might lead to impaired cognitive function in patients with first-episode schizophrenia as a result of reduced left OFC H-shaped sulci volume. This phenomenon has rarely been investigated in previous studies. The present study included patients with first-episode schizophrenia who were not taking medications (or had taken medications for less than 2 weeks) and had a total course of disease of less than 3 years, to reduce the potential influence of antipsychotic drugs or disease course on OFC H-shaped sulci volume. Interventions to address childhood trauma — especially emotional neglect — may be a strategy for alleviating cognitive deficits in patients with schizophrenia.
Supplementary Material
Acknowledgement
The authors thank Editage (www.editage.cn) for English-language editing of the pre-accept version of the manuscript.
Footnotes
Competing interests: None declared.
Contributors: L. Wang, P. Zhang, S. Tan and Y. Tan designed the study. W. Feng, J. Huang and S. Chen acquired the data, which Y. Yin, Y. Zhou, H. Fan, Y. Cui, X. Luo, Z. Wang, B. Tian, L. Tian and C. Li analyzed. W. Feng, P. Zhang and S. Chen wrote the article, which L. Wang, Y. Yin, Y. Zhou, J. Huang, S. Chen, Y. Cui, X. Luo, S. Tan, Z. Wang, B. Tian, L. Tian, C. Li and Y. Tan reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Funding: Supports were received from the National Natural Science Foundation of China (82171507, 81761128021, 81771452) and the National Institute of Health (R01MH112180 and R01MH116948).
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon request.
References
- 1.Parsons CE, Stark EA, Young KS, et al. Understanding the human parental brain: a critical role of the orbitofrontal cortex. Soc Neurosci 2013;8:525–43. [DOI] [PubMed] [Google Scholar]
- 2.Walton E, Hibar DP, van Erp TGM, et al. Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychol Med 2018;48:82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cropley VL, Bartholomeusz CF, Wu P, et al. Investigation of orbitofrontal sulcogyral pattern in chronic schizophrenia. J Psychiatr Res 2015;234:280–3. [DOI] [PubMed] [Google Scholar]
- 4.Lavoie S, Bartholomeuz CF, Nelson B, et al. Sulcogyral pattern and sulcal count of the orbitofrontal cortex in individuals at ultra high risk for psychosis. J Schizophr Res 2014;154:93–9. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi T, Nakamura M, Nishikawa Y, et al. Decreased number of orbital sulci in schizophrenia spectrum disorders. Psychiatry Res Neuroimaging 2016;250:29–32. [DOI] [PubMed] [Google Scholar]
- 6.Takayanagi Y, Takahashi T, Orikabe L, et al. Volume reduction and altered sulco-gyral pattern of the orbitofrontal cortex in first-episode schizophrenia. J Schizophr Res 2010;121:55–65. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura M, Nestor PG, McCarley RW, et al. Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain 2007;130:693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi T, Nakamura M, Nishikawa Y, et al. Potential role of orbitofrontal surface morphology on social and cognitive functions in high-risk subjects for psychosis and schizophrenia patients. Psychiatry Res Neuroimaging 2019;283:92–5. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura M, Nestor PG, Levitt JJ, et al. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain 2008;131:180–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.And M, Petrides M. Orbitofrontal sulci of the human and macaque monkey brain. J Comp Neurol 2000;422:35–54. [PubMed] [Google Scholar]
- 11.Chi JG, Dooling EC. Gyral development of the human brain. Ann Neurol 2010;1:86–93. [DOI] [PubMed] [Google Scholar]
- 12.Ganella EP, Burnett A, Cheong J, et al. Abnormalities in orbitofrontal cortex gyrification and mental health outcomes in adolescents born extremely preterm and/or at an extremely low birth weight. Hum Brain Mapp 2015;36:1138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilian S, Asmal L, Chiliza B, et al. Childhood adversity and cognitive function in schizophrenia spectrum disorders and healthy controls: evidence for an association between neglect and social cognition. Psychol Med 2018;48:2186–93. [DOI] [PubMed] [Google Scholar]
- 14.Li X-B, Bo Q-J, Zhang G-P, et al. Effect of childhood trauma on cognitive functions in a sample of Chinese patients with schizophrenia. Comprehensive Psychiatry 2017;76:147–52. [DOI] [PubMed] [Google Scholar]
- 15.Rokita KI, Dauvermann MR, Mothersill D, et al. Childhood trauma, parental bonding, and social cognition in patients with schizophrenia and healthy adults. J Clin Psychol 2021;77:241–53. [DOI] [PubMed] [Google Scholar]
- 16.Wells R, Jacomb I, Swaminathan V, et al. The impact of childhood adversity on cognitive development in schizophrenia. Schizophr Bull 2020;46:140–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lysaker P, Meyer P, Evans J, et al. Neurocognitive and symptom correlates of self-reported childhood sexual abuse in schizophrenia spectrum disorders. Ann Clin Psychiatry 2001;13:89–92. [DOI] [PubMed] [Google Scholar]
- 18.Hanson JL, Chung MK, Avants BB, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci 2010;30:7466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Brito SA, Viding E, Sebastian CL, et al. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J Child Psychol Psychiatry 2013;54:105–12. [DOI] [PubMed] [Google Scholar]
- 20.Gianaros PJ, Jennings JR, Lei KS, et al. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage 2007;35:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson S, Phillips L, Baksheev G, et al. Stability of retrospective self-reports of childhood trauma in first episode psychosis. Early Interv Psychiatry 2018;13:908–13. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 2003;27:169–90. [DOI] [PubMed] [Google Scholar]
- 23.Yizhuang Z, Jiefeng C, Jian W, et al. Clinical reliability and validity of the Chinese version of measurement and treatment research to improve cognition in schizophrenia consensus cognitive battery. Chin J Psychiatry 2009;42:29–33. [Google Scholar]
- 24.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–55. [DOI] [PubMed] [Google Scholar]
- 25.Xie P, Wu K, Zheng Y, et al. Prevalence of childhood trauma and correlations between childhood trauma, suicidal ideation, and social support in patients with depression, bipolar disorder, and schizophrenia in southern China. J Affect Disord 2018;228:41–8. [DOI] [PubMed] [Google Scholar]
- 26.Li X-B, Li Q-Y, Liu J-T, et al. Childhood trauma associates with clinical features of schizophrenia in a sample of Chinese inpatients. J Psychiatr Res 2015;228:702–7. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Wang Z, Hou Y, et al. Effects of childhood trauma on personality in a sample of Chinese adolescents. Child Abuse Negl 2014;38:788–96. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Yin Y, Zhou Y, et al. The mediating effect of brain-derived neurotrophic factor levels on childhood trauma and psychiatric symptoms in patients with first-episode schizophrenia. Aust N Z J Psychiatry 2021;48674211031478. [DOI] [PubMed] [Google Scholar]
- 29.Holleran L, Kelly S, Alloza C, et al. The relationship between white matter microstructure and general cognitive ability in patients with schizophrenia and healthy participants in the ENIGMA consortium. Am J Psychiatry 2020;177:537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, Tian L, Chen N, et al. More dampened monocytic Toll-like receptor 4 response to lipopolysaccharide and its association with cognitive function in Chinese Han first-episode patients with schizophrenia. J Schizophr Res 2019;206:300–6. [DOI] [PubMed] [Google Scholar]
- 31.Lewis SJ, Koenen KC, Ambler A, et al. Unravelling the contribution of complex trauma to psychopathology and cognitive deficits: a cohort study. Br J Psychiatry 2021;219:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding R, He P. Associations between childhood adversities and late-life cognitive function: potential mechanisms. Soc Sci Med 2021;291:114478. [DOI] [PubMed] [Google Scholar]
- 33.Yenilmez DO, Atagun MI, Altun IK, et al. relationship between childhood adversities, emotion dysregulation and cognitive processes in bipolar disorder and recurrent depressive disorder. Turk Psikiyatri Derg; 2021;32:8–16. [DOI] [PubMed] [Google Scholar]
- 34.Biedermann SV, Meliss S, Simmons C, et al. Sexual abuse but not posttraumatic stress disorder is associated with neurocognitive deficits in South African traumatized adolescents. Child Abuse Negl 2018;80:257–67. [DOI] [PubMed] [Google Scholar]
- 35.Gervasio M, Beatty A, Kavanaugh B, et al. The association between neurocognition and sexual abuse within a children’s psychiatric inpatient program. J Clin Neuropsychol 2022;36:189–206. [DOI] [PubMed] [Google Scholar]
- 36.Womersley JS, Hemmings SMJ, Ziegler C, et al. Childhood emotional neglect and oxytocin receptor variants: association with limbic brain volumes. World J Biol Psychiatry 2020;21:513–28. [DOI] [PubMed] [Google Scholar]
- 37.Hanson JL, Nacewicz BM, Sutterer MJ, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry 2015;77:314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seitz R, Vracotas N, Bechard-Evans L, et al. The Trier Social Stress Test in first episode psychosis patients: impact of perceived stress, protective factors and childhood trauma. Psychoneuroendocrinology 2019;105:155–63. [DOI] [PubMed] [Google Scholar]
- 39.Law R, Clow A. Stress, the cortisol awakening response and cognitive function. Int Rev Neurobiol 2020;150:187–217. [DOI] [PubMed] [Google Scholar]
- 40.Law R, Evans P, Thorn L, et al. The cortisol awakening response predicts a same-day index of executive function in healthy young adults. Int J Psychophysiol 2020;158:27–33. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Liu S, Cao X, et al. Disassociated and concurrent structural and functional abnormalities in the drug-naive first-episode early onset schizophrenia. Brain Imaging Behav 2022. Feb 18 [Epub ahead of print]. doi 10.1007/s11682-021-00608-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Athanassiou M, Dumais A, Tikasz A, et al. Increased cingulo-orbital connectivity is associated with violent behaviours in schizophrenia. J Psychiatr Res 2022;147:183–9. [DOI] [PubMed] [Google Scholar]
- 43.Asmal L, Kilian S, du Plessis S, et al. Childhood trauma associated white matter abnormalities in first-episode schizophrenia. Schizophr Bull 2019;45:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armio RL, Laurikainen H, Ilonen T, et al. Amygdala subnucleus volumes in psychosis high-risk state and first-episode psychosis. J Schizophr Res 2020;215:284–92. [DOI] [PubMed] [Google Scholar]
- 45.Poletti S, Vai B, Smeraldi E, et al. Adverse childhood experiences influence the detrimental effect of bipolar disorder and schizophrenia on cortico-limbic grey matter volumes. J Affect Disord 2016;189:290–7. [DOI] [PubMed] [Google Scholar]
- 46.Li A, Jing D, Dellarco DV, et al. Role of BDNF in the development of an OFC-amygdala circuit regulating sociability in mouse and human. J Mol Psychiatry 2021;26:955–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chat IK, Nusslock R, Moriarity DP, et al. Goal-striving tendencies moderate the relationship between reward-related brain function and peripheral inflammation. Brain Behav Immun 2021;94:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adler SM, Girotti M, Morilak DA. Optogenetically-induced long term depression in the rat orbitofrontal cortex ameliorates stress-induced reversal learning impairment. Neurobiol Stress 2020; 13:100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Assari S, Boyce S, Saqib M, et al. Parental education and left lateral orbitofrontal cortical activity during N-back task: an fMRI study of american adolescents. Brain Sci 2021;11:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frey S, Petrides M. Orbitofrontal cortex and memory formation. Neuron 2002;36:171–6. [DOI] [PubMed] [Google Scholar]
- 51.Viskontas IV, Possin KL, Miller BL. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann N Y Acad Sci 2007;1121:528–45. [DOI] [PubMed] [Google Scholar]
- 52.Hornberger M, Savage S, Hsieh S, et al. Orbitofrontal dysfunction discriminates behavioral variant frontotemporal dementia from Alzheimer’s Disease. Dement Geriatr Cogn Disord 2011;30:547–52. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Leri F, Rizvi SJ. Anhedonia as a central factor in depression: neural mechanisms revealed from preclinical to clinical evidence. Prog Neuropsychopharmacol Biol Psychiatry 2021;110:110289. [DOI] [PubMed] [Google Scholar]
- 54.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn 2004; 55:11–29. [DOI] [PubMed] [Google Scholar]
- 55.Steffens DC, McQuoid DR, Welsh-Bohmer KA, et al. Left orbital frontal cortex volume and performance on the benton visual retention test in older depressives and controls. Neuropsychopharm 2003;28:2179–83. [DOI] [PubMed] [Google Scholar]
- 56.Saleh A, Potter GG, McQuoid DR, et al. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol Med 2017;47:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher HL, Craig TK, Fearon P, et al. Reliability and comparability of psychosis patients’ retrospective reports of childhood abuse. Schizophr Bull 2011;37:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vrijsen JN, van Amen CT, Koekkoek B, et al. Childhood trauma and negative memory bias as shared risk factors for psychopathology and comorbidity in a naturalistic psychiatric patient sample. Brain Behav 2017;7:e00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alameda L, Ferrari C, Baumann PS, et al. Childhood sexual and physical abuse: age at exposure modulates impact on functional outcome in early psychosis patients. Psychol Med 2015;45:2727–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.