Abstract
A mutant Rhodococcus strain lacking the ability to utilize 1-chlorohexadecane was found to cis-desaturate aliphatic compounds, such as 1-chlorohexadecane, n-hexadecane, and heptadecanonitrile, yielding corresponding products with a double bond mainly at the ninth carbon from the terminal methyl groups. A new oxidative pathway involving the cis-desaturation step was suggested for alkane utilization by Rhodococcus spp.
Hydrocarbon-utilizing microorganisms have been examined for their ability to produce industrially important products, such as vitamins (7), surfactant-like lipids (5, 8), antibiotics (9), perfumes (15), and organic acids (12). If hydrocarbon-utilizing microorganisms are able to hyperproduce ω-hydroxy and dicarboxylic fatty acids (FAs) from alkanes, these FAs may be used as basic industrial materials for the synthesis of new homopolymers with unidentified properties. Moreover, synthetic musks can be prepared from ω-hydroxypalmitic acid (FA16) and 1,15-dicarboxypentadecane by intramolecular cyclization. Since it is known that ω-chloro-FAs (ω-Cl-FAs) can be easily converted to ω-hydroxy-FAs by chemical procedures (10), they are also potential precursors of commercially valuable products.
According to this scenario, we screened for microorganisms that were able to utilize both n-hexadecane and 1-chlorohexadecane (each at 5.0% [vol/vol]) as a sole source of carbon and energy in semisynthetic medium A composed of 1.0% (NH4)2SO4, 0.2% KH2PO4, 0.02% MgSO4 · 7H2O, 0.002% FeSO4 · 7H2O, 0.002% MnSO4 · 7H2O, 0.02% yeast extract, 0.0005% (vol/vol) Tween 80, and distilled water (pH 7.0). Isolates obtained were grown in soybean-casein digest broth (Nippon Pharmaceutical, Tokyo, Japan), with shaking, at 30°C for 2 days, collected by centrifugation, and washed with chilled saline twice. The washed cells (resting cells) were then examined for the production of ω-hydroxy or ω-Cl-FAs in a reaction mixture composed of 1.0 ml (or 1.0 g) of an aliphatic substrate, 1 g (wet weight) of resting cells, and 20 ml of 0.25 M phosphate buffer (pH 7.0) and placed in 500-ml flasks. The flasks were incubated at 30°C for an appropriate number of days on a rotary shaker (120 rpm). Products and residual substrates were then extracted from the spent media with three volumes of n-hexane. Compounds in the solvent were measured with n-heptadecane as a calibration standard by using a Hewlett Packard 5880 A gas chromatograph (GC) equipped with a flame ionization detector and a cross-linked methyl silicone column (0.2 mm by 25 m). Free FAs were esterified with a boron trifluoride methanol complex (BF3-MeOH; Wako Pure Chemical, Kyoto, Japan) before GC analysis. Purification of products was done by high-performance liquid chromatography with ethanol (95%) as a mobile phase by using an Inertsil ODS-2 column (4.6 mm by 25 cm; Gasukurokogyo, Tokyo, Japan) and a UV detector set at 210 nm. The following spectroscopes and spectrometers were used: a Hitachi 270-30 infrared (IR) spectroscope (KBr) or a Nicolet 20 SXB GC-Fourier transform-IR machine for recording IR spectra; a Varian EM-360L nuclear magnetic resonance (NMR) spectroscope (60 MHz) for recording 1H-NMR spectra with (CH3)4Si as an internal standard; and a Hewlett Packard 5995 A GC-mass spectrometer for performing high-resolution mass spectrometry (GC-MS).
Among the isolates, resting cells of a strain designated KSM-B-3, isolated from a soil sample collected in Okinawa, Japan, were found to accumulate various short chain ω-hydroxy-FAs after a 3-day incubation with 5.0% (vol/vol) 1-chlorohexadecane as the substrate, i.e., in addition to 0.04 g of ω-Cl-FA16 per liter, 0.2 g of ω-Cl-FA6 per liter, 0.5 g of ω-Cl-FA8 per liter, 0.4 g of ω-Cl-FA10 per liter, 0.03 g of ω-Cl-FA12 per liter, and 0.03 g of ω-Cl-FA14 per liter. The isolate was a gram-positive, immotile, rod-shaped (0.5 by 1.0 μm) bacterium, which produced neither aerial mycelia nor conidia. It was an obligate aerobe and could grow neither below pH 5 nor above pH 10; the temperature range for growth was 20 to 37°C. Thiamine was required for growth. The cell wall contained arabinose, galactose, and meso-diaminopimeric acid as the cross-linked amino acid, and the glycolyl type of N-acyl residue (11, 16). MK-8(H2) was identified as the major menaquinone (4). The G+C content of the DNA was 67.5 mol%. Furthermore, as determined by 16S ribosomal DNA (rDNA) gene sequencing (3, 14), isolate KSM-B-3 may be a relative of Rhodococcus globerulus or Rhodococcus erythropolis. Gene sequence data are available from the DDBJ, EMBL, and GenBank databases under the accession no. AB032365.
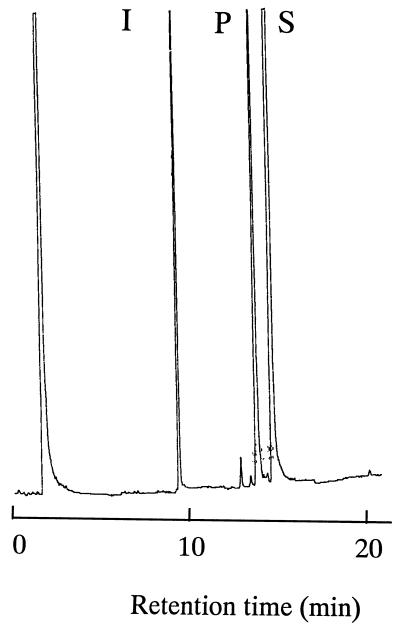
To improve the productivity of ω-Cl-FA16, Rhodococcus sp. strain KSM-B-3 was mutated by UV irradiation, and hundreds of mutants showing no or less ability to utilize 1-chlorohexadecane were obtained as follows. Cells suspended in chilled saline such that the final cell concentration was 106 to 108 viable cells/ml were irradiated by a UV light for 1 to 3 min so that 0.01 to 0.1% of the initial cell number survived. The UV-irradiated cells were transferred into 50 ml of medium A containing 1.0% (vol/vol) 1-chlorohexadecane and cultivated, with shaking, at 30°C for 4 to 6 h. To enrich the negative mutants, ampicillin (5 mg) was added to the culture. After a further 20-h incubation, the culture was spread over and grown on soybean-casein digest agar. Colonies formed after 2 days were each checked for growth on 1.0% (vol/vol) 1-chlorohexadecane or n-hexadecane in medium A, and mutants showing no or weak growth on these carbon sources but good growth on FA16 and glucose were selected. However, no mutants showing enhanced productivity of ω-Cl-FA16 were obtained. Instead, resting cells of a mutant designated KSM-B-3M were found to produce an unidentified product from 1-chlorohexadecane, as shown in Fig. 1. As determined by analysis with a GC, the concentrations of the product were 4.6, 8.5, and 10 g/liter after 1 day, 2 days, and 3 days, respectively, of incubation in the presence of 5.0% (vol/vol) 1-chlorohexadecane.
FIG. 1.
Gas chromatogram of an unidentified product generated from 1-chlorohexadecane by resting cells of Rhodococcus sp. strain KSM-B-3M. Resting cells (1 g [wet weight]) were added to the reaction medium containing 20 ml of 0.25 M phosphate buffer (pH 7.0) and 1 ml of 1-chlorohexadecane, and the cell suspension was placed in a 500-ml flask. The flask was incubated, with shaking, at 30°C for 3 days on a rotary shaker. Residual substrate (S) and an unidentified product (P) were extracted with n-hexane and detected by analysis with a GC with n-heptadecane (I) as an internal standard.
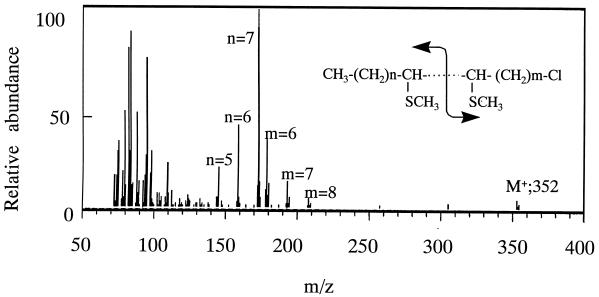
The unidentified product from 1-chlorohexadecane was deduced from the following physicochemical data: mass spectra, m/z 260 (M+) 258 (M+ − H2); 1H-NMR δ, 5.34 ppm (olefinic H); IR (KBr), 3,013, 2,943, and 2,864 cm−1 (cis form). The double bond position of the product was determined, after alkylthiolation with dimethyl disulfide (Wako Pure Chemical) (6, 17), by GC-MS. As shown in Fig. 2, in addition to the M+ ion at m/z 352 of the alkylthiolated product, several pairs of fragment ions, such as m/z 173 and 179, m/z 159 and 193, and m/z 145 and 207, were observed in a GC-MS spectrum. From the relative abundance of these fragment ion pairs, the double bond was located at the ninth (60%), the eighth (25%), and the seventh (15%) carbons from the terminal methyl group of the product. All the data obtained indicate that the main structure of the product is 1-chloro-cis-7-hexadecene [CH3(CH2)7CH⩵CH(CH2)5CH2Cl]. When the reaction was done in a stoppered test tube filled with nitrogen gas, no products were formed from 1-chlorohexadecane (data not shown). To our knowledge this is the first finding of microbial desaturation of haloalkanes.
FIG. 2.
Mass spectrum of the alkylthiolated product generated from 1-chlorohexadecane by resting cells of Rhodococcus sp. strain KSM-B-3M. The product extracted with n-hexane was evaporated. Approximately 0.2 mg of product was treated with 2.0 ml of dimethyl disulfide containing 6 mg of I2 for 30 min at room temperature under argon gas atmosphere. The alkylthiolated product was then analyzed by GC-MS.
n-Hexadecane (M+, m/z 226) was also desaturated by KSM-B-3M cells to yield hexadecene [M+, m/z 224; CH3(CH2)7CH⩵CH(CH2)5CH3] at a level of 12.5 g/liter after a 3-day incubation (Table 1). A GC-MS spectrum of the alkylthiolated product (M+, m/z 318) showed two fragment pairs, m/z 145 and 173 (90%) and two m/z 159 (10%), indicating that the double bond was introduced mainly at the seventh (or the ninth) carbon from the terminal methyl group of n-hexadecane. Heptadecanonitrile (M+, m/z 251) was desaturated to yield heptadecenonitrile [M+, m/z 249; CH3(CH2)7CH⩵CH(CH2)6CN] at a level of 2.1 g/liter after a 3-day incubation. A GC-MS spectrum of the alkylthiolated product of heptadecenonitrile (M+, m/z 343) showed a single fragment pair of m/z 170 and 173, indicating that the desaturation occurred exclusively at the ninth carbon from the terminal methyl group. IR spectra of the products from n-hexadecane and heptadecanonitrile showed that the double bonds were of cis configuration. Methyl palmitate, 1,2-epoxyhexadecane, cetyl alcohol, hexadecyl benzene, and hexadecyl chloroformate were also desaturated by the rhodococcal cells as judged by their GC-MS spectra (data not shown), although amounts of the products were small. Unesterified FA14, FA16, and FA18, palmitochloride, and tripalmityl glycerol were inert in the reaction.
TABLE 1.
Desaturation of aliphatic compounds by Rhodococcus sp. strain KSM-B-3M
| Substrate | Amt of product (g/liter)a | Desaturated positionb |
|---|---|---|
| n-Hexadecane | 12.5 | cis-9 |
| 1-Chlorohexadecane | 10.0 | cis-9 |
| Cetyl alcohol | 0.4 | NDc |
| Heptadecanonitrile | 2.1 | cis-9 |
| 1,2-Epoxyhexadecane | 0.2 | ND |
| Hexadecyl benzene | 0.4 | ND |
| Hexadecyl chloroformate | 0.2 | ND |
| Palmitochloride | 0.0 | |
| Myristic acid | 0.0 | |
| Palmitic acid | 0.0 | |
| Stearic acid | 0.0 | |
| Methyl palmitate | 0.1 | ND |
| Tripalmityl glycerol | 0.0 |
Resting cell reaction was carried out for 3 days at 30°C, and the products were quantified by analysis with a GC.
Double bond position was numbered from the terminal methyl group of the substrate.
ND, not determined due to small amounts produced.
These results suggest that a novel oxidative pathway may be involved in alkane degradation by Rhodococcus. As the initial step in utilization of alkanes, a coenzyme A-independent internal cis-desaturation may be involved, where an unsaturated metabolite generated would be then split at the double bond into two shorter chain FAs and ultimately oxidized via β-oxidation. This postulated pathway is different from the well-known subterminal oxidation system that converts alkanes initially to their alkyl alcohols and then to FAs, which are further degraded by a β-oxidation system (13), as in yeast (2) and other microorganisms (1). The hyperproduction of unsaturated products by mutant KSM-B-3M suggests that the mutation might occur on the gene encoding a double bond-splitting enzyme, since the mutant strain grows poorly on n-hexadecane but well on FA16, and converts aliphatic substrates mainly to cis-9-unsaturated products having the same chain length.
Acknowledgments
This study was financially supported by the Ministry of Trades and Industries of Japan, the New Energy and Industrial Technology Development Organization of Japan, and the Japan Association of Biotechnology.
REFERENCES
- 1.Ascenzi J M, Vestal J R. Regulation of fatty acid biosynthesis by hydrocarbon substrates in Mycobacterium convoltum. J Bacteriol. 1979;137:384–390. doi: 10.1128/jb.137.1.384-390.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasig R, Schunck W-H, Jockisch W, Franke P, Muller H-G. Degradation of long chain n-alkanes by the yeast Lodderomyces elongisporus. Appl Microbiol Biotechnol. 1984;19:241–246. [Google Scholar]
- 3.Briglia M, Rainey F A, Stackebrandt E, Schraa G, Salkinoja-Salonen M S. Rhodococcus percolatus sp. nov., a bacterium degrading 2,4,6-trichlorophenol. Int J Syst Bacteriol. 1996;46:23–30. doi: 10.1099/00207713-46-1-23. [DOI] [PubMed] [Google Scholar]
- 4.Collins M D. A note on the separation of natural mixtures of bacterial menaquinones using reverse-phase high-performance liquid chromatography. J Appl Bacteriol. 1982;52:457–460. doi: 10.1111/j.1365-2672.1980.tb01227.x. [DOI] [PubMed] [Google Scholar]
- 5.Cooper D G, Zajic J E, Gerson D F. Production of surface-active lipids by Corynebacterium lepus. Appl Environ Microbiol. 1979;37:4–10. doi: 10.1128/aem.37.1.4-10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis G W, Veland K. Alkylthiolation for the determination of double-bond positions in linear alkenes. J Chromatogr. 1981;219:379–384. [Google Scholar]
- 7.Fukui S, Tanaka A. Proceedings of the 8th World Petroleum Congress. Vol. 5. London, England: Applied Science Publishers Ltd.; 1971. Production of vitamins and coenzymes from hydrocarbons by microorganisms; pp. 157–163. [Google Scholar]
- 8.Ito S, Inoue S. Sophorolipids from Torulopsis bombicola: possible relation to alkane uptake. Appl Environ Microbiol. 1983;43:1278–1283. doi: 10.1128/aem.43.6.1278-1283.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitano K, Kintaka K, Suzuki S, Katamoto K, Nara K, Nakao Y. Studies on the production of β-lactam antibiotics. III. Screening of microorganisms capable of producing β-lactam antibiotics from n-paraffins. Hakko Kogaku Zasshi. 1976;54:683–695. . (In English). [Google Scholar]
- 10.Kohnstam G, Williams D L. Directive and activating effects of CO2H and CO2R group. In: Patai S, editor. The chemistry of carboxylic acids and esters. New York, N.Y: Interscience Publishers; 1969. pp. 810–815. [Google Scholar]
- 11.Lechevalier H A. Nocardioforms. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 1458–1506. [Google Scholar]
- 12.Nout M J R. Screening methods for citric acid production by yeasts, using glucose- and hydrocarbon-containing media as substrates, and Candida strains as test organisms. Antonie Leeuwenhoek J Microbiol Serol. 1972;38:633–636. [Google Scholar]
- 13.Rehm H J, Reiff I. Mechanisms and occurrence of microbial oxidation of long-chain alkanes. Adv Biochem Eng. 1981;19:175–215. [Google Scholar]
- 14.Ruimy R, Riegel P, Boiron P, Monteil H, Christen R. Phylogeny of the genus Corynebacterium deduced from analyses of small-subunit ribosomal DNA sequences. Int J Syst Bacteriol. 1995;45:740–746. doi: 10.1099/00207713-45-4-740. [DOI] [PubMed] [Google Scholar]
- 15.Takigawa H, Kubota H, Sonohara H, Okuda M, Tanaka S, Fujikura Y, Ito S. Novel allylic oxidation of α-cedrene to sec-cedrenol by a Rhodococcus strain. Appl Environ Microbiol. 1993;59:1336–1341. doi: 10.1128/aem.59.5.1336-1341.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchida K, Aida K. Acyl type of bacterial cell wall: its simple identification by colorimetric method. J Gen Appl Microbiol. 1977;23:249–260. [Google Scholar]
- 17.Yamamoto K, Shibahara A, Nakayama T, Kajimoto G. Determination of double-bond positions in methylene-interrupted dienoic fatty acids by GC-MS as their dimethyl disulfide adducts. Chem Phys Lipids. 1991;60:39–50. [Google Scholar]