Abstract
This study aimed to determine the incidence of leptospirosis and melioidosis in long-tailed macaques (Macaca fascicularis) in Thailand. Serum samples from 223 monkeys were subjected to the Lepto Latex Test and indirect hemagglutination (IHA) test to detect antibodies against Leptospira spp. and Burkholderia pseudomallei. The microagglutination test (MAT) was used to identify serovars of Leptospira spp. Conventional PCR for the LipL32 gene of L. interogans and the BPSS0120 and btfc-orf18 genes of B. pseudomallei was used for molecular detection. The overall seroprevalence of leptospirosis and melioidosis was 2.69% (95% confidence interval (CI): 0.99–5.76%) and 14.35% (95% CI: 10.03–19.65%), respectively. Six samples that showed positive MAT results were also positive for IHA. The serovars of Leptospira were Ranarum (5/6), Shermani (6/6), and both (5/6). Conventional PCR for the LipL32 gene of Leptospira spp. was positive in 10.31% of the samples (95% CI: 5.56–13.51%). However, there were no positive results for BPSS0120 and btfc-orf18 in B. pseudomallei. Active infection was detected only for leptospirosis; however, it can be assumed that pathogen exposure occurred in this group of animals because immunity could be detected. The routes of infection and elimination pathways of both bacteria remain unclear, and the mechanism of protection in non-human primates needs to be elucidated in further studies. Moreover, this health issue should be considered to prevent human infections in monkeys and their environment.
Keywords: leptospirosis, long-tailed macaque, melioidosis, natural infection, Thailand
Leptospirosis and melioidosis are important anthropozoonotic diseases in Thailand. Leptospirosis is an infection caused by the spirochete bacterium Leptospira spp. Melioidosis is a severe infectious disease caused by the gram-negative rod-shaped bacterium, Burkholderia pseudomallei. Both diseases are considered emerging and re-emerging infectious diseases and are additionally considered as neglected tropical diseases in many regions of the world with tropical climates. In Thailand, leptospirosis and melioidosis are major endemic diseases, and frequent reports of outbreaks are associated with exposure to contaminated water [8, 13, 15, 34]. Animals are often a source of pathogens. In Thailand, seroprevalence of Leptospira spp. has been found in companion animals, including hospitalized dogs, stray dogs, and stray cats [21, 24]. It has also been recorded in many species of livestock, including cattle, buffaloes, pigs, sheep, and goats [26, 33]. Interestingly, some studies have reported the presence of antibodies against different Leptospira serovars in 6.12% of terrapins [4]. Endemic areas of B. pseudomallei are Northern Australia and Southeast Asia, including Thailand [7, 16, 38]. Seroprevalence of melioidosis has been reported in dairy cattle, indigenous cattle, goats, and pigs [19, 32]. In humans, septicemia melioidosis mainly occurs in farmers during the rainy season and is a common cause of death in northeast Thailand [36].
Both pathogens infect humans and other animals, usually via direct contact with the source of the disease or indirect contact with a contaminated environment, such as the urine of an infected animal. Symptoms of leptospirosis and melioidosis in both humans and animals are characterized by non-specific signs of acute undifferentiated fever. Therefore, it is difficult to rule out other diseases that contribute to under-reporting and neglected disease status. However, these pathogens have not been investigated in non-human primates in Thailand. This might become a public health concern because non-human primates surround human settlements in the same environment, as some of them are free living near the urban areas of Thailand. However, they may release the pathogen into the human environment in the future. Therefore, this study aimed to investigate infections with Leptospira spp. and B. pseudomallei in free-living long-tailed macaques (Macaca fascicularis) in three regions of Thailand. These results may promote monkey health care and awareness of zoonosis transmission from monkeys to humans.
MATERIALS AND METHODS
Ethical statement
This study was approved by the Animal Ethics Committee of Kasetsart University, Thailand (Approval No. ACKU60-VET-049).
Sample collection
Blood samples were randomly collected from wild monkeys (Macaca fascicularis) free-living near urban areas in three regions of Thailand during catching for castration and health checks. A total of 223 blood samples were obtained from 214 males and nine females aged between 2 and 4 years. The samples included 19 from the southern region (Tang Kuan Mountain, Maung Songkhla District), 87 from the central region (Hua Hin District, Prachuap Khiri Khan), and 117 from the eastern region (Laem Chabang, Chon Buri) (Fig. 1). Serum and DNA samples were obtained from all blood samples in the laboratory of the Faculty of Veterinary Science, Prince of Songkla University. DNA extraction was performed using a commercial Blood DNA Isolation Kit (Geneaid, Teipei, Taiwan), according to the manufacturer’s instructions, and kept at −20°C until molecular detection.
Fig. 1.
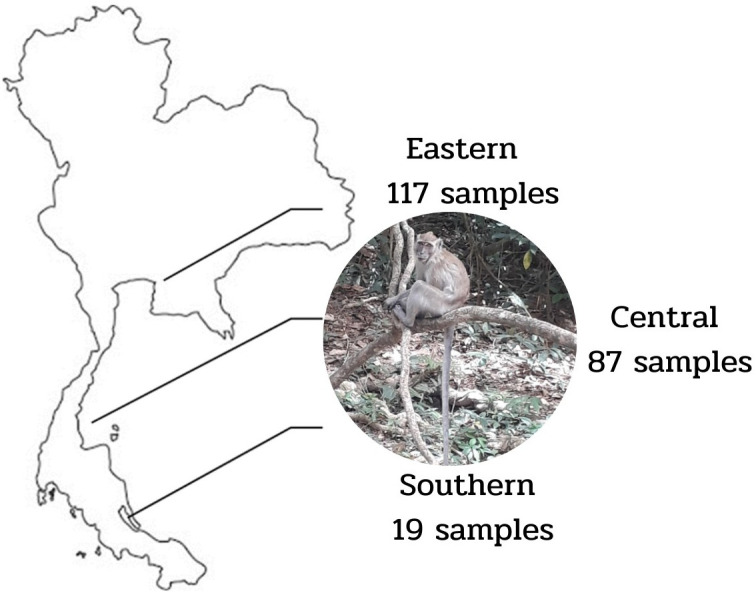
Sampling areas and the number of samples: 19 from the Southern region (Tang Kuan Mountain, Maung Songkhla district), 87 from the Central region (Hua Hin district, Prachuap Khiri Khan), and 117 from the Eastern region (Laem Chabang, Chon Buri).
Serological assessment
Lepto latex test according to the instructions of the Department of Medical Sciences, Ministry of Public Health, Thailand, which has a sensitivity and specificity of 94% and 93%, respectively (http://webdb.dmsc.moph.go.th/ifc_nih/a_nih_2_003c.asp?info_id=1007, accessed December 2, 2021), was used to screen for antibodies against Leptospira infection. The long-tailed macaque antibody response to B. pseudomallei was detected by an indirect hemagglutination test (IHA) using a melioidosis-IHA kit (Department of Medical Sciences, Ministry of Public Health, Thailand). The cutoff titer for animals was ≥1:160 [23], defined as a melioidosis-seropositive result.
Microagglutination test (MAT)
Positive sera from the Lepto Latex Test were subjected to the microagglutination test (MAT) at the National Institute of Animal Health, Thailand to evaluate antibodies against 24 Leptospira serovars at a titer ≥1:100. The Leptospira serovars included in this study were Autumnalis, Ballum, Bratisalava, Bataviae, Canicola, Celledoni, Cynopteri, Djasiman, Grippotyphosa, Hebdomatis, Icterohaemorrhagiae, Javanica, Louisiana, Manhao, Mini, Panama, Pomona, Pyrogenes, Ranarum, Shermin, Sejroe, Shermani, Tarrassovi, and Pathoc.
Molecular analysis
DNA samples were subjected to PCR amplification to detect the LipL32 gene of Leptospira spp. as previously described [28] (Table 1). The reaction was prepared in 12 µl and included 5.0 µl of master mix with Taq polymerase (KAPA®, Tokyo, Japan), 0.25 µl of each primer, 4.0 µl of ultra-pure water, and 2.0 µl of the DNA template. The PCR cycle consisted of an initial denaturation step at 95°C for 5 min followed by 35 cycles of denaturation at 94°C, 1 min at 60°C, and extension at 72°C. The final extension phase was conducted at 72°C for 7 min. The PCR products were electrophoresed on 2% agarose gels at 100 V for 30 min to identify 423-bp fragments. The PCR reaction for detecting B. pseudomallei was performed using specific primer pairs, according to a previous study [37] (Table 1). The DNA amplification reactions were performed in a 20-µl volume, containing 1x TopTaq Master Mix Kit (Qiagen, Hilden, Germany), 10 pmol of each primer, and 100–200 ng of template DNA that was extracted from the monkey blood samples. The cycling conditions consisted of an initial denaturation step at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C, 1 min at 60°C, and extension at 72°C. The product extension phase was prolonged to 5 min at 72°C in the last cycle. The PCR products of B. pseudomallei were visualized by gel electrophoresis on 2% agarose gels. The pattern of the PCR amplicon was either 350 bp for the BPSS0120 gene target in the YLF cluster or 115 bp for the btfc-orf18 target in the BTFC cluster.
Table 1. Primer set for detecting Leptospira spp. and Burkholderia pseudomallei.
| Pathogen | Primer name | Primer order | Product length (bp) | Reference | |
|---|---|---|---|---|---|
| Leptospira spp. | LipL32 | F | 5′ CGC GCT GCA GTT ACT TAG TCG CGT CAG AAG 3′ | 423 | [17] |
| R | 5′CCA ACAGATGCAACGAAAGATCCT 3′ | ||||
| B. pseudomallei | BPSS0120 | F | 5-TGA CCC ATT CAG GCA AGG GAT TCT-3 | 350 | [18] |
| R | 5-TCC GTC CTG TTC GGT GAT TTC GAT-3 | ||||
| btfc-orf18 | F | 5-GTC GAT TTC GGC TGC GAA ACA ACA-3 | 115 | ||
| R | 5-ATG CCG TCG CAA CCA TTG ATG ATG-3 | ||||
Statistical analysis
Raw data were used to summarize the positive results and were analyzed as a percentage with both binomial and normal approximations to the proportion. The total number of positive samples was divided by the total number of samples with 95% confidence intervals (CI) on a free website (https://sample-size.net/confidence-interval-proportion). The Fisher exact test was used to identify significant differences between proportions using the social science statistics website (https://www.socscistatistics.com/tests/fisher/default2.aspx, accessed December 2, 2021).
RESULTS
Serological assessments identified that 48 of 223 serum samples contained antibodies against Leptospira spp. (21.52%, 95% CI: 16.32–27.51%) by the Lepto Latex Test. The overall seropositivity by MAT was 2.96% (95% CI: 0.99–5.76%). Of 223 serum samples, 32 contained antibodies against B. pseudomallei (14.35%, 95% CI: 10.03–19.65%) by IHA. Regarding the region, leptospirosis seropositivity was identified in the southern 21.05% (95% CI: 6.05–45.57%), central 22.99% (95% CI: 14.64–33.25%), and eastern 20.51% (95% CI: 13.61–28.97%) regions. Melioidosis seropositivity in the southern, central, and eastern regions was 15.79% (95% CI: 3.38–39.58%), 25.29% (95% CI: 16.58–35.75%), and 5.98% (95% CI: 2.44–11.94%), respectively. The results of this study showed that all cases positive by MAT (6) were positive for IHA. Therefore, overall co-seropositivity for leptospirosis and melioidosis was 2.96% (95% CI: 0.99–5.76%), including 3.45% (95% CI: 0.72–9.75%) in the central region, and 2.56% (95% CI: 0.53–7.31) in the eastern region (Table 2). The analysis by Fisher exact test revealed that the difference in proportion between the southern and central regions (P=1.0) and between the southern and eastern regions (P=0 .0567) was not significantly different. However, co-infection between the central and eastern populations was significantly different (P=0.043).
Table 2. Total percentage of positive samples detected by serological tests; indirect hemagglutination test (IHA) detected antibodies against Burkholderia pseudomallei, Lepto Latex test, and microagglutination test (MAT) detected antibodies against Leptospira spp.
| Region | Total samples | IHA |
Lepto Latex Test |
MAT |
|||
|---|---|---|---|---|---|---|---|
| No of positive | % (95% CI) | No of positive | % (95% CI) | No of positive | % (95% CI) | ||
| Southern | 19 | 3 | 15.79% (3.35–39.58) | 4 | 21.05% (6.05–45.57) | 0 | 0 |
| Central | 87 | 22 | 25.29% (16.58–35.75) | 20 | 22.99% (14.64–33.25) | 3 | 3.45% (0.72–9.75) |
| Eastern | 117 | 7 | 5.98% (2.44–11.94) | 24 | 20.51% (13.61–28.97) | 3 | 2.56% (0.53–7.31) |
| Total | 223 | 32 | 14.35% (10.03–19.65) | 48 | 21.52% (16.32–27.51) | 6 | 2.69% (0.99–5.76) |
In total, 48 positive samples from the Lepto Latex Test were subjected to MAT to detect antibodies against Leptospira spp. at a titer of >1:100; only 6 samples were above this cutoff. The test identified Ranarum (5/6), Shermani (6/6), and both (5/6) serovars of Leptospira spp. Five samples infected with both serovars were from the eastern region (3) and central region (2). The remaining one, infected with only Shermani, was from the central region. No serovars were found at a titer of >1:100 by MAT from the southern region, likely because of the small number of samples.
Molecular detection revealed positive results for LipL32 at 423 bp in 2% gel electrophoresis. The overall positive result rate was 10.31% (23/223) (95% CI: 16.58–35.75%). The most positive results were found in the southern region (21.05%, 95% CI: 6.05–45.57%), followed by the eastern region (11.97%, 95% CI: 6.70–19.26%), and central region (5.75%, 95% CI: 1.89–12.90). There were no positive PCR results for BPSS0120 and btfc-orf18 in B. pseudomallei (Table 3).
Table 3. Total percentage of positive sample by molecular detection; BPSS0120 and btfc-orf18 PCR detected Burkholderia pseudomallei and LipL32 PCR detected Leptospira spp.
| Region | Total samples | BP PCR |
LipL32 PCR |
||
|---|---|---|---|---|---|
| No of positive | Total % (95% CI) | No of positive | Total % (95% CI) | ||
| Southern | 19 | 0 | 0 | 4 | 21.05% (6.05–45.57) |
| Central | 87 | 0 | 0 | 5 | 5.75% (1.89–12.90) |
| Eastern | 117 | 0 | 0 | 14 | 11.97% (6.70–19.26) |
| Total | 223 | 0 | 0 | 23 | 10.31% (6.65–15.07) |
CI, confidence interval.
DISCUSSION
In Thailand, there have been no reports of seroprevalence and DNA detection of leptospirosis and melioidosis in non-human primates. In this study, the seroprevalence of leptospirosis and melioidosis was 2.69% and 14.35%, respectively. Active infection was identified for leptospirosis by detecting the LipL32 gene in approximately 10.31% of cases; however, active infection of melioidosis by detecting the BTFC and YLF genes was not identified. The overall seropositivity of leptospirosis was lower than that of melioidosis. This might be because the antibody from the active infection was not established at the time of sampling.
In comparison, the differentiation between the Lepto Latex Test and MAT results. It indicates that the sensitivity of the Lepto Latex Test is quite low compared with the standard sensitivity (MAT), possibly because the sample was randomly collected only once. Moreover, human bias may have occurred, according to the evaluation based on visual inspection. According to a previous study on human patients in Thailand, the sensitivity and specificity of the Lepto Latex Test were 94.1% and 97.0%, respectively; however, for the first sera obtained from the patients, the sensitivity of the test for acute infection in sera was only 17.6% [25]. The application of a latex agglutination assay to detect human leptospirosis in the Netherlands indicated a mean overall sensitivity of 82.3% and a mean overall specificity of 94.6% [30].
Interestingly, this result demonstrated the co-seropositivity of leptospirosis and melioidosis to be 8.97%. The detection of seropositivity and active infection of leptospirosis was higher than that of melioidosis. This might be because many species of animal reservoirs harbor Leptospira spp., such as rodents, cats, dogs, cattle, goats, and sheep [5, 12, 24]. This pathogen can easily spread in the environment, especially in the humid and flooded areas of Thailand. According to a previous report in 2020, the proportion of human cases of leptospirosis in the southern region increased from the previous year (36.5% to 55.6%), with the most frequent occurrence in Southern Thailand [10]. Human melioidosis has been reported to result in 2,800 deaths yearly in Thailand [15]. From January 1, 2021, to August 7, 2021, the number of patients with melioidosis reached 1,426 with one death, mostly found in the northeastern region, followed by the north, central, and southern regions [11]. This might be related to the fact that seroprevalence and active infection of leptospirosis was higher than that of melioidosis in long-tailed macaques in Southern Thailand. Both pathogens spread during the rainy season and are often found contaminating the water where animals are living [6, 35]. Therefore, it should be encouraged to evaluate the status of both diseases in wild monkeys that are freely ranging near the urban area because they might play an important role as a reservoir.
These results indicate that the overall seroprevalence of leptospirosis in monkeys is lower than that in other animals, such as cats, cattle, pigs, and goats. As previously reported, the seroprevalence of animal leptospirosis in Thailand was 32.48% in stray dogs, 10.93% in stray cats, 9.9–12.5% in cattle, 30.5% in buffaloes, 10.8% in pigs, 4.7% in sheep, and 7.9% in goats [8, 9, 16]. These macaques inhabit endemic areas. Therefore, this might imply that DNA detection is increasing. Moreover, the results of this study indicate that macaques can play a role in leptospirosis transmission to other species and humans. Using MAT at a cutoff titer of ≥1:100, we identified that the seroprevalence in wild monkeys in Thailand was 2.96%. In Japan, antibodies against L. interrogans were found in 2.9% of samples from monkeys [2]. In rescue monkeys in a wildlife rehabilitation center in Colombia, the attack and motility rates of leptospirosis were approximately 27% and 71%, respectively, and L. interrogans sequence type 17 from rats was identified as the source of infection [34]. In African green monkeys, 48% were positive for Leptospira antibodies by MAT and 4% by DNA detection in kidney samples [27]. Moreover, seroprevalence is higher than that in humans (23.7%), buffaloes (24.8%), cattle (28.1%), and pigs (11.3%) [5].
Based on the present study, all monkeys were seropositive for Leptospira, but an active infection status (detected of DNA) was low. This might be because the timing of sample collection was not the period of bacteremia or because they were resistant to the pathogen. However, these monkeys may also play an important role in disease transmission to humans. The serovars identified in monkeys used in this study were Ranarum and Shermani. This finding coincides with a previous study that identified the most common serovar in humans and pigs as Shermani and Ranarum/Shermani in cattle in all regions of Thailand [5]. In northeastern Thailand between 2001 and 2012, Leptospira interrogans serogroup Autumnalis was the major infection in patients [36]. The serovar Icterohaemorrhagiae, which is the most prevalent serovar reported in Rattus spp. worldwide [3], was not detected in this study. A previous study identified 19 different serovars of Leptospira in animals in Thailand, including Anhoa, Australis, Ballum, Bataviae, Bratislava, Broomi, Canicola, Copenhageni, Coxi, Grippotyphosa, Haemolytica, Icterohaemorrhagiae, Khorat, Paidjan, Patoc, Pyrogenes, Rachmati, Saxkoebing, and Sejroe [1]. In neighboring countries, Malaysia has detected more than 30 serovars of Leptospira in a variety of domestic and wild animal species [14]. MAT titers of 1:20 to 1:160 were used to detect Leptospira sp. antibodies in pet cats, found in about 5.4% (95% CI: 3.0–8.6%) of cases, including the serovars Autumnalis, Anhoa, Celledoni, Djasiman, Copenhageni, Icterohaemorrhagiae, and Patoc [31]. Leptospiral infection in non-human primates in Thailand may be related to humans because the same serovars were found. This may be because the forest areas were cut off into parts. The living area of the monkeys was near the urban community, and they were treated with humans. The colony became large with a high number of monkeys. The lack of proper population control is another factor contributing to the increase in the number of monkeys. This resulted in a lack of food and in their invasion of human homes for food. Humans and monkeys live in the same environment and share diseases that can infect mammals. However, this study did not send positive samples for sequencing analysis in the cases of active infection.
In all regions of Thailand, a previous melioidosis infection was observed. A study of animal melioidosis from 2006 to 2015 estimated incidence rates in northeastern, northern, eastern, central, southern, and western areas at about 9.05, 1.87, 1.60, 0.57, 0.53, and 0.36 per 100,000 populations, respectively [19]. A retrospective study estimated the incidence of melioidosis in animals in Thailand between 2006 and 2010, with the highest incidence in goats (1.63/100,000/year), followed by pigs and cattle [20]. In non-human primates, melioidosis has been diagnosed in many species in other countries, such as in stump-tailed macaque monkeys (Macaca arctoides), cynomolgus monkeys (Macaca fascicularis), and rhesus macaques (Macaca mulatta) [18, 19, 39]. However, there have been no reports of wild monkeys in Thailand. The incidence has been reported in non-human primates in zoos at approximately 28%. The highest prevalence of the disease has been reported in a zoo in northeastern Thailand, followed by a zoo in southern Thailand, at approximately 47% and 38%, respectively [17]. The rates reported in this study were lower than those of previous studies because they were not captive monkeys. Importantly, active infection was not detected, and the patients were asymptomatic despite seropositivity.
According to the results of this study, the co-seroprevalence for leptospirosis and melioidosis was 8.97%, indicating that both diseases circulate in the same environment. However, a comparison among the regions found that only the co-infection rate in the central region was significantly different from the co-infection rate in the eastern region. The co-infection rate in the eastern region was low, possibly because the monkeys live near Laem Chabang Port, which is not an urban area with a crowded population as in the central and southern regions. Moreover, it might be correlated with the human cases report, which found that the rate of leptospirosis and melioidosis among the population in Southern and Central region was higher than that in the Eastern region (Department of Disease Control, Ministry of Public Health, Thailand). Co-infection with leptospirosis and melioidosis was previously reported in professional rescuers and villagers involved in rescue operations in Malaysia. At that time, 4 cases were confirmed as melioidosis co-infected with leptospirosis [22]. In another case report of coinfection, the DNA sequences of Leptospira sp. and B. pseudomallei were detected in a diabetic 40-year-old woman with a history of visits to a freshwater camping site in northern Malaysia [29]. The incidence of both infections usually increase during the wet season. Non-human primates may contact with contaminated soil or water while finding food on the ground. The Leptospira serovars that were identified in six monkeys were the same serovars detected in humans; therefore, this might be a close species or one living in the same environment as humans.
Therefore, wild long-tailed macaques in Thailand may act as reservoirs for Leptospira spp. and B. pseudomallei, because they live freely near urban areas. They may play an important role in the spread of disease to the environment and other species of animals, including humans. Hence, wildlife organizations should focus on healthcare in long-tailed macaque herds. Vaccination is difficult because there is no vaccine for melioidosis and vaccines are not cost-effective for leptospirosis. As these monkeys live in tourist attractions, travelers should be aware of and protect themselves from infection. To prevent the risk of infection, people should wear protective footwear and gloves, and wash their hands after contacting with animals and before eating. To prevent leptospirosis, rubbish, food scraps, and junk should be cleaned to keep rodents away. When traveling into the monkey’s territory, they should not be fed in order to prevent cross-contamination with the pathogen.
From these results, it can be inferred that Leptospira spp. and B. pseudomallei exposure occurred in one group of these animals because antibodies could be detected even without showing signs or symptoms. This provides information on a new possible reservoir of mammalian species that maintains these diseases in these three areas. Infected monkeys play an important role in human infection, which may affect public health, the economy (tourism), and conservation efforts. The routes of infection and the elimination pathways of both bacteria are still unclear, and understanding the protective mechanism active in non-human primates as well as the serovars of Leptospira spp. needs to be elucidated in further studies.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
Acknowledgments
We thank the partially support (Grant No. VET6405007S) received from National Science, Research and Innovation Fund (NRSF) and Prince of Songkla University for the microagglutination test and EXT601064S for staff support. We thank for the samples supported by the non-human primate research group at the Faculty of Veterinary Medicine, Kasetsart University, and the Department of National Park, Wildlife, and Plant Conservation, Bangkok. We gratefully acknowledge all those who collected samples from monkeys in Thailand.
REFERENCES
- 1.Altheimer K., Jongwattanapisan P., Luengyosluechakul S., Pusoonthornthum R., Prapasarakul N., Kurilung A., Broens E. M., Wagenaar J. A., Goris M. G. A., Ahmed A. A., Pantchev N., Reese S., Hartmann K.2020. Leptospira infection and shedding in dogs in Thailand. BMC Vet. Res. 16: 89. doi: 10.1186/s12917-020-2230-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai T., Kinjo T., Minamoto N., Sugiyama M., Matsubayashi N., Narama I.1991. Prevalence of antibodies to five selected zoonosis agents in monkeys. J. Vet. Med. Sci. 53: 553–559. doi: 10.1292/jvms.53.553 [DOI] [PubMed] [Google Scholar]
- 3.Boey K., Shiokawa K., Rajeev S.2019. Leptospira infection in rats: a literature review of global prevalence and distribution. PLoS. Negl. Trop. Dis. 9: e0007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonacina E., Oltolina M., Robbiati R., Pinzauti P., Ebani V. V.2021. Serological survey on the occurrence of anti-Leptospira spp. antibodies in red-eared terrapins (Trachemys scripta elegans) living in a natural park of northern Italy. Animals (Basel) 11: 602 (Basel). doi: 10.3390/ani11030602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chadsuthi S., Bicout D. J., Wiratsudakul A., Suwancharoen D., Petkanchanapong W., Modchang C., Triampo W., Ratanakorn P., Chalvet-Monfray K.2017. Investigation on predominant Leptospira serovars and its distribution in humans and livestock in Thailand, 2010–2015. PLoS Negl. Trop. Dis. 11: e0005228. doi: 10.1371/journal.pntd.0005228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chadsuthi S., Chalvet-Monfray K., Wiratsudakul A., Suwancharoen D., Cappelle J.2018. A remotely sensed flooding indicator associated with cattle and buffalo leptospirosis cases in Thailand 2011–2013. BMC Infect. Dis. 18: 602. doi: 10.1186/s12879-018-3537-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaowagul W., White N. J., Dance D. A., Wattanagoon Y., Naigowit P., Davis T. M., Looareesuwan S., Pitakwatchara N.1989. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J. Infect. Dis. 159: 890–899. doi: 10.1093/infdis/159.5.890 [DOI] [PubMed] [Google Scholar]
- 8.Currie B. J., Mayo M., Anstey N. M., Donohoe P., Haase A., Kemp D. J.2001. A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Am. J. Trop. Med. Hyg. 65: 177–179. doi: 10.4269/ajtmh.2001.65.177 [DOI] [PubMed] [Google Scholar]
- 9.Dance D. A., King C., Aucken H., Knott C. D., West P. G., Pitt T. L.1992. An outbreak of melioidosis in imported primates in Britain. Vet. Rec. 130: 525–529. doi: 10.1136/vr.130.24.525 [DOI] [PubMed] [Google Scholar]
- 10.Department of Disease Control Leptospirosis. 2021. https://ddc.moph.go.th/brc/news.php?news=16719&deptcode=brc[cited on December 9, 2021].
- 11.Department of Disease Control Melliodosis. 2021.https://ddc.moph.go.th/brc/news.php?news=20212&deptcode=brc [cited on December 9, 2021].
- 12.Doungchawee G., Phulsuksombat D., Naigowit P., Khoaprasert Y., Sangjun N., Kongtim S., Smythe L.2005. Survey of leptospirosis of small mammals in Thailand. Southeast Asian J. Trop. Med. Public Health 36: 1516–1522. [PubMed] [Google Scholar]
- 13.Gallardo C., Williams-Smith J., Jaton K., Asner S., Cheseaux J. J., Troillet N., Manuel O., Berthod D.2015. Leptospirosis in a family after whitewater rafting in Thailand. Rev. Med. Suisse 11: 872–876 (in French). [PubMed] [Google Scholar]
- 14.Garba B., Bahaman A. R., Bejo S. K., Zakaria Z., Mutalib A. R., Bande F.2018. Major epidemiological factors associated with leptospirosis in Malaysia. Acta Trop. 178: 242–247. doi: 10.1016/j.actatropica.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 15.Hinjoy S., Hantrakun V., Kongyu S., Kaewrakmuk J., Wangrangsimakul T., Jitsuronk S., Saengchun W., Bhengsri S., Akarachotpong T., Thamthitiwat S., Sangwichian O., Anunnatsiri S., Sermswan R. W., Lertmemongkolchai G., Tharinjaroen C. S., Preechasuth K., Udpaun R., Chuensombut P., Waranyasirikul N., Anudit C., Narenpitak S., Jutrakul Y., Teparrukkul P., Teerawattanasook N., Thanvisej K., Suphan A., Sukbut P., Ploddi K., Sirichotirat P., Chiewchanyon B., Rukseree K., Hongsuwan M., Wongsuwan G., Sunthornsut P., Wuthiekanun V., Sachaphimukh S., Wannapinij P., Chierakul W., Chewapreecha C., Thaipadungpanit J., Chantratita N., Korbsrisate S., Taunyok A., Dunachie S., Palittapongarnpim P., Sirisinha S., Kitphati R., Iamsirithaworn S., Chaowagul W., Chetchotisak P., Whistler T., Wongratanacheewin S., Limmathurotsakul D.2018. Melioidosis in Thailand: present and future. Trop. Med. Infect. Dis. 3: 38. doi: 10.3390/tropicalmed3020038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inglis T. J., Garrow S. C., Adams C., Henderson M., Mayo M.1998. Dry-season outbreak of melioidosis in Western Australia. Lancet 352: 1600. doi: 10.1016/S0140-6736(05)61047-1 [DOI] [PubMed] [Google Scholar]
- 17.Kasantikul T., Sommanustweechai A., Polsrila K., Kongkham W., Chaisongkram C., Sanannu S., Kongmakee P., Narongwanichgarn W., Bush M., Sermswan R. W., Banlunara W.2016. Retrospective study on fatal melioidosis in captive zoo animals in Thailand. Transbound. Emerg. Dis. 63: e389–e394. doi: 10.1111/tbed.12315 [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann A. F., Alexander A. D., Allen M. A., Cronin R. J., Dillingham L. A., Douglas J. D., Moore T. D.1970. Melioidosis in imported non-human primates. J. Wildl. Dis. 6: 211–219. doi: 10.7589/0090-3558-6.4.211 [DOI] [PubMed] [Google Scholar]
- 19.Kongkaew W., Thiptara A., Kaewkalong S., Hinjoy S.2017. Situation of melioidosis in Thailand, 2006–2015. Thai-NIAH eJournal 12: 80–102. [Google Scholar]
- 20.Limmathurotsakul D., Thammasart S., Warrasuth N., Thapanagulsak P., Jatapai A., Pengreungrojanachai V., Anun S., Joraka W., Thongkamkoon P., Saiyen P., Wongratanacheewin S., Day N. P., Peacock S. J.2012. Melioidosis in animals, Thailand, 2006–2010. Emerg. Infect. Dis. 18: 325–327. doi: 10.3201/eid1802.111347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meeyam T., Tablerk P., Petchanok B., Pichpol D., Padungtod P.2006. Seroprevalence and risk factors associated with leptospirosis in dogs. Southeast Asian J. Trop. Med. Public Health 37: 148–153. [PubMed] [Google Scholar]
- 22.Mohd Ali M. R., Mohamad Safiee A. W., Thangarajah P., Fauzi M. H., Muhd Besari A., Ismail N., Yean Yean C.2017. Molecular detection of leptospirosis and melioidosis co-infection: A case report. J. Infect. Public Health 10: 894–896. doi: 10.1016/j.jiph.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 23.National Institute of Animal Health (NIAH). 2009. Manual of Standard Methods for Serological Diagnosis of Melioidosis. p. 11. National Institute of Animal Health, Bangkok (in Thai). [Google Scholar]
- 24.Ngasaman R., Saechan V., Prachantasena S., Yingkajorn M., Sretrirutchai S.2020. Investigation of Leptospira infection in stray animals in Songkhla, Thailand: leptospirosis risk reduction in human. Vector Borne Zoonotic Dis. 20: 432–435. doi: 10.1089/vbz.2019.2549 [DOI] [PubMed] [Google Scholar]
- 25.Pradutkanchana S., Nakarin J.2005. The use of latex agglutination for the diagnosis of acute human leptospirosis. J. Med. Assoc. Thai. 88: 1395–1400. [PubMed] [Google Scholar]
- 26.Pumipuntu N., Suwannarong K.2016. Seroprevalence of Leptospira spp. in cattle and dogs in Mahasarakham Province, Thailand. J. Health Res. 30: 223–226. [Google Scholar]
- 27.Rajeev S., Bolfa P., Shiokawa K., Beierschmitt A., Palmour R.2020. Leptospira infection in African green monkeys in an endemic area: an opportunity for comparative studies in a natural environment. Pathogens 9: 9. doi: 10.3390/pathogens9060474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero-Vivas C. M., Thiry D., Rodríguez V., Calderón A., Arrieta G., Máttar S., Cuello M., Levett P. N., Falconar A. K.2013. Molecular serovar characterization of Leptospira isolates from animals and water in Colombia. Biomedica 33 Suppl 1: 179–184. [PubMed] [Google Scholar]
- 29.Sapian M., Khair M. T., How S. H., Rajalingam R., Sahhir K., Norazah A., Khebir V., Jamalludin A. R.2012. Outbreak of melioidosis and leptospirosis co-infection following a rescue operation. Med. J. Malaysia 67: 293–297. [PubMed] [Google Scholar]
- 30.Smits H. L., van der Hoorn M. A., Goris M. G., Gussenhoven G. C., Yersin C., Sasaki D. M., Terpstra W. J., Hartskeerl R. A.2000. Simple latex agglutination assay for rapid serodiagnosis of human leptospirosis. J. Clin. Microbiol. 38: 1272–1275. doi: 10.1128/JCM.38.3.1272-1275.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sprißler F., Jongwattanapisan P., Luengyosluechakul S., Pusoonthornthum R., Prapasarakul N., Kurilung A., Goris M., Ahmed A., Reese S., Bergmann M., Dorsch R., Klaasen H. L. B. M., Hartmann K.2019. Leptospira infection and shedding in cats in Thailand. Transbound. Emerg. Dis. 66: 948–956. doi: 10.1111/tbed.13110 [DOI] [PubMed] [Google Scholar]
- 32.Srikitjakarn L., Sirimalaisuwan A., Khattiya R., Krueasukhon K., Mekaprateep M.2002. Seroprevalence of melioidosis in dairy cattle in Chiang Mai Province, northern Thailand. Southeast Asian J. Trop. Med. Public Health 33: 739–741. [PubMed] [Google Scholar]
- 33.Suwancharoen D., Chaisakdanugull Y., Thanapongtharm W., Yoshida S.2013. Serological survey of leptospirosis in livestock in Thailand. Epidemiol. Infect. 141: 2269–2277. doi: 10.1017/S0950268812002981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szonyi B., Agudelo-Flórez P., Ramírez M., Moreno N., Ko A. I.2011. An outbreak of severe leptospirosis in capuchin (Cebus) monkeys. Vet. J. 188: 237–239. doi: 10.1016/j.tvjl.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thaipadungpanit J., Chierakul W., Pattanaporkrattana W., Phoodaeng A., Wongsuvan G., Huntrakun V., Amornchai P., Chatchen S., Kitphati R., Wuthiekanun V., Day N. P. J., Peacock S. J., Limmathurotsakul D.2014. Burkholderia pseudomallei in water supplies, southern Thailand. Emerg. Infect. Dis. 20: 1947–1949. doi: 10.3201/eid2011.140832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thipmontree W., Suputtamongkol Y., Tantibhedhyangkul W., Suttinont C., Wongswat E., Silpasakorn S.2014. Human leptospirosis trends: northeast Thailand, 2001–2012. Int. J. Environ. Res. Public Health 11: 8542–8551. doi: 10.3390/ijerph110808542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuanyok A., Auerbach R. K., Brettin T. S., Bruce D. C., Munk A. C., Detter J. C., Pearson T., Hornstra H., Sermswan R. W., Wuthiekanun V., Peacock S. J., Currie B. J., Keim P., Wagner D. M.2007. A horizontal gene transfer event defines two distinct groups within Burkholderia pseudomallei that have dissimilar geographic distributions. J. Bacteriol. 189: 9044–9049. doi: 10.1128/JB.01264-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiersinga W. J., Currie B. J., Peacock S. J.2012. Melioidosis. N. Engl. J. Med. 367: 1035–1044. doi: 10.1056/NEJMra1204699 [DOI] [PubMed] [Google Scholar]
- 39.Yingst S. L., Facemire P., Chuvala L., Norwood D., Wolcott M., Alves D. A.2014. Pathological findings and diagnostic implications of a rhesus macaque (Macaca mulatta) model of aerosol-exposure melioidosis (Burkholderia pseudomallei). J. Med. Microbiol. 63: 118–128. doi: 10.1099/jmm.0.059063-0 [DOI] [PubMed] [Google Scholar]


