Abstract
PURPOSE
Wilms tumor (WT) is associated with (epi)genetic predisposing factors affecting a growing number of WT predisposing genes and loci, including those causing Beckwith-Wiedemann spectrum (BWSp) or WT1-related syndromes. To guide genetic counseling and testing, we need insight into the prevalence of WT predisposing (epi)genetic factors.
PATIENTS AND METHODS
All children diagnosed with WT in the Netherlands between 2015 and 2020 were referred to a clinical geneticist. Phenotypic data, disease characteristics, and diagnostic test results were collected. If no genetic predisposition was identified by targeted diagnostic testing, germline (trio-)whole-exome sequencing and BWSp testing on normal kidney-derived DNA were offered.
RESULTS
A total of 126 cases were analyzed of 128 identified patients. (Epi)genetic predisposing factors were present in 42 of 126 patients (33.3%) on the basis of a molecular diagnosis in blood-derived DNA (n = 26), normal kidney-derived DNA (n = 12), or solely a clinical diagnosis of BWSp (n = 4). Constitutional, heterozygous DIS3L2 variants were identified as a recurrent predisposing factor in five patients (4%), with a second somatic hit in 4 of 5 tumors. Twenty patients (16%) were diagnosed with BWSp while four additional patients without BWSp features harbored chromosome 11p15 methylation defects in normal kidney tissue. Remaining findings included WT1-related syndromes (n = 10), Fanconi anemia (n = 1), neurofibromatosis type 1 (n = 1), and a pathogenic REST variant (n = 1). In addition, (likely) pathogenic variants in adult-onset cancer predisposition genes (BRCA2, PMS2, CHEK2, and MUTYH) were identified in 5 of 56 (8.9%) patients with available whole-exome sequencing data. Several candidate WT predisposition genes were identified, which require further validation.
CONCLUSION
(Epi)genetic WT predisposing factors, including mosaic aberrations and recurrent heterozygous DIS3L2 variants, were present in at least 33.3% of patients with WT. On the basis of these results, we encourage standard genetic testing after counseling by a clinical geneticist.
INTRODUCTION
Wilms tumor (WT, nephroblastoma) arises from a developmental arrest in the embryonic kidney1 and is frequently associated with (epi)genetic predisposing factors.2,3 Our understanding of WT predisposition continues to evolve, as illustrated by the identification of novel WT predisposition genes (TRIM28, REST, and CTR9),4-8 the role of mosaic aberrations,9 and the range of phenotypic variability. With various study designs and definitions, previous reports identified WT predisposition syndromes in 5%-24% of children with WT.10-13
CONTEXT
Key Objective
To determine the prevalence and distribution of (epi)genetic predisposing factors in children with Wilms tumor (WT) and identify novel WT predisposition genes.
Knowledge Generated
A Dutch national cohort of children with WT (2015-2020) was referred for genetic evaluation. If no genetic predisposition was identified by targeted diagnostic testing, (trio-)whole-exome sequencing was offered. (Epi)genetic predisposing factors, including mosaic aberrations, were found to be present in 33.3% of the patients. We identified an important role for constitutional heterozygous DIS3L2 variants.
Relevance
On the basis of these results, standard genetic testing after counseling by a clinical geneticist is encouraged for all children with WT.
We hypothesized that the prevalence may be even higher when evaluating a cohort of patients with WT for all currently known predisposing factors. Therefore, we performed a phenotypic and genomic characterization of a 5-year nationwide WT cohort by a stepwise approach including targeted diagnostic testing and, after informed consent, whole-exome sequencing (WES) of germline and parental DNA (trio-analysis). We aimed to determine the prevalence of (epi)genetic predisposing factors, to correlate germline findings with patients' phenotypic and tumor characteristics, and to identify novel WT predisposition genes.
PATIENTS AND METHODS
Patients and Data Collection
From 2015 onwards, Dutch hospitals referred all patients with (suspected) WT to the Princess Máxima Center for Pediatric Oncology. All patients diagnosed between January 1, 2015, and January 1, 2020, were retrospectively (2015-2018) or prospectively (2018-2020) invited for participation in this study. The study was referred to as the WES-KidTs study (whole-exome sequencing in children with kidney tumors). Parents, patients, and/or legal representatives were asked to give written informed consent for biomaterial and data collection (Medical Research Ethics Committee Utrecht: METC 18-033/M). This study conforms to the Declaration of Helsinki as revised in 2013.
In the definition of WT, we included all patients with WT and/or nephrogenic rests (WT precursor lesions14). Bilateral disease was defined as bilateral WT, bilateral nephrogenic rests, or WT with contralateral nephrogenic rests. Detailed data were collected, including patient characteristics (sex, birthweight, age at diagnosis, and medical and family history), tumor characteristics (stage, histology, and presence of nephrogenic rests as specified in the pathology report), and phenotypic findings during the clinical genetic consultation.
In the definition of (epi)genetic WT predisposition, we did not include (likely) pathogenic variants in adult-onset cancer predisposition genes nor genetic diagnoses, which are unrelated to WT development on the basis of current knowledge.
Diagnostic Procedures
Pediatric oncologists were instructed to refer all patients with WT to a clinical geneticist. Testing for Beckwith-Wiedemann spectrum (BWSp) was recommended for all patients, except for those with an alternative (suspected) diagnosis. BWSp testing was performed by methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA), primarily using blood-derived DNA. On a research basis, MS-MLPA was additionally performed using healthy kidney-derived DNA and tumor tissue, if this material was available after nephrectomy (Data Supplement, online only). Targeted WT1 analysis was recommended for patients with a urogenital malformation, bilateral/multifocal disease, and/or age < 2 years at diagnosis (Data Supplement). Other targeted genetic testing was performed according to the judgment of the clinical geneticist (Data Supplement).
Whole-Exome Sequencing
Patients in whom a clinical or molecular diagnosis of a WT predisposition syndrome was identified upon standard diagnostic testing were included for data collection only. In all remaining patients, informed consent for germline WES was requested. Patients were eligible if standard diagnostic testing had been completed by September 1, 2020.
Patients' germline DNA was assessed using a WES-based 30-gene WT gene panel (Data Supplement), including single-nucleotide variant, small indel, and copy number analyses (Data Supplement). If no causative variant was identified after panel analysis, exome-wide (trio-)analysis was performed using the patients' and (if available) parents' DNA. Participants could choose to limit the analysis to the WES-based WT gene panel only.
Variants were filtered on the basis of population frequency (gnomAD v3.1.1), quality metrics, protein effect, and in silico conservation and prediction scores. For genes included in the WT gene panel (Data Supplement), only (likely) pathogenic variants were communicated with the families. When variants of unknown significance were identified in the gene panel, tumor tissue (if available) was assessed by WES and/or single-nucleotide polymorphism array analysis (Data Supplement) for loss of heterozygosity (LOH) or somatic variants in this gene.
In the exome-wide trio-analysis, variants were additionally filtered on the basis of inheritance mode, prioritizing de novo, homozygous, and compound heterozygous variants. Genes that were considered strong candidates were submitted to GeneMatcher15, and if available, tumor tissue was assessed for LOH or somatic variants. A subset of genes was selected for meta-analysis on the basis of criteria specified in the Data Supplement. Germline sequencing data of all WT patients with informed consent for exome-wide analysis were combined, and variants in selected genes were extracted. In the resulting variant list, genes with multiple rare truncating and/or missense variants were prioritized.
Unsolicited findings were communicated with the families only after approval by a local multidisciplinary committee installed for this purpose at the Department of Genetics of the University Medical Center Utrecht.
RESULTS
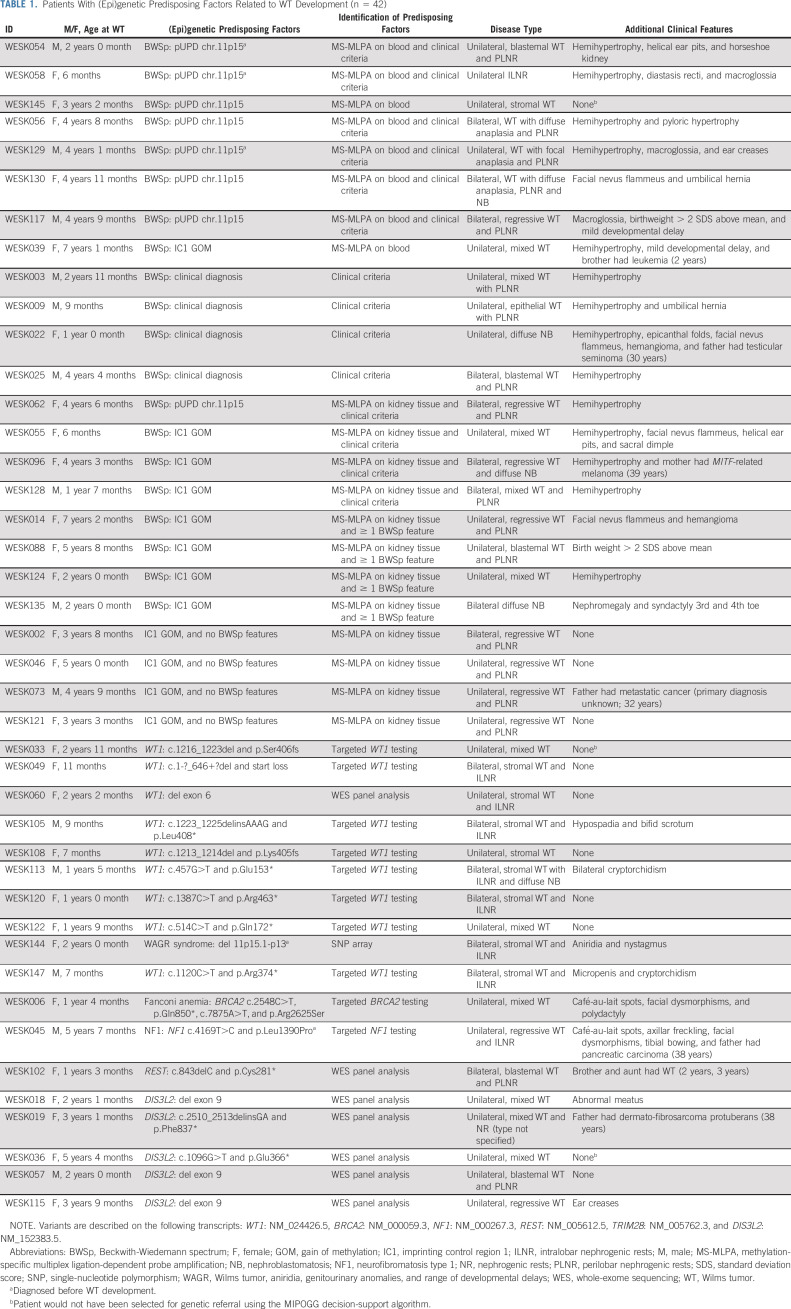
A total of 128 patients with WT were identified. Two patients did not give informed consent for data collection (including one patient who died before 2018), leaving 126 patients (71 females and 55 males) available for analysis (Data Supplement). The median age at WT diagnosis was 3.0 years (range, 0-18.9 years). Five patients (4.0%) had a molecularly confirmed diagnosis of a WT predisposition syndrome at the time of WT diagnosis, including BWSp (n = 3), Wilms tumor, aniridia, genitourinary anomalies, and range of developmental delays syndrome (n = 1), and neurofibromatosis type 1 (NF1; n = 1; Table 1). One patient had a family history of WT.
TABLE 1.
Patients With (Epi)genetic Predisposing Factors Related to WT Development (n = 42)
Genetic Examination and Diagnostic Testing
Of the 121 patients without a prior diagnosis of a WT predisposition syndrome, 111 (91.7%) were examined by a clinical geneticist (Data Supplement). Seven patients were not referred, and three families refused referral for a clinical genetic consultation. For these patients, phenotypic data were extracted from the medical records. Targeted WT1 testing was performed in 56 of 126 (44.4%) patients and diagnostic BWSp testing on blood-derived DNA in 97 of 126 (77.0%) patients. Additional MS-MLPA on normal kidney tissue was performed in 53 of 97 (54.6%) patients. Other targeted genetic testing was performed with various indications in six patients (Data Supplement).
Consent for Germline WES
Forty-three patients were not eligible for WES because a genetic predisposition had already been identified by germline-targeted testing and/or clinical criteria (n = 27) by MS-MLPA on kidney tissue (n = 3) or because diagnostic genetic testing had not been performed (n = 13). Of the 83 patients who were eligible for germline WES after diagnostic testing, we were able to approach 80 patients for WES, of whom (parents of) 57 patients (71.3%) gave informed consent. The consent was limited to the WT gene panel in four patients. DNA collection failed in one patient, and WES data were ultimately available for 56 patients. WES-based copy number variant analysis was informative in 52 of 56 patients (93%).
(Epi)genetic Predisposing Factors
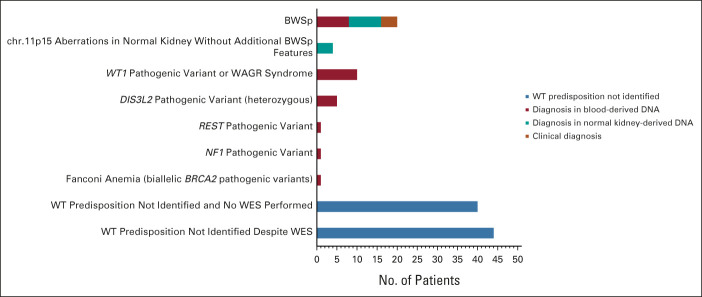
When combining the results of standard diagnostic testing, BWSp testing on normal kidney tissue and WES panel analysis, an (epi)genetic WT predisposition was identified in 42 of 126 patients (33.3%; Fig 1 and Table 1). This included 26 patients with a molecular diagnosis in blood-derived DNA, 12 patients with a diagnosis in normal kidney-derived DNA, and four patients with solely a clinical diagnosis of BWSp. In seven patients (16.7%), the diagnosis was established by WES analysis (WT gene panel). Additionally, several variants of unknown significance were identified in the WT gene panel (Data Supplement) which were not considered to be causative on the basis of inheritance mode and lack of LOH/somatic variants in the tumor.
FIG 1.
(Epi)genetic predisposing factors in patients with WT and/or nephroblastomatosis (N = 126). BWSp, Beckwith-Wiedemann spectrum; WAGR, Wilms tumor, aniridia, genitourinary anomalies, and range of developmental delays syndrome; WES, whole-exome sequencing; WT, Wilms tumor.
BWSp/11p15 aberrations.
Twenty patients (15.9%) were diagnosed with BWSp (Table 1), including eight patients with a molecular diagnosis in blood-derived DNA. In eight more patients who had at least one additional feature of BWSp, a molecular diagnosis could not be confirmed in blood but was established in normal kidney-derived DNA (Data Supplement). Finally, in four patients, for whom no resected kidney tissue was available for analysis, a clinical diagnosis of BWSp was established according to the criteria of the 2018 consensus statement by Brioude et al.16 Four patients were not diagnosed with BWSp because they lacked additional BWSp features, but they did display a gain of methylation of imprinting control region 1 in normal kidney-derived DNA (Data Supplement).
The 20 patients with BWSp had a median age of 3.6 years at WT diagnosis (range, 0.5-7.2 years), and 14 of 20 patients (70%) displayed lateralized overgrowth (hemihypertrophy), which was frequently subtle. WTs in patients with BWSp were not characterized by any specific histological subtype but frequently accompanied by perilobar nephrogenic rests (12 of 20, 60%). Among the eight patients with a confirmed molecular diagnosis in blood-derived DNA, one patient lacked BWSp features other than her WT diagnosis.
WT1 aberrations.
Germline WT1 aberrations were identified in 10 patients (7.9%), including one patient with Wilms tumor, anirida, genitourinary anomalies, and range of developmental delays syndrome (Table 1). These 10 patients were characterized by a young age at diagnosis (median 1.3 years, range, 0.6-3.0), stromal type WT (8 of 10 patients, 80%), and intralobar nephrogenic rests (7 of 10 patients, 70%). Seven patients (70%) had bilateral disease (n = 6) or unilateral WT with nephrogenic rests (n = 1), and 3 of 10 (30%) patients (all XY males) had urogenital malformations, including hypospadias, bifid scrotum, micropenis, and/or cryptorchidism.
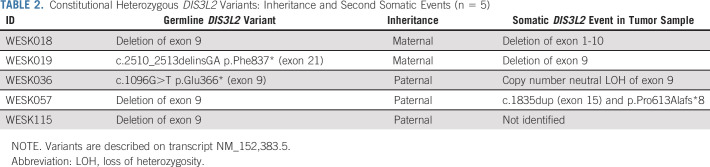
Heterozygous DIS3L2 variants.
Constitutional heterozygous variants in DIS3L2, which was in our WT gene panel because of the associated autosomal recessive Perlman syndrome, were identified in 5 of 126 patients (4%; Table 2). Among patients with available WES data, DIS3L2 variants were identified in 4 of 56 (7.1%), including two truncating (stopgain) variants and two deletions of exon 9. The fifth constitutional variant, again a deletion of exon 9, was identified by single-nucleotide polymorphism array analysis performed for clarifying an ambiguous MS-MLPA result. A second somatic hit was identified in 4 of 5 tumors, including a deletion of exon 9, deletion of exons 1-10, and a somatic truncating DIS3L2 variant.
TABLE 2.
Constitutional Heterozygous DIS3L2 Variants: Inheritance and Second Somatic Events (n = 5)
All five patients with constitutional DIS3L2 variants had inherited the variant from an unaffected parent. The median age at diagnosis was 3.1 years (range, 2.1-5.4). Two patients presented with metastatic WT while a third patient developed a metastatic relapse. None of the patients had bilateral disease, but one patient had multifocal WT with perilobar nephrogenic rests. Histological WT subtypes included mixed type WT (n = 3), regressive type WT (n = 1), and blastemal and regressive type WTs in the patient with multifocal disease. Minor phenotypic abnormalities were observed in two patients, including an abnormal meatus (n = 1) and ear creases (n = 1).
Other aberrations in known WT predisposition genes.
Other (likely) pathogenic, germline variants in known WT predisposition genes were diagnosed in three patients. In these patients, the presence of a germline variant was suspected on the basis of the patient's phenotype or family history, and the findings included a familial REST variant, Fanconi anemia, and NF1 (Table 1).
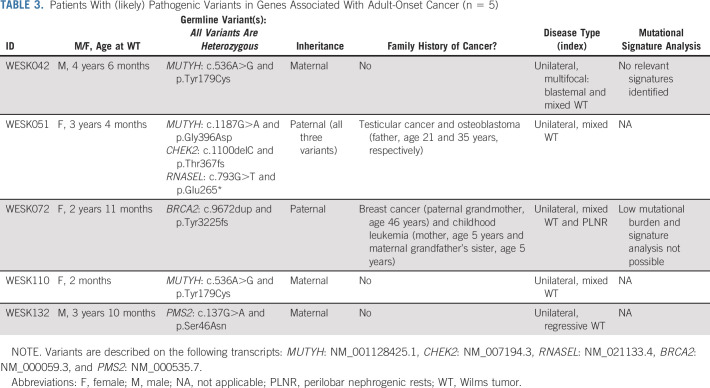
Findings in adult cancer predisposition genes.
(Likely) pathogenic variants in adult-onset cancer predisposition genes were identified in 5 of 56 (8.9%) patients with available WES data (Table 3). Two patients had heterozygous variants in BRCA2 or PMS2 (WESK132), the genes included in the WT gene panel because of the associated recessive conditions that predispose to WT. No somatic variant in the wildtype BRCA2 allele was identified, and the mutational burden was too low to perform a mutational signature analysis. The tumor of WESK132 showed retained protein expression of PMS2 (immunohistochemical staining), and there were no signs of microsatellite instability (Idylla MSI v.1.4, seven MSI markers).
TABLE 3.
Patients With (likely) Pathogenic Variants in Genes Associated With Adult-Onset Cancer (n = 5)
In one patient, exome-wide analysis revealed (likely) pathogenic heterozygous variants in three genes (CHEK2, MUTYH, and RNASEL), all inherited from her father who had a history of testicular cancer and osteoblastoma. Heterozygous MUTYH variants were identified in two additional patients. A single WT sample was available to assess the presence of a second-hit or MUTYH-related mutational signature (COSMIC signature SBS36)17,18 which was not identified, suggesting that the MUTYH variant did not drive WT development in this patient.
Meta-analysis: novel candidate genes.
On the basis of the exome-wide trio-analysis, 77 genes were selected for meta-analysis (Data Supplement). These included 31 genes with verified de novo variants and 46 genes with inherited variants (Data Supplement). For none of the genes, de novo variants were identified in more than one patient. Missense or truncating variants in the ubiquitin gene USP45 were detected in four unrelated patients, including a de novo missense variant (WESK007) and three inherited variants (Data Supplement). For none of these patients, tumor tissue was available to assess LOH or second-hit somatic variants. Notably, WESK007 had additional clinical characteristics including developmental delay, multiple dysmorphisms, and a urogenital malformation (shawl scrotum). In this patient, a second (mosaic) de novo variant affecting the MTA1 gene was observed. Variants in other candidate genes were assessed in the meta-analysis but not considered to be convincing on the basis of the inheritance pattern, in silico conservation and prediction scores, and/or lack of LOH or second somatic variants in tumor tissue.
Unrelated genetic diagnoses.
Four patients had a genetic diagnosis unrelated to WT development on the basis of current knowledge, including 47,XYY syndrome (n = 1), KAT6A syndrome (n = 1), and spondylodysplastic Ehlers-Danlos syndrome (biallelic B3GALT6 variants, n = 1). The fourth patient was found to have PHIP-related developmental delay (de novo truncating variant in PHIP) and 16p12.2 deletion syndrome.
Family history of cancer.
Apart from the patient with familial WT, 12 of 126 patients had a suspicious family history as defined in Jongmans' criteria.19 Recurrent cancer types in affected relatives included childhood leukemia (4 relatives in three families), testicular cancer (three relatives in three families), melanoma (two relatives in two families), and neuroblastoma (three relatives in two families). In these families, we did not identify variants that could explain both the WT and the relative's cancer diagnosis.
DISCUSSION
With a comprehensive and stepwise approach of diagnostic genetic testing and research-based WES analysis in a unique national unselected cohort of children with WT, we determined the prevalence of (epi)genetic predisposing factors, including mosaic aberrations and clinical BWSp diagnoses, to be at least 33.3%. This level of (epi)genetic predisposition is higher than 5%-24% that has been reported in previous studies.10-13
BWSp was diagnosed in 16% of all patients, compared with only 1%-8% in earlier reports.10-12 This higher frequency was due to the fact that we applied clinical criteria16 and performed MS-MLPA on resected healthy kidney tissue in addition to blood-derived DNA. It can be argued that chromosome 11p15 aberrations detected in resected kidney tissue, represent tissue-specific, somatic events.9,20 However, for patients who had at least one additional feature of BWSp, we consider it likely that these aberrations were also present in other tissues. In these patients, methylation changes in blood-derived DNA may have been present below the detection threshold of our MS-MLPA (approximately 10%). In the future, the development of more sensitive molecular techniques may increase the yield of BWSp testing in blood-derived DNA.21
Constitutional, heterozygous DIS3L2 variants were identified in 4% of all patients with WT (7% of patients with WES data), indicating that this gene is a bonafide WT predisposition gene. These children lacked a clearly recognizable phenotype. Biallelic DIS3L2 pathogenic variants cause Perlman syndrome,22 a congenital overgrowth syndrome with a high risk of WT development.23 Somatic DIS3L2 variants have been demonstrated in 1%-5% of WTs22,24,25 and deletions or LOH in 4%-30%.22,25,26 On the basis of incidental reports, heterozygous germline variants in DIS3L2 were previously suggested to cause an increased WT risk.24-27 Additionally, patients with rare constitutional deletions of 2q37.1/DIS3L2 have been reported to develop WT.28 In our cohort, three of five constitutional DIS3L2 aberrations were exon 9 deletions, which are predicted to cause an in-frame deletion of 58 amino acids, resulting in reduced ribonuclease activity as demonstrated in transfected HEK293 cells.22 Exon 9 is flanked by two approximately 5 Kb LINE-1 repeats causing genomic instability.29 Homozygous exon 9 deletions have been reported in Perlman syndrome,22 whereas heterozygous exon 9 deletions are present in 0.05% of healthy individuals (11 of 21,364 alleles in gnomAD SVs v.2.1). The identified second somatic hits strongly suggest that constitutional heterozygous DIS3L2 variants contribute to WT development. However, their presence in unaffected parents and population databases implies a reduced penetrance.
Similar to previous childhood cancer studies,27,30-33 we identified heterozygous, pathogenic germline variants in adult-onset cancer predisposition genes (BRCA2, PMS2, CHEK2, and MUTYH) in 8.9% of patients with available WES data. It remains unclear whether these variants contributed to WT development. For comparison, in WES data of 1,640 healthy Dutch individuals, pathogenic variants in dominant cancer predisposition genes were identified in 0.7% and heterozygous pathogenic MUTYH variants in 1.9%.34 Analysis of the mutational profile extracted from a single available WT sample did not reveal a contribution of MUTYH to tumor development.
Similarly, the contribution of NF1 to WT development is not entirely clear. An association has been suggested in a report in which 3 of 342 children with WT were found to have NF1.35 Since then, several case reports have been published supporting this association.36-39 However, the risk of WT development in patients with NF1 is considered too low (< 1%) to recommend WT surveillance.40
This study was limited by the fact that not all patients underwent (complete) genetic testing and/or WES analysis because of physicians' and families' personal choices. Moreover, future reanalysis of the WES data may provide novel insights, when, for instance, even better tools for splice effect prediction and copy number variant detection become available. This study reflects (epi)genetic aberrations in a Dutch population of children with WT and does not account for the differences in (epi)genetic predisposing factors which appear to exist between different geographical populations.41-43
Our exome-wide trio-analysis approach did not yield strong candidate WT predisposition genes outside the gene panel, which illustrates the complexity of searching for novel WT predisposition genes. In contrast to unsolved familial WT pedigrees, where a monogenic cause is suspected,4 epigenetic factors and postzygotic mosaicism play an important role in isolated (nonfamilial) WT. Moreover, yet to be identified WT predisposition genes may exhibit reduced penetrance as demonstrated for DIS3L2.
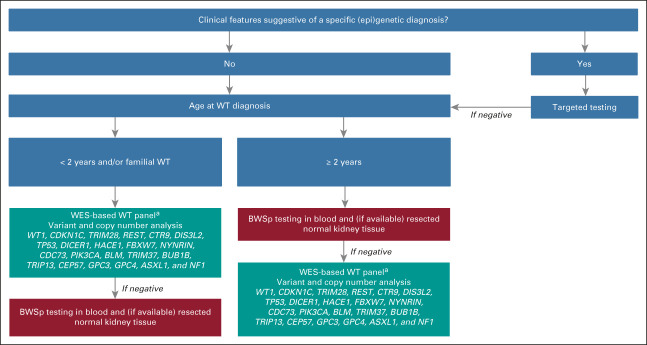
On the basis of the results of this study, we encourage standard genetic testing after counseling by a clinical geneticist for all children with WT. In settings where this is not feasible, decision-support algorithms such as the MIPOGG tool12 can be used to prioritize children for genetic testing. Using such a tool reduces the rate of genetic referrals, although our findings indicate that some diagnoses are missed with this approach. Among the 42 patients with identified WT predisposition in this study, three (7%) would not have been selected for genetic testing using MIPOGG, including a patient with a germline WT1 variant, germline DIS3L2 variant, and molecularly confirmed BWSp. Targeted testing is advised if a child has clinical features suggestive of a specific (epi)genetic diagnosis. For all other patients, we propose a diagnostic strategy (Fig 2) which includes (mosaic) BWSp testing and/or WES-based panel analysis. This is justified by the high prevalence of (epi)genetic predisposing factors, including mosaic aberrations and recurrent heterozygous DIS3L2 variants, as demonstrated in this study.
FIG 2.
Suggested strategy for germline genetic testing in children with WT. aAdult-onset cancer predisposition genes were excluded for ethical reasons and may be assessed by targeted testing in children who are clinically suspected of Fanconi anemia (BRCA2 and PALB2) or constitutional mismatch repair deficiency (PMS2, MSH2, MSH6, and MLH1). BWSp, Beckwith-Wiedemann spectrum; WES, whole-exome sequencing; WT, Wilms tumor.
Jarno Drost
Patents, Royalties, Other Intellectual Property: WO2016/083613; culture medium for epithelial stem cells and Organoids comprising said stem cells. WO2016/083612; culture medium for expanding breast epithelial stem cells
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 1853
PRIOR PRESENTATION
Presented in part at the 53rd Annual Congress of the International Society of Paediatric Oncology (SIOP) Virtual Congress, October 21-24, 2021 and the European Human Genetics Conference (ESHG) Virtual Conference, August 28-31, 2021.
SUPPORT
Supported by Stichting Kinderen Kankervij (Foundation KiKa), Grant No. 278 to M.M.v.d.H.E., M.C.J.J., and R.P.K. The support of the renal tumor team members in the Princess Máxima Center for Pediatric Oncology is highly appreciated.
AUTHOR CONTRIBUTIONS
Conception and design: Janna A. Hol, Roland P. Kuiper, Marry M. van den Heuvel-Eibrink, Marjolijn C.J. Jongmans
Provision of study materials or patients: Esmé Waanders, Saskia Hopman, Jarno Drost, Ronald R. de Krijger, Marry M. van den Heuvel-Eibrink, Marjolijn C.J. Jongmans
Collection and assembly of data: Janna A. Hol, Roland P. Kuiper, Esmé Waanders, Reno Bladergroen, Simon V. van Reijmersdal, Jet Bliek, Saskia Hopman, Jarno Drost, Marry M. van den Heuvel-Eibrink, Marjolijn C.J. Jongmans
Data analysis and interpretation: Janna A. Hol, Roland P. Kuiper, Freerk van Dijk, Esmé Waanders, Sophie E. van Peer, Marco J. Koudijs, Simon V. van Reijmersdal, Lionel M. Morgado, Jet Bliek, Maria Paola Lombardi, Ronald R. de Krijger, Marry M. van den Heuvel-Eibrink, Marjolijn C.J. Jongmans
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prevalence of (Epi)genetic Predisposing Factors in a 5-Year Unselected National Wilms Tumor Cohort: A Comprehensive Clinical and Genomic Characterization
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jarno Drost
Patents, Royalties, Other Intellectual Property: WO2016/083613; culture medium for epithelial stem cells and Organoids comprising said stem cells. WO2016/083612; culture medium for expanding breast epithelial stem cells
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hohenstein P, Pritchard-Jones K, Charlton J: The yin and yang of kidney development and Wilms' tumors. Genes Dev 29:467-482, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott RH, Stiller CA, Walker L, et al. : Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet 43:705-715, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RW, Fraumeni JF, Jr, Manning MD: Association of Wilms's tumor with aniridia, hemihypertrophy and other congenital malformations. N Engl J Med 270:922-927, 1964 [DOI] [PubMed] [Google Scholar]
- 4.Mahamdallie S, Yost S, Poyastro-Pearson E, et al. : Identification of new Wilms tumour predisposition genes: An exome sequencing study. Lancet Child Adolescent Health 3:322-331, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliday BJ, Fukuzawa R, Markie DM, et al. : Germline mutations and somatic inactivation of TRIM28 in Wilms tumour. PLoS Genet 14:e1007399, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diets IJ, Hoyer J, Ekici AB, et al. : TRIM28 haploinsufficiency predisposes to Wilms tumor. Int J Cancer 145:941-951, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Mahamdallie SS, Hanks S, Karlin KL, et al. : Mutations in the transcriptional repressor REST predispose to Wilms tumor. Nat Genet 47:1471-1474, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Hanks S, Perdeaux ER, Seal S, et al. : Germline mutations in the PAF1 complex gene CTR9 predispose to Wilms tumour. Nat Commun 5:4398, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coorens THH, Treger TD, Al-Saadi R, et al. : Embryonal precursors of Wilms tumor. Science 366:1247-1251, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumoucel S, Gauthier-Villars M, Stoppa-Lyonnet D, et al. : Malformations, genetic abnormalities, and Wilms tumor. Pediatr Blood Cancer 61:140-144, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Segers H, Kersseboom R, Alders M, et al. : Frequency of WT1 and 11p15 constitutional aberrations and phenotypic correlation in childhood Wilms tumour patients. Eur J Cancer 48:3249-3256, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Cullinan N, Villani A, Mourad S, et al. : An eHealth decision-support tool to prioritize referral practices for genetic evaluation of patients with Wilms tumor. Int J Cancer 146:1010-1017, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Merks JH, Caron HN, Hennekam RC: High incidence of malformation syndromes in a series of 1,073 children with cancer. Am J Med Genet A 134A:132-143, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Beckwith JB: Nephrogenic rests and the pathogenesis of Wilms tumor: Developmental and clinical considerations. Am J Med Genet 79:268-273, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Gene Matcher. https://genematcher.org/
- 16.Brioude F, Kalish JM, Mussa A, et al. : Expert consensus document: Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: An international consensus statement. Nat Rev Endocrinol 14:229-249, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tate JG, Bamford S, Jubb HC, et al. : COSMIC: The catalogue of somatic mutations in cancer. Nucleic Acids Res 47:D941-D947, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COSMIC, Catalogue Of Somatic Mutations In Cancer; signature SBS36. cancer.sanger.ac.uk
- 19.Jongmans MC, Loeffen JL, Waanders E, et al. : Recognition of genetic predisposition in pediatric cancer patients: An easy-to-use selection tool. Eur J Med Genet 59:116-125, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Foulkes WD, Polak P: Bilateral tumors—Inherited or acquired? N Engl J Med 383:280-282, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Fiala EM, Ortiz MV, Kennedy JA, et al. : 11p15.5 epimutations in children with Wilms tumor and hepatoblastoma detected in peripheral blood. Cancer 126:3114-3121, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Astuti D, Morris MR, Cooper WN, et al. : Germline mutations in DIS3L2 cause the Perlman syndrome of overgrowth and Wilms tumor susceptibility. Nat Genet 44:277-284, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Alessandri JL, Cuillier F, Ramful D, et al. : Perlman syndrome: Report, prenatal findings and review. Am J Med Genet A 146A:2532-2537, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Gadd S, Huff V, Walz AL, et al. : A Children's Oncology Group and TARGET initiative exploring the genetic landscape of Wilms tumor. Nat Genet 49:1487-1494, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciceri S, Gamba B, Corbetta P, et al. : Genetic and epigenetic analyses guided by high resolution whole-genome SNP array reveals a possible role of CHEK2 in Wilms tumour susceptibility. Oncotarget 9:34079-34089, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wegert J, Ishaque N, Vardapour R, et al. : Mutations in the SIX1/2 pathway and the DROSHA/DGCR8 miRNA microprocessor complex underlie high-risk blastemal type Wilms tumors. Cancer Cell 27:298-311, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Parsons DW, Roy A, Yang Y, et al. : Diagnostic yield of clinical tumor and germline whole-exome sequencing for children with solid tumors. JAMA Oncol 2:616-624, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falk RE, Casas KA: Chromosome 2q37 deletion: Clinical and molecular aspects. Am J Med Genet C Semin Med Genet 145C:357-371, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Higashimoto K, Maeda T, Okada J, et al. : Homozygous deletion of DIS3L2 exon 9 due to non-allelic homologous recombination between LINE-1s in a Japanese patient with Perlman syndrome. Eur J Hum Genet 21:1316-1319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Walsh MF, Wu G, et al. : Germline mutations in predisposition genes in pediatric cancer. N Engl J Med 373:2336-2346, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grobner SN, Worst BC, Weischenfeldt J, et al. : The landscape of genomic alterations across childhood cancers. Nature 555:321-327, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Diets IJ, Waanders E, Ligtenberg MJ, et al. : High yield of pathogenic germline mutations causative or likely causative of the cancer phenotype in selected children with cancer. Clin Cancer Res 24:1594-1603, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Byrjalsen A, Hansen TVO, Stoltze UK, et al. : Nationwide germline whole genome sequencing of 198 consecutive pediatric cancer patients reveals a high incidence of cancer prone syndromes. PLoS Genet 16:e1009231, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haer-Wigman L, van der Schoot V, Feenstra I, et al. : 1 in 38 individuals at risk of a dominant medically actionable disease. Eur J Hum Genet 27:325-330, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stay EJ, Vawter G: The relationship between nephroblastoma and neurofibromatosis (Von Recklinghausen's disease). Cancer 39:2550-2555, 1977 [DOI] [PubMed] [Google Scholar]
- 36.Shvartsbeyn M, Bassani L, Mikolaenko I, et al. : Brain metastasis of Wilms tumor with diffuse anaplasia and complex cytogenetic phenotype in a child with neurofibromatosis type 1. J Neurosurg Pediatr 8:353-356, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Ito F, Watanabe Y, Ito T: Synchronous occurrence of Wilms tumor and ganglioneuroblastoma in a child with neurofibromatosis. Eur J Pediatr Surg 7:308-310, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Perilongo G, Felix CA, Meadows AT, et al. : Sequential development of Wilms tumor, T-cell acute lymphoblastic leukemia, medulloblastoma and myeloid leukemia in a child with type 1 neurofibromatosis: A clinical and cytogenetic case report. Leukemia 7:912-915, 1993 [PubMed] [Google Scholar]
- 39.Chu JY, O'Connor DM, Danis RK: Neurofibrosarcoma at irradiation site in a patient with neurofibromatosis and Wilms' tumor. CA Cancer J Clin 31:333-335, 1981 [DOI] [PubMed] [Google Scholar]
- 40.Hol JA, Jewell R, Chowdhury T, et al. : Wilms tumour surveillance in at-risk children: Literature review and recommendations from the SIOP-Europe Host Genome Working Group and SIOP Renal Tumour Study Group. Eur J Cancer 153:51-63, 2021 [DOI] [PubMed] [Google Scholar]
- 41.Haruta M, Arai Y, Watanabe N, et al. : Different incidences of epigenetic but not genetic abnormalities between Wilms tumors in Japanese and Caucasian children. Cancer Sci 103:1129-1135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuzawa R, Breslow NE, Morison IM, et al. : Epigenetic differences between Wilms' tumours in white and east-Asian children. Lancet 363:446-451, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Kaneko Y, Okita H, Haruta M, et al. : A high incidence of WT1 abnormality in bilateral Wilms tumours in Japan, and the penetrance rates in children with WT1 germline mutation. Br J Cancer 112:1121-1133, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]