Abstract
Primary hepatocellular carcinoma (HCC) in patients <30 years old is extremely rare. In younger patients, HCC develops against a background of persistent hepatitis B virus infection. We herein report a 23-year-old woman with HCC with all-negative hepatitis virus markers developing in an apparently healthy liver. Imaging studies showed a 50-mm hypervascular mass in segment 4 of the left liver lobe, compatible with HCC. The patient underwent surgical resection. A histological examination showed the presence of poorly differentiated HCC. The patient was diagnosed with HCC developing in a healthy liver. This is an extremely rare case of non-B non-C HCC.
Keywords: hepatocellular carcinoma, non-B non-C, healthy liver, juvenile hepatocellular carcinoma
Introduction
In Japan, primary hepatocellular carcinoma (HCC) often develops against a background of chronic hepatitis, resulting from persistent infection with hepatitis B virus (HBV) or hepatitis C virus (HCV). Individuals over 50 years old are considered the most susceptible. However, the onset of primary HCC at a younger age is often associated with persistent HBV infection (1).
We herein report a 23-year-old woman with primary HCC who showed no findings of chronic hepatitis and for whom all hepatitis-related markers remained consistently negative.
Case Report
A 23-year-old healthy Japanese woman had normal vaginal delivery at 21 years old. One year before this presentation, she noticed an abdominal mass, visited a local doctor, and underwent a follow-up examination. On admission because of dysuria, a tumor was detected by abdominal ultrasonography (US) in her upper abdomen, and she was referred to our hospital for a further examination of the mass. She had no family history of HCC or any other malignancy. Furthermore, she was a non-smoker and non-drinker, with no history of medications including pills or supplements. The patient also had no history of blood transfusion or trauma, such as hepatic trauma. She did not have any metabolic abnormalities, including her thyroid function based on the findings obtained either at the clinic of her own obstetrician at the time of her first child's birth or at our hospital.
On visiting our hospital, her physical characteristics were as follows: height, 159 cm; weight, 35 kg; and body mass index (BMI), 13.8 kg/m2. She had a soft abdomen with no palpable liver or spleen, no abdominal distension, no tenderness, and no edema. Blood test results showed normal results for liver function tests (Table 1). However, some tumor markers were elevated: alpha fetoprotein, 941.8 ng/mL; protein induced by vitamin K absence-II, 1,170 mAU/mL. These findings suggested HCC (Table 1). All HBV markers, including HBsAg, HBsAb, and HBcAb, were negative (Table 1). Results for anti-HCV antibody were also negative. Serum concentrations of IgG, IgM, copper, and ferritin were all normal (Table 1). The laboratory findings for the patient were negative for antinuclear antibody and anti-mitochondrial antibody (Table 1). The albumin level, total bilirubin level, prothrombin time, and indocyanine green (ICG) R15 value were within normal ranges, suggesting a good hepatic reserve (Table 1).
Table 1.
Results of Laboratory Blood Examination on Admission.
| TP | 7.8 | g/dL | AFP | 941.8 | ng/mL |
| Albumin | 4.8 | g/dL | AFP-L3 | 1.80 | % |
| BUN | 11 | mg/dL | PIVKA-II | 1,170 | mAU/mL |
| Cre | 0.49 | mg/dL | CEA | 0.5 | ng/mL |
| AST | 18 | U/L | CA19-9 | 13.2 | U/mL |
| ALT | 12 | U/L | HBsAg | (-) | |
| γ-GTP | 16 | U/L | HBsAb | (-) | |
| ALP | 216 | U/L | HBcAb | (-) | |
| T-bil | 0.6 | mg/dL | HCVAb | (-) | |
| LDH | 161 | U/L | IgG | 1,179 | mg/dL |
| Na | 140 | mmol/L | IgM | 294 | mg/dL |
| K | 3.8 | mmol/L | ANA | <40 | |
| Cl | 106 | mmol/L | AMA | <20 | |
| ChE | 263 | U/L | |||
| HbA1c | 5.3 | % | Fe | 35.8 | ng/mL |
| WBC | 9,320 | /μL | Cu | 107 | μg/dL |
| RBC | 498×104 | /μL | TC | 204 | mg/dL |
| Hb | 13.8 | g/dL | TG | 55 | mg/dL |
| Plt | 26.4×104 | /μL | |||
| PT-INR | 1.06 | % | |||
| APTT | 42.2 | s |
TP: total protein, BUN: blood urea nitrogen, Cre: creatinine, AST: aspartate aminotransferase, ALT: alanine aminotransferase, γ-GTP: γ-glutamyl transferase, ALP: alkaline phosphatase, T-bil: total bilirubin, LDH: lactate dehydrogenase, ChE: cholinesterase, WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, Plt: platelet, PT-INR: prothrombin time-international normalized ratio, APTT: activated partial thromboplastin time, AFP: alpha-fetoprotein, PIVKA-II: protein induced by vitamin K absence or antagonist II, HBsAg: hepatitis B surface antigen, HBsAb: hepatitis B surface antibody, HBcAb: hepatitis B core antibody, HCVAb: hepatitis C virus antibody, ANA: antinuclear antibody, AMA: anti-mitochondrial antibody, TC: total cholesterol, TG: triglyceride
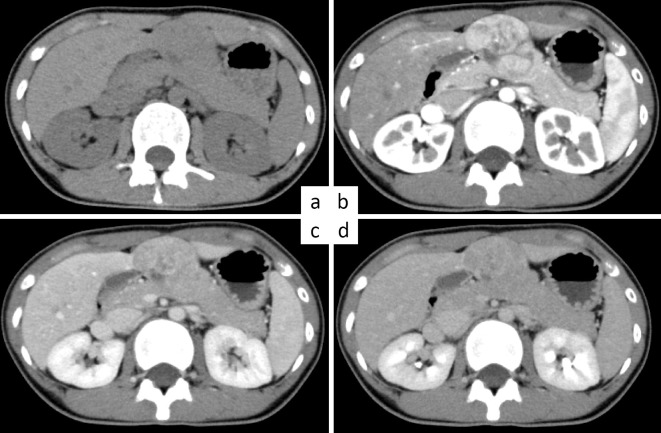
Abdominal US showed a solitary hypoechoic tumor with clear boundaries in segment 4 of the left liver lobe. Abdominal computed tomography (CT) showed a 5-cm tumor in S4 of the liver (Fig. 1a). Multiphase CT showed a strong uptake of the contrast agent in the hepatic arterial phase (Fig. 1b), followed by washout in the portal venous phase (Fig. 1c) and the equilibrium phase (Fig. 1d). However, CT showed no findings of a central scar. Magnetic resonance imaging (MRI) showed decreased and increased signal intensities for the lesion on T1- and T2-weighted imaging, respectively, whereas diffusion restriction with a high signal intensity and a low apparent diffusion coefficient were apparent on diffusion-weighted imaging. Dynamic contrast-enhanced MRI showed strong enhancement in the hepatic artery phase with subsequent washout in the portal venous and equilibrium phases and typical hypointensity in the hepatobiliary phase in segment 4 of the liver. Based on these findings, HCC of cT2N0M0 cStage II (Union for International Cancer Control Seventh Edition, UICC 7th edition) was diagnosed.
Figure 1.
Multiphase computed tomography (CT) on admission showing juvenile hepatocellular carcinoma that developed in a normal liver. a) Image taken before the administration of contrast medium shows a tumor in the S4 segment of the liver. b-d) Findings of contrast-enhanced CT. A solid hepatic mass is seen in the S4 segment of the liver. The uptake of the contrast agent in the hepatic arterial phase (b) is strong, with subsequent washout in the portal venous phase (c) and the equilibrium phase (d).
The patient underwent surgical resection of segment 4, as her hepatic reserve was considered adequate for resection. The operation and postoperative clinical course were uneventful, and the patient was discharged on postoperative day 7.
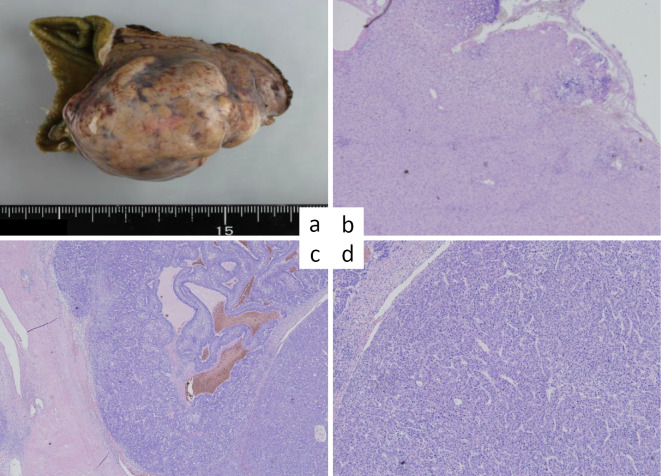
A postoperative examination of the tumor specimen showed a 50×30-mm lesion with expansive growth (Fig. 2a). A histological examination of the non-tumorous portions of the specimen showed no fibrosis (Fig. 2b), and no infiltration of inflammatory cells into the portal areas or hepatic lobules was evident (Fig. 2b). No fatty deposits were identified in hepatocytes (Fig. 2b). The pathological report indicated poorly differentiated HCC, as follows: fc (capsule formation) (+), fc-inf (infiltration) (+), sf (septum formation) (+), s0, n0, Vp1, Vv1, Va0, b0, p0, sm (-) pT2 N0 M0 p Stage II (UICC, 7th edition) (Fig. 2c).
Figure 2.
A postoperative examination shows a tumor 50×30 mm in size with expansive growth. a) Resected tumor. b) Hematoxylin and Eosin (H&E) staining of the noncancerous portions showing no fibrosis and no inflammatory infiltration to the portal areas and hepatic lobules. c, d) H&E staining of the tumor showing partial fatty changes, a solid nest structure, and a false duct structure, indicating poorly differentiated hepatocellular carcinoma. The tumor cells show invasion into a capsule with partial vascular invasion.
She gave birth to her second child 15 months after surgery, and at the time of writing this manuscript, at 5 years post-operation, she has had no recurrence of the disease. We conducted follow-up investigations such as blood tests, including tumor marker evaluations, every month, abdominal US every three months, and abdominal CT every six months.
Discussion
Approximately 80% of primary liver cancers are HCC, and 80% of all HCC patients have chronic hepatitis or liver cirrhosis with persistent HBV or HCV infection and a history of alcohol intake (1-6). Recently, the incidence of non-B non-C-type HCC, which may be due to alcoholic hepatitis, nonalcoholic steatohepatitis, hemochromatosis, primary biliary cirrhosis (PBC), or drugs such as oral contraceptive pill (7-11), has been reported to be increasing. In Japan, HCV infection often causes chronic hepatitis or liver cirrhosis, and HCC caused by HCV often develops in middle-aged patients (12,13). The frequency of HCC in patients <35 years old is 0.89%, and only 0.14% are <25 years old (6,12). In carcinogenesis of HCC in young patients, the roles of HBV DNA integration or the HBV HBx protein have been reported (14,15). HCC that occurs unrelated to hepatitis or liver cirrhosis is extremely rare (3.2%) in Japan (12). Fibrolamellar HCC (FLC) is a special type of liver cancer (16,17) that develops in young patients and is unrelated to hepatitis or liver cirrhosis. It is characterized by a large tumor with calcification and a central scar (17,18).
In the present case, the patient was a young woman, at only 23 years old. Furthermore, in this case, hepatitis virus-related markers such as HBsAg, HBsAb, HBcAb, HCVAb were all negative, and neither HBV-DNA nor HCV-RNA was detected. In the postoperative examination of pathological specimens, the background liver appeared normal and no findings suggestive of chronic hepatitis were detected and the pathological findings were also different from FLC. As far as we search in Pubmed and medical journal ‘Igaku Chuo Zasshi (ICHUSHI)’ up to 2021, only 17 cases of non-B non-C HCC without chronic hepatitis or liver cirrhosis in the liver have been reported (Table 2). Although approximately half of those cases involved patients who were under thirty years old, only two cases, showed HCC without previous history, family history, blood transfusion history, drinking history or oral administration other than this patient. The patient was definitively underweight and BMI was low. However, her weight did not change preoperatively, and she had not experienced any changes in her body shape during her life. Further, blood testing revealed no evidence of malnutrition, and no evidence of fatty liver from malnutrition was seen in the background liver on postoperative pathology, so we do not believe that underweight status affected the development of HCC in this case. Therefore, we consider this case as extremely rare.
Table 2.
Juvenile Hepatocellular Carcinoma without Hepatitis Infection: Summary of Case Reports.
| No. | Age (years) | Sex | HBsAg/ Ab |
HBcAb | HBV-DNA | HCV-Ab | Family history |
Past history | Drinking/ smoking |
|
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22) | 25 | F | -/- | - | - | - | - | Mycoplasma pneumonia |
-/- |
| 2 | 23) | 23 | F | -/- | - | - | - | Chronic hepatitis |
Congenital cataract |
-/- |
| 3 | 8) | 17 | M | -/- | - | - | - | - | Bone fracture |
-/- |
| 4 | 24) | 28 | M | -/- | ND | - | - | - | - | -/+ |
| 5 | 25) | 20 | M | -/- | - | - | - | - | - | ND/ND |
| 6 | 26) | 20 | M | -/- | - | - | - | - | Precocious puberty |
ND/ND |
| 7 | 27) | 25 | F | -/- | - | ND | - | ND | Epilepsy | ND/ND |
| 8 | 28) | 25 | F | -/- | - | - | - | - | Ovarian cyst | -/- |
| 9 | 29) | 26 | F | -/- | - | ND | ND | ND | ND | ND |
| 10 | 30) | 39 | F | -/- | - | - | - | ND | ND | -/- |
| 11 | 31) | 41 | M | -/- | - | - | - | - | - | -/- |
| 12 | 32) | 31 | F | -/- | - | - | - | ND | Pyogenic spondylitis |
ND |
| 13 | 33) | 31 | M | -/- | - | - | - | - | Appendicitis | +/- |
| 14 | 34) | 40 | F | -/- | ND | - | - | - | - | -/ND |
| 15 | 35) | 26 | M | -/- | - | - | - | - | - | +/+ |
| 16 | 36) | 30 | M | -/- | - | - | - | - | Asthma | -/ND |
| 17 | 37) | 20’s | M | -/- | - | - | - | - | - | -/- |
| 18 | Onishi et al. | 23 | F | -/- | - | - | - | - | - | -/- |
| No. |
Transfusion
recording |
Drug | AFP | PIVKAII | Treatment | Recurrence |
Outcome
(after treatment) |
|||
| 1 | 22) | - | ND | 35,200 | 14.4 | TAE | No recurrence | Alive 11 months |
||
| 2 | 23) | - | - | 10,320 | Normal | TAE | Brain metastasis (2 years) |
Died 2 years |
||
| 3 | 8) | + | - | <3.0 | <1.0 | TAE, Ope | No recurrence | Alive 19 months |
||
| 4 | 24) | - | - | 37,000 | 18,100 | ND | No recurrence | Died 16 days |
||
| 5 | 25) | - | - | 181.8 | 75,000 | Ope, TAE, PEIT |
Intrahepatic (7 months) |
Alive 36 months |
||
| 6 | 26) | ND | ND | 25,100 | 292 | Ope | No recurrence | Alive 30 months |
||
| 7 | 27) | ND | Phenytoin | 5,011 | 6,022 | TAE, Ope | No recurrence | Alive 21 months |
||
| 8 | 28) | - | - | 1.2 | 329 | Ope | No recurrence | Alive 108 months |
||
| 9 | 29) | ND | ND | >300 | ND | Palliative chemo |
No recurrence | Died 3 months |
||
| 10 | 30) | - | - | Normal | Normal | Ope | ND | ND | ||
| 11 | 31) | - | - | ND | ND | BSC | ND | Died 3 months |
||
| 12 | 32) | ND | - | 4.1 | 39 | Ope | No recurrence | Alive 31 months |
||
| 13 | 33) | - | - | 4.8 | 41 | Ope | ND | Alive 24 months |
||
| 14 | 34) | + | ND | ND | ND | BSC | ND | Died 8 days |
||
| 15 | 35) | - | - | Normal | Normal | Ope | ND | ND | ||
| 16 | 36) | - | - | 2.3 | 24 | Ope | - | Alive 10 months |
||
| 17 | 37) | ND | - | 76,860 | 74,849 | Chemotherapy | ND | Died 9 months |
||
| 18 | Onishi et al. | - | - | 941.8 | 1,170 | Ope | - | Alive 60 months |
||
M: male, F: female, ND: not described, TAE: transcatheter arterial embolization, Ope: operation, PEIT: percutaneous ethanol injection therapy, BSC: best supportive care
Recently, methods for genome-wide association analyses have advanced dramatically, and some reports have provided genomic information for HCC. However, HCC is a heterogeneous disease, with characteristics not only due to the cause, but also due to the genetic characteristics of the tumor, as the process of multicentric carcinogenesis differs between individual cases. In addition to identifying gene mutations for whole-genome sequencing, viral integration can also be identified. In recent years, not only the HBV genome, but also the adeno-associated virus type 2 genome has been reported to become integrated into HCC, and many cases without known risk factors have been reported. In our case, genome sequencing thus appears important for clarifying the genetic characteristics of carcinogenesis.
In general, HCC that develops in young patients often progresses and shows a low degree of differentiation on histology (19,20). Recurrence within one year or pathological vascular invasion at the time of initial resection is said to be a factor contributing to poor prognosis at the time of recurrence (21) and follow-up within the short term using various modalities is desirable after surgical resection. In our case, vascular invasion (Vp1, Vv1) was seen in the postoperative pathological tissue, so the risk of recurrence was considered high. We conducted follow-up observations such as blood tests including tumor markers every month, abdominal ultrasonography every three months, and abdominal CT every 6 months. As of 5 years after surgery, she is undergoing follow-up with no recurrence, and we will continue follow-up observation in the future.
In conclusion, we experienced present an extremely rare case of non-B non-C HCC occurred in normal liver in at 23 years old. No recurrence has been observed for 5 years, however, careful follow-up is required due to the risk of recurrence.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Herbst DA, Reddy KR. Risk factors for hepatocellular carcinoma. Clin Liver Dis 1: 180-182, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56: 908-943, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Nowicki TK, Markiet K, Szurowska E. Diagnostic imaging of hepatocellular carcinoma - a pictorial essay. Curr Med Imaging Rev 13: 140-153, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 379: 1245-1255, 2012. [DOI] [PubMed] [Google Scholar]
- 5. El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol 35: S72-S78, 2002. [DOI] [PubMed] [Google Scholar]
- 6. Shiratori Y, Yoshida H, Omata M. Different clinicopathological features of hepatocellular carcinoma: recent trends in Japan. Gastroenterology 127: S17-S26, 2001. [DOI] [PubMed] [Google Scholar]
- 7. Farinati F, De Maria N, Marafin C, Fagiuoli S, Della Libera G, Naccarato R. Hepatocellular carcinoma in alcoholic cirrhosis: is sex hormone imbalance a pathogenetic factor? Eur J Gastroenterol Hepatol 7: 145-150, 1995. [PubMed] [Google Scholar]
- 8. Farinati F, Floreani A, De Maria N, Fagiuoli S, Naccarato R, Chiaramonte M. Hepatocellular carcinoma in primary biliary cirrhosis. J Hepatol 21: 315-316, 1994. [DOI] [PubMed] [Google Scholar]
- 9. Kosaka A, Takahashi H, Yajima Y, et al. Hepatocellular carcinoma associated with anabolic steroid therapy: report of a case and review of the Japanese literature. J Gastroenterol 31: 450-454, 1996. [DOI] [PubMed] [Google Scholar]
- 10. Tavani A, Negri E, Parazzini F, et al. Female hormone utilization and risk of hepatocellular carcinoma. Br J Cancer 67: 635-637, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol 28: 753-770, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Liver Cancer Study Group of Japan. [The 19th national primary liver cancer follow-up report (2006-2007)]. Kanzo (Acta Hepatol Jpn) 57: 45-73, 2016(in Japanese). [Google Scholar]
- 13. Churiki M, Ikai I, Yamamoto M, et al. A case of juvenile hepatocellular carcinoma without hepatitis infection. Nihon Shoukakibyou Gakkai Zasshi (J Jpn Soc Gastroenterol) 30: 2292-2296, 1997. [Google Scholar]
- 14. Ali A, Abdel-Hafiz H, Suhail M, et al. Hepatitis B virus, HBx mutants and their role in hepatocellular carcinoma. World J Gastroenterol 20: 10238-10248, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Minor MM, Slagle BL. Hepatitis B virus HBx protein interactions with the ubiquitin proteasome system. Viruses 6: 4683-4702, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lafaro KJ, Pawlik TM. Fibrolamellar hepatocellular carcinoma: current clinical perspectives. J Hepatocell Carcinoma 2: 151-157, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ganeshan D, Szklaruk J, Kundra V, Kaseb A, Rashid A, Elsayes KM. Imaging features of fibrolamellar hepatocellular carcinoma. AJR Am J Roentgenol 202: 544-552, 2014. [DOI] [PubMed] [Google Scholar]
- 18. Sergi CM. Hepatocellular carcinoma, fibrolamellar variant: diagnostic pathologic criteria and molecular pathology update. A primer. Diagnostics (Basel) 6: 3, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ng IO, Ng MM, Lai EC, Fan ST. Pathologic features and patients survival in hepatocellular carcinoma in relation to age. J Surg Oncol 61: 134-137, 1996. [DOI] [PubMed] [Google Scholar]
- 21. Hanazaki K, Kajikawa S, Shimozawa N, et al. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg 191: 381-388, 2000. [DOI] [PubMed] [Google Scholar]
- 22. Fujita N, Yoshikawa T, Uemura M, et al. A case of juvenile hepatocellular carcinoma arising from normal liver. Nihon Shokakibyo Gakkai Zasshi (J Jpn Soc Gastroenterol) 92: 1092-1097, 1995. (Abstract in English). [PubMed] [Google Scholar]
- 23. Sato S, Tanioka H, Nagata H, et al. A case of non-B, non-C, juvenile hepatocellular carcinoma with brain metastasis. Nihon Shokakibyo Gakkai Zasshi (J Jpn Soc Gastroenterol) 93: 758-762, 1996. (Abstract in English). [PubMed] [Google Scholar]
- 24. Nakano M, Ohtsuka Y, Sugaya H, Terano A, Sasai T, Monma T. A case of rapidly progressive hepatocellular carcinoma in a juvenile patient with no serum hepatitis virus marker. Dokkyo J Med Sci 29: 177-181, 2002. (Abstract in English). [Google Scholar]
- 25. Fukui K, Taniguchi H, Ikoma H, et al. A case with juvenile hepatocellular carcinoma without hepatitis underwent right hepatic trisegmentectomy. Gan to Kagaku Ryoho (Jpn J Cancer Chemother) 38: 2475-2477, 2011. (Abstract in English). [PubMed] [Google Scholar]
- 26. Kaibori M, Ishizaki M, Uchida I, et al. A case of non-B, non-C, juvenile hepatocellular carcinoma. Nihon Shokakibyo Gakkai Zasshi (J Jpn Soc Gastroenterol) 40: 617-622, 2007. (Abstract in English). [Google Scholar]
- 27. Okamoto N, Yamafuji K, Kubochi K, et al. A case of ruptured hepatocellular carcinoma having neither hepatitis B nor hepatitis C virus infection in a young adult patient. Nihon Shokakibyo Gakkai Zasshi (J Jpn Soc Gastroenterol) 43: 929-934, 2010. (Abstract in English). [Google Scholar]
- 28. Kitagawa H, Tsuda S, Tuboi K, et al. A case of well-differentiated hepatocellular carcinoma that developed in a normal liver on a young woman. Nihon Rinsho Gega Igakkai Zasshi (J Jpn Soc Clin Surg) 75: 190-196, 2014. (Abstract in English). [Google Scholar]
- 29. Assed C, Marchiori E, Zanetti G, et al. Pulmonary metastases from primary hepatocellular carcinoma in a 26-year-old patient: a case report. Cases J 2: 6256, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka T, Hoshino Y, Hayashi S, et al. A case of hepatocellular carcinoma in normal liver with no evidence of HBV or HCV infection. Hepatogastroenterology 43: 1390-1394, 1996. [PubMed] [Google Scholar]
- 31. Herath HM, Kulatunga A. Large hepatocellular carcinoma in a non-cirrhotic liver with peritoneal and omental metastasis in a healthy man: a case report. J Med Case Rep 11: 34, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanada H, Sueda T, Nakai S, et al. Hepatocellular carcinoma in a young woman which occurred in the liver without virus infection. Nihon Rinsho Gega Igakkai Zasshi (J Jpn Soc Clin Surg) 65: 1918-1923, 2004. [Google Scholar]
- 33. Kubo Y, Itubo M, Koike K, et al. A young male patient with nonB-nonC hepatocellular carcinoma and concomitant focal nodular hyperplasia developed in the normal liver. Kanzo (Acta Hepatol Jpn) 48: 233-239, 2007. (Abstract in English). [Google Scholar]
- 34. Sharma ZD, Gokhale VS, Chaudhari N, Kakrani AL. An acute unusual presentation of hepatocellular carcinoma. J Cancer Res Ther 10: 401-403, 2014. [DOI] [PubMed] [Google Scholar]
- 35. Sunagawa H, Ogura K, Orokawa T, et al. A case of juvenile hepatocellular carcinoma without viral hepatitis. Liver Cancer 19: 23-27, 2013. (Abstract in English). [Google Scholar]
- 36. Abe Y, Nojiri K, Mogaki M, et al. A case of hepatocellular carcinoma (with the maximum diameter of 12 cm) arisen in the normal liver of a 30-year-old man. Nihon Rinsho Gega Igakkai Zasshi (J Jpn Soc Clin Surg) 80: 2067-2072, 2019. (Abstract in English). [Google Scholar]
- 37. Hashimoto A, Hattori A, Tanaka T, et al. A case of young hepatocellular carcinoma with non-B, non-C, non-cirrhotic background. Nihon Shokakibyo Gakkai Zasshi (J Jpn Soc Gastroenterol) 118: 348-357, 2021. [DOI] [PubMed] [Google Scholar]