Abstract
Background and Objective
The concern surrounding the association between Guillain–Barré syndrome (GBS) and vaccination has increased with the widespread use of COVID-19 vaccines. The aim of this study was to assess the potential association of GBS with mRNA-based or adenovirus-vectored COVID-19 vaccines.
Methods
Reports of GBS associated with mRNA-based or adenovirus-vectored COVID-19 vaccines were extracted from the WHO pharmacovigilance database, exposure data from the Our World in Data website, and the background rates of GBS from published data. For countries contributing to VigiBase and with available data on COVID-19 vaccine exposure, reporting rates were estimated and observed-to-expected (OE) analyses were performed.
Results
A total of 2499 cases were included: 1157 (46.3%) cases with adenovirus-vectored COVID-19 vaccines and 1342 (53.7%) with mRNA-based COVID-19 vaccines. The male-to-female sex ratio was 1.09 and the median (IQR) age was 57 (45–66) years. The reporting rates (95% CI) per 100,000 person-years within the 42-day window were 5.57 (5.13–6.03) for adenovirus-vectored COVID-19 vaccines and 1.39 (1.31–1.47) for mRNA-based COVID-19 vaccines, while the background incidence was 1.2–3.1 per 100,000 person-years. For mRNA-based COVID-19 vaccines, the OE ratio was <1 for both time windows in all European countries and slightly elevated for the 21-day window in the USA. For adenovirus-vectored COVID-19 vaccines, the OE ratio was consistently > 2.0 for all countries. Sensitivity analyses minimally altered these results.
Conclusions
These findings suggest both the absence of safety concern for GBS with mRNA-based COVID-19 vaccines and an increased risk with adenovirus-vectored COVID-19 vaccines.
Back to top
Key Points
| Guillain Barré syndrome (GBS) is commonly feared with vaccines and of particular concern as new technologies such as mRNA-based or adenovirus-vectored COVID-19 vaccines have emerged. |
| Data from VigiBase, an international adverse drug reaction database, evidenced a lower reporting rate of GBS associated with mRNA-based COVID-19 vaccines compared with adenovirus-vectored COVID-19 vaccines, as the observed-to-expected ratio was consistently < 1.0 for mRNA-based COVID-19 vaccines and > 2.0 for adenovirus-vectored COVID-19 vaccines. |
| These results suggesting no safety concern for GBS with mRNA-based COVID-19 vaccines and an increased risk with adenovirus-vectored COVID-19 vaccines were in accordance with other data, and suggested spontaneous reporting may be convenient to quantify risks during a vaccination campaign. |
Introduction
Owing to the prompt development of COVID-19 vaccines, initial concerns regarding the safety of mRNA- or adenovirus-based technologies and their massive use within a few months have been raised, and an intensive pharmacovigilance monitoring program has been put together worldwide during the early phases of the vaccination campaign. A priority list of Adverse Events of Special Interest (AESI) based on proven associations or theoretical concerns previously encountered in the context of immunization in general and against COVID-19 has been generated by the Safety Platform for Emergency vACcines (SPEAC) [1].
Among the immunologically based AESI, Guillain–Barré syndrome (GBS) has been historically feared since the discontinuation of the 1976 US influenza immunization program due to an excess of GBS cases among vaccine recipients [2]. Since then, numerous epidemiological studies have provided no evidence for a causal relationship between most vaccines and GBS, except for a small increased risk of GBS with the influenza A/H1N1/2009 vaccine, which was nonetheless still considerably lower than the one observed after natural influenza infection [3, 4]. A recent nested case-control study performed in three Chinese cities also found no increased risk of GBS or its recurrence with commonly used non-COVID-19 vaccines [5]. Even though most studies provided no evidence for a causal association between GBS and immunization, these were frequently underpowered to detect a small relative risk and to properly reject a causal association.
Soon after the COVID-19 vaccine campaign started, a number of spontaneously reported severe AESI were temporally associated with immunization; however, only a few, such as myocarditis with mRNA-based COVID-19 vaccines [6] and thrombotic thrombocytopenia syndrome [7] or GBS [8, 9] with adenovirus-vectored COVID-19 vaccines, were convincingly attributed to these vaccines. Besides conventional pharmacoepidemiological studies, the intensive worldwide safety monitoring program that took place during the early phases of the COVID-19 vaccination campaign has offered unique opportunities to assess the risk of GBS associated with COVID-19 vaccines by using pharmacovigilance data and to provide quantitative information for both types of vaccines.
In order to compare the risk of GBS and its variant Miller Fisher syndrome (MFS) associated with mRNA-based or adenovirus-vectored COVID-19 vaccines, we performed a comparative analysis using data from VigiBase, the WHO international pharmacovigilance database. Our aims were to describe the features of GBS cases between vaccines, to estimate the reporting rate for each vaccine between countries, and to perform an observed-to-expected (OE) analysis in order to assess the potential excess in risk compared with the background rate.
Methods
Source of Data
VigiBase contains spontaneous reports on adverse drug reactions (ADRs) from 149 countries. A listing of de-duplicated Individual Case Safety Reports (ICSRs) of COVID-19 vaccines associated with acute polyneuropathies was extracted on October 15, 2021. COVID-19 vaccines were selected using the filter on drug groups ‘Vaccines for COVID-19 (SDG—Narrow)’, and the ADRs of interest were selected using the High Level Term ‘Acute polyneuropathies’, which included acute neuropathy, GBS, MFS, subacute inflammatory demyelinating polyneuropathy, and intensive care unit weakness. The term ‘GBS’ encompassing related disorders will be used throughout the article.
ICSRs contain data related to patients (age, sex), reporter description (healthcare professional or not), all reported medications (suspect, interacting, or concomitant) including dose and date of administration, and all ADRs coded according to the Medical Dictionary for Regulatory Activities (MedDRA®, version 22.1), with date of occurrence and outcome of the event.
Case Selection
Only cases involving mRNA-based SARS-CoV-2 vaccines (Pfizer/BioNTech BNT162b2 [tozinameran] and Moderna mRNA-1273 [elasomeran]) or adenovirus-vectored SARS-CoV-2 vaccines (Oxford/AstraZeneca ChAdOx1nCoV-19 and Janssen/Johnson & Johnson Ad26.COV2-S) were selected. As the elapsed time between the date of vaccination and the diagnosis of GBS may reach as long as 42 days, and as a 2-week period is usually required for the complete export of ICSRs to VigiBase, only cases in which the vaccine was administered before August 15, 2021 were retained. This was expected to provide more complete and stable data on both the number of cases and the number of doses administered for each vaccine.
Cases were excluded if (i) the time to onset (TTO) between vaccination and the occurrence of GBS was missing, (ii) the TTO was > 42 days after the most proximal dose, because this time interval reasonably excludes the role of immunization in GBS acute polyneuropathies [10], (iii) concomitant infections known to be associated with the occurrence of GBS were identified in the ICSR (COVID-19 or positive SARS-CoV-2 test, bacterial or viral infection such as Campylobacter, Chlamydia, Epstein Barr, cytomegalovirus, Herpes simplex, and upper respiratory tract infections), and (iv) the report only mentioned ‘Intensive care unit weakness’. Even though a TTO of < 3 days after vaccination weakens the plausibility of a causal vaccine involvement, these cases were retained for analysis as recommended by the Brighton Collaboration criteria [10].
Handling of Data
As the vaccine dose number or exact TTO of GBS after immunization were not always accurately recorded in the line listing, individual ICSRs were scrutinized to retrieve this information. Other data such as the presence of positive SARS-CoV-2 test and concomitant pregnancy were also completed from the ICSRs. If only the month of vaccination or the ADR was recorded, the date of vaccination was set as the 15th of the month. When GBS occurred after the second dose of a heterologous vaccine schedule, it was attributed to the vaccine used for the first dose if the TTO was both < 3 days after the second dose and < 42 days after the first dose. In other cases, the TTO was calculated from the date of the second dose. Cases associated with facial palsy were identified by the MedDRA Preferred Terms “Bell’s palsy, diplegia, facial paralysis, facial nerve disorders, facial paresis, and oculofacial paralysis”. All other immunizations that took place within 6 weeks before the diagnosis were recorded.
Statistical Analysis
The number of doses of COVID-19 vaccine distributed by each manufacturer was retrieved from the Our Word in Data website that contains full data for 39 countries [11]. Data extraction was limited to the cumulative data available up to August 15, 2021 and to the countries that actually contributed to VigiBase. As data combining the manufacturer with sex or age were not available, only unstratified analysis could be performed. Data from the UK doses distributed according to manufacturer/brand name were unavailable despite request and not found elsewhere. Based on these data, the reporting rates per million doses administered or per 100,000 person-years was estimated and OE analyses performed for the selected countries.
The OE analysis comprised comparing the number of spontaneous reports of GBS with the expected number of cases issued from published background rates for each country. A 21-day and a 42-day risk window were used for this purpose. The analysis was restricted to countries contributing to at least 20 reports of GBS, and for which both the number of doses administered as of August 15, 2021 and the national background incidence rate of GBS were available. As the estimated reported incidence of GBS between each mRNA-based COVID-19 vaccine and each adenovirus-vectored COVID-19 vaccine was close, the OE analysis was performed by grouping vaccines from the same platform. Data on the background annual incidence rates of GBS per 100,000 patients were obtained from different sources. For Germany, Italy, the Netherlands, Spain, and the European Union (EU) as a whole, we used data derived from the European ADVANCE project [12]. For each of these countries, we extracted the crude estimates for both the most recent year and from the largest database. For France, data were issued from the nationwide hospital discharge database [13]. For the USA, crude rates were derived from the largest available study [14]. These rates were applied to estimate the number of cases of GBS expected to occur within 21 or 42 days after vaccine administration. The expected number of GBS cases was calculated by multiplying the background incidence rate per 100,000 by the person-time at risk for both the 21-day and 42-day risk windows, regardless of the dose number. The OE ratio was obtained by dividing the observed by the expected number of GBS cases for each vaccine platform.
Sensitivity analyses were performed by using scenarios based on under-reporting rates, estimates of confirmed cases of GBS, and both. These analyses were limited to the USA and grouped EU countries. As limited data on the underreporting rate between countries were available, we referred to estimates based on the review of 37 studies indicating a median (interquartile range [IQR]) under-reporting rate for serious ADRs of 80% (77–99) [15]. However, owing to the need for close monitoring of the safety of COVID-19 vaccines, most countries actively organized comprehensive and unprecedented intensive campaigns on ADR reporting and promoted direct patient reporting schemes combined with large media coverage and public weekly surveillance reports, likely resulting in better spontaneous reporting. The under-reporting rate was therefore arbitrarily set at 50% for the purpose of this analysis (worst-case scenario). On the other hand, definite confirmation of GBS cannot be obtained from reports transmitted to VigiBase, and a number of cases could have been miscoded as GBS. Uncertainty regarding the formal diagnosis issued from VigiBase was therefore taken into account. A US study based on the Vaccine Safety Datalink found that only 20% and 55% of GBS reports identified from outpatient and inpatient/emergency department settings, respectively, met the Brighton Collaboration criteria for confirmed or probable cases [14]. Accordingly, a rate of 55% of confirmed diagnosis was assumed for US cases. In two independent studies from Germany and the Netherlands, GBS was ultimately confirmed according to these criteria in 72% and 80% of the examined cases, respectively [16, 17]. This led us to consider an intermediate estimate of 76% for EU countries. This scenario used the most conservative estimate of the potential association with the vaccines. Supplemental analyses were performed after adding cases previously excluded because of unknown TTO or onset > 42 days.
All the results for the reporting rates and OE ratio were expressed with their 95% confidence interval (CI). Statistical analyses (ANOVA, chi-2) were performed using R (version 4.0.5).
Results
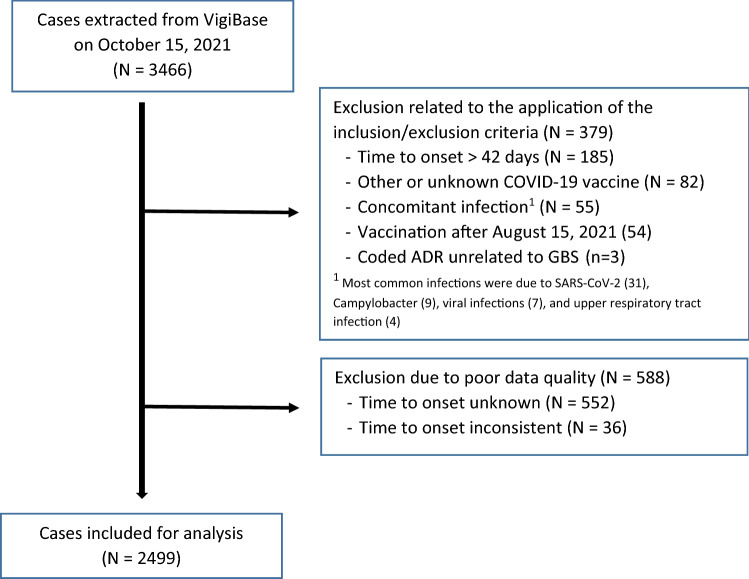
On October 15, 2021, 3466 cases of GBS associated with COVID-19 vaccine administration were extracted from VigiBase, of which 967 (27.9%) were excluded. The most common causes of exclusion were an unevaluable TTO (552, 57.1%) and a TTO > 42 days after the last dose (185, 19.1%; Fig. 1). Among the most frequent contributors (> 50 cases), the rates of exclusion due to poor quality data was the highest for Australia (34.4%), Brazil (31.8%), the US (21.6%), and the UK (15.9%) and the lowest for Germany (8.7%), Italy (5.4%), France (3.6%), Mexico (3.1%), and Spain (2.1%).
Fig. 1.
Selection of cases included in the analysis. ADR adverse drug reactions, GBS Guillain Barré syndrome
A total of 2499 reports were included in the final analysis (Table 1). Among the 49 countries that contributed to the data, nine accounted for 90% of cases. Reporters were physicians or pharmacists in 839/1438 (58.3%) cases (for which the identification was reported). A total of 1948 (78.0%) cases were included in the database between May and September 2021.
Table 1.
Baseline characteristics of COVID-19 vaccine-related reports of acute polyneuropathies in VigiBase as of 15 August 2021
| Characteristic | Tozinameran, N (%) | Elosameran, N (%) | ChAdOx1 nCoV-19, N (%) | Ad26.CoV2.S, N (%) |
|---|---|---|---|---|
| Number of cases | 1058 (42.3) | 284 (11.4) | 882 (35.3) | 275 (11.0) |
| Region of origin | ||||
| Australia | 11 (1.0) | 78 (8.8) | ||
| European Union | 293 (27.7) | 44 (15.5) | 345 (39.1) | 89 (32.4) |
| United Kingdom | 47 (4.4) | 3 (1.1) | 358 (0.6) | |
| United States of America | 640 (60.5) | 229 (80.6) | 170 (61.8) | |
| Central/South America | 47 (4.4) | 1 (0.4) | 77 (8.7) | 13 (4.7) |
| Other regionsa | 20 (1.9) | 7 (2.5) | 24 (2.7) | 3 (1.1) |
| Sex | ||||
| Female | 531 (50.2) | 148 (52.1) | 398 (45.1) | 108 (39.3) |
| Male | 525 (49.6) | 136 (47.9) | 472 (53.5) | 166 (60.4) |
| Unknown | 2 (0.2) | 12 (1.4) | 1 (0.4) | |
| Age (years) | ||||
| Median [IQR] | 54 [38–68] | 57 [44–68] | 59 [50–65] | 56 [46–62] |
| 12–29 | 90 (8.5) | 25 (8.8) | 15 (1.7) | 14 (5.1) |
| 30–49 | 258 (24.4) | 73 (24.4) | 179 (20.3) | 75 (27.3) |
| 50–69 | 262 (24.8) | 118 (41.5) | 511 (57.9) | 164 (59.6) |
| 70–89 | 175 (16.5) | 65 (22.9) | 135 (15.3) | 20 (7.3) |
| 90+ | 9 (0.9) | 40 (0.5) | ||
| Not recorded | 264 (25.0) | 3 (1.1) | 38 (4.3) | 2 (0.7) |
| Dose number involved | ||||
| First dose | 251 (23.7) | 56 (19.7) | 531 (60.3) | 275 (100) |
| Second dose | 102 (9.6) | 16 (5.6) | 64 (7.2) | |
| Unknown | 705 (66.7) | 212 (74.6) | 287 (32.6) | |
| Time to onset (days), median [IQR] | ||||
| After dose 1 | 9 [3–16] | 10 [3–19] | 14 [9–20] | 14 [7–19] |
| After dose 2 | 11 [3–19] | 13 [3–24] | 10 [2–18] | |
| D ose number unknown | 7 [2–16] | 8 [3–17] | 14 [9–20] | |
| ≤ 21 days after any dose | 888 (83.9) | 219 (77.1) | 709 (80.4) | 228 (82.9) |
| Type of polyneuropathy | ||||
| GBS | 1039 (98.2) | 280 (98.6) | 852 (96.6) | 274 (99.6) |
| MFS | 19 (1.8) | 4 (1.4) | 30 (3.4) | 1 (0.4) |
| Facial palsy | 41 (3.9) | 21 (7.4) | 118 (13.4) | 61 (22.2) |
| Recent other immunizations | 2 (0.2) | 2 (0.7) | 20 (2.3) | 0 (0) |
| Outcome at the time of recording | ||||
| Recovery | 69 (6.5) | 21 (7.4) | 43 (4.9) | 20 (7.3) |
| Recovery with sequelae | 10 (0.9) | 2 (0.7) | 23 (2.6) | 7 (2.5) |
| Improvement | 131 (12.4) | 20 (7.0) | 305 (34.6) | 30 (10.9) |
| Death | 22 (2.1) | 2 (0.7) | 10 (1.1) | 1 (0.4) |
| Not recovered or unknown | 826 (78.1) | 239 (84.25) | 501 (56.8) | 217 (78.9) |
GBS Guillain Barré syndrome, IQR interquartile range, MFS Miller Fisher syndrome
aOther regions include Asia (18), North Africa (6), and other European countries (30)
The included cases concerned 1299 (52.0%) males, 1185 (47.4%) females, and sex was unknown in 15 (0.6%) cases. Information about age was available for 2192 (87.7%) patients, their median (IQR) age was 57 (45–66) years, 652 (29.7%) were older than 64 years, and 144 (5.6%) were younger than 26 years (Table 1). Three patients were pregnant at the time of GBS, six were noted to have disease recurrence without further information, and 14 had a history of chronic inflammatory demyelinating polyradiculoneuropathy. The suspected vaccines were mRNA-based COVID-19 vaccines in 1342 (53.7%) cases and adenovirus-vectored COVID-19 vaccines in 1157 (46.3%). Among the six reports involving a heterologous ChAdOx1 nCoV-19/mRNA vaccine, five were attributed to the mRNA-based vaccine as the TTO after ChAdOx1 nCoV-19 administration was > 42 days (Table 1). Concomitant immunization within 42 days of diagnosis was noted in ten patients and involved influenza vaccine in seven, pneumococcal vaccine in two, diphtheria-Pertussis-tetanus toxoid, meningococcal, and varicella vaccines in one each (two vaccines in two patients).
GBS occurred after the first dose in 1113 (44.5%) cases, after the second dose in 182 (7.3%), while the dose number was undetermined in 1204 (48.2%). The median (IQR) TTO was 13 (7–19) days after the first dose, 10 (3–19) days after the second dose, and 9 (3–17) days when the dose number was unknown. The TTO after the first dose was comparable between mRNA-based vaccines and between adenovirus-vectored COVID-19 vaccines, and significantly shorter after mRNA-based vaccines (mean ± standard deviation [SD] 10.9 ± 9.3 days) compared with adenovirus-vectored vaccines (mean ± SD 14.7 ± 8.8 days). After excluding the recipients of the Ad26.COV2-S COVID-19 vaccine administered in a single dose, significantly more patients developed GBS within < 3 days after the second dose (23.1%) compared with the first dose (13.0%).
The subtype of acute polyneuropathy was GBS in 2445 (97.8%) patients and MFS in 54 (2.2%). Facial palsy was found in 241 (9.6%) patients and was more frequently reported with adenovirus-vectored COVID-19 vaccines (n = 179, 15.5%) compared with recipients of mRNA-based COVID-19 vaccines (n = 62, 4.6%; p < 0.001; Table 1). For adenovirus-vectored COVID-19 vaccines, facial palsy was more frequently coded in the UK (22.2%) and the US (24.1%) than in other countries (12.2%).
An outcome at the time of reporting was completed for 716 (28.7%) patients, of whom 153 (21.4%) recovered, 486 (67.9%) improved, 42 (5.9%) recovered with sequelae, and 35 (4.9%) died (Table 1). Death occurred in 22 males and 13 females at a median (IQR) age of 73 (63–78) years (age was unknown for eight patients). Information about the suspected cause of death was available for 14 patients (respiratory failure for 9, acute arterial ischemia for 3, pulmonary thromboembolism for 1, and septic shock for 1) and unavailable for 21 patients.
The global reporting rates of GBS per 1,000,000 doses administered and reporting rates for selected countries are shown in Table 2. When expressed by using the amount of person-time at risk per 100,000 person-years, the reporting rates for the 42-day window were 1.39 (95% CI 1.31–1.47) for mRNA-based COVID-19 vaccines (1227 per 88,701,869) and 5.57 (95% CI 5.13–6.03) for adenovirus-vectored COVID-19 vaccines (609 per 10,959,824), whereas the background incidence was 1.2 to 3.1 per 100,000 person-years. The highest reporting rates were found for Ad26.COV2-S (9.84, 95% CI 8.68–11.11). Overall, the reporting rates were four times higher with adenovirus-vectored COVID-19 vaccines (6.40, 95% CI 5.91–6.93) compared with mRNA-based COVID-19 vaccines (1.60, 95% CI 1.51–1.69). For a given vaccine, the reporting rates varied between EU countries and the lowest rates were reported in south Europe. Compared with EU countries considered as a whole, 1.7 and 3 times higher reporting rates were observed in the US for Ad26.COV2-S and mRNA-based COVID-19 vaccines, respectively.
Table 2.
Rates of spontaneous reporting of Guillain Barré syndrome between countries
| All mRNA-based COVID-19 vaccines | Tozinameran | Elosameran | All adenovirus-based COVID-19 vaccines | ChAdOx1 nCoV-19 | Ad26.CoV2.S | |
|---|---|---|---|---|---|---|
| All countriesa | ||||||
| No. cases | 1232 | 952 | 280 | 610 | 349 | 261 |
| No. doses | 770,856,446 | 573,575,606 | 197,280,840 | 95,246,095 | 68,721,497 | 26,524,598 |
| Reporting rate [95% CI] per million dose administered | 1.60 [1.51–1.69] | 1.66 [1.56–1.77] | 1.42 [1.26–1.60] | 6.40 [5.91–6.93] | 5.08 [4.56–5.64] | 9.84 [8.68–11.11] |
| All EU countries | ||||||
| No. cases | 337 | 293 | 44 | 434 | 345 | 89 |
| No. doses | 406,708,824 | 358,078,048 | 48,630,776 | 79,304,511 | 66,730,990 | 12,573,521 |
| Reporting rate [95% CI] per million dose administered | 0.83 [0.74–0.92] | 0.82 [0.74–0.92] | 0.90 [0.66–1.21] | 5.47 [4.97–6.01] | 5.17 [4.64–5.75] | 7.08 [5.69–8.71] |
| Selected EU countriesb | ||||||
| Austria | 0.61 [0.20–1.43] | 0.70 [0.23–1.63] | 8.37 [4.69–13.81] | 8.31 [4.42–14.21] | 8.81 [1.07–31.84] | |
| Belgium | 0.76 [0.35–1.44] | 0.66 [0.27–1.37] | 1.56 [0.19–5.62] | 3.48 [1.74–6.23] | 3.90 [1.95–6.98] | –c |
| France | 1.17 [0.94–1.45] | 1.21 [0.96–1.51] | 0.87 [0.35–1.80] | 6.52 [4.94–8.45] | 6.44 [4.78–8.49] | 7.21 [2.90–14.85] |
| Germany | 1.28 [1.05–1.55] | 1.26 [1.02–1.54] | 1.44 [0.77–2.46] | 8.27 [6.89–9.84] | 7.37 [5.95–9.03] | 12.56 [8.65–17.64] |
| Italy | 0.53 [0.36–0.75] | 0.52 [0.34–0.75] | 0.61 [0.20–1.42] | 4.85 [3.74–6.18] | 4.33 [3.24–5.68] | 9.23 [4.92–15.79] |
| Netherlands | 1.05 [0.63–1.64] | 0.93 [0.52–1.53] | 2.09 [0.57–5.34] | 5.58 [3.41–8.62] | 4.64 [2.47–7.94] | 8.96 [3.60–18.45] |
| Portugal | 0.67 [0.27–1.39] | 0.56 [0.18–1.31] | 1.33 [0.16–4.81] | 6.70 [4.15–10.24] | 5.94 [3.17–10.17] | 8.45 [3.65–16.64] |
| Spain | 0.49 [0.31–0.73] | 0.48 [0.29–0.74] | 0.58 [0.16–1.48] | 5.25 [4.01–6.74] | 5.15 [3.82–6.79] | 5.75 [2.87–10.29] |
| Sweden | 0.98 [0.47–1.81] | 0.90 [0.39–1.77] | 1.54 [0.19–5.55] | 9.01 [4.66–15.75] | 9.01 [4.66–15.75] | –d |
| USA | ||||||
| No. cases | 869 | 640 | 229 | 170 | 170 | |
| No. doses | 342,208,275 | 200,551,649 | 141,656,626 | 13,892,386 | –e | 13,892,386 |
| Reporting rate [95% CI] per million dose administered | 2.54 [2.37–2.7] | 3.19 [2.95–3.45] | 1.62 [1.41–1.84] | 12.24 [10.47–14.22] | 12.24 [10.47–14.22] | |
CI confidence interval, EU European Union
aData include regions or countries with available cumulative data on the number of doses of each vaccine administered as of 15 August 2021 [11] and that contributed to reporting in VigiBase (at least one case, regardless of the vaccine). Besides the European Union and the US, five other countries contributed to the data (Ecuador, Iceland, Norway, Switzerland, and Uruguay)
bFor EU countries, reporting rates per million doses administered were given only for those that contributed to at least 20 cases of Guillain Barré syndrome associated with COVID-19 vaccines
cNo report in VigiBase
dNot used in Sweden
eNot used in the US
The reported cases from six countries were used for the OE analyses. The background rate estimates of GBS substantially varied between countries and ranged from 1.20 to 3.13 per 100,000 person-years, the highest rate was reported for the USA. For mRNA-based COVID-19 vaccines, the OE ratios were systematically lower than 1.0 in all selected EU countries; for EU as a whole, the OE ratio was 0.54 (95% CI 0.47–0.63) for the 21-day window and 0.35 (95% CI 0.31–0.40) for the 42-day window. For the USA, the OE ratio for mRNA-based COVID-19 vaccines was elevated for the 21-day window (OE ratio 1.20, 95% CI 1.07–1.33) but not for the 42-day window (0.71, 95% CI 0.65–0.77; Table 3). Sensitivity analyses assuming an under-reporting rate of 50% for the EU failed to significantly increase the OE ratio for both time windows; conversely, assuming that only 55% of the cases are validated GBS in the USA, the OE decreased below 1.0 for the 21-day window (OE ratio 0.66, 95% CI 0.58–0.75; Table 4).
Table 3.
Observed-to-expected (OE) analysis of Guillain Barré syndrome after mRNA-based and adenovirus-vectored COVID-19 vaccine administration
| Countriesa | Doses administered | Background rates per 100,000 Py [95% CI] | Observed cases within 21 days after vaccination (n) | Expected cases within 21 days (n) | OE ratio [95% CI] for onset within 21 days | Observed cases within 42 days after vaccination (n) | Expected cases within 42 days | OE ratio [95% CI] for onset within 42 days |
|---|---|---|---|---|---|---|---|---|
| mRNA-based vaccines | ||||||||
| All EU countriesa | 406,708,824 | 2.06 [2.0–2.10] | 261 | 482 | 0.54 [0.47–0.63) | 337 | 964 | 0.35 [0.31–0.40] |
| France | 72,425,406 | 2.42 [2.37–2.47] | 65 | 101 | 0.64 [0.47–0.88] | 85 | 202 | 0.42 [0.33–0.54] |
| Germany | 82,839,917 | 2.32 [1.16–4.14] | 76 | 111 | 0.69 [0.51–0.92] | 106 | 221 | 0.48 [0.38–0.60] |
| Italy | 60,441,763 | 2.88 [2.24–3.64] | 29 | 100 | 0.29 [0.19–0.44] | 32 | 200 | 0.16 [0.11–0.23] |
| Netherlands | 18,053,536 | 1.21 [0.99–1.46] | 11 | 13 | 0.88 [0.39–1.97] | 19 | 25 | 0.76 [0.42–1.37] |
| Spain | 48,902,604 | 1.20 [0.95–1.49] | 21 | 34 | 0.62 [0.36–1.07] | 24 | 68 | 0.36 [0.22–0.57] |
| USA | 342,208,275 | 3.13 [2.98–3.28] | 737 | 616 | 1.20 [1.07–1.33] | 869 | 1233 | 0.71 [0.65–0.77] |
| Adenovirus-vectored vaccines | ||||||||
| All EU countries | 79,304,511 | 2.06 [2.0–2.10] | 343 | 94 | 3.65 [2.90–4.58] | 434 | 188 | 2.31 [1.95–2.74] |
| France | 8,739,417 | 2.42 [2.37–2.47] | 36 | 12 | 2.96 [1.54–5.67] | 57 | 24 | 2.34 [1.46–3.76] |
| Germany | 15,244,863 | 2.32 [1.16–4.14] | 99 | 20 | 4.87 [3.02–7.84] | 126 | 41 | 3.10 [2.17–4.41] |
| Italy | 13,409,450 | 2.88 [2.24–3.64] | 54 | 22 | 2.43 [1.48–3.98] | 65 | 44 | 1.46 [1.0–2.14] |
| Netherlands | 3,582,336 | 1.21 [0.99–1.46] | 18 | 2 | 7.22 [1.92–27.14] | 20 | 5 | 4.01 [1.50–10.69] |
| Spain | 11,625,273 | 1.20 [0.95–1.49] | 52 | 8 | 6.48 [3.08–13.62] | 61 | 16 | 3.80 [2.19–10.59] |
| USA | 13,892,386 | 3.13 [2.98–3.28] | 144 | 25 | 5.76 [3.76–8.80] | 170 | 50 | 3.40 [2.48–4.66] |
CI confidence interval, EU European Union, OE observed to expected, Py patient-years
aFor the EU, all cases regardless of the country and the total number of doses administered in the EU as of 15 August 2021 (extracted from Our World in Data website) were taken into account[11]
Table 4.
Sensitivity analyses of the observed-to-expected (OE) analysis of Guillain Barré syndrome after mRNA-based or adenovirus vectored COVID-19 vaccinationa
| Countriesb | Observed cases within 21 days (n) | Expected cases within 21 days (n) | OE ratio [95% CI] for the 21-day window | Observed cases within 42 days (n) | Expected cases within 42 days (n) | OE ratio [95% CI] for onset within 42 days |
|---|---|---|---|---|---|---|
| mRNA-based vaccines | ||||||
| Assuming a 50% under-reporting rate in both regions | ||||||
| EU | 522 | 482 | 1.08 [0.96–1.23] | 674 | 964 | 0.70 [0.63–0.77] |
| USA | 1474 | 616 | 2.39 [2.18–2.63] | 1738 | 1233 | 1.41 [1.31–1.52] |
| Assuming that only 55% of the USA and 76% of the EU cases are confirmed GBSc | ||||||
| EU | 198 | 482 | 0.41 [0.35–0.49] | 256 | 964 | 0.27 [0.23–0.30] |
| USA | 405 | 616 | 0.66 [0.58–0.75] | 478 | 1233 | 0.39 [0.35–0.43] |
| Combining both hypotheses | ||||||
| EU | 397 | 482 | 0.82 [0.72–0.94] | 512 | 964 | 0.53 [0.48–0.59] |
| USA | 811 | 616 | 1.32 [1.18–1.46] | 956 | 1233 | 0.78 [0.71–0.84] |
| After adding cases excluded for missing TTOd (N = 137) | ||||||
| EU | NR | NR | 365 | 964 | 0.38 [0.34–0.43] | |
| USA | NR | NR | 978 | 1233 | 0.79 [0.73–0.86] | |
| Adenovirus-vectored vaccines | ||||||
| Assuming a 50% under-reporting rate in both regions | ||||||
| EU | 686 | 94 | 7.30 [5.86–9.09] | 868 | 188 | 4.62 [3.94–5.41] |
| USA | 288 | 25 | 11.51 [7.65–17.32] | 340 | 50 | 6.80 [5.05–9.14] |
| Assuming that only 55% of the USA and 76% of the EU cases are confirmed GBSc | ||||||
| EU | 261 | 94 | 2.77 [2.17–3.54] | 330 | 188 | 1.75 [1.47–2.10] |
| USA | 79 | 25 | 3.17 [2.02–4.96] | 94 | 50 | 1.87 [1.33–2.63] |
| Combining both hypotheses | ||||||
| EU | 521 | 94 | 5.55 [4.43–6.95] | 660 | 188 | 3.51 [2.98–4.13] |
| USA | 158 | 25 | 6.33 [4.15–9.65] | 187 | 50 | 3.74 [2.74–5.11] |
| After adding cases excluded for missing TTOd (N = 223) | ||||||
| EU | NR | NR | 464 | 188 | 2.47 [2.08–2.92] | |
| USA | NR | NR | 363 | 50 | 7.25 [5.40–9.75] | |
CI confidence interval, GBS Guillain Barré syndrome, NR not relevant, OE observed to expected, TTO time to onset
aRefer to Table 3 for the number of doses administered and the background rates of GBS for EU and the USA
bFor EU, all cases regardless of the country and the total number of doses administered in EU as of 15 August 2021
cAccording to Brighton Collaborative Criteria [10]
dAssuming that all cases with missing TTO occurred within 42 days after vaccination
Conversely, OE analyses for adenovirus-vectored COVID-19 vaccines consistently indicated significant increases in the OE ratio across all countries for both time windows, with the lower bound of the 95% CI > 1.5 in most strata. In the sensitivity analyses in which only 55% of the US and 75% of the EU cases are confirmed GBS, an elevated OE ratio > 1.7 persisted for both time windows (Table 4).
Adding cases excluded for missing TTO data (Table 4) or those occurring > 42 days after vaccination (data not shown) did not substantially alter the OE ratio for mRNA-based COVID-19 vaccines. For adenovirus-vectored COVID-19 vaccines, the OE ratio remained unchanged for the EU countries and consistently increased for the US (Table 4).
Discussion
This study, based on a high number of cases of GBS or its variants mostly reported by Western countries, evidenced that both adenovirus-vectored COVID-19 vaccines are associated with higher reporting rates of GBS compared with mRNA-based COVID-19 vaccines. Consistently higher reporting rates for the US compared with EU countries were also found. These results are supported by an elevated OE ratio across all countries for adenovirus-vectored COVID-19 vaccines but not for mRNA-based COVID-19 vaccines. Moreover, the reporting rate expressed in person-years for the 42-day window for mRNA-based COVID-19 vaccines was within the background incidence of GBS, suggesting there is no association between GBS and these vaccines.
GBS is a rare severe event that typically falls into the category of AESI established for the safety surveillance of any vaccine. The global age-standardized rate is 0.8–1.9 (median 1.1 [1.5–2.4]) cases per 100,000 patients per year, and these figures vary between countries and increase with age [18]. Although mostly attributed to Campylobacter jejuni or other infections, this acute immune-mediated polyradiculoneuropathy is commonly feared with any vaccines, albeit consistent causal relationship remains controversial [3].
GBS resulting from SARS-CoV-2 infection was discussed early on. A retrospective analysis led in parallel to a prospective cohort study conducted in the UK during the early period of the COVID-19 pandemic found a decreased incidence of GBS, possibly attributable to a decreased incidence of pathogen-associated GBS during the first national UK lockdown [21]. The same study compared the clinical and neurophysiological features of GBS patients and found no difference between COVID-19 positive and negative patients. Later on, another UK self-controlled case series study evidenced a 5.28-fold increased risk of GBS after a SARS-CoV-2-positive test, resulting in 145 excess cases per 10 million patients [19]. Finally, a meta-analysis of observational and case series studies estimated that 15 in 100,000 SARS-CoV-2-infected patients developed GBS [20].
Early after the COVID-19 vaccination campaign started, numerous isolated reports described the occurrence of GBS after the administration of adenovirus-vectored COVID-19 vaccines [22, 23] or mRNA-based COVID-19 vaccines [24, 25]. However, this could only reflect a temporally coincidental and not a causal association in this particular context of active surveillance. Two earlier studies investigating disproportionality analyses previously used data from the WHO international pharmacovigilance database. One found lower reporting of GBS with mRNA-based COVID-19 vaccines compared with influenza vaccines, but data extraction was limited to January 17, 2021 [26]. The other was performed later and failed to confirm a signal of disproportionate reporting for GBS with COVID-19 vaccines compared with all other viral vaccines, but the analysis was performed by grouping all types of COVID-19 vaccines [27].
For mRNA-based COVID-19 vaccines, the few available epidemiological evidence argue against an association with GBS. A study using safety surveillance data from the Vaccine Safety Datalink encompassing data from more than 11.8 million doses of elosameran or tozinameran administered has found no significant increase in the risk of GBS in recipients of either mRNA-based COVID-19 vaccine administered 1–21 days before diagnosis compared with those vaccinated 22–42 days before [28]. However, this time window is debatable as it postulated that GBS is unlikely to be related to vaccine exposure beyond 21 days. In addition, there were only ten and six events in the two vaccine groups. Another study using a self-controlled case series design and a higher number of GBS cases has confirmed these reassuring results for tozinameran and found no increased risk of GBS after vaccination [8]. Finally, one study involving 539 recipients of tozinameran previously diagnosed with GBS has identified only one patient who experienced a moderate relapse after each of the two doses [29].
In contrast, adenovirus-vectored COVID-19 vaccines have been repeatedly associated with an increased risk of GBS. Using reports of GBS cases associated with the Ad26.COV2.S COVID-19 vaccine to the US Vaccine Adverse Event Reporting System, the estimated reporting rate was one case of GBS per 100,000 doses administered [9]. A significant association between ChAdOx1nCoV-19 and the occurrence of GBS has also been found in two independent cohort studies conducted in England and Scotland, with a similar incidence rate ratio in both cohorts [8]. In addition, a particular phenotype associating frequent facial palsy with GBS has also been consistently reported with both adenovirus-vectored COVID-19 vaccines, thereby reinforcing a specific causal relationship with these vaccines [30, 31]. Owing to the increased number of reports after adenovirus-vectored COVID-19 vaccines, GBS has been recently added to the product information of both vaccines worldwide.
Strengths
This study, based on a high number of GBS cases and on exposure data from a number of countries, offers unique opportunities to address the relevance of the spontaneous reporting of rare events for epidemiological purposes. As there was no reason for differential reporting between vaccines in the early period of the campaign, comparisons were considered appropriate.
The reporting rates and results of the OE analysis were in line with those reported by other studies using more classical epidemiological approaches. Even though the reporting rates calculated from spontaneously reported ADRs only approximate the incidence, our findings for tozinameran in terms of reporting rate for the 42-day risk window (1.66 per million doses administered or 14.3 per million person-years) were close to the incidence of GBS reported within 30 days after vaccination in a large Mexican cohort of more than 3.8 million recipients of tozinameran (1.8 per million doses administered) [32] and to those found in a large US population-based study using a 21-day risk window (15.1 per million person-years) [28]. In addition, our calculated reporting rate expressed in person-years for the 42-day window with mRNA-based COVID-19 vaccines was in line with the background incidence of GBS.
Conversely, we confirmed particular features for both adenovirus-vectored COVID-19 vaccines with a high prevalence of facial palsy associated with GBS, a higher incidence of GBS than expected in the general population, and an elevated OE ratio consistent with the increased incidence rate ratio found at 15–21 days after ChAdOx1 nCoV-19 vaccination (IRR 2.9; 95% CI 2.15–3.92) in a self-controlled case series study [8] or close to the elevated rate ratio calculated at 21 days for Ad26.CoV2.S (RR 6.98; 95% CI 5.71–8.45) using a similar OE approach [9].
Both results suggest that an approach using data from spontaneous reporting is relevant in the particular setting of active pharmacovigilance of COVID-19 vaccines when data on the number of doses administered are available.
Limitations
Studies based on spontaneous reports are subject to numerous uncertainties including under- or differential reporting between countries, misclassification of the ADR, unexpectedness of the ADR, and type of reporter [33]. For example, the more frequent coding of facial palsy in the UK or the US than in any other countries might reflect differential coding of associated symptoms between countries. As limited clinical data are available from VigiBase, several reports were classified as unevaluable and case ascertainment with respect to the Brighton Collaboration criteria unfeasible [10]. This resulted in a 17% rate of excluded cases for poor quality data, mostly for missing TTO. As the incidence of GBS is higher in men than in women and increased with age, the lack of exposure data stratified by age group and sex may also limit the generalizability of our results. Finally, OE analyses were based on several assumptions due to uncertainty regarding the total number of cases over the risk period [34].
Although we found no important variability of reporting between EU countries, there was a two to three times higher reporting rate for adenovirus and mRNA-based COVID-19 vaccines in the US resulting in an elevated OE ratio for mRNA-based vaccines for the 21-day window. Differential spontaneous reporting regardless of the causality assessment or a higher rate of misclassification might account for it.
Using the most conservative estimate assuming that only 55% of the US cases are confirmed GBS, the OE ratio for the 21-day window for mRNA-based COVID-19 vaccines decreased below 1, while the lower bound of the 95% CI remained >2 for adenovirus-vectored COVID-19 vaccines for both the US and EU. Conversely, and even after applying an under-reporting rate of 50% for EU countries, the OE ratio for mRNA-based COVID-19 vaccines slightly increased above 1 for the 21-day window only. Similar results were found when considering both scenarios for mRNA-based COVID-19 vaccines or after taking into account excluded cases in the OE analyses for mRNA-based COVID-19 vaccines. On the other hand, the potential misclassification of the cases only marginally affected the results for adenovirus-vectored COVID-19 vaccines, for which the OE ratio was still significantly elevated. Overall, the sensitivity analysis mostly affected the results for mRNA-based COVID-19 vaccines and the results obtained by combining under-reporting and misclassification remained reassuring.
Conclusions
Results of the present study both support the moderate increased risk of GBS associated with adenovirus-vectored COVID-19 vaccines found by other investigators and suggest an absence of safety concern for the recipients of mRNA-based COVID-19 vaccines. Despite some evidence of differential reporting between countries, our study suggests that spontaneous reporting may be of value to quantify rare events in specific situations.
Acknowledgments
We are grateful to Giuseppe Ballice, MD (Hospices Civils de Lyon) for technical assistance for the statistical analyses, Gaelle Simeon (Hospices Civils de Lyon) for improving the English quality of the manuscript, and Hélène Boyer (Hospices Civils de Lyon) for help in manuscript revision. We thank VigiBase for giving us access to the data. The data supplied to VigiBase come from a variety of sources, and the likelihood of causal relationship is not the same in all reports. The conclusions of the present study do not represent the opinions of the Uppsala Monitoring Centre or the World Health Organization.
Declarations
Funding
No source of funding.
Conflict of interest
All the authors have no conflict of interest relevant to the present study.
Ethics approval
This research study was performed in accordance with the ethical standards of our institutions and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Due to the retrospective and non-interventional design of this study, and as data were fully anonymized, the approval of the local Ethics Committee was not required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data used for the present analysis are available at https://vigilyze.who-umc.org and are accessible by national pharmacovigilance centers. Other professional users can apply to use specialized services licensed as a subscription with an annual fee. The raw data used for the purpose of this article are stored in an Excel file available upon reasonable request to the corresponding author.
Standards of reporting
Reporting of the data follows the STROBE Statement for observational studies.
Author contributions
All authors contributed to the study. Study conception and design were performed by MA, AP, and TV; acquisition and analysis by MA, BB, CK, and TV. The first draft of the manuscript was written by MA and TV, and all authors commented on all versions of the manuscript. All authors read and approved the final version of the manuscript.
Code availability
Code can be provided to interested parties upon reasonable request to the corresponding author.
Contributor Information
Marina Atzenhoffer, Email: marina.atzenhoffer@chu-lyon.fr.
Marine Auffret, Email: marine.auffret@chu-lyon.fr.
Antoine Pegat, Email: antoine.pegat@chu-lyon.fr.
Kamel Masmoudi, Email: masmoudi.kamel@chu-amiens.fr.
Charles Khouri, Email: ckhouri@chu-grenoble.fr.
Blandine Bertin, Email: blandine.bertin@chu-lyon.fr.
Thierry Vial, Email: thierry.vial@chu-lyon.fr.
References
- 1.Priority list of Adverse Events of Special Interest: Covid 19. Updated December 23, 2020. https://brightoncollaboration.us/priority-list-aesi-covid/. Accessed 31 Oct 2021.
- 2.Principi N, Esposito S. Vaccine-preventable diseases, vaccine and Guillain–Barré syndrome. Vaccine. 2019;37:5544–5550. doi: 10.1016/j.vaccine.2018.05.119. [DOI] [PubMed] [Google Scholar]
- 3.Petras M, Lesna IK, Danova J, Celko AM. Is an increased risk of developing Guillain–Barré syndrome associated with seasonal Influenza vaccination? A systematic review and meta-analysis. Vaccines (Basel). 2020;8:150. doi: 10.3390/vaccines8020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stowe J, Andrews N, Miller E. Do vaccines trigger neurological diseases? Epidemiological evaluation of vaccination and neurological diseases using examples of multiple sclerosis, Guillain–Barré syndrome and narcolepsy. CNS Drugs. 2020;34:1–8. doi: 10.1007/s40263-019-00670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Zhang J, Chu X, Xu Y, Ma F. Vaccines and the risk of Guillain–Barré syndrome. Eur J Epidemiol. 2020;35:3663–3670. doi: 10.1007/s10654-019-00596-1. [DOI] [PubMed] [Google Scholar]
- 6.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok FA, Pai M, Huisman MV, Makris M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2021 doi: 10.1016/S2352-3026(21)00306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patone M, Handunnetthi L, Saatci D, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27:2144–2153. doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo EJ, Mba-Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of receipt of the Ad26.COV2.S COVID-19 vaccine with presumptive Guillain–Barré syndrome, February–July 2021. JAMA. 2021;326:1606–1613. doi: 10.1001/jama.2021.16496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain–Barre syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599–612. doi: 10.1016/j.vaccine.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Mathieu E, Ritchie H, Ortiz-Ospina E et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947–53. 10.1038/s41562-021-01122-8. https://ourworldindata.org/covid-vaccinations. Accessed 31 Oct 2021. [DOI] [PubMed]
- 12.Willame C, Dodd C, Gini R, Durán CE, Thomsen RM, Lei W et al. Background rates of adverse events of special interest for monitoring COVID-19 vaccines, version 2.0, June 30, 2021. https://zenodo.org/record/5255870#.YadwNPlKiUl. Accessed 1 Dec 2021.
- 13.Delannoy A, Rudant J, Chaignot C, Bolgert F, Mikaeloff Y, Weill A. Guillain–Barre syndrome in France: a nationwide epidemiological analysis based on hospital discharge data (2008–2013) Peripher Nerv Syst. 2017;22:51–58. doi: 10.1111/jns.12202. [DOI] [PubMed] [Google Scholar]
- 14.Shui IM, Rett MD, Weintraub E, et al. Guillain–Barré syndrome incidence in a large United States cohort (2000–2009) Neuroepidemiology. 2012;39:109–115. doi: 10.1159/000339248. [DOI] [PubMed] [Google Scholar]
- 15.Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29:385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- 16.Prestel J, Volkers P, Mentzer D, Lehmann HC, Hartung HP, Keller-Stanislawski B. Risk of Guillain–Barré syndrome following pandemic influenza A(H1N1) 2009 vaccination in Germany. Pharmacoepidemiol Drug Saf. 2014;23:1192–1204. doi: 10.1002/pds.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain–Barré syndrome and validation of Brighton criteria. Brain. 2014;137(1):33–43. doi: 10.1093/brain/awt285. [DOI] [PubMed] [Google Scholar]
- 18.Willison HJ, Jacobs BC, van Doorn PA. Guillain–Barré syndrome. Lancet. 2016;388:717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 19.Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain–Barré syndrome. Brain J Neurol. 2021;144:682–693. doi: 10.1093/brain/awaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain–Barre syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2021;268:1133–1170. doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palaiodimou L, Stefanou MI, Katsanos AH, et al. Prevalence, clinical characteristics and outcomes of Guillain–Barré syndrome spectrum associated with COVID-19: a systematic review and meta-analysis. Eur J Neurol. 2021;28:3517–3529. doi: 10.1111/ene.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao SC, Wang CH, Chang KC, Hung MJ, Chen HY, Liao SC. Guillain–Barre syndrome associated with COVID-19 vaccination. Emerg Infect Dis. 2021;27:3175–3178. doi: 10.3201/eid2712.211634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenblum HG, Hadler SC, Moulia D et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices—United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021;13;70:1094–9. 10.15585/mmwr.mm7032e4. [DOI] [PMC free article] [PubMed]
- 24.Bouattour N, Hdiji O, Sakka S, et al. Guillain–Barré syndrome following the first dose of Pfizer-BioNTech COVID-19 vaccine: case report and review of reported cases. Neurol Sci. 2021 doi: 10.1007/s10072-021-05733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain–Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13:e13426. doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MS, Jung SY, Ahn JG, et al. Comparative safety of mRNA COVID-19 vaccines to influenza vaccines: a pharmacovigilance analysis using WHO international database. J Med Virol. 2021 doi: 10.1002/jmv.27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noseda R, Ripellino P, Ghidossi S, Bertoli R, Ceschi A. Reporting of acute inflammatory neuropathies with COVID-19 vaccines: subgroup disproportionality analyses in VigiBase. Vaccines. (Basel) 2021;9:1022. doi: 10.3390/vaccines9091022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben Simon SS, Potasman I, Rahamim-Cohen D. Rate of recurrent Guillain–Barre syndrome after mRNA COVID-19 vaccine BNT162b2. JAMA Neurol. 2021;78:1409–1411. doi: 10.1001/jamaneurol.2021.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen CA, Ramsamy S, Tarr AW, Tighe PJ, Irving WL, Radu Tanasescu R, Evans JR. Guillain–Barré syndrome variant occurring after SARS-CoV-2 vaccination. Ann Neurol. 2021;90:315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 31.Pegat A, Vogrig A, Khouri C, Masmoudi K, Vial T, Bernard E. Adenovirus COVID-19 vaccines and Guillain–Barré syndrome with facial paralysis. Ann Neurol. 2021 doi: 10.1002/ana.26258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Grimshaw M, Michel-Chávez A, Vera-Zertuche JM, et al. Guillain–Barre syndrome is infrequent among recipients of the BNT162b2 mRNA COVID-19 vaccine. Clin Immunol. 2021 doi: 10.1016/j.clim.2021.108818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore N, Hall G, Sturkenboom M, Mann R, Lagnaoui R, Begaud B. Biases affecting the proportional reporting ratio (PPR) in spontaneous reports pharmacovigilance databases: the example of sertindole. Pharmacoepidemiol Drug Saf. 2003;12:271–281. doi: 10.1002/pds.848. [DOI] [PubMed] [Google Scholar]
- 34.Mahaux O, Bauchau V, van Holle L. Pharmacoepidemiological considerations in observed-to-expected analyses for vaccines. Pharmacoepidemiol Druf Saf. 2016;25:215–222. doi: 10.1002/pds.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]