Abstract
Background
In recent years, it has been demonstrated that ferroptosis can be involved in a variety of kidney injury processes, but the role played by ferroptosis in hypertensive kidney injury is still unclear. The aim was to explore the mechanism of ferroptosis playing a role in hypertensive kidney disease and related signalling pathways.
Methods
GSE37455 microarray data was downloaded from the Gene Expression Omnibus (GEO) database and preprocessed. Batch correction and differential analysis were performed on the normal population and the hypertensive nephropathy samples using the “sva” and “limma” packages in R software. Ferroptosis-related genes were obtained from the FerrDb database and normalized and processed using UniProt. Ferroptosis-related differentially expressed genes were obtained using Venny 2.1. and imported into the Search Tool for the Retrieval of Interacting Genes (STRING) to obtain protein-protein interactions (PPIs). The data were imported into Cytoscape 3.7.2 for processing to identify the core differential genes of ferroptosis based on nodes. Gene set enrichment analysis (GSEA) was performed on the core differential genes of ferroptosis to infer the pathway of ferroptosis action in hypertensive nephropathy.
Results
The R software processing yielded 37 differential genes, including 13 upregulated genes and 24 downregulated genes. 202 ferroptosis-related genes were obtained by screening, and 3 ferroptosis-related differentially expressed genes were obtained after taking the intersection. The ferroptosis-related core differentially expressed gene albumin (ALB) was obtained by PPI network analysis and Cytoscape processing. GSEA analysis revealed that ferroptosis may act in hypertensive nephropathy through pathways such as drug metabolism-cytochrome P450, branched-chain amino acid (BCAA) metabolism, retinol metabolism, and biological processes (BPs) such as organic and amino acid metabolism and humoral immunity.
Conclusions
Ferroptosis may act in the development of hypertensive nephropathy through pathways such as BCAA metabolism and retinol metabolism and BPs such as organic and amino acid metabolism and humoral immunity.
Keywords: Bioinformatics, ferroptosis, hypertensive nephropathy, mechanism of action
Introduction
The phenomenon of ferroptosis, which was introduced into the field of biology in 2003 and formally conceptualized in 2012, is a novel, iron ion–dependent mode of cell death that occurs due to lipid peroxidation resulting in the massive accumulation of reactive oxygen species (ROS) (1,2). Studies have shown that oxidative stress is a common phenomenon in the development of hypertensive disease, with specific mechanisms being changes in redox status and excessive production of ROS (3,4). ROS induction is also one of the key factors in renal injury (5), which allows inflammation and immune cell recruitment and promotes the process of renal fibrosis (6). Damage associated molecular pattern (DAMP), a danger signal for the immune system, has been shown to be a mediator of inflammation induced by ferroptosis (7), and the resulting inflammatory response can initiate adaptive repair and lead to chronic changes in renal disease (8). Previously, through data mining and network pharmacology research methods, we found that treatment of hypertension can be accompanied by reduced or delayed kidney damage, which involves the process of ferroptosis. Also given the similar mechanisms of action of ferroptosis and hypertensive nephropathy, such as massive accumulation of ROS and inflammatory response, this paper aimed to explore the possible mechanisms and pathways of action of ferroptosis in the development and progression of hypertensive nephropathy, using key genes as a bridge. We present the following article in accordance with the STREGA reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-276/rc).
Methods
Data collection and preprocessing
GSE37455 microarray data were downloaded from the publicly available Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/), along with data from the GPL11670 platform (18 normal population samples and 0 hypertensive nephropathy samples) and the GPL14663 platform (3 normal population samples and 20 hypertensive nephropathy samples). The gene IDs were converted to gene symbols based on the corresponding matrix information from the 2 platforms. The 2 datasets were merged by a Perl script, and the “sva” and “limma” packages of R (The R Foundation for Statistical Computing, Vienna, Austria) were used for batch correction and preprocessing, including data reading and background correction. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Analysis of variances
All genes were analyzed by classical Bayesian analysis with the support of the R “limma” package to obtain the corresponding P and logFC values and the Benjamini-Hochberg (BH) adjusted P values. The results were evaluated at both the fold and significance levels, and the screening criteria for differentially expressed genes were as follows: false discovery rate (FDR) <0.05 and log absolute value of the fold difference (|log2fold change|, |log2FC|) >1. Volcano and heat maps were drawn based on the results.
Acquisition of differentially expressed genes for ferroptosis
Ferroptosis-related genes were obtained from the FerrDb database (http://www.zhounan.org/ferrdb/legacy/index.html). The genes were screened for “Validated”, and duplicate genes were removed. The genes were then normalized to “Reviewed (Swiss-Prot)” and “Human” by the UniProt (https://www.uniprot.org/) database. Hypertensive nephropathy dataset after analysis of variance and ferroptosis dataset obtained after screening were imported into Venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/) to obtain a Venn diagram of ferroptosis-related differentially expressed genes.
Construction of protein-protein interaction (PPI) networks and acquisition of core genes
The differentially expressed genes were imported into the Search Tool for the Retrieval of Interacting Genes (STRING; https://string-db.org/) database for PPI analysis. The PPI network of differentially expressed genes was obtained by restricting the study species to “human” (Homo sapiens) and setting the minimum interaction score to medium confidence (0.400), with the remaining parameters set to the default. The data were imported into Cytoscape 3.7.2 for processing to determine which proteins to use as cores, and the top 10 gene proteins were ranked according to the number of nodes (degrees) on the gene proteins. A bar chart was created to screen the core genes for ferroptosis-related differentially expressed genes.
Gene set enrichment analysis (GSEA)
GSEA is a method for determining the statistical significance of a defined set of genes and whether there is a consistent difference between 2 biological states (9,10). In this experiment, GSEA generated an initial list of gene classifications based on their correlation with core gene expression, resulting in high and low expression groups. This calculation elaborated on the significant differences we observed between the high and low core gene groups. For genomic alignments where 1,000 replicates were performed for each analysis, the phenotype labels were the expression levels of the core genes. In addition, to classify the enrichment pathways in each phenotype, we used the nominal (NOM) P value and the normalized enrichment score (NES) (11). Genomes with an FDR less than 0.05 was considered significantly enriched.
Statistical analysis
R 4.1.1 software supported by classical Bayesian analysis, FDR <0.05 and log absolute value of the fold difference (|log2fold change|, |log2FC|) >1. PPI analysis by restricting the study species to “human” (Homo sapiens) and setting the minimum interaction score to medium confidence (0.400). GSEA analysis was performed for each analysis with 1,000 replicate genomic comparisons. FDR less than 0.05 was considered significantly enriched.
Results
Results of differentially expressed gene acquisition
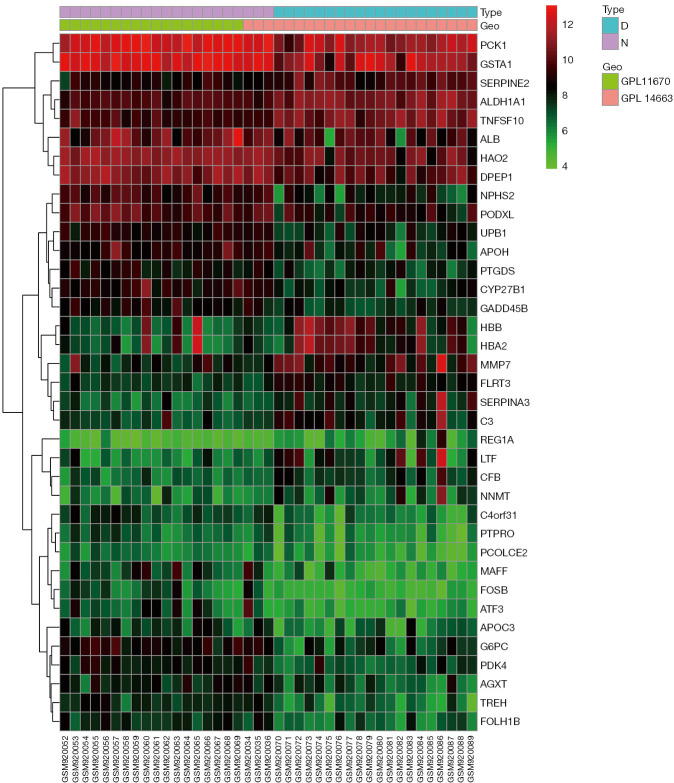
The GSE37455 microarray data and the GPL11670 and GPL14663 platform data were downloaded from the GEO database. A total of 41 samples were obtained, including 21 samples from the control group and 20 samples from the experimental group with hypertensive nephropathy. A correlation analysis was performed with the R “limma” package, and 37 genes were obtained, including 13 upregulated genes and 24 downregulated genes. A volcano plot (Figure 1) and a heat map (Figure 2) were plotted.
Figure 1.
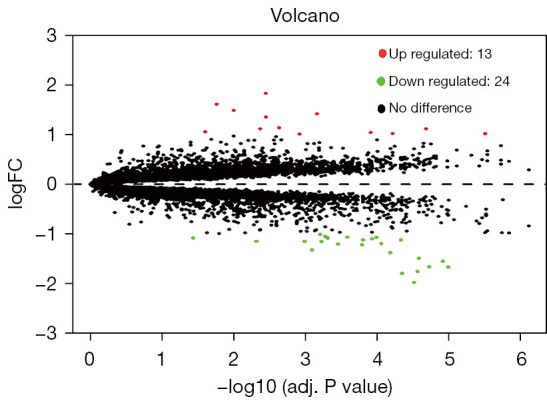
Volcano map of differentially expressed genes. FC, Fold change.
Figure 2.
Heat map of differentially expressed genes. Type D, disease samples; Type N, normal samples.
Ferroptosis-related differentially expressed gene acquisition
A total of 382 ferroptosis-related genes were obtained from the FerrDb ferroptosis database. Among these genes, 123 duplicate genes were removed, 57 nonhuman genes were removed using “Human” as the screening condition, and 202 eligible ferroptosis-related genes were obtained. The genes were imported into Venny 2.1 along with the 37 differentially expressed genes. The 3 genes in the intersection of the Venn diagram were albumin (ALB), nicotinamide N-methyltransferase (NNMT), and activating transcription factor 3 (ATF3; Figure 3).
Figure 3.
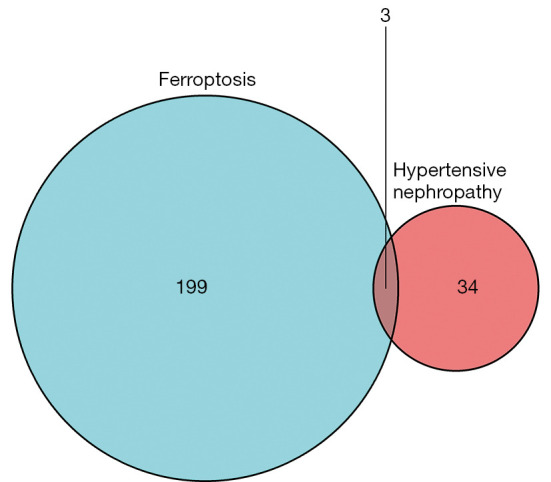
Venn diagram of differentially expressed genes in hypertensive nephropathy and ferroptosis-related genes.
PPI network construction and core gene screening
The differentially expressed genes were imported into the STRING database with a medium confidence protein interaction parameter score of >0.4. A total of 8 unrelated genes, including NNMT, serpin family E member 2 (SERPINE2), neuron derived neurotrophic factor (NDNF), procollagen C-endopeptidase enhancer 2 (PCOLCE2), trehalase (TREH), regenerating family member 1 alpha (REG1A), fibronectin leucine rich transmembrane protein 3 (FLRT3), and beta-ureidopropionase 1 (UPB1), were removed to ensure the reliability of the data and to obtain the PPI network graph (Figure 4). The results showed that there were 37 nodes, 50 edges, and an average node degree of 2.7. The data were imported into Cytoscape to create a bar chart of the top 10 gene proteins according to the number of nodes on the gene protein (Figure 5). The NNMT gene is a free gene protein, and the ATF3 gene has a degree of 3, which is much smaller than the ALB gene, so it can be assumed that the ALB gene is the core gene in the differential expression of ferroptosis.
Figure 4.
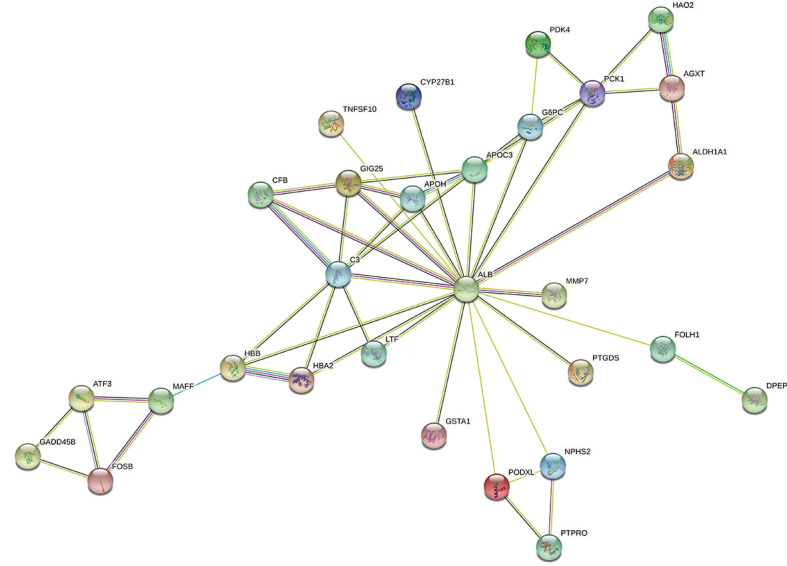
Protein interactions of differentially expressed genes.
Figure 5.
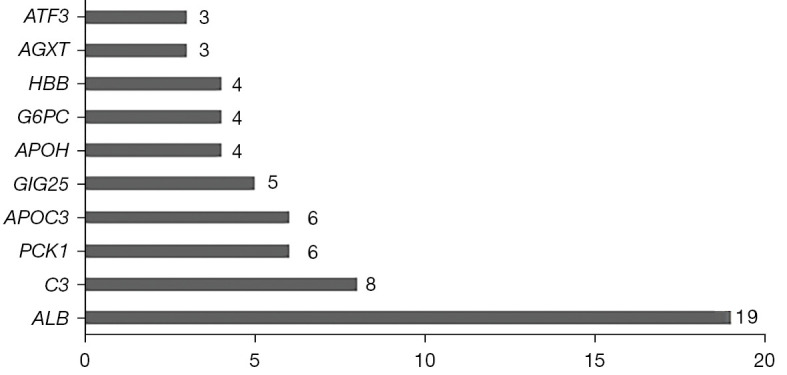
Interaction node relationship diagram for differentially expressed genes.
GSEA analysis of the ALB gene
The 41 samples were divided into a high expression group (24 samples) and a low expression group (17 samples) based on the mean expression of ALB genes in the dataset samples. GSEA-based ALB-related signaling pathways were used to create the high and low expression datasets, and Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed to identify the mechanism of action of ferroptosis in hypertensive nephropathy and related signaling pathways. The top 5 high and low expression KEGG and GO enrichment pathways were listed based on NES values and FDR screening (Table 1) and visualized for analysis.
Table 1. KEGG and GO results in GSEA (top 5 for each of the high and low expression groups).
| Designation | NES | NOM | FDR | |
|---|---|---|---|---|
| p-val | q-val | |||
| High expression | ||||
| KEGG_DRUG_METABOLISM_CYTOCHROME_P450 | 2.25 | 0 | 0 | |
| KEGG_VALINE_LEUCINE_AND_ISOLEUCINE_DEGRADATION | 2.04 | 0 | 0.001 | |
| KEGG_RETINOL_METABOLISM | 2.02 | 0.002 | 0.001 | |
| KEGG_GLYCINE_SERINE_AND_THREONINE_METABOLISM | 2 | 0 | 0.001 | |
| KEGG_DRUG_METABOLISM_OTHER_ENZYMES | 1.99 | 0 | 0.001 | |
| GOBP_ORGANIC_ACID_CATABOLIC_PROCESS | 2.55 | 0 | 0 | |
| GOBP_CELLULAR_AMINO_ACID_CATABOLIC_PROCESS | 2.37 | 0 | 0 | |
| GOBP_ALPHA_AMINO_ACID_CATABOLIC_PROCESS | 2.36 | 0 | 0 | |
| GOBP_CELLULAR_AMINO_ACID_METABOLIC_PROCESS | 2.33 | 0 | 0 | |
| GOBP_XENOBIOTIC_METABOLIC_PROCESS | 2.33 | 0 | 0 | |
| Low expression | ||||
| KEGG_SYSTEMIC_LUPUS_ERYTHEMATOSUS | −2.29 | 0 | 0 | |
| KEGG_GRAFT_VERSUS_HOST_DISEASE | −2.23 | 0 | 0 | |
| KEGG_CELL_ADHESION_MOLECULES_CAMS | −2.16 | 0 | 0 | |
| KEGG_LEISHMANIA_INFECTION | −2.16 | 0 | 0 | |
| KEGG_PATHOGENIC_ESCHERICHIA_COLI_INFECTION | −2.14 | 0 | 0 | |
| GOBP_HUMORAL_IMMUNE_RESPONSE | −2.28 | 0 | 0.001 | |
| GOBP_POSITIVE_REGULATION_OF_INTERLEUKIN_1_PRODUCTION | −2.26 | 0 | 0.001 | |
| GOCC_LUMENAL_SIDE_OF_MEMBRANE | −2.24 | 0 | 0 | |
| GOCC_IMMUNOGLOBULIN_COMPLEX | −2.24 | 0 | 0 | |
| GOBP_GRANULOCYTE_MIGRATION | −2.24 | 0 | 0 |
Gene sets with NOM P value <0.05 and FDR q-value <0.05 are considered significant. KEGG, Kyoto Encyclopedia of Genes and Genomes; GO, Gene Ontology; GSEA, gene set enrichment analysis; NES, normalized enrichment score; NOM, nominal; FDR, false discovery rate.
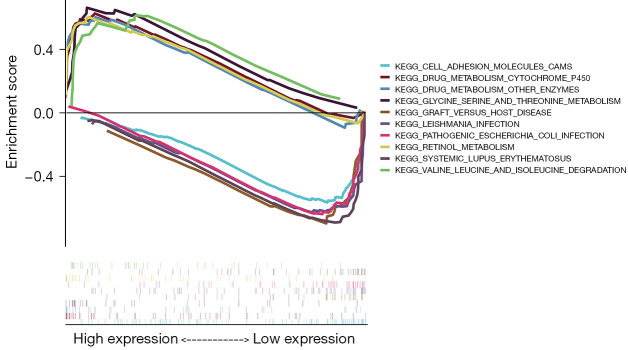
Five KEGG items, including drug metabolism-cytochrome P450; degradation of valine, leucine, and isoleucine; retinol metabolism; metabolism of glycine, serine and threonine; and drug metabolism—other enzymes, showed significant differential enrichment in high ALB expression genotypes. Five KEGG items, including systemic lupus erythematosus, graft-versus-host disease, cell adhesion molecules (CAMs), leishmaniasis infection, and pathogenic Escherichia coli infection, showed significant differential enrichment in low ALB expression genotypes (Figure 6).
Figure 6.
KEGG enrichment map in GSEA. KEGG, Kyoto Encyclopedia of Genes and Genomes; GSEA, gene set enrichment analysis.
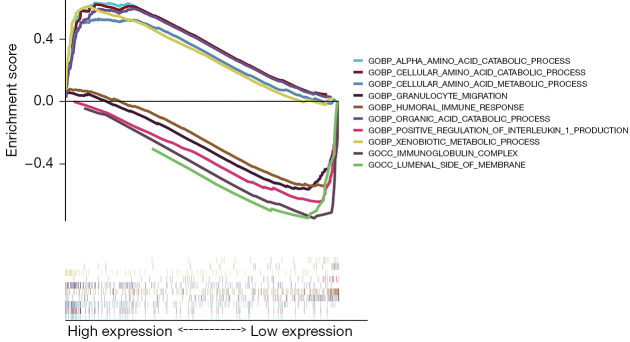
Five GO items, including organic acid catabolic processes, cellular amino acid catabolic processes, amino acid catabolic processes, cellular amino acid metabolic processes, and xenobiotic metabolic processes, showed significant differential enrichment in the high ALB expression genotypes. Five GO items, including humoral immune response, positive regulation of interleukin 1 production, inner luminal side of the membrane, immunoglobulin complex, and granulocyte migration, showed significant differential enrichment in the low ALB expression genotypes (Figure 7). This evidence suggested a potential mechanism for the role of ferroptosis ALB genes in hypertensive nephropathy.
Figure 7.
GO enrichment map in GSEA. GO, Gene Ontology; GSEA, gene set enrichment analysis.
Discussion
Ferroptosis is a recently identified form of programmed cell death characterized by the iron-dependent accumulation of lipid ROS and the depletion of plasma membrane polyunsaturated fatty acids. Ferroptosis is involved in biological processes (BPs) such as iron metabolism, lipid metabolism, and amino acid metabolism (12-14) and is associated with cardiovascular disease, ischemia-reperfusion injury, and kidney degeneration (15). Hypertensive nephropathy is a condition in which primary hypertension results in structural and functional damage to the kidney. The early stages of the disease can be asymptomatic and may manifest as microproteinuria and rising blood creatinine, which can increase the risk of end-stage renal disease, adverse cardiovascular events, and sudden death, thus posing a serious threat to a patient’s life (16). A study has shown that hypertensive nephropathy is involved in renal tubular cell death in diabetic nephropathy (DN) and can be inhibited by upregulation of nuclear factor erythroid 2-related factor 2 (NRF2) to slow the progression of DN (17). However, the mechanism by which ferroptosis plays a role in hypertensive nephropathy is unclear.
In this paper, using a bioinformatics method supported by the GEO database, we performed differential gene expression analysis on 41 samples (21 normal population samples and 20 hypertensive nephropathy population samples) and obtained 13 upregulated genes and 24 downregulated genes. After intersecting this dataset with the ferroptosis gene set, we obtained 3 candidate ferroptosis-related core differentially expressed genes. PPI network analysis and Cytoscape screening were performed to obtain the ferroptosis-related core differentially expressed gene ALB. Finally, the dataset was divided into a high ALB expression group and a low ALB expression group by single gene GSEA analysis, and the possible mechanism of ferroptosis in the pathogenesis of hypertensive nephropathy was explored under enrichment analysis.
Using the FerrDb ferroptosis database, we discovered that the ALB gene plays a role in the ferroptosis model. Its expressed protein (ALB) is a nonspecific transport protein that can bind many inorganic ions and insoluble small molecules and convert them into soluble substances, which play a physiological function in the body. Pathological damage to the glomerulus can lead to proteinuria, which lowers the levels of ALB in the serum, increases the compensatory synthesis of hepatocytes and the metabolic burden on the kidneys while aggravating hypoproteinemia, induces infections, causes loss of trace elements and endocrine dysfunction, lowers immune function and hypercoagulation (18), and causes disorders of the body’s metabolism. A previous study has shown that massive local aggregation of ALB (e.g., in the kidney) can cause further kidney damage through activation of the inflammatory factor nuclear factor kappa B (NFκB) (19).
We performed a GSEA analysis of the ALB gene and found that drug metabolism-cytochrome P450; degradation of valine, leucine, and isoleucine; retinol metabolism; glycine, serine, and threonine metabolism; drug metabolism—other enzymes; systemic lupus erythematosus; graft-versus-host disease; CAMs; leishmaniasis infection; and pathogenic Escherichia coli infection showed significant differential enrichment in the high and low expression groups. Branched-chain amino acids (BCAAs), which consist of leucine, isoleucine, and valine, are essential amino acids that are abundant in the human body and account for approximately 35% of all essential amino acids (20). Guan et al. found that plasma BCAA levels were closely related to the severity of cardiovascular disease (21), and that the prevalence of hypertensive disease increased if the intake of BCAAs was increased. After adjusting for variables such as age, gender, body mass index, and diabetes, Mirmiran et al. found that BCAA intake remained a risk factor for the development of hypertension (22). Another study (23) showed that leucine and isoleucine had a significant positive association with the lower quartile of the glomerular filtration rate. In a study by Zhu et al. (24) on mice with acute paraquat poisoning, isoleucine and leucine were found to be significantly negatively correlated with blood creatinine levels (both P<0.05), suggesting a correlation between BCAA metabolism and renal function. Among the metabolic pathways of glycine, serine, and threonine, it has been shown that other amino acids such as glycine have a close relationship with renal pathology, not only by regulating the excretion of oxalic and citric acids in the urine and inhibiting the deposition of calcium oxalate crystals in the kidney (25), but also by protecting against inflammatory damage in the kidney (26). This provides a basis for exploring hypertensive kidney injury in ferroptosis model. In the retinol metabolic pathway, retinol and retinol-binding proteins, which are synthesized and secreted in the liver, are paired and enter the bloodstream to form a complex with prealbumin in equal molar ratios to maintain the transport and metabolism of retinol. Zhang et al. (27) found that retinol binding protein levels were abnormally high in patients with hypertensive nephropathy, and that the levels of retinol binding protein increased gradually as the degree of kidney damage increased. In a study by Shu et al. (28) on hypertensive patients during pregnancy, retinol binding protein values also increased with increasing renal injury and had a significant diagnostic role in early renal injury, suggesting that the ALB gene in the mechanism of ferroptosis may act through the retinol metabolic pathway in hypertensive renal injury. Studies by Lin (29) and Yin et al. (30) also found that hypertensive renal damage was associated with various factors such as macrophages, T lymphocytes, interleukin-6, tumor necrosis factor alpha, and chemokines.
Our KEGG pathway analysis suggested that the mechanism of action of ferroptosis in hypertensive nephropathy may involve the metabolism of retinol, drugs, and amino acids such as valine and leucine, while Leishmania and bacterial infections may also be involved. In the GO enrichment analysis, BPs accounted for most of the enriched terms, including organic acid catabolic processes, cellular amino acid catabolic processes, amino acid catabolic processes, cellular amino acid metabolic processes, xenobiotic metabolic processes, humoral immune responses, positive regulation of interleukin 1 production, and granulocyte migration, while cellular components (CCs) accounted for 2 terms, including inner luminal side of the membrane and immunoglobulin complexes. The KEGG and GO analyses suggested that amino acid metabolism may play an important role in the ferroptosis mechanism in hypertensive nephropathy, and that organismal immune responses, such as the migration process of granulocytes and immunoglobulin complexes, may also be involved.
In summary, ferroptosis and hypertensive nephropathy share similar regulatory mechanisms related to the massive accumulation of ROS and inflammatory response. Using bioinformatics methods, including GSEA analysis of ALB (the core differential gene for ferroptosis), we hypothesized that KEGG pathways such as retinol metabolism and amino acid metabolism and BPs such as organic and amino acid catabolism and humoral immunity are important pathways for ferroptosis to act in hypertensive nephropathy, thereby providing a reference for further experimental validation.
The discovery of ferroptosis provides a new platform for the treatment and prevention of kidney disease. As it differs from other modes of cell death, to a certain extent, it provides feasibility for the combined application of existing therapeutic regimens. However, research on the specific molecular mechanisms of kidney injury and related signalling pathways is still in its infancy; therefore, future research directions could explore specific markers of ferroptosis based on mechanistic studies, offering the possibility of targeted therapy for kidney injury under the ferroptosis mechanism.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81804061), the Shandong Province ‘Taishan Scholar’ Construction Project Funds (No. 2018-35), and the Ji’nan Science and Technology Project (No. 201805078).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Reporting Checklist: The authors have completed the STREGA reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-276/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-276/coif). All authors report that this work was supported by the National Natural Science Foundation of China (No. 81804061), the Shandong Province ‘Taishan Scholar’ Construction Project Funds (No. 2018-35), and the Ji’nan Science and Technology Project (No. 201805078). The authors have no other conflicts of interest to declare.
References
- 1.Hirschhorn T, Stockwell BR. The development of the concept of ferroptosis. Free Radic Biol Med 2019;133:130-43. 10.1016/j.freeradbiomed.2018.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hao S, Liang B, Huang Q, et al. Metabolic networks in ferroptosis. Oncol Lett 2018;15:5405-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Touyz RM, Rios FJ, Alves-Lopes R, et al. Oxidative Stress: A Unifying Paradigm in Hypertension. Can J Cardiol 2020;36:659-70. 10.1016/j.cjca.2020.02.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Ding Y, Ramprasath T, et al. Oxidative Stress, GTPCH1, and Endothelial Nitric Oxide Synthase Uncoupling in Hypertension. Antioxid Redox Signal 2021;34:750-64. 10.1089/ars.2020.8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Z, Zhang H, Yang SK, et al. Emerging Role of Ferroptosis in Acute Kidney Injury. Oxid Med Cell Longev 2019;2019:8010614. 10.1155/2019/8010614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuadrado A, Manda G, Hassan A, et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol Rev 2018;70:348-83. 10.1124/pr.117.014753 [DOI] [PubMed] [Google Scholar]
- 7.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun 2019;510:278-83. 10.1016/j.bbrc.2019.01.090 [DOI] [PubMed] [Google Scholar]
- 8.Gong M, Li J. Mechanism of cellular ferroptosis and its role in kidney disease. Journal of Clinical Nephrology 2021;21:1028-33. [Google Scholar]
- 9.Subramanian A, Kuehn H, Gould J, et al. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics 2007;23:3251-3. 10.1093/bioinformatics/btm369 [DOI] [PubMed] [Google Scholar]
- 10.Tu Z, Xiong J, Xiao R, et al. Loss of miR-146b-5p promotes T cell acute lymphoblastic leukemia migration and invasion via the IL-17A pathway. J Cell Biochem 2019;120:5936-48. 10.1002/jcb.27882 [DOI] [PubMed] [Google Scholar]
- 11.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545-50. 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Li D. Ferroptosis and its research progress in cardiovascular and cerebrovascular diseases. Chinese Bulletin of Life Sciences 2019,31:886-93. [Google Scholar]
- 13.Song S, Gao Y, Sheng Y, et al. Targeting NRF2 to suppress ferroptosis in brain injury. Histol Histopathol 2021;36:383-97. [DOI] [PubMed] [Google Scholar]
- 14.Hassannia B, Van Coillie S, Vanden Berghe T. Ferroptosis: Biological Rust of Lipid Membranes. Antioxid Redox Signal 2021;35:487-509. 10.1089/ars.2020.8175 [DOI] [PubMed] [Google Scholar]
- 15.Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017;171:273-85. 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson DL. Immune mechanisms of salt-sensitive hypertension and renal end-organ damage. Nat Rev Nephrol 2019;15:290-300. 10.1038/s41581-019-0121-z [DOI] [PubMed] [Google Scholar]
- 17.Li S, Zheng L, Zhang J, et al. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic Biol Med 2021;162:435-49. 10.1016/j.freeradbiomed.2020.10.323 [DOI] [PubMed] [Google Scholar]
- 18.Zhang MJ, Zhu B, Zhu CF, et al. Risk Factors for Primary Nephrotic Syndrome Complicated by Acute Kidney Injury Analysis. Chinese Journal of Integrated Traditional and Western Nephrology 2018;19:960-4. [Google Scholar]
- 19.Carney EF. Chronic kidney disease: Key role of exosomes in albumin-induced inflammation. Nat Rev Nephrol 2018;14:142. 10.1038/nrneph.2018.6 [DOI] [PubMed] [Google Scholar]
- 20.Neinast M, Murashige D, Arany Z. Branched Chain Amino Acids. Annu Rev Physiol 2019;81:139-64. 10.1146/annurev-physiol-020518-114455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan YJ, Li BL, Huang GR. Clinical study on plasma BCAAs level in patients with different degrees of ischemic heart diseases. Journal of Guangdong Medical College 2020;38:446-8. [Google Scholar]
- 22.Mirmiran P, Teymoori F, Asghari G, et al. Dietary Intakes of Branched Chain Amino Acids and the Incidence of Hypertension: A Population-Based Prospective Cohort Study. Arch Iran Med 2019;22:182-8. [PubMed] [Google Scholar]
- 23.Mahbub MH, Yamaguchi N, Nakagami Y, et al. Association of Plasma Branched-Chain and Aromatic Amino Acids with Reduction in Kidney Function Evaluated in Apparently Healthy Adults. J Clin Med 2021;10:5234. 10.3390/jcm10225234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu KK, Xie JX, Yu HB, et al. The characteristics of amino acid metabolism in mice with acute paraquat poisoning. Journal of Tongji University (Medical Edition) 2021;42:604-11. [Google Scholar]
- 25.Lan Y, Zhu W, Duan X, et al. Glycine suppresses kidney calcium oxalate crystal depositions via regulating urinary excretions of oxalate and citrate. J Cell Physiol 2021;236:6824-35. 10.1002/jcp.30370 [DOI] [PubMed] [Google Scholar]
- 26.Park S, Lee J, Yang SH, et al. Comprehensive metabolomic profiling in early IgA nephropathy patients reveals urine glycine as a prognostic biomarker. J Cell Mol Med 2021;25:5177-90. 10.1111/jcmm.16520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang ZB, Wen QH, Zhang XB, et al. Detection and clinical significance of serum Cys C, urinary β2-microglobulin, N-acetyl-β-D-aminoglucosidase, and retinol binding protein levels in early stage of hypertensive nephropathy. Hainan Medical Journal 2021;32:3024-27. [Google Scholar]
- 28.Shu YZ, Quan H, Zeng ZR, et al. The Diagnostic Value of Serum Amyloid A, Retinol Binding Protein and Urine β2 Microglobulin in Early Renal Injury During Pregnancy Hypertension. Hebei Medicine 2022;28:66-71. [Google Scholar]
- 29.Lin WP, Wu Y. Association of coagulation function changes with inflammatory endothelial factors and oxidative stress in patients with chronic kidney disease. Acta Aacademiae Medicinae Qingdao Universitatis 2019;55:99. [Google Scholar]
- 30.Yin HH, Wang JC, Ma XC, et al. Effect of Shenqi Bushen Granule on Clinical Curative Efficacy, Inflammatory Factors and Microcirculation Index of patients with Early Renal Damage in Essential Hypertension. Pharmacology and Clinics of Chinese Materia Medica 2019;35:159. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as