Abstract
A novel bacteriocin-like substance produced by vaginal Lactobacillus salivarius subsp. salivarius CRL 1328 with activity against Enterococcus faecalis, Enterococcus faecium, and Neisseria gonorrhoeae was characterized. The highest level of production of this heat-resistant peptide or protein occurred during the late exponential phase. Its mode of action was shown to be bactericidal. L. salivarius subsp. salivarius CRL 1328 could be used for the design of a probiotic to prevent urogenital infections.
Lactobacilli are the dominant microorganisms isolated from the vaginas of healthy premenopausal women (11, 19). They interfere with the colonization of pathogens by different mechanisms, such as the production of organic acids, H2O2, and bacteriocins (2, 6, 9, 10–12). Bacteriocins are proteinaceous, bactericidal substances synthesized by bacteria and usually have a narrow spectrum of activity (7). The term bacteriocin-like substance is applied to antagonistic substances which are not completely defined or do not fit the typical criteria of bacteriocins. They have been reported to inhibit a wide range of both gram-positive and gram-negative bacteria as well as fungi (11). Although bacteriocin production has been described as an antagonistic mechanism that could be exerted by lactobacilli in the vaginal tract, no bacteriocin has been characterized to date for Lactobacillus strains isolated from the vagina.
At present, bacteriocin-producing lactic acid bacteria are widely used for the elaboration of probiotics for the gastrointestinal tract (4, 5, 13, 17). To select probiotic strains for local application in the human vagina, isolation, identification, and certain characteristics (e.g., adhesion and production of H2O2) of vaginal Lactobacillus strains were previously reported (14–16). In the present paper, the screening of bacteriocin production in 134 human vaginal lactobacilli is reported. Characterization, production kinetics, and mode of action of a bacteriocin produced by Lactobacillus salivarius subsp. salivarius CRL 1328 are described in more detail.
Strains and culture conditions.
Lactobacillus strains were isolated from vaginal swabs as described before (16). The other microorganisms employed are indicated in Table 1.
TABLE 1.
Sensitive and resistant microorganisms tested for inhibition by a bacteriocin-like substance produced by L. salivarius subsp. salivarius CRL 1328
| Strain and response |
|---|
| Sensitive |
| E. faecalisa |
| E. faecalis ATCC 19433 |
| E. faecalis CRL 318b |
| E. faecalis CRL 341b |
| Enterococcus faecium ATCC 19434 |
| L. paracasei subsp. paracasei CRL 1289b |
| N. gonorrhoeaea |
| Resistant |
| Candida sp.a |
| Escherichia colia |
| Gardnerella vaginalisa |
| Klebsiella sp.a |
| Lactobacillus crispatus CRL 1266b |
| L. paracasei subsp. paracasei CRL 1251b |
| S. aureusa |
| Streptococcus group B spp.a |
| Streptococcus agalactiae ATCC 27956 |
From the Instituto de Microbiología of the Universidad Nacional de Tucumán, Tucumán, Argentina. Microorganisms were isolated from vaginal swabs and identified by standard techniques.
From the CERELA Culture Collection, Tucumán, Argentina.
Detection of antimicrobial activity.
Inhibitory substances from Lactobacillus culture supernatants were studied according to standard methods (7). Lactobacilli were grown in Laptg broth (18), and supernatants were separated, neutralized, and filter sterilized. The pathogens tested were vaginal Staphylococcus aureus, Enterococcus faecalis, and group B Streptococcus spp. Agar plates were sown with the pathogens, and Lactobacillus supernatant aliquots were assayed on them. After incubation for 24 h at 37°C, the diameters of the inhibition halos were measured.
L. salivarius subsp. salivarius CRL 1328 was selected because it was able to inhibit the growth of E. faecalis. Although L. salivarius subsp. salivarius CRL 1328 is not the predominant species isolated from the vagina, its isolation from this environment was reported previously (3). The same method was used to test its inhibitory activity against other microorganisms. Different Enterococcus strains, Neisseria gonorrhoeae, and Lactobacillus paracasei subsp. paracasei CRL 1289 were inhibited (Table 1).
Characterization of antimicrobial substance.
Aliquots of supernatant were treated with catalase (1,000 U ml−1), proteases (1 mg ml−1), α-amylase (0.1 M), sodium metaperiodate (10 mM) and lipase (10 g ml−1). Activity was lost after treatment with chymotrypsin and proteinase K, but the substance was resistant to catalase, trypsin, pepsin, type II protease, type XV protease, lipase, and α-amylase. The effect of sodium periodate could not be evaluated because the reagent control was positive.
Effects of temperature, lyophilization, and pH on antimicrobial activity.
Heat resistance was studied after the supernatants were heated to 60, 80, and 100°C for 10 min each and also after autoclaving (121°C; 15 min). They were cooled and tested for activity. The activity was also evaluated by modifying the pH (between 2 and 8) of the supernatants with 2 N NaOH. Furthermore, resistance to lyophilization was studied. The supernatant fluid of a 12-h L. salivarius subsp. salivarius CRL 1328 culture was filter sterilized. Fractions of 10 ml each were lyophilized and later resuspended in the same volume of Laptg broth. The bacteriocin activity of the resuspended lyophilized powder was determined.
The substance responsible for the inhibition of E. faecalis retained activity after heating and even after autoclaving. Antagonistic activity was observed at all tested pH levels. Lyophilization did not alter the activity.
Kinetics of bacteriocin production.
Laptg broth was inoculated with a 2% concentration of 12-h L. salivarius subsp. salivarius CRL 1328 culture and incubated at 37°C. Samples were taken every 3 h to determine the titer of the bacteriocin and the number of CFU per milliliter. For the titration, supernatants were filter sterilized and serially diluted in Laptg broth. Twenty-five microliters of each dilution was poured into the holes of agar plates containing vaginal E. faecalis. The titer was expressed in arbitrary units (AU), defined as the inverse of the dilution in milliliters. The number of CFU per milliliter was determined by using Laptg agar plates.
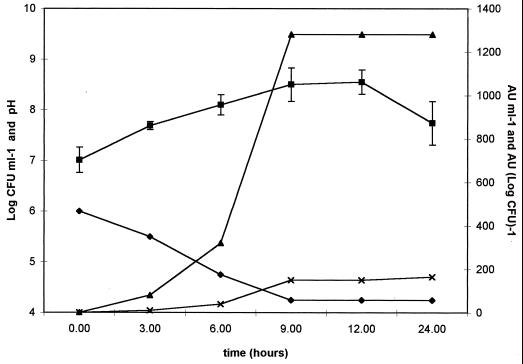
Activity was detected after 3 h of incubation. The highest concentration was obtained between 9 and 12 h of incubation, with titers of 1,280 AU ml−1 of supernatant. The production kinetics are shown in Fig. 1.
FIG. 1.
Kinetics of bacteriocin-like substance production during the growth of Lactobacillus salivarius subsp. salivarius CRL 1328. Growth of L. salivarius subsp. salivarius CRL 1328 was determined by the plate count method (■) and culture pH (⧫); bacteriocin concentration is expressed as arbitrary units per milliliter (▴) and per log CFU (×).
Mixed cultures of L. salivarius subsp. salivarius CRL 1328 and vaginal E. faecalis.
The effect of L. salivarius subsp. salivarius CRL 1328 on E. faecalis growth in mixed cultures in Laptg broth at 37°C was studied. The initial inocula were 106 CFU ml−1 for lactobacilli and 105 or 107 CFU ml−1 for enterococci. The number of CFU was determined with selective media, Lactobacillus selective medium, and Streptococcus faecalis medium agar plates. The plates were incubated for 48 h at 37°C.
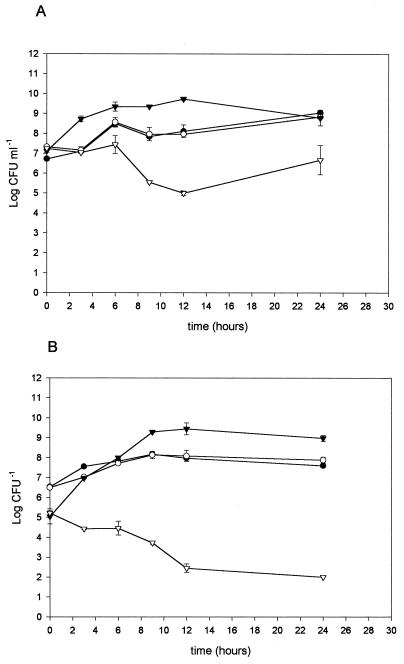
L. salivarius subsp. salivarius CRL 1328 inhibited E. faecalis, depending on the initial inoculum of the pathogen. At an inoculum of 107 CFU ml−1, the growth rate was 4.74 log units lower than that in the pure culture (Fig. 2A). The decrease was observed in a 12-h culture when the bacteriocin concentration was the highest (1,280 AU ml−1). With an inoculum of 105 CFU ml−1, the growth rate was 7.02 log units lower (Fig. 2B). L. salivarius subsp. salivarius CRL 1328 growth was not affected in mixed cultures and no antagonistic substances from E. faecalis against lactobacilli were found (data not shown). Even though complete inhibition of the pathogen growth was not obtained, the number of viable cells decreased significantly. Control of the pathogen overgrowth by L. salivarius subsp. salivarius CRL 1328 would be of interest in the prevention of genitourinary infections. Reid et al. (20) have suggested that the dominance of inhibitor-producing lactobacilli on the urogenital epithelium and the ability of these organisms to interact closely with uropathogens would constitute an important host defense mechanism against infection.
FIG. 2.
Pure and mixed cultures of L. salivarius subsp. salivarius CRL 1328 and E. faecalis. Lactobacilli were inoculated at 107 CFU ml−1; enterococci were inoculated at 107 (A) and 105 (B) CFU ml−1. Lactobacilli in pure (○) and mixed (●) cultures and enterococci in pure (▾) and mixed (▿) cultures are shown.
Mode of action.
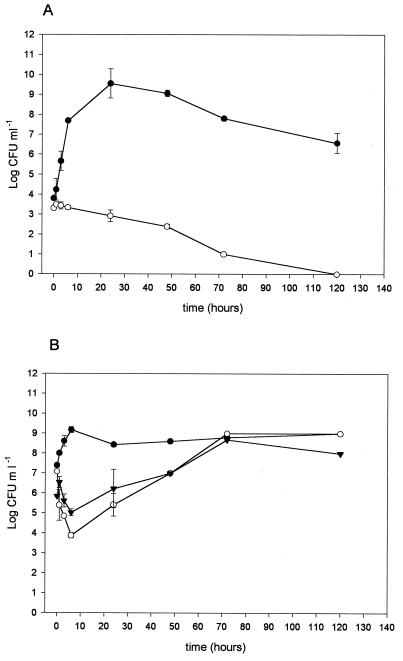
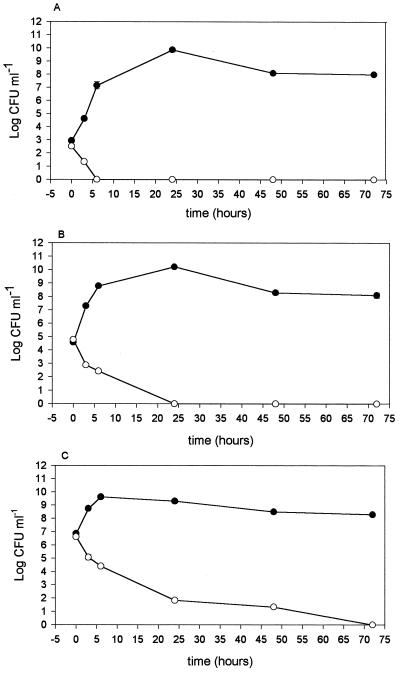
Five milliliters of supernatant of a 12-h L. salivarius subsp. salivarius CRL 1328 culture was lyophilized, resuspended in the same volume of Laptg broth, inoculated with E. faecalis, and incubated at 37°C. Samples were taken at different times, and the number of CFU per milliliter was determined by using S. faecalis medium agar plates. The bacteriocin-like substance was shown to be bactericidal when 103 CFU ml−1 of E. faecalis was inoculated in Lactobacillus spent supernatant with 1,280 AU ml−1 of bacteriocin during 120 h of culture (Fig. 3A). With larger inocula (105 and 107 CFU ml−1), a bacteriostatic effect during the first 24 h of growth was observed and was then reestablished (Fig. 3B). Another set of experiments was performed with a twofold concentration of bacteriocin. Larger amounts of the E. faecalis inoculum were completely inhibited after incubation times were increased (Fig. 4), thus indicating a bactericidal mode of action.
FIG. 3.
Mode of action of a L. salivarius subsp. salivarius CRL 1328 bacteriocin-like substance against E. faecalis. (A) E. faecalis inoculated at 103 CFU ml−1 without (●) and with (○) bacteriocin. (B) E. faecalis inoculated at 107 CFU ml−1 without bacteriocin (●) and inoculated at 107 (▾) and 105 (○) CFU ml−1 with bacteriocin.
FIG. 4.
Mode of action of an L. salivarius subsp. salivarius CRL 1328 bacteriocin-like substance against E. faecalis. E. faecalis was inoculated at 103 (A), 105 (B), and 107 (C) CFU ml−1 without bacteriocin (●) and with a twofold bacteriocin concentration (○).
Electron microscopy.
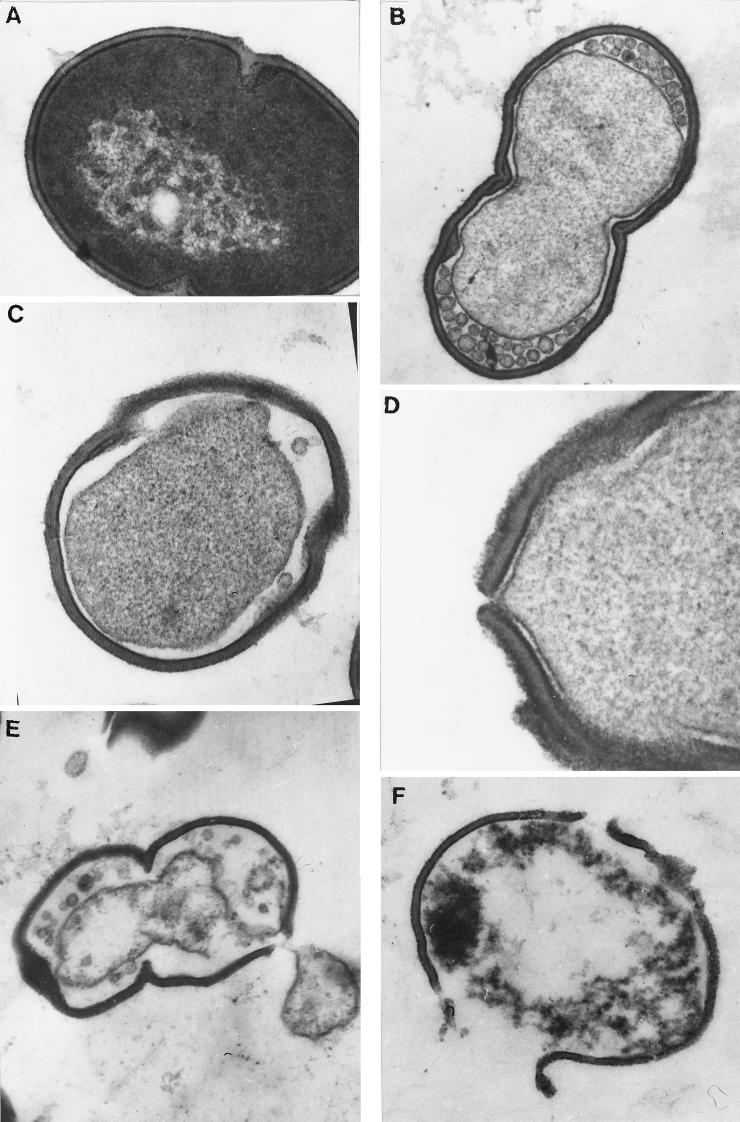
E. faecalis harvested from an early-stationary-phase culture (108 CFU ml−1) was incubated for 12 h at 37°C in the reconstituted supernatant of L. salivarius subsp. salivarius CRL 1328 with a bacteriocin titer of 2,560 AU ml−1. After incubation, the microorganisms were separated and prepared for transmission electron microscopy. E. faecalis showed vesiculization of protoplasm, formation of pores, and complete disintegration of cells (Fig. 5).
FIG. 5.
Electron photomicrographs of the effect of L. salivarius subsp. salivarius CRL 1328 bacteriocin-like substance on E. faecalis. The Enterococcus control cell (A), vesiculization of protoplasm (B), vesiculization of protoplasm and a damaged cell wall (C), pore formation in the cell wall (D), a disintegrated cell with loss of the protoplasmic material through a cell wall pore (E), and a disintegrated cell (F) are shown. The method is described in the text.
Characterization and purification studies of the bacteriocin-like substance are still in progress. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed with a high-titer bacteriocin extract (21), showing that only one band was responsible for the inhibitory effect (data not shown). These results would indicate a single compound substance.
Many bacteriocins for lactobacilli from different environments have been described (8). However, no bacteriocin for an L. salivarius strain has been reported, except Salivacin 140, which is produced by a Lactobacillus salivarius subsp. salicinius strain isolated from Japanese grass leaves (1).
The properties of L. salivarius subsp. salivarius CRL 1328 allow us to further study its probable application in a probiotic for the prevention of urogenital infections. The inhibition of Enterococcus would prevent the recurrence of urinary tract infections (of significant incidence in elderly women) while the effect on N. gonorrhoeae suggests its potential use in keeping gonorrhea under control. Thermotolerance and retention of activity after lyophilization are desired characteristics if its use as an additive in a probiotic is to be considered. The activity of the substance at low pH is also important because the effect would be exerted in the vagina, where the pH is between 3.8 and 4.5.
Acknowledgments
This paper was supported by CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas, Buenos Aires, Argentina) (PID 0359-1998-2000) and CIUNT (Consejo de Investigación de la Universidad Nacional de Tucumán, Tucumán, Argentina) (D-128) grants.
We kindly thank Clara Silva and Olga Aulet for providing the pathogenic strains from the Culture Collection of the Instituto de Microbiología Luis C. Verna from the Universidad Nacional de Tucumán. We also thank Beatriz Winik for her technical assistance in the electron microscopy studies and Raúl Raya for critical review of the manuscript.
REFERENCES
- 1.Arihara K, Ogihara S, Mukai T, Itoh M, Kondo Y. Salivacin 140, a novel bacteriocin from Lactobacillus salivarius subsp. salicinius T140 active against pathogenic bacteria. Lett Appl Microbiol. 1996;22:420–424. doi: 10.1111/j.1472-765x.1996.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 2.Baerheim S, Larsen E, Di Granes A. Vaginal application of lactobacilli in the prophylaxis of recurrent lower urinary tract infections in women. Scand J Prim Health Care. 1994;12:239–242. doi: 10.3109/02813439409029247. [DOI] [PubMed] [Google Scholar]
- 3.Giori A, Torrani S, Dellaglio F, Bo G, Stola E, Bernuzzi L. Identification of vaginal lactobacilli from asymptomatic women. Microbiologica. 1987;10:377–384. [PubMed] [Google Scholar]
- 4.Guillilan S E. Beneficial interrelationships between certain microorganisms and humans: candidate microorganisms for use as dietary adjuncts. J Food Prot. 1979;42:164–167. doi: 10.4315/0362-028X-42.2.164. [DOI] [PubMed] [Google Scholar]
- 5.Havenaar R, Brink B T, Huis In’T Veld J H J. Selection of strains for probiotics use. In: Fuller R, editor. Probiotics. The scientific basis. London, England: Chapman & Hall; 1992. pp. 209–223. [Google Scholar]
- 6.Hawes S E, Hillier S L, Benedetti J, Stevens C E, Kousty L A, Wolner-Hanssen P, Holmes K K. Hydrogen-peroxide producing lactobacilli and acquisition of vaginal infections. J Infect Dis. 1996;174:1058–1063. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- 7.Jack R W, Tagg J R, Ray B. Bacteriocins of Gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klaenhammer T. Bacteriocin of lactic acid bacteria. Biochimie. 1988;70:337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- 9.Klebanoff S J, Hillier S L, Eschenbach D A, Waltersdorph A M. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J Infect Dis. 1991;164:94–100. doi: 10.1093/infdis/164.1.94. [DOI] [PubMed] [Google Scholar]
- 10.Larsen B. Vaginal flora in health and disease. Clin Obstet Gynecol. 1993;36:107–121. doi: 10.1097/00003081-199303000-00016. [DOI] [PubMed] [Google Scholar]
- 11.McGoarty J. Probiotic use of lactobacilli in the human female urogenital tract. FEMS Immunol Med Microbiol. 1993;6:251–264. doi: 10.1111/j.1574-695X.1993.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 12.McGoarty J A, Tomeczek L, Pond D, Reid G, Bruce A. Hydrogen peroxide production by Lactobacillus species: correlation with susceptibility to the spermicidal compound Nonoxynol-9. J Infect Dis. 1992;165:1142–1144. doi: 10.1093/infdis/165.6.1142. [DOI] [PubMed] [Google Scholar]
- 13.Nader-Macías M E, Romero N C, Apella M C, González S N, Oliver G. Prevention of infections produced by E. coli and L. monocytogenes feeding milk fermented with lactobacilli. J Food Prot. 1993;56:401–405. doi: 10.4315/0362-028X-56.5.401. [DOI] [PubMed] [Google Scholar]
- 14.Ocaña V S, de Ruiz Holgado A P, Nader-Macías M E. Selection of vaginal H2O2-generating Lactobacillus for probiotic use. Curr Microbiol. 1999;38:279–284. doi: 10.1007/pl00006802. [DOI] [PubMed] [Google Scholar]
- 15.Ocaña V S, de Ruiz Holgado A P, Nader-Macías M E. Growth inhibition of Staphylococcus aureus by H2O2-producing Lactobacillus paracasei subsp. paracasei isolated from the human vagina. FEMS Immunol Med Microbiol. 1999;23:87–92. doi: 10.1111/j.1574-695X.1999.tb01227.x. [DOI] [PubMed] [Google Scholar]
- 16.Ocaña, V. S., E. Bru, A. P. de Ruiz Holgado, and M. E. Nader-Macías. Surface characteristics of lactobacilli isolated from human vagina. J. Gen. Appl. Microbiol., in press. [DOI] [PubMed]
- 17.Okereke A, Montville T. Bacteriocin-mediated inhibition of Clostridium botulinum spores by lactic acid bacteria at refrigeration and abuse temperatures. Appl Environ Microbiol. 1991;57:3423–3428. doi: 10.1128/aem.57.12.3423-3428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raibaud P, Galpin J V, Ducluzeau R, Mocquot F, Oliver G. Le genre Lactobacillus dans le tube digestif du rat. II. Caracters de souches heterofermentaires isolates de rats “holo” et “gnotoxeniques.”. Ann Microbiol. 1973;124:223–235. [PubMed] [Google Scholar]
- 19.Redondo-López V, Cook R L, Sobel J D. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990;12:856–872. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- 20.Reid G, McGoarty J A, Angotti R, Cook R L. Lactobacillus inhibitor production against Escherichia coli and coaggregation ability with uropathogens. Can J Microbiol. 1988;34:344–351. doi: 10.1139/m88-063. [DOI] [PubMed] [Google Scholar]
- 21.Yang R, Johnson M C, Ray B. Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl Environ Microbiol. 1992;58:3355–3359. doi: 10.1128/aem.58.10.3355-3359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]