Abstract
Anthrax is a zoonotic infection caused by the gram-positive, aerobic, spore-forming bacterium Bacillus anthracis. Depending on the origin of the infection, serious health problems or mortality is possible. The virulence of B. anthracis is reliant on three pathogenic factors, which are secreted upon infection: protective antigen (PA), lethal factor (LF), and edema factor (EF). Systemic illness results from LF and EF entering cells through the formation of a complex with the heptameric form of PA, bound to the membrane of infected cells through its receptor. The currently available anthrax vaccines have multiple drawbacks, and recombinant PA is considered a promising second-generation vaccine candidate. However, the inherent chemical instability of PA through Asn deamidation at multiple sites prevents its use after long-term storage owing to loss of potency. Moreover, there is a distinct possibility of B. anthracis being used as a bioweapon; thus, the developed vaccine should remain efficacious and stable over the long-term. Second-generation anthrax vaccines with appropriate adjuvant formulations for enhanced immunogenicity and safety are desired. In this article, using protein engineering approaches, we have reviewed the stabilization of anthrax vaccine candidates that are currently licensed or under preclinical and clinical trials. We have also proposed a formulation to enhance recombinant PA vaccine potency via adjuvant formulation.
Keywords: Anthrax, Bacillus anthracis, Protective antigen, Instability, Deamidation, Adjuvant formulations, Adjuvants, Aluminum adjuvants
Abbreviations: PA, Protective antigen; LF, Lethal factor; EF, Edema factor; CMG2, Capillary morphogenesis protein; TEM8, Tumor endothelial marker 8; rPA, Recombinant PA; AVA, Anthrax Vaccine Adsorbed; cAMP, cyclic AMP; dm, Double mutants; WT, Wild type; Gln, Glutamine; Gly, Glycine; Ser, Serine; Asn, Asparagine; CpG-ODN, Synthetic oligodeoxynucleotides containing unmethylated cytosine phosphoguanine; MPLA, Monophosphoryl lipid A; AVP, Anthrax vaccine precipitated; TLR, Toll-like receptor; AlPO4, Aluminum phosphate; Al(OH)3, Aluminum hydroxide; AlOH, Aluminum oxyhydroxide
1. Introduction
Anthrax is a deadly infection caused by the gram-positive, aerobic, spore-forming bacterium B anthracis. The associated mortality rates depend on the origin of infection; untreated respiratory, gastrointestinal, and cutaneous infections are reportedly associated with mortality rates of 100%, 25–75%, and 20%, respectively (Plotkin et al., 2017). The world has survived multiple intentional and nonintentional anthrax-related incidents. For example, in 1979, Russia reported an intentional leakage of anthrax spores from a military laboratory, where 80 individuals were infected, and 42 fatalities were reported (Abramova et al., 1993). The largest and most recent anthrax-related epidemic occurred in Rhodesia, Zimbabwe, with a total of 10,738 infections and 200 fatalities reported after contracting the disease from infected cattle (Wilson et al., 2016). More recently, in October 2001, the US government reported multiple cases of inhalational anthrax in Florida and New York. Subsequent investigations revealed that all these incidences were linked to a single individual who deliberately disseminated B. anthracis spores to multiple government officials using mailing services Update: Investigation of anthrax associated with intentional exposure and interim public health guidelines (2001). Given that the associated mortality rates are high and the ease with which the spores get dispersed, B. anthracis is one of the most frightening bioweapons. Although unlikely, the possible use of B. anthracis as a bioweapon is distinctly possible, and this has led many governments to stockpile anthrax vaccines for emergency use. The challenge, however, lies in maintaining the stockpiled vaccines such that they remain efficacious and stable over the long-term. This warrants the development of a second-generation vaccine candidate with preserved potency and lasting stability over long-term storage (Verma et al., 2016).
1.1. First-generation anthrax vaccine protective antigen (PA)
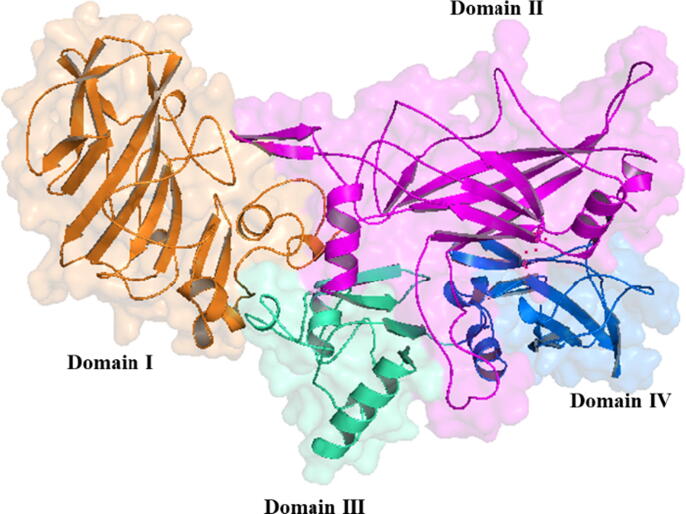
PA is a single-chained protein comprising 735 amino acids; it has a total molecular mass of 83 kDa. According to crystal structure analysis, the monomeric form of PA comprises four domains organized primarily as antiparallel β-sheets (Fig. 1). The domain 1(residues 1–258) comprises a pair of calcium ions, a furin cleavage site, and the LF-or EF-binding sites, whereas the domain 2 (residues 259–487) plays a crucial role in transmembrane pore formation (Petosa et al., 1997). The domain 3 (residues 488–595) is implicated in PA heptamerization and stabilization of the outcome oligomer (Mogridge et al., 2001), whereas the domain 4 (residues 596–735) harbors regions for binding host cell membrane receptors [capillary morphogenesis protein (CMG2) and tumor endothelial marker 8 (TEM8)](Rosovitz et al., 2003), (Chen et al., 2016).
Fig. 1.
View of protective antigen protein with colors representing the different domains. Domain I –IV are depicted respectively as follow, brown, pink, green and blue.
PA83, the 83-kDa form of PA, is considered as the major anthrax immunogen, and all four PA domains have neutralizing epitopes, which are sufficient for toxin neutralization (McComb and Martchenko, 2016). A previous report (Reason et al., 2011a) has shown that mice immunized with all four recombinant PA (rPA) domains generate elevated toxin-neutralizing antibody titers than those immunized with single or double rPA domains. PA20 does not appear to play a functionally important role in the pathogenesis of anthrax toxin; however, the monoclonal antibody 47F12, which was secluded from a human vaccinated with anthrax vaccine adsorbed (AVA; AVA BioThraxTM), presented the ability to neutralize LF–PA (LT) in vitro (Reason et al., 2011b). According to multiple studies protection and vaccine potency are mediated by the production of anti-PA neutralizing antibodies (Weiss et al., 2006, Chitlaru et al., 2011). Passive immunization of animals with polyclonal or monoclonal antibodies has demonstrated that PA neutralization might be sufficient as anthrax prophylaxis (Migone et al., 2009).
The three virulence elements of B. anthracis strains are encoded within the pXO1 megaplasmid. This megaplasmid controls the synthesis of the three major virulence factors of B. anthracis: PA, LF, and EF(Okinaka et al., 1999). Each of the virulence factors plays crucial role in infected cells. LF is a zinc-dependent metalloprotease that specifically targets mitogen-activated protein kinase in cells, mediating vascular pathologies in the form of hemorrhage and septic shock (Bromberg-White et al., 2010). EF is a calmodulin-dependent adenylate cyclase that stimulates the transformation of intracellular ATP to cyclic AMP (cAMP), resulting in water homeostasis and edema formation (Leppla, 1982). The entry of LF and EF into cells is mediated by the formation of a complex structure with the heptameric form of PA, which acts as a “shuttle” macrostructure for LF and EF factors. Subsequently, individual complexes (PA–EF and PA–LF) become internalized through endocytosis and are then transported to endosomes for processing and release in their individual forms (free LF and EF) into the cytosol, where they exert their cytotoxic activities and manifest systemic illness (Plotkin et al., 2017).
The currently available anthrax vaccines are produced from a sterile filtrate of an attenuated, the unencapsulated strain of the pathogen, comprising mainly the PA antigen and very little amounts of LF and EF. These vaccines, however, suffer from multiple drawbacks, including the undefined nature of the filtrate adsorbed to the adjuvant, the less than optimum purity of the final product, the loss of activity during long-term storage, and the requirement of 6 doses in the first 18 months followed by an annual booster to generate a protective immune response. These drawbacks have prompted defense agencies, health authorities, and their partners in the biotechnology industry to develop next-generation vaccine candidates for use in humans (Gorantala et al., 2011).
1.2. Second-generation anthrax vaccine recombinant protective antigen (rPA)
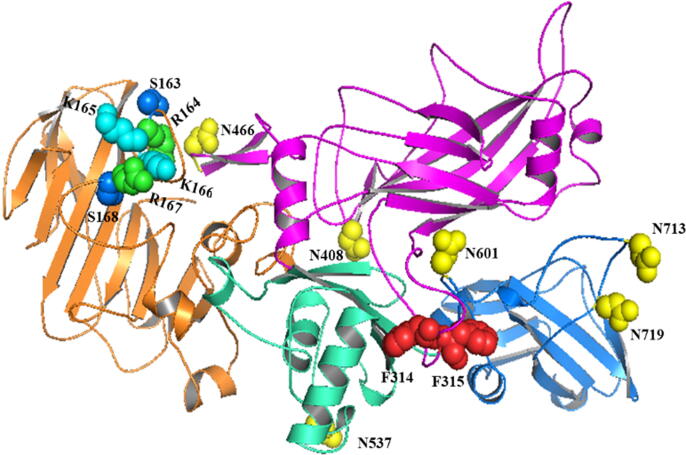
Of the many second-generation anthrax vaccine platforms proposed, subunit vaccines produced through recombinant DNA approaches (rPA) are considered as one of the most attractive platforms. This can be attributed to their capability to stimulate a protective immune response, the ease of manufacturing and development, their defined nature compared with older vaccines, and their nontoxicity, despite the essential contribution to the pathogenicity of infective agents (Leffel et al., 2012). Multiple clinical studies have been conducted to evaluate the immunogenicity of rPA and its safety in humans. All of those studies concluded that rPA-based vaccines could elicit protective immune response against anthrax, characterized by the induction of neutralizing anti-PA antibodies (Chi et al., 2015). However, the further development of rPA as a vaccine candidate got hampered owing to its inherent chemical instability, which has been attributed to the spontaneous deamidation of multiple Asn residues, resulting in the gradual loss of immunogenicity over time (Powell et al., 2007). Of the 68 Asn residues present in rPA, seven “hot spots” have been reported to spontaneously deamidate at different rates. These residues, ranked according to the rate of deamidation (fastest to slowest), are as follows: Asn537, Asn713, Asn466, Asn719, Asn601, Asn408, and Asn162(Verma et al., 2013).
2. Effect of site-directed mutagenesis approaches on the immunogenicity and stability of anthrax PA
Although rPA is considered a promising second-generation anthrax vaccine candidate, its inherent chemical instability (Asn deamidation at multiple sites) negatively impacts its potency, thereby precluding its use after long-term storage. Different site-directed mutagenesis approaches evaluated previously to mitigate this problem have been sumurised in Table 1.
Table 1.
Summary of Site-directed mutagenesis.
| Site-directed mutagenesis approaches | Protein Susceptibility | Functionality | |
|---|---|---|---|
|
|
rPA variant was not cleaved by either trypsin or the cell-surface protease. (Singh, Chaudhary et al., 1989) | Loss of toxicity when administered with lethal factor (Singh, Ivins et al., 1998) |
|
Not tested | 100% protection against aerosol challenge in a Rabbit Model, but no immunogenicity in clinical trials. (Hermanson, Whitlow et al., 2004) | |
|
|
Mutated rPA variants show that PA was not cleaved by either trypsin or the cell-surface protease. Also, the absences of two proteolysis-sensitive sites increased (dm) (rPA) stability (Ramirez, Leppla et al., 2002). | Safe and high immunogenicity profile. (Bellanti, Lin et al., 2012). |
|
Deamidation hot spots (Asn 408, Asn 466, Asn 537, Asn 601, Asn 713, and Asn 719) were mutated to either Asp, Gln. | The structure of the mutant rPA was not significantly altered compared to that of the WT PA. | Loss of immunogenicity (Verma, McNichol et al., 2013). |
|
Substitution of Asn713 and Asn719 by Gln. | The improved inherent stability of (rPA). | Insignificant loss in immunogenicity (Verma and Burns, 2018). |
2.1. Deletions in the furin cleavage site
The Arg164-Lys165-Lys166-Arg167 residues located on the domain I of the rPA protein harbor a cleavage site recognizable by the furin proteases. Once bound to the cell surface receptor, PA83 is cleaved into the active form (PA63), which in turn heptameric and shuttles the other pathogenic factors inside the cell. The designing of rPA variants for use as vaccine antigens that lack biological activity and the ability to be cleaved by the furin has been performed previously. In one report, researchers deleted the entire recognition site (163–168) and evaluated the activity and safety of the protein (Fig. 2). Their results confirmed that this rPA variant was not cleavable by either trypsin or cell surface protein and that the variant was not toxic when given with other pathogenic factors because it failed to bind either LF or EF (Singh et al., 1989). Potency evaluation of native and mutated rPA preparations in guinea pigs revealed that immunization provides high anti-PA titers. Complete protection was elicited after the immunization of mutant rPA either alone or in combination with LF and EF (Singh et al., 1998).
Fig. 2.
Representation of all rPA amino acid site deletions or mutation made in previous studies.
In another study, Hermanson et al. (2004) designed an rPA lacking the Ser192-Arg193-Lys194-Lys195-Arg196-Ser197 amino acid sequence located within the furin cleavage site and the LF domains I–III. High titers of anti-PA, anti-LF, and neutralizing antibodies against the lethal toxin were measured. Additionally, 100% protection was achieved against aerosolized anthrax spores in a rabbit model.
2.2. Mutations at the furin and chymotrypsin cleavage sites
Ramirez et al. (2002) designed an rPA with double mutants (dm) at both the furin and chymotrypsin cleavage sites. A strand of four amino acids within the furin cleavage site (Arg164-Lys165-Lys166-Arg167) was substituted with Ser-Asp-Lys-Glu; on the other hand, a deletion of two phenylalanine residues was made within the chymotrypsin cleavage site at positions 314–315 (Fig. 2). A comparison of both wild type (WT) rPA and dm rPA in terms of stability indicated that dm rPA is superior. Additionally, the phase I clinical trial of dm rPA conducted in 186 healthy individuals using three different formulations, dm rPA treated with formalin, and dm rPA adsorbed onto aluminum hydroxide, revealed that the vaccine tested is safe and immunogenic under all formulations. No statistical differences were observed between the antibody titers measured for the three different formulations. Enzyme-linked immunosorbent assay was used to determine the levels of anti-rPA induced by dm rPA immunization, and the findings indicated a good correlation of the findings with toxin neutralization assay (r = 0.97) (Bellanti et al., 2012).
2.3. Replacement of six Asn residues with Asp, Gln, or Ala residues
According to the study conducted by Verma et al. (2013) to reduce the extent of rPA deamidation using a mutational approach. Several deamidation hot spots (Asn408, Asn466, Asn537, Asn601, Asn713, and Asn719) were mutated to either Asp, Gln, or Ala, and the different rPA variants generated were then evaluated in terms of their functionality and stability. Conformational analysis of WT rPA and mutated rPA variants indicated similarities in terms of high-order structures. However, similar to that of WT rPA, the immunogenicity profile of mutated variants were found to undergo a significant decline during storage over 4 weeks at 25 °C.
2.4. Replacement of Asn713and Asn719 residues with Gln residues
Asn residues located within domain 4 (binding domain) of PA (Asn713 and Asn719) were substituted with glutamine (Gln) to generate an rPA(N713Q/N719Q) variant to eliminate/reduce the deamidation rate significantly because Gln is not suspected to undergo deamination easily. The modified rPA variant adsorbed onto aluminum hydroxide showed a good stability profile during 4 weeks of storage at room temperature, with no significant loss in immunogenicity (Verma and Burns, 2018).
2.5. Predictive analysis of site-directed mutagenesis approaches
Owing to the lack of steric hindrance upon cyclic imide intermediate formation, amino acids with small side chains preceding an Asn deamidation site (n + 1 position) are known to accelerate the rate of deamidation. Amino acids with hydrogen-donor side groups in their side chains that precede a deamidation site can also accelerate deamidation through hydrogen bonding to the carbonyl oxygen of Asn, thereby rendering it more electronegative and prone to nucleophilic attack and the formation of a cyclic imide intermediate (Kempkes et al., 2018). Closer examination of the seven deamidation hot spots revealed a pattern in terms of neighboring amino acids, in which the four Asn residues with the fastest deamidation rates (i.e., residues 537, 713, 466, and 719) all have a glycine (Gly) as a neighboring (n + 1) amino acid. The n + 1 sites of the remaining three hot spots (i.e., Asn residues 408, 601, and 162) are either serine (Ser) or asparagine (Asn), which are characterized by harboring hydrogen bond donor side chains. In addition to the effect of the neighboring amino acids, conformational and environmental factors also contribute to the deamidation rates of rPA. Mapping the positions of hot spot Asn residues within the three-dimensional structure of rPA reveals that almost all of these residues, particularly those with the fastest degradation properties are dispersed across unstructured, flexible loops that are exposed to external solvents and, thus, a polar environment, thereby rendering them even more prone to deamidation.
We believe that the root cause of these high deamidation rates lies in the fact that Asn is preceded by Gly at multiple sites. Among the seven deamidation sites available in rPA, four with the highest rates accommodate Gly at the n + 1 position.
2.6. Effects of different adjuvants on the immunogenicity and stability of anthrax PA
Passive immunization with antisera from other animals has been used to treat anthrax among livestock and humans (Plotkin et al., 2017). While in the era after antibiotics, humans have been depending heavily on active vaccination to eradicate or reduce the incidence of numerous dreaded diseases such as anthrax, cholera, polio, tetanus, typhoid, and rabies. Therefore, vaccination has been considered an instrumental public health intervention since the past century, sparing nations from infectious diseases; this has made vaccination crucial in public health practice (Ehreth, 2003). Extremely purified recombinant antigens are weakly or moderately immunogenic; adjuvants are often mandatory to increase the level and duration of protection induced by vaccines. Historically, in terms of human and veterinary vaccines, aluminum adjuvants are the most frequently used adjuvants. Aluminum adjuvants are often referred to as “alum” in the literature; however, this might cause ambiguity due to the different physical characteristics between the two most widely used adjuvants from this group––aluminum hydroxide and aluminum phosphate (Hem and White, 1995). Aluminum adjuvants have been combined into a variety of vaccines and administered to millions of people perhaps because they are effective with the antigens present in currently marketed vaccines, with exceptional safety profiles, minimal unwanted immunogenicity, and affordable prices (Seder et al., 2015). Synthetic oligodeoxynucleotides containing unmethylated cytosine phosphoguanine (CpG-ODN)-based adjuvants, which mimic bacterial and viral genetic material, are also good adjuvants for optimization to further potentiation. In case of a pandemic or widespread exposure to anthrax as a bioweapon, a vaccine providing single immunization protection would be the best choice. Therefore, (CpG-ODN)-based adjuvants might outweigh aluminum hydroxyl gel in terms of rapidity and potency (Kachura et al., 2016). PA encapsulated in liposomes containing monophosphoryl lipid A (MPLA) also shows great induction to lethal toxin-neutralizing titers in comparison to PA adsorbed onto aluminum hydroxide (Peachman et al., 2012). Currently available, licensed vaccines include AVA in the United States and anthrax vaccine precipitated (AVP) in the UK. There also exist other vaccines that are licensed in Russia and China; all four of these vaccines are created from a sterile filtrate of attenuated, the unencapsulated strains of the pathogen. Differences between AVA and AVP comprise adsorption to aluminum hydroxide gel versus precipitation with aluminum potassium sulfate and the use of various preservatives (Kondakova et al., 2019). Definitely, the selection of an inappropriate adjuvant might hamper the adequacy of the vaccine antigen. Therefore, at early developmental stages, the benefit/risk of the adjuvant must be considered to avoid the rejection of effective vaccine candidates.
2.7. The pivotal role of adjuvants
Adjuvants play a significant role in enhancing the translocation of an antigen from the injection site to the lymph node, a process that activates T-cells. Also, adjuvants provide protection to antigens and are considered an antigen reservoir to grant prolonged delivery (Schijns, 2000). Active immunization with a purified antigen usually induces insignificant antibody and T-cell response; therefore, vaccine producers need to use adjuvants to improve the immune response. In the case of pathogens that display a major antigen drift and strain variations, the need for an adjuvant becomes crucial to broaden an immune response by expanding B-cell diversity (Draper et al., 2013). Adjuvants might induce a great amount of antibodies with a higher affinity to the antigen (Kasturi et al., 2011). In the case of a pandemic, supplying vaccines to cover the world’s population will be a challenge for vaccine producers. Pairing purified protein antigens with an adjuvant can significantly reduce the amount of purified protein antigens required to increase the antibody titer. Single-shot vaccines are an optimum goal in both biodefense and pandemics and could be achieved by pairing the antigen with an adjuvant (Reed et al., 2013). The above mentioned roles lead us to classify adjuvants into four groups: the delivery system for antigens; depot effect; immunostimulation; or combination of delivery, storage, and immunostimulation.
2.8. Adjuvants employed in human vaccines
Each vaccine has specific desired features that necessitate specific adjuvant properties. Literature has shown that adjuvants enhance vaccine efficiency, durability, and potency. However, efforts to develop new generations of adjuvants to improve the currently available vaccines or new vaccines have been hampered by unproved health risks or lack of information but not safety issues. However, it is extremely important to utilize all available data on the mode of action, safety, potency, tolerability, and physical and chemical properties of adjuvants (Reed et al., 2013).
Adjuvants represent various materials that are either synthetic or extracted from natural products. Insoluble or aluminum-based adjuvants have been used since the beginning of the last century and incorporated into different vaccines with desired efficiency and safety profiles. However, aluminum alone as the first generation of adjuvants has shown insufficient immunogenicity against complex pathogens (O’Hagan et al., 2017). The second generation of adjuvants that combined alum with toll-like receptor (TLR) agonists offer a genuine advantage of activating innate and adaptive immune cells to overcome such a challenge. In terms of the next generation of adjuvants, alum with TLRs and MPLA is considered the most advanced (Seder et al., 2015). Overall, according to firmly established records, experts suggest that all new adjuvant candidates should be compared with aluminum adjuvants as the “gold standard” in terms of safety and efficacy (HogenEsch et al., 2018).
3. Adjuvants employed in anthrax vaccines
3.1. Aluminum compounds as adjuvants
Aluminum compounds can be used as adjuvants in licensed vaccines in the US, in limited amounts (0.85–0.125 mg/dose)(Baylor et al., 2002). There are three main aluminum compounds that are used as adjuvants: aluminum hydroxide [Al(OH)3], aluminum phosphate (AlPO4), and aluminum precipitate in either sodium (Na) or potassium (K)(Gupta and Rost, 2000). Al(OH)3 has a crystalline structure with a 500-m2/g adsorption surface, with a particle diameter of approximately 10 µm (Johnston et al., 2002). AlPO4 is a heterogeneous salt and has an adsorption surface of 250 m2/g, with an average diameter of 2 µm (Glemza et al., 1992). In the literature, the word alum has been commonly used as an abbreviation for aluminum adjuvants. Al(OH)3 adjuvants are often referred to in the literature as (AH).
Adsorption of antigens to adjuvants occurs via electrostatic interaction, hydrophobic interaction, or ligand exchange mechanisms (which is the strongest)(HogenEsch et al., 2018). The isoelectric point (pI) of aluminum oxyhydroxide (AlOH) is 11.4 (Hsu, 1989); as such, its surface will be positively charged at a neutral pH and would bind the acidic PA antigen (pI ∼ 5). Alhydrogel-bound antigen or the (rPA–AlOH) complex preserves secondary (Agopian et al., 2007), tertiary (Soliakov et al., 2012), and quaternary structures (Harris et al., 2012) but shows reduced thermal stability compared with their free counterparts in solution (Jones et al., 2005). Basic surface antigens are known to bind AlPO4 adjuvants, which have a pI of 4.6–5.6, yielding a negative surface charge to it at neutral pH (Rowe et al., 2009). The storage of aluminum adjuvants at room temperature results in deprotonation and dehydration processes and leads to decreases in the surface area, consequently reducing the adsorptive capacity within 15 months (Burrell et al., 2000); moreover, there is an inverse relationship between the strength of adsorption and the immune response (Hansen et al., 2011). Furthermore, the rate of disassociation of freshly prepared antigen–adjuvant complexes in interstitial fluid is larger than that of the older formulated samples (Jiang et al., 2006).
Alum adjuvants act as a reservoir for antigens to cause prolonged exposure and improve the transfer of antigen to antigen-presenting cells and the activation of dendritic cells via the induction of uric acid (Kool et al., 2008). However, they also have some limitations, such as pore augmentation of IgE antibody responses, incompetence to augment cell-mediated immune responses (specifically T-killer cell responses), induction of local reactions at the injection site, erythema, contact hypersensitivity (Baylor et al., 2002) and lack of efficacy in some antigens due to weak electrostatic forces between the antigen and alum adjuvant, hydrophobic interactions, Van der Waals forces and hydrogen bonding that contribute to proper adsorption between the antigen and alum adjuvant, or inappropriate range of pH in which the charge on the gel and the antigens would change and thus affect adsorption. Moreover, the size and surface area of alum adjuvant gel particles play a crucial role in antigen adsorption (Gupta and Rost, 2000). There are contradictions in the literature regarding the integrity and stability of antigens after adsorption onto (AH), but some researchers have demonstrated the structural integrity of the protein even after exposing recombinant protein adsorbed onto Al(OH)3with accelerated heat conditions (Colaprico et al., 2020). According to Jendrek et al. (2003) the anthrax rPA interacts with aluminum hydroxide adjuvant via electrostatic interaction. A study to evaluate the immunogenicity of anthrax rPA adsorbed onto Al(OH)3 and AlPO4 concluded that the latter is not essential for adjuvanticity, but the amount of adjuvant does induce antibody response (Berthold et al., 2005). Moreover, in terms of immunogenicity, stored formulations of an rPA–Alhydrogel combination for around 3 weeks caused loss of the capability to induce toxin-neutralizing antibodies (Wagner et al., 2012).
The phosphate buffer used in anthrax vaccine formulation would have a significant effect on the binding capacity and adsorption coefficient. In the case of the (rPA–AlOH) complex, phosphate ions of the buffer bind the exposed surface of hydroxyl groups to yield an aluminum phosphate surface that reduces the adsorption between (rPA) and AlOH by forming a water layer between the antigen and the adjuvant, thereby revealing that phosphate is an advantageous modeling agent for adsorption between (rPA) and AlOH. Moreover, the hypothesis of the strongest immunogenic effects to an aluminum-adjuvanted (rPA) is likely due to the reduced binding capacity and the adsorption coefficient (Watkinson et al., 2013).
3.2. Cholera toxin (CT)
An exotoxin from Vibrio cholerae is used as a powerful mucosal vaccine adjuvant in mice. Scientists believe its mechanism of action as an adjuvant could be via increasing epithelial permeability, modifying antigen presentation (Sijun and Yong, 2009), inducing Th2-type immune responses, and producing immunoglobulins such as IgA, IgG, and IgE (Holmgren et al., 2006). When rPA is adjuvanted into CT and administered nasally to mice, it generates systemic and mucosal immune responses. Furthermore, a study showed that B. anthracis associated with 1-µg CT protects the mice against inhalation anthrax (Datta et al., 2010). However, due to safety issues and the toxicity of intranasal administration of CT as an adjuvant, other immunopotentiators are needed in clinical trials (Sijun and Yong, 2009).
3.3. Polyriboinosinic-polyribocytidylic acid [poly (I: C)]
poly (I: C) is synthetic double-stranded RNA used as an adjuvant in the nasal vaccines because it is recognized by TLR 3 that is located in the endosomal compartment of myeloid DCs. Therefore, it has the ability to induce interferon (IFN1), inflammatory cytokine production and antigen-presenting cell development (Matsumoto and Seya, 2008). In the PA–poly (I: C) complex used as the nasal immunization vaccine against anthrax in mice, the outcomes exhibited abundant IgG and IgA production and a higher anthrax toxin-neutralizing antibody (TNA) when compared to CT (Sloat and Cui, 2006). Another study used a triantigen nasal vaccine that contained poly γ-D-glutamic acid (PGA), which is a B. anthracis capsule linked to the protein carrier bovine serum albumin, anthrax LF, and poly (I:C) as an adjuvant. The study showed the ability of the complex to induce strong immunization responses against all three antigens (Sloat et al., 2008).
3.4. (CpG-ODN)
The unmethylated CpG sequence is a part of bacterial DNA and is considered as an immunostimulatory sequence. Different studies have used synthetic oligodeoxynucleotides containing unmethylated (CpG-ODN) as an immunostimulatory oligonucleotide adjuvant to potentiate both humoral and cellular immune responses to vaccines. (CpG-ODN) induces human plasmacytoid dendritic cells and B-cells to express TLR9, produce Th1and proinflammatory cytokines, and improve the functional maturation/activation of professional antigen-presenting cells. Both preclinical and clinical trials have proven that (CpG-ODN)s enhance the activity and immunogenicity of vaccines (Bode et al., 2011). Combining (CpG-ODN) in the AVA vaccine potentiates the immunity of macaques, specifically via subcutaneous administration (Klinman et al., 2004). A double-blind phase II clinical study to evaluate the safety and immunogenicity of a combination of AVA and CPG 7909 candidate (known as AV7909) has proven the safety and good tolerance of the AV7909 candidate. A similar immune response to AV7909 candidates and AVA has been noted specifically at 0/14 days of the vaccines schedule; however, the AV7909 candidate has shown a higher and earlier peak (Hopkins et al., 2016).
Cationic hydrophilic polymers such as chitosan were employed to encapsulate rPA and (CpG-ODN) due to their capacity to encapsulate antigens and (CpG-ODN) in the same particle (Jesus and Borges, 2011); investigators have assessed the adjuvant potential of three different formulations containing PA encapsulated within trimethyl–chitosan nanoparticles in combination with either (CpG-ODN) or [poly (I: C)]. The designed routes of administration for all formulations in mice are intraperitoneal, subcutaneous, or intramuscular. The study revealed that regardless of the route of administration, (CpG-ODN)-and [poly (I: C)]-conjugated TMC–PA nanoparticles elicited a strong humoral response. In contrast, TMC–PA revealed a cellular immune response. Furthermore, TMC–PA and CpG TMC–PA formulations were the most effective when administered via subcutaneous and intramuscular routes of administration in mice (Malik et al., 2018). Moreover, anthrax rPA adjuvanted within (CpG-ODN) on a Ficoll scaffold combination (rPA/DV230-Ficoll) used against aerosolized anthrax as immunization in monkeys augmented rapid immunogenicity compared with rPA alone, revealing their capability in antigen delivery and increasing adjuvant activity (Kachura et al., 2016).
3.5. Poly (lactic-co-glycolic acid) (PGLA)
PLGA-based delivery systems have been well studied in subunit-based vaccines and have revealed their capability in encapsulating antigens, targeting antigen-presenting cells, and subsequently potentiating immune responses (Silva et al., 2016). The domain 4 of anthrax PA has been combined into a PLGA nanoparticle (PAD4–NP) complex. A single dose of this complex generates PA domain 4-specific antibodies and produces both Th1/Th2 responses, ultimately protecting mice against B. anthracis (Manish et al., 2013).
3.6. MPLA with liposomes
MPLA is a detoxified endotoxin lipid A fraction that lacks one phosphate group and has no physiological toxicity. However, it holds adjuvanticity. It upregulates costimulatory molecules on dendritic cells and macrophages through TLR4 (Han et al., 2013) and induces strong Th1 responses (Moingeon et al., 2001). Limited reactogenicity with high immunogenicity and a safety profile of liposomal MPLA has been confirmed in human phase I clinical trials using various candidates vaccine formulations (Alving, 2002). Anthrax PA as an antigenic material encapsulated in liposomal adjuvant systems comprising MPLA contains amplified titers of binding and neutralizing antibodies to anthrax PA compared to the (AH)-adsorbed antigen in macaques, which reveals the high safety and efficiency of liposomal MPLA in monkeys and humans (Rao et al., 2011). Different anthrax PA-adjuvant vaccine formulations have undergone comparative studies. The formulation comprises PA encapsulated in liposomes containing monophosphoryl lipid AL(PA + MPLA), stable liposomal oil-in-water emulsion (PA-emulsion), PA presented on the T4 nanoparticle antigen delivery system (T4-PA), and PA with heat-labile Escherichia coli enterotoxin (PA + HLT) tested on New Zealand white rabbits. Two L(PA + MPLA) and (PA + HLT) formulations induce complete protection against B. anthracis Ames strain spores, whereas only limited protection was observed in the other two formulations. Moreover, L(PA + MPLA) ensured long-term protection of the PA-antibody complex (Peachman et al., 2012).
3.7. Mast cell activators
One of the major mast cell activators widely used in bioresearch is compound 48/80 (C48/80), which is an end product derived from the condensation process of N-methyl p-methoxyphenethylamine and formaldehyde to induce mast cells, which are scattered throughout the epithelium of connective tissue (Koibuchi et al., 1985). A study to evaluate the immune response induced by rPA with C48/80 delivered intranasally as a dry powder formulation in rabbits showed elevated anti-rPA IgG levels and more significant lethal toxin neutralization antibody titers, similar to that elicited by intramuscular immunization with rPA alone. Surprisingly, rPA and the C48/80 dry powder formulation remain stable after 2.5 years of storage at ambient room temperature, which is comparable with the stability of freshly prepared formulations (Wang et al., 2012). Chitosan nanoparticles have been incorporated with mast cell activator compound 48/80 (C48/80) and used as adjuvants for anthrax rPA in the nasal vaccine; these have shown a reduction in the rPA dose required to induce high neutralizing antibody titers, potentiating the production of IgG1 and IgG2 (Bento et al., 2015).
3.8. Virus-like particles (VLPs)
Manayani et al. (2007) combined anthrax PA with a chimeric VLPs, which has been used to display the anthrax toxin receptor ANTXR2. The study showed that the complex produced a potent TNA response and prevented lethal toxin action. Loading domain 4 of anthrax PA and VLPs produced potent and rapid immune responses against anthrax PA (Venter et al., 2011).
3.9. Polysaccharide-derived adjuvants
AdvaxTM is a polysaccharide derived from the Inulin plan (Cooper and Petrovsky, 2011)and is used as an adjuvant in a wide variety of vaccines due to its ability to enhance immunogenicity against different antigens such as the Japanese encephalitis virus (Lobigs et al., 2010) and human immunodeficiency virus (Cristillo et al., 2011). A combination of anthrax PA plus Advax adjuvant was studied to assess the immune response in mice and its ability to induce PA immunogenicity; the study concluded that there was a significant protection with a single subcutaneous dose of PA-AdvaxTM complex against aerosolized B. anthracis than with three immunizations with PA alone. Furthermore, the formulation of AdvaxTM with Murabutide, a synthetic derivative of the bacterial peptidoglycan cell wall, elicited a double effect on immunogenicity and 100% protection after just two doses (Feinen et al., 2014).
3.10. Incorporation of acetylated dextran and TLR 7/8 agonist
Association of acetylated dextran (Ac-DEX) (71-kDa) with imiquimod TLR 7/8 agonist revealed dose-sparing activation for dendritic cells (Bachelder et al., 2010), and the incorporation of anthrax rPA within resiquimod Ac-DEX particles to evaluate rapid immune response in mice showed a significantly higher level of antibodies compared to the response elicited from rPA and the alum group. However, antibodies were not required for fast neutralization, and the protection might have been due to the T-cell response (Schully et al., 2013, Gallovic et al., 2016).
3.11. Predictive analysis of different adjuvants on the immunogenicity and stability of anthrax PA
Despite the contradictions in the literature in terms of the integrity and stability of antigens after adsorption to (AH), recent studies have the structural integrity of proteins (Colaprico et al., 2020). Long-range electrostatic interaction is considered of the major ways of adsorption of antigen to adjuvants (HogenEsch et al., 2018). At a neutral pH, aluminum oxyhydroxide (AlOH) has a positive surface charge owing to its high pI of 11.4, and rPA is negatively charged due to its acidic pI of 5.6. rPA complexes with AlOH and protect the secondary, tertiary, and quaternary structures. In addition to the structural and conformational factors influencing chemical stability, environmental factors play a substantial role in deamidation degradation kinetics. Known factors that influence deamidation include pH, peptide chain flexibility, and the presence of excipients and buffers with deamidation catalytic activities.
4. Conclusions
In summary, rPA is considered a primary candidate for a second-generation anthrax vaccine. It has multiple domains that play critical role in protease activation, heptomerization, membrane insertion, and binding to cell receptors. Most importantly, all domains contain neutralizing epitopes. Adjuvants employed in anthrax vaccine formulations, specifically those used in clinical or preclinical trials, undergo adequate safety or quality standards. However, in terms of alhydrogel, strong alhydrogel-bound antigens might reflect low vaccine immunogenicity. Moreover, only the freshly prepared formulations of the rPA–alhydrogel combination elicit a strong immune response. Innovations in the design of safe and immunogenic adjuvants come with combinations of immunopotentiators such as CPG, TLR agonists, liposomal MPLAs, and the nanoparticles adjuvant platform. The majority of combinations shows rapid induction of both memory and protective immunity. The main problem with rPA-based vaccines is protein instability, which is significantly enhanced when aluminum adjuvants and other novel adjuvants are used. Attempts are made to overcome the instability by spray freeze–dried formulations.
Countless studies are being conducted worldwide to develop safe and effective anthrax vaccines and to prevent inherent chemical instability as a result of Asn deamidation at multiple sites of the protein. However, all studies to reduce deamidation extent of rPA have not found success to avert inherent chemical instability. rPA vaccine with improved long term stability and enhanced immunogenicity could be attained in future via substitution of Gly residues preceding Asn by Ala at four different deamidation hotspot sites (538, 714, 467, and 720).
5. Disclaimer
The contents of this manuscript are solely the authors’ views and may not be understood or quoted as being made on behalf of or reflecting the position of the Department of Pharmaceutics, College of Pharmacy, King Saud University.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We extend our appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work (project number: DRI-KSU-293).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abramova F.A., Grinberg L.M., Yampolskaya O.V., Walker D.H. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc. Natl. Acad. Sci. U. S. A. 1993;90(6):2291–2294. doi: 10.1073/pnas.90.6.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agopian A., Ronzon F., Sauzéat E., Sodoyer R., El Habib R., Buchet R., Chevalier M. Secondary structure analysis of HIV-1-gp41 in solution and adsorbed to aluminum hydroxide by Fourier transform infrared spectroscopy. BBA. 2007;1774(3):351–358. doi: 10.1016/j.bbapap.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Alving C.R. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine. 2002;20(Suppl 3):S56–S64. doi: 10.1016/s0264-410x(02)00174-3. [DOI] [PubMed] [Google Scholar]
- Bachelder E.M., Beaudette T.T., Broaders K.E., Fréchet J.M.J., Albrecht M.T., Mateczun A.J., Ainslie K.M., Pesce J.T., Keane-Myers A.M. In vitro analysis of acetalated dextran microparticles as a potent delivery platform for vaccine adjuvants. Mol. Pharm. 2010;7(3):826–835. doi: 10.1021/mp900311x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor N.W., Egan W., Richman P. Aluminum salts in vaccines—US perspective. Vaccine. 2002;20:S18–S23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- Bellanti J.A., Lin F.-Y., Chu C., Shiloach J., Leppla S.H., Benavides G.A., Karpas A., Moayeri M., Guo C., Robbins J.B., Schneerson R. Phase 1 study of a recombinant mutant protective antigen of Bacillus anthracis. Clin. Vaccine Immunol.: CVI. 2012;19(2):140–145. doi: 10.1128/CVI.05556-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento D., Staats H.F., Borges O. Effect of particulate adjuvant on the anthrax protective antigen dose required for effective nasal vaccination. Vaccine. 2015;33(31):3609–3613. doi: 10.1016/j.vaccine.2015.06.037. [DOI] [PubMed] [Google Scholar]
- Berthold I., Pombo M.-L., Wagner L., Arciniega J.L. Immunogenicity in mice of anthrax recombinant protective antigen in the presence of aluminum adjuvants. Vaccine. 2005;23(16):1993–1999. doi: 10.1016/j.vaccine.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Bode C., Zhao G., Steinhagen F., Kinjo T., Klinman D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccine. 2011;10(4):499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-White J., Lee C.-S., Duesbery N. Consequences and utility of the zinc-dependent metalloprotease activity of anthrax lethal toxin. Toxins. 2010;2(5):1038–1053. doi: 10.3390/toxins2051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell L.S., White J.L., Hem S.L. Stability of aluminium-containing adjuvants during aging at room temperature. Vaccine. 2000;18(21):2188–2192. doi: 10.1016/s0264-410x(00)00031-1. [DOI] [PubMed] [Google Scholar]
- Chen K.-H., Liu S., Leysath C.E., Miller-Randolph S., Zhang Y.i., Fattah R., Bugge T.H., Leppla S.H. Anthrax Toxin Protective Antigen Variants That Selectively Utilize either the CMG2 or TEM8 Receptors for Cellular Uptake and Tumor Targeting. J. Biol. Chem. 2016;291(42):22021–22029. doi: 10.1074/jbc.M116.753301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Li J., Liu W., Wang X., Yin K., Liu J.u., Zai X., Li L., Song X., Zhang J., Zhang X., Yin Y., Fu L., Xu J., Yu C., Chen W., Burns D.L. Generation and Characterization of Human Monoclonal Antibodies Targeting Anthrax Protective Antigen following Vaccination with a Recombinant Protective Antigen Vaccine. Clin. Vaccine Immunol. 2015;22(5):553–560. doi: 10.1128/CVI.00792-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitlaru T., Altboum Z., Reuveny S., Shafferman A. Progress and novel strategies in vaccine development and treatment of anthrax. Immunol. Rev. 2011;239(1):221–236. doi: 10.1111/j.1600-065X.2010.00969.x. [DOI] [PubMed] [Google Scholar]
- Colaprico A., Senesi S., Ferlicca F., Brunelli B., Ugozzoli M., Pallaoro M., O'Hagan D.T. Adsorption onto aluminum hydroxide adjuvant protects antigens from degradation. Vaccine. 2020;38(19):3600–3609. doi: 10.1016/j.vaccine.2020.02.001. [DOI] [PubMed] [Google Scholar]
- Cooper P.D., Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising β-D-[2 -> 1] poly(fructo-furanosyl) α-D-glucose polymers. Glycobiology. 2011;21(5):595–606. doi: 10.1093/glycob/cwq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristillo A.D., Ferrari M.G., Hudacik L., Lewis B., Galmin L., Bowen B., Thompson D., Petrovsky N., Markham P., Pal R. Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. J. Gen. Virol. 2011;92(Pt 1):128–140. doi: 10.1099/vir.0.023242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S.K., Sabet M., Nguyen K.P.L., Valdez P.A., Gonzalez-Navajas J.M., Islam S., Mihajlov I., Fierer J., Insel P.A., Webster N.J., Guiney D.G., Raz E. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc. Natl. Acad. Sci. 2010;107(23):10638–10643. doi: 10.1073/pnas.1002348107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper E., Bissett S.L., Howell-Jones R., Waight P., Soldan K., Jit M., Andrews N., Miller E., Beddows S. A randomized, observer-blinded immunogenicity trial of Cervarix(®) and Gardasil(®) Human Papillomavirus vaccines in 12–15 year old girls. PLoS ONE. 2013;8(5):e61825. doi: 10.1371/journal.pone.0061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehreth J. The global value of vaccination. Vaccine. 2003;21(7-8):596–600. doi: 10.1016/s0264-410x(02)00623-0. [DOI] [PubMed] [Google Scholar]
- Feinen B., Petrovsky N., Verma A., Merkel T.J., Wilkins P.P. Advax-adjuvanted recombinant protective antigen provides protection against inhalational anthrax that is further enhanced by addition of murabutide adjuvant. Clin. Vaccine Immunol.: CVI. 2014;21(4):580–586. doi: 10.1128/CVI.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallovic M.D., Schully K.L., Bell M.G., Elberson M.A., Palmer J.R., Darko C.A., Bachelder E.M., Wyslouzil B.E., Keane-Myers A.M., Ainslie K.M. Acetalated Dextran Microparticulate Vaccine Formulated via Coaxial Electrospray Preserves Toxin Neutralization and Enhances Murine Survival Following Inhalational Bacillus Anthracis Exposure. Adv. Healthcare Mater. 2016;5(20):2617–2627. doi: 10.1002/adhm.201600642. [DOI] [PubMed] [Google Scholar]
- Glemza R., Parent Y.O., Welsh W.A. Amorphous aluminum phosphate gels. Catal. Today. 1992;14(2):175–188. [Google Scholar]
- Gorantala J., Grover S., Goel D., Rahi A., Jayadev Magani S.K., Chandra S., Bhatnagar R. A plant based protective antigen [PA(dIV)] vaccine expressed in chloroplasts demonstrates protective immunity in mice against anthrax. Vaccine. 2011;29(27):4521–4533. doi: 10.1016/j.vaccine.2011.03.082. [DOI] [PubMed] [Google Scholar]
- Gupta R.K., Rost B.E. Springer; 2000. Aluminum Compounds as Vaccine Adjuvants. Vaccine Adjuvants; pp. 65–89. [Google Scholar]
- Han Y., Li Y., Chen J., Tan Y., Guan F., Wang X. Construction of monophosphoryl lipid A producing Escherichia coli mutants and comparison of immuno-stimulatory activities of their lipopolysaccharides. Mar. Drugs. 2013;11(2):363–376. doi: 10.3390/md11020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B., Malyala P., Singh M., Sun Y., Srivastava I., Hogenesch H., Hem S.L. Effect of the strength of adsorption of HIV 1 SF162dV2gp140 to aluminum-containing adjuvants on the immune response. J. Pharm. Sci. 2011;100(8):3245–3250. doi: 10.1002/jps.22555. [DOI] [PubMed] [Google Scholar]
- Harris J.R., Soliakov A., Lewis R.J., Depoix F., Watkinson A., Lakey J.H. Alhydrogel(R) adjuvant, ultrasonic dispersion and protein binding: a TEM and analytical study. Micron. 2012;43(2–3):192–200. doi: 10.1016/j.micron.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Hem S.L., White J.L. Structure and properties of aluminum-containing adjuvants. Pharm. Biotechnol. 1995;6:249–276. doi: 10.1007/978-1-4615-1823-5_9. [DOI] [PubMed] [Google Scholar]
- Hermanson G., Whitlow V., Parker S., Tonsky K., Rusalov D., Ferrari M., Lalor P., Komai M., Mere R., Bell M., Brenneman K., Mateczun A., Evans T., Kaslow D., Galloway D., Hobart P. A cationic lipid-formulated plasmid DNA vaccine confers sustained antibody-mediated protection against aerosolized anthrax spores. Proc. Natl. Acad. Sci. U.S.A. 2004;101(37):13601–13606. doi: 10.1073/pnas.0405557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HogenEsch H., O’Hagan D.T., Fox C.B. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. npj Vaccines. 2018;3(1):51. doi: 10.1038/s41541-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren, J., Harandi, A.M., Lebens, M., Sun, J.-B., Anjuère, F., Czerkinsky, C., 2006. 14 – Mucosal adjuvants based on cholera toxin and E. coli heat-labile enterotoxin. In: Schijns, V.E.J.C., O'Hagan, D.T. (Eds.), Immunopotentiators in Modern Vaccines. London, Academic Press, pp. 235–252.
- Hopkins R.J., Kalsi G., Montalvo-Lugo V.M., Sharma M., Wu Y., Muse D.D., Sheldon E.A., Hampel F.C., Lemiale L. Randomized, double-blind, active-controlled study evaluating the safety and immunogenicity of three vaccination schedules and two dose levels of AV7909 vaccine for anthrax post-exposure prophylaxis in healthy adults. Vaccine. 2016;34(18):2096–2105. doi: 10.1016/j.vaccine.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, P., 1989. luminium hydroxides and oxyhydroxides. In: Dixon, J.B., Weed, S.B. (Eds.), Minerals in soil environments. Madison, WI: Soil Science Society of America, pp. 331–378.
- Jendrek S., Little S.F., Hem S., Mitra G., Giardina S. Evaluation of the compatibility of a second generation recombinant anthrax vaccine with aluminum-containing adjuvants. Vaccine. 2003;21(21–22):3011–3018. doi: 10.1016/s0264-410x(03)00109-9. [DOI] [PubMed] [Google Scholar]
- Jesus S., Borges O. Recent developments in the nasal immunization against anthrax. World J. Vaccine. 2011;1:79–91. [Google Scholar]
- Jiang D., Morefield G., Hogenesch H., Hem S. Relationship of adsorption mechanism of antigens by aluminum-containing adjuvants to in vitro elution in interstitial fluid. Vaccine. 2006;24(10):1665–1669. doi: 10.1016/j.vaccine.2005.09.048. [DOI] [PubMed] [Google Scholar]
- Johnston C.T., Wang S.-L., Hem S.L. Measuring the surface area of aluminum hydroxide adjuvant. J. Pharm. Sci. 2002;91(7):1702–1706. doi: 10.1002/jps.10166. [DOI] [PubMed] [Google Scholar]
- Jones L.S., Peek L.J., Power J., Markham A., Yazzie B., Middaugh C.R. Effects of adsorption to aluminum salt adjuvants on the structure and stability of model protein antigens. J. Biol. Chem. 2005;280(14):13406–13414. doi: 10.1074/jbc.M500687200. [DOI] [PubMed] [Google Scholar]
- Kachura M.A., Hickle C., Kell S.A., Sathe A., Calacsan C., Kiwan R., Hall B., Milley R., Ott G., Coffman R.L., Kanzler H., Campbell J.D. A CpG-Ficoll nanoparticle adjuvant for anthrax protective antigen enhances immunogenicity and provides single-immunization protection against inhaled anthrax in monkeys. J. Immunol. 2016;196(1):284–297. doi: 10.4049/jimmunol.1501903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturi S.P., Skountzou I., Albrecht R.A., Koutsonanos D., Hua T., Nakaya H.I., Ravindran R., Stewart S., Alam M., Kwissa M., Villinger F., Murthy N., Steel J., Jacob J., Hogan R.J., García-Sastre A., Compans R., Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470(7335):543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempkes L.J.M., Boles G.C., Martens J., Berden G., Armentrout P.B., Oomens J. Deamidation of protonated asparagine-valine investigated by a combined spectroscopic, guided ion beam, and theoretical study. J. Phys. Chem. A. 2018;122(9):2424–2436. doi: 10.1021/acs.jpca.7b12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman D., Xie H., Little S., Currie D., Ivins B. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine. 2004;22:2881–2886. doi: 10.1016/j.vaccine.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Koibuchi Y., Ichikawa A., Nakagawa M., Tomita K. Histamine release induced from mast cells by active components of compound 48/80. Eur. J. Pharmacol. 1985;115(2–3):163–170. doi: 10.1016/0014-2999(85)90687-9. [DOI] [PubMed] [Google Scholar]
- Kondakova O.A., Nikitin N.A., Evtushenko E.A., Ryabchevskaya E.M., Atabekov J.G., Karpova O.V. Vaccines against anthrax based on recombinant protective antigen: problems and solutions. Expert Rev. Vaccine. 2019;18(8):813–828. doi: 10.1080/14760584.2019.1643242. [DOI] [PubMed] [Google Scholar]
- Kool M., Soullie T., van Nimwegen M., Willart M.A., Muskens F., Jung S., Hoogsteden H.C., Hammad H., Lambrecht B.N. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008;205(4):869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffel E.K., Bourdage J.S., Williamson E.D., Duchars M., Fuerst T.R., Fusco P.C. Recombinant protective antigen anthrax vaccine improves survival when administered as a postexposure prophylaxis countermeasure with antibiotic in the New Zealand white rabbit model of inhalation anthrax. Clin. Vaccine Immunol. 2012;19(8):1158–1164. doi: 10.1128/CVI.00240-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S.H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. U. S. A. 1982;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobigs M., Pavy M., Hall R.A., Lobigs P., Cooper P., Komiya T., Toriniwa H., Petrovsky N. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J. Gen. Virol. 2010;91(Pt 6):1407–1417. doi: 10.1099/vir.0.019190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A., Gupta M., Mani R., Gogoi H., Bhatnagar R. Trimethyl Chitosan Nanoparticles Encapsulated Protective Antigen Protects the Mice Against Anthrax. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manayani D.J., Thomas D., Dryden K.A., Reddy V., Siladi M.E., Marlett J.M., Rainey G.J.A., Pique M.E., Scobie H.M., Yeager M., Young J.A.T., Manchester M., Schneemann A., Hultgren S.J. A viral nanoparticle with dual function as an anthrax antitoxin and vaccine. PLoS Pathog. 2007;3(10):e142. doi: 10.1371/journal.ppat.0030142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manish M., Rahi A., Kaur M., Bhatnagar R., Singh S. A single-dose PLGA encapsulated protective antigen domain 4 nanoformulation protects mice against Bacillus anthracis spore challenge. PLoS ONE. 2013;8(4):e61885. doi: 10.1371/journal.pone.0061885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv. Drug Deliv. Rev. 2008;60(7):805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- McComb R.C., Martchenko M. Neutralizing antibody and functional mapping of Bacillus anthracis protective antigen-The first step toward a rationally designed anthrax vaccine. Vaccine. 2016;34(1):13–19. doi: 10.1016/j.vaccine.2015.11.025. [DOI] [PubMed] [Google Scholar]
- Migone T.-S., Subramanian G.M., Zhong J., Healey L.M., Corey A.l., Devalaraja M., Lo L., Ullrich S., Zimmerman J., Chen A., Lewis M., Meister G., Gillum K., Sanford D., Mott J., Bolmer S.D. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 2009;361(2):135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- Mogridge J., Mourez M., Collier R.J. Involvement of domain 3 in oligomerization by the protective antigen moiety of anthrax toxin. J. Bacteriol. 2001;183(6):2111–2116. doi: 10.1128/JB.183.6.2111-2116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moingeon P., Haensler J., Lindberg A. Towards the rational design of Th1 adjuvants. Vaccine. 2001;19(31):4363–4372. doi: 10.1016/s0264-410x(01)00193-1. [DOI] [PubMed] [Google Scholar]
- O’Hagan D.T., Friedland L.R., Hanon E., Didierlaurent A.M. Towards an evidence based approach for the development of adjuvanted vaccines. Curr. Opin. Immunol. 2017;47:93–102. doi: 10.1016/j.coi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Okinaka R.T., Cloud K., Hampton O., Hoffmaster A.R., Hill K.K., Keim P., Koehler T.M., Lamke G., Kumano S., Mahillon J., Manter D., Martinez Y., Ricke D., Svensson R., Jackson P.J. Sequence and organization of pXO1, the large Bacillus anthracis plasmid harboring the anthrax toxin genes. J. Bacteriol. 1999;181(20):6509–6515. doi: 10.1128/jb.181.20.6509-6515.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachman K.K., Li Q., Matyas G.R., Shivachandra S.B., Lovchik J., Lyons R.C., Alving C.R., Rao V.B., Rao M. Anthrax vaccine antigen-adjuvant formulations completely protect New Zealand white rabbits against challenge with Bacillus anthracis Ames strain spores. Clin. Vaccine Immunol. 2012;19(1):11–16. doi: 10.1128/CVI.05376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petosa C., Collier R.J., Klimpel K.R., Leppla S.H., Liddington R.C. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385(6619):833–838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- Plotkin, S.A., Orenstein, W., Offit, P.A., Edwards, K.M., 2017. Vaccines E-Book, Elsevier Health Sciences.
- Powell B.S., Enama J.T., Ribot W.J., Webster W., Little S., Hoover T., Adamovicz J.J., Andrews G.P. Multiple asparagine deamidation of Bacillus anthracis protective antigen causes charge isoforms whose complexity correlates with reduced biological activity. Proteins. 2007;68(2):458–479. doi: 10.1002/prot.21432. [DOI] [PubMed] [Google Scholar]
- Ramirez D.M., Leppla S.H., Schneerson R., Shiloach J. Production, recovery and immunogenicity of the protective antigen from a recombinant strain of Bacillus anthracis. J. Ind. Microbiol. Biotechnol. 2002;28(4):232–238. doi: 10.1038/sj/jim/7000239. [DOI] [PubMed] [Google Scholar]
- Rao M., Peachman K.K., Li Q., Matyas G.R., Shivachandra S.B., Borschel R., Morthole V.I., Fernandez-Prada C., Alving C.R., Rao V.B. Highly effective generic adjuvant systems for orphan or poverty-related vaccines. Vaccine. 2011;29(5):873–877. doi: 10.1016/j.vaccine.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reason D., Liberato J., Sun J., Camacho J., Zhou J. Mechanism of Lethal Toxin Neutralization by a Human Monoclonal Antibody Specific for the PA20 Region of Bacillus anthracis Protective Antigen. Toxins. 2011;3:979–990. doi: 10.3390/toxins3080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reason D., Liberato J., Sun J., Camacho J., Zhou J. Mechanism of lethal toxin neutralization by a human monoclonal antibody specific for the PA(20) region of Bacillus anthracis protective antigen. Toxins (Basel) 2011;3(8):979–990. doi: 10.3390/toxins3080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S.G., Orr M.T., Fox C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19(12):1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- Rosovitz M.J., Schuck P., Varughese M., Chopra A.P., Mehra V., Singh Y., McGinnis L.M., Leppla S.H. Alanine-scanning mutations in domain 4 of anthrax toxin protective antigen reveal residues important for binding to the cellular receptor and to a neutralizing monoclonal antibody. J. Biol. Chem. 2003;278(33):30936–30944. doi: 10.1074/jbc.M301154200. [DOI] [PubMed] [Google Scholar]
- Rowe R.C., Sheskey P., Quinn M. Libros Digitales-Pharmaceutical Press; 2009. Handbook of Pharmaceutical Excipients. [Google Scholar]
- Schijns V.E. Immunological concepts of vaccine adjuvant activity: commentary. Curr. Opin. Immunol. 2000;12(4):456–463. doi: 10.1016/s0952-7915(00)00120-5. [DOI] [PubMed] [Google Scholar]
- Schully K.L., Sharma S., Peine K.J., Pesce J., Elberson M.A., Fonseca M.E., Prouty A.M., Bell M.G., Borteh H., Gallovic M., Bachelder E.M., Keane-Myers A., Ainslie K.M. Rapid vaccination using an acetalated dextran microparticulate subunit vaccine confers protection against triplicate challenge by bacillus anthracis. Pharm. Res. 2013;30(5):1349–1361. doi: 10.1007/s11095-013-0975-x. [DOI] [PubMed] [Google Scholar]
- Seder R., Reed S.G., O’Hagan D., Malyala P., D’Oro U., Laera D., Abrignani S., Cerundolo V., Steinman L., Bertholet S. Gaps in knowledge and prospects for research of adjuvanted vaccines. Vaccine. 2015;33:B40–B43. doi: 10.1016/j.vaccine.2015.03.057. [DOI] [PubMed] [Google Scholar]
- Sijun H., Yong X. Helicobacter pylori vaccine : mucosal adjuvant & delivery systems. Indian J. Med. Res. 2009;130(2):115–124. [PubMed] [Google Scholar]
- Silva A.L., Soema P.C., Slütter B., Ossendorp F., Jiskoot W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Human Vaccines Immunother. 2016;12(4):1056–1069. doi: 10.1080/21645515.2015.1117714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y., Chaudhary V.K., Leppla S.H. A deleted variant of Bacillus anthracis protective antigen is non-toxic and blocks anthrax toxin action in vivo. J. Biol. Chem. 1989;264(32):19103–19107. [PubMed] [Google Scholar]
- Singh Y., Ivins B.E., Leppla S.H. Study of Immunization against Anthrax with the Purified Recombinant Protective Antigen of <em>Bacillus anthracis</em>. Infect. Immun. 1998;66(7):3447–3448. doi: 10.1128/iai.66.7.3447-3448.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloat B.R., Cui Z. Nasal Immunization with Anthrax Protective Antigen Protein Adjuvanted with Polyriboinosinic-Polyribocytidylic Acid Induced Strong Mucosal and Systemic Immunities. Pharm. Res. 2006;23(6):1217–1226. doi: 10.1007/s11095-006-0206-9. [DOI] [PubMed] [Google Scholar]
- Sloat B.R., Shaker D.S., Le U.M., Cui Z. Nasal immunization with the mixture of PA63, LF, and a PGA conjugate induced strong antibody responses against all three antigens. FEMS Immunol. Med. Microbiol. 2008;52(2):169–179. doi: 10.1111/j.1574-695X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Soliakov A., Kelly I.F., Lakey J.H., Watkinson A. Anthrax sub-unit vaccine: The structural consequences of binding rPA83 to Alhydrogel®. Eur. J. Pharmac. Biopharmac.: Off. J. Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2012;80(1):25–32. doi: 10.1016/j.ejpb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Update: Investigation of anthrax associated with intentional exposure and interim public health guidelines, October 2001. MMWR Morb Mortal Wkly Rep, vol. 50, no. 41, p. 889–993. [PubMed]
- Venter P.A., Dirksen A., Thomas D., Manchester M., Dawson P.E., Schneemann A. Multivalent display of proteins on viral nanoparticles using molecular recognition and chemical ligation strategies. Biomacromolecules. 2011;12(6):2293–2301. doi: 10.1021/bm200369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Burns D.L. Improving the stability of recombinant anthrax protective antigen vaccine. Vaccine. 2018;36(43):6379–6382. doi: 10.1016/j.vaccine.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Verma A., McNichol B., Domínguez-Castillo R.I., Amador-Molina J.C., Arciniega J.L., Reiter K., Meade B.D., Ngundi M.M., Stibitz S., Burns D.L., Blanke S.R. Use of site-directed mutagenesis to model the effects of spontaneous deamidation on the immunogenicity of bacillus anthracis protective antigen. Infect. Immun. 2013;81(1):278–284. doi: 10.1128/IAI.00863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Ngundi M.M., Burns D.L., Wilkins P.P. Mechanistic analysis of the effect of deamidation on the immunogenicity of anthrax protective antigen. Clin. Vaccine Immunol. 2016;23(5):396–402. doi: 10.1128/CVI.00701-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner L., Verma A., Meade B.D., Reiter K., Narum D.L., Brady R.A., Little S.F., Burns D.L. Structural and immunological analysis of anthrax recombinant protective antigen adsorbed to aluminum hydroxide adjuvant. Clin. Vaccine Immunol. 2012;19(9):1465–1473. doi: 10.1128/CVI.00174-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.H., Kirwan S.M., Abraham S.N., Staats H.F., Hickey A.J. Stable dry powder formulation for nasal delivery of anthrax vaccine. J. Pharm. Sci. 2012;101(1):31–47. doi: 10.1002/jps.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson A., Soliakov A., Ganesan A., Hirst K., LeButt C., Fleetwood K., Fusco P.C., Fuerst T.R., Lakey J.H. Increasing the potency of an alhydrogel-formulated anthrax vaccine by minimizing antigen-adjuvant interactions. Clin. Vaccine Immunol. 2013;20(11):1659–1668. doi: 10.1128/CVI.00320-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Kobiler D., Levy H., Marcus H., Pass A., Rothschild N., Altboum Z. Immunological correlates for protection against intranasal challenge of Bacillus anthracis spores conferred by a protective antigen-based vaccine in rabbits. Infect. Immun. 2006;74(1):394–398. doi: 10.1128/IAI.74.1.394-398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.M., Brediger W., Albright T.P., Smith-Gagen J. Reanalysis of the anthrax epidemic in Rhodesia, 1978–1984. PeerJ. 2016;4:e2686. doi: 10.7717/peerj.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]