Graphical abstract
Keywords: Simvastatin, Aspirin, SLS, Tablets, In vitro dissolution, Cytokines, Ulcer index
Abstract
According to the American College of Cardiology/American Heart Association (ACC/AHA), both aspirin and statin are used in the primary prevention of cardiovascular diseases. Aspirin (ASA) is contraindicated if there is gastrointestinal bleeding because it will exaggerate the condition. In this study, the effect of surfactant; sodium lauryl sulfate (SLS), in enhancing the in vitro dissolution of simvastatin (SIM) and ASA, as well as gastric irritation and upset, was studied. Oral tablets containing both ASA and SIM with and without the SLS were manufactured using the direct compression technique. The prepared tablets were characterized with respect to hardness, friability, uniformity of dosage units, in vitro disintegration, and dissolution. The effect of the addition of SLS in reducing the in vivo irritation and protection of gastric mucosa were also investigated. The results showed that the compressed tablets possessed sufficient hardness, acceptable friability, and are uniform with respect to disintegration, drugs contents, and tablet weight. The results showed that SIM alone exhibited a gastroprotective effect on the induced irritation. In addition, the incorporation of the SLS in the tablets containing SIM and ASA significantly enhanced the dissolution rates of both drugs and significantly decreased the gastric irritation and the ulcer index. The ulcer index of aspirin was decreased from 2.3 for tablets manufactured without SLS to 0.8 for tablets containing SLS. In a conclusion, the addition of pH modifier surfactant; SLS could enhance the dissolution rate of poorly soluble acidic drugs, reduce gastric upset and irritation without any effect on the main characters of the tablets. Moreover, the addition of SLS is very useful in improving the therapeutic activities and reducing the side effects of ASA and SIM for patients who require long-term administration of these drugs.
1. Introduction
Cardiovascular diseases (CVDs) are considered one of the primary causes of death globally. According to World Health Organization (WHO) survey in 2019, 32% of death in that year is due to CVDs (cardiovascular diseases, WHO, 2021). Several risk factors can act as a determinant for CVDs. These are smoking and alcohol consumption, obesity, and overweight. These factors, separately or collectively, may lead to some chronic disease such as hypertension, diabetes, high cholesterol level which all leads to the end of CVDs (cardiovascular diseases, WHO, 2021). Moreover, several studies showed that the mortality rate due to coronary artery disease is higher in diabetic patients than in non-diabetic patients (Wienbergen et al., 2008). American College of Cardiology/American Heart Association (ACC/AHA) provides protocol guidelines for primary prevention of CVDs and they recommend the use of statin with a small dose of ASA (Arnett et al., 2019). Simvastatin is a member of the statin group, which is classified as β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase inhibitors that inhibit the synthesis of cholesterol. High cholesterol level is one of the risk factors in the development of CVDs. A low dose of aspirin acts as antiplatelet aggregation by irreversibly acetylating the active site of cyclooxygenase-1 (COX-1), which is required for the production of thromboxane A2, a powerful stimulus of platelets aggregation (Arnett et al., 2019). For the primary prevention, ASA was beneficial in reducing the first cardiovascular disease by 26% in an elderly woman while statins reduce the cardiovascular risk by 37%. A meta-analysis done by Hennekens et al. (2004) showed that combination therapy of the hypolipidemic agent; SIM with ASA had a significant effect in reducing the CVDs. More interestingly, aspirin provides an additional 24% reduction in CVDs when added to simvastatin monotherapy.
For the development of oral dosage forms, the researchers should provide great attention to the API aqueous solubility and its dissolution. The aqueous drug solubility is the rate-determining step in the dissolution process of poorly soluble drugs, and consequently, affects their absorption and bioavailabilities (Alshora et al., 2016). According to Noyes–Whitney equation (Eq. (1)), the dissolution rate is depending mainly on the surface area (S), saturation solubility (Cs), and concentration of the drug in the bulk fluid (C).
| (1) |
Additionally, modifying the pH of dissolution fluid could alter the surface saturation solubility (Cs) and finally affect the dissolution behavior of drugs with pH-dependent solubility. Modifying the pH of the environment surrounding the drug could increase or decrease the saturation solubility of the drug depending on the physicochemical properties of the drug (Martin, 1993). Based on these facts, introducing a pH modifier in an oral solid dosage form containing weakly acidic or weakly basic drugs could promote the dissolution throughout the gastrointestinal tract by reducing the degree of supersaturation and thus decrease the rate of crystallization in the microenvironment (Badawy and Hussain, 2007). Moreover, the incorporation of a solubilizing agent within the solid dosage form, which increases the microenvironment pH to be higher than the pKa of the drug, will increase the solubility of the weakly acidic drug such as SIM and ASA (Gouardhane et al., 2014)
Sodium lauryl sulfate (SLS) is an anionic surfactant, which is categorized as Generally Regarded as Safe (GRAS) excipient according to the FDA database and EMA (European Medicine Agency, 2015). SLS is mainly used as a lubricant and wetting agent in solid dosage forms. SLS acts also as a wetting agent with alkalinizing properties, as it has a pH range from 7 to 9.5. Therefore, incorporation of a small amount of SLS in semi-solid and solid preparations may slightly raise the pH of the final preparation during drug dissolution from the dosage form (European Medicine Agency, 2015). SLS is adopted in several functional uses in pharmaceutical formulations as an emulsifying agent, release modifying agent, penetration enhancer, solubilizing agent, tablet, and capsule lubricant orally. In addition, it is used externally in several cosmetic preparations such as skin cleansers, mouthwashes, and shampoos (European Medicine Agency; EMA, 2015). Moreover, SLS is widely used as a solubility and dissolution rates enhancer for poorly water-soluble drugs. Cyclosporin A was dispersed in a system containing SLS and dextrin to improve its bioavailability (Lee et al., 2001). It was observed that incorporating SLS with a certain concentration significantly promotes the dissolution rate of the drug to reach 100% after 30 min compared with 30% only for pure drugs (Lee et al., 2001). Several researchers found that incorporating a surfactant in the formulation could have an enhancing effect on the dissolution of poorly water-soluble drugs (Nokhodchi et al., 2002; Taupitz and Klein, 2013, Desai et al., 2014, Alizadehet al., 2018).
As mentioned previously, the long-term oral administration of SIM and ASA, as poorly water-soluble and weakly acidic drugs, can cause gastrointestinal irritation and ulcer. Several techniques have been developed to enhance solubility and consequently reduce their gastrointestinal side effects. Co-crystal formation of aspirin with valine was found to enhance the dissolution rate significantly where 100% dissolution was reached after 1 h compared to only about 59% dissolution was observed for pure drug (Shanthala et al., 2021). Chaudhari et al., (2015) showed that the dissolution rate of aspirin prepared by solid dispersion (fusion method) using polyethylene glycol (PEG 6000) as a carrier was enhanced compared to the physical mixture and untreated drug (Chaudhari et al., 2015) Simvastatin (SIM) is an anti-hyperlipidemic drug belongs to statin group. It is classified as a Class II drug, according to the Biopharmaceutical Classification System (BCS), which means that the drug is characterized by high permeability and low solubility. The oral bioavailability of the drug is less than 5% which is due to its poor water solubility (Schachter, 2005). Accordingly, enhancement of the aqueous solubility of SIM will improve the drug bioavailability. Different methods and techniques have been applied to significantly promote the dissolution rate of the drug (Margulis-Goshen and Magdassi, 2009; Jiang et al., 2012).
The current study is aiming to formulate and evaluate directly compressed tablets containing ASA and SIM and incorporate SLS as an alkalinizing agent to enhance the dissolution rates of the two acidic drugs and at the same time, increase the pH of the media and thus decrease the irritation caused by the two APIs. The prepared tablets will be tested in vitro for uniformity of weight and drug content, hardness, friability, disintegration, and dissolution. Additionally, in vivo studies including gastric irritation, histopathology and immunohistochemistry, and morphometric analysis will be performed.
2. Materials and methods
2.1. Materials
Simvastatin (SIM) was obtained from Riyadh Pharma Pharmaceutical Company, Riyadh, KSA. Acetylsalicylic acid; Aspirin (ASA) was obtained from Fluka (Buchs SG, Switzerland) Sodium lauryl sulphate (SLS) was purchased from WINLAB, Leicestershire, UK. Microcrystalline cellulose (Avicel® PH101) was purchased from Serva Feinbiochemica (Heidelberg, Germany). Magnesium stearate was purchased from Riedel-de Haën (Seelze, Germany). Potassium dihydrogen orthophosphate and Disodium hydrogen phosphate were purchased from Merk, Darmstadt, Germany.
2.2. Methodology
2.2.1. Direct compression of tablets containing SIM and ASA
Table 1 shows the composition of the directly compressed tablets containing SIM and ASA with and without SLS. The formula weights of SIM, ASA, Avicel PH101 with and without SLS were weighed accurately and blended for 10 min using a Turbula mixer (Erweka, S2Y, Heusenstamm, Germany). Thereafter, the lubricant (magnesium stearate) was added to the powder mix and further mixed in the Turbula mixer for 2 min. Finally, the powder mix was compressed into tablets weighing 200 mg using Korsh single punch machine (Erweka, EKO, Germany) with 9 mm shallow concave punches.
Table 1.
Composition of different tablet formulations containing SIM and ASA.
| Ingredient | Weight (mg) |
|
|---|---|---|
| SA Tablets (without SLS) | SAS Tablets (with SLS) | |
| Simvastatin (SIM) | 20 | 20 |
| Aspirin (ASA) | 81 | 81 |
| Sodium Lauryl Sulfate (SLS) | – | 1 |
| Magnesium stearate | 2 | 2 |
| Avicel pH 101® | to 200 mg | to 200 mg |
2.2.2. Evaluation of the prepared tablets
2.2.2.1. Weight variation
Ten tablets from each formulation were weighed individually (Analytical balance, Shimadzu, EB-3200D, Tokyo, Japan) and the average weight, the standard deviation was calculated.
2.2.2.2. Uniformity of drug content
Ten directly compressed tablets weighing 200 mg and containing 81 mg ASA and 20 mg SIM were accurately weighed, finely powdered, and transferred into a 250 mL volumetric flask. About 20 mL methanol was added, and the dispersion was sonicated for 30 min. Thereafter, the volume was completed using phosphate buffer (pH 6.8) and was then sonicated and filtered. The drug content was determined spectrophotometrically at 268 nm for ASA and 238 mm for SIM. The test was performed and repeated in triplicates.
2.2.2.3. Hardness
The braking force was determined with the Hardness Tester (Pharma test GmbH, Hainburg, Germany). Ten tablets (with known weight and thickness) were selected from each batch and the average hardness, the standard deviation was reported.
2.2.2.4. Friability
The friability of the manufactured tablet was measured per USP30-NF25. Twenty tablets were cleaned, weighed (W1), and loaded to the friabilator (Erweka, TA3R, Heusenstamm, Germany) that was rotated at a constant speed (25 rpm) for 4 min. At the end of the rotation time, the tablets were brushed to remove fines, and reweighed (W2). The friability % was calculated from the equation
2.2.2.5. In vitro disintegration
The USP30-NF25 guidelines for the In vitro disintegration test were followed. One dosage unit was placed into each of the six tubes of the basket (Electrolab, ED-21, Mumbai, India). The apparatus was operated, using phosphate buffer (pH 6.8) as the immersion fluid, maintained at 37 °C ± 2 °C. Disintegration time was reported when no tablet residue is left, and the standard deviation of six tablets was calculated.
2.2.2.6. In vitro dissolution studies
USP dissolution apparatus II, paddle method, (Caleva Ltd., Model 85 T) with a continuous automated monitoring system, was used to perform the in vitro dissolution test in triplicate. This system consists of an IBM computer PK8620 series and PU 8605/60 dissolution test software, Philips VIS/UV/NIR single beam eight cell spectrophotometer Model PU 8620, Epson FX 850 printer, and Watson-Marlow peristaltic pump using in each flask a 500 mL 0.1 N HCl, pH 1.2, 4.6, and 6.8. The temperature was maintained at 37 ± 0.5 °C and the dissolution media were stirred at 100 rpm. At preplanned times intervals (5, 10, 15, 20, and 30 min), the absorbance was recorded automatically at 268 nm for ASA and 238 mm for SIM nm, and the percentage of drug dissolution was determined as a function of time. The dissolution efficiency (DE%) was calculated using a trapezoidal rule, by measuring the area under the dissolution curve at a time and expressed as a percentage of the area of the rectangle described by 100% dissolution in the same time (Khan, 1975).
2.2.2.7. Calculation of f1 and f2
The dissolution profiles will be compared to each other in a pairwise model (Moore and Flanner, 1996). The difference factor (f1) and the similarity factor (f2) will be calculated.
The f1 calculates the percent difference between the two dissolution profiles at each time point. As the value of f1 is close to zero (0), this indicates the difference between the two dissolution profiles and can be calculated as follow:
| (3) |
While f2 measure the similarity between the two dissolution profiles. As the value of f2 is close to 100, this indicates the similarity of the profiles. The f2 can be calculated as follow:
where n is the number of time points, Rt is the mean dissolution value for the reference product at time t, and Tt is the mean dissolution value for the test product at that same time point (Zayed, 2014).
2.2.3. In vivo anti-ulcer activity
Thirty adult male Sprague Dawley rats were obtained from Assiut University Animal House, Assiut. Food was not allowed for 18–24 h before the experiment water access was free. The rats were randomly assigned into five groups, each group consisting of 6 rats, and the treatment was given by gastric tube. The stomachs were removed after the rats were sacrificed and opened along the greater curvature. The animal study protocol followed the ‘Guide for Care and Use of Laboratory Animals of the Laboratory Animal Centre at the College of Pharmacy at Al Azhar University (Assiut, Egypt). The study protocol was approved by Al Azhar University Ethical Committee for Experimental Animals (IRB approval number: ZA-AS/PH/7/C/2021). Group I (control gp) administered Saline solution daily for 7 days. In Group II (ASA GP), the rats were administered aspirin in a dose of 350 mg/kg and started on the 5th day of the experiment. The aspirin was suspended in saline just before the administration (Clara et al., 2012). In Group III (SIM gp), the rats were administered SIM in a dose of 60 mg/kg daily for 7 days (Carvalho et al., 2016). Group IV (SA-Tablets treated gp (without SLS)), each rat in this group was administered 60 mg/kg of SIM for 7 days and on the 5th day of the experiment, they will receive 350 mg/kg of ASA in concomitants with SIM. In the fifth group (SAS-Tablets treated gp (with SLS)), rats were given (2 cm) sodium dodecyl sulfate (Shay et al., 1947) and (60 mg /kg) Simvastatin daily for 7 days, and the ASA was added in the same regimen as gp II.
2.2.3.1. Sampling
At the end of the experimental period, blood samples were collected, sera were separated by centrifugation and stored at < -20˚c until assaying of the cytokines. Rats were anesthetized using diethyl ether, their stomach was fixed in 10% formalin, embedded in the paraffin section for histopathological and immunostaining.
2.2.3.2. Methods
2.2.3.2.1. Determination of plasma TNF- α and IL-6 levels
Serum samples were collected and centrifuged at 1000 × g for 10 min at 4 ˚C. The levels of IL-6 and TNF- were measured by ELISA using rat TNF alpha ELISA Kit (ab46070) and rat IL-6 ELISA Kit: ab100772).
2.2.3.2.2. Gross examination
After sacrificing the rats from different groups, the stomach of rats was removed and opened along the greater curvature to assess the degree of damage and ulceration. Different parts of the gastric mucosa were grossly inspected for the presence of various lesions to record their numbers and severity.
The grade of lesions for each rat was scored according to the following ulcer score (MacAllister et al., 1997); 0 = no lesions, 1= (1–2) superficial localized lesions, 2= (3–5) deep localized lesions, 3= (6–10) Multiple lesions, 4= > 10 or deep hyperemic and/or darkened diffuse lesions. The ulcer index for each group of rats was calculated according to the following equation (Swarnakar et al., 2005):
2.2.3.2.3. Histopathological examination
Sections of the stomach flattened on a piece of photographic paper with mucosal surface up containing lesions. Stomach fixed in 10% neutral buffered formalin solution for 24 h. After fixation, all tissue samples were routinely processed for conventional histopathological examination. Five-micron sections were cut and stained with hematoxylin and eosin stain (Bancroft and Stevens, 1982) for histopathological examination by light microscopy (CX31; Olympus, Tokyo Japan) and photographed using a digital camera (Toupview, LCMos10000KPA, china) in the Photomicrograph Lab. of Pathology & Clinical Pathology Department, Faculty of Veterinary Medicine, Assiut University (Suvarna, 2018).
The microscopic findings for each group were presented in a table to demonstrate the type of lesion, severity, and percentage of animals.
2.2.3.2.4. Morphometric analysis
The H&E-stained slides of the stomach in all examined rats were subjected to morphometric analysis to measure gastric mucosa. The images were obtained and measured digitally using an Axiostar plus microscope (Carl Zeiss, Thornwood, NY, USA) interfaced with an Axiostar plus digital camera and Axiovision 4.1 software (Carl Zeiss). For each rat multiple gastric segments were examined with a 10x objective lens and photographs were taken and then 3 fields were selected for analysis.
2.2.3.2.5. Immunohistochemistry examination
Paraffin sections from the stomach were used for immunohistochemical detection of Cox-1 at the end of the study. The tissue sections (3 μm thick) were deparaffinized and washed by distal water. Heat-induced antigen retrieval was applied in a water bath using citrate buffer (pH 6) for 20 min. The endogenous peroxidase activities were removed with 3% hydrogen peroxide (H2O2). Sections were then incubated in primary antibody overnight at 4 °C in a humidified chamber for Cox-1 (obtained from USBiological life sciences) diluted in phosphate-buffered saline (PBS). The primary antibodies were detected in all experimental groups. Econo Tek biotinylated Anti polyvalent was applied and incubated for 30 min. Then the sections were rinsed four times for 5 min each with Phosphate-buffered saline, and the sections were incubated in Econo Tek HRP Conjugate for 30 min at room temperature. A mixture of DAB chromogen was visualized in the sections, and the DAB substrate was then incubated for 10 min. Sections were washed with distilled water then counterstained with hematoxylin and dehydrated and mounted. Positive immunoreactions looked at the brown coloration. Negative controls were performed by neglecting the primary antibody, which resulted in negative immunoreactivity.
3.6. Statistical analysis
The software Graphpad Prism version 5 (GraphPad Software, CA, USA) was used for analyzing data. One-way analysis of variance was performed to compare means among groups followed by Tukey's post-hoc test. Significance was considered at p-value < 0.05.
3. Results
3.1. Tablet evaluation
Tablets containing 20 mg SIM and 81 mg ASA without SLS (SA-Tablets) and with SLS (SAS-Tablets) were successfully prepared using the direct compression method and were evaluated for weight variation, content uniformity of the incorporated drug, hardness, and friability. The obtained tablets' evaluation results are listed in Table 2.
Table 2.
Properties of different tablet formulations containing SIM and ASA.
| Tablet | Weight (mg) |
Content Uniformity (AV) |
Hardness (KP) | Friability (%) | Disintegration (s) |
|---|---|---|---|---|---|
| SA-Tablets | 199.5 ± 7.8 | 10.21 (SIM) | 6.87 ± 0.76 | 0.49 | 237.4 ± 10.2 |
| 12.76 (ASA) | |||||
| SAS-Tablets containing 0.5% SLS | 205.4 ± 6.5 | 8.87 (SIM) | 5.60 ± 0.51 | 0.54 | 165.7 ± 5.7 |
| 13.26 (ASA) | |||||
| Zocor® 20 mg | 200.0 ± 5.4 | 9.84 | 9.37 ± 0.37 | 0.34 | 217.33 ± 17.27 |
3.1.1. Weight variation and content uniformity
The weight of SA-tablets prepared without SLS was 199.5 ± 7.8 mg, while the weight of SAS-tablet containing 0.5% SLS showed a weight of 205.4 ± 6.5 mg. Moreover, the commercial tablets (Zocor®) showed an average weight of 200.0 ± 5.4 mg.
The content uniformity for SIM and ASA in the prepared tablets; based on the calculation of acceptance value (AV) according to USP Pharmacopeia, (USP 34) was performed, and the results are tabulated in Table 2. The calculated AV values for the SA-Tablets formula were 10.21 for SIM, and 12.76 for ASA. For SAS-tablets containing 0.5% SLS, the AV values were 8.87 and 13.26 for SIM and ASA, respectively. In addition, the AV for commercial tablets product was 9.84. The obtained results for drugs content uniformity for both APIs in the tested tablet formulations revealed AV values less than 15, indicating that content uniformity meets the criteria for 10 individual units in the first stage, L1 (USP 34,).
3.1.2. Hardness
The hardness of the SA-tablets formula was 6.87 ± 0.76 KP, while that of the SAS-Tablets formula was 5.60 ± 0.51 KP, Table 2. The results showed that SAS-Tablets formulations (that contain 0.5% SLS) exhibited a slightly low hardness value in comparison to the hardness of SA-Tablets and commercial tablets (9.37 ± 0.37).
3.1.3. Friability
All tested tablet formulations, showed % friability less than 0.55, Table 2, which meet the compendia guidelines.
3.2. In vitro disintegration
The in vitro disintegration of the prepared SIM-ASA tablets using phosphate buffer (pH 6.8) was performed and the results are shown in Table 2. The commercial SIM tablets disintegrated within 217.33 ± 17.27 s, while the tested SAS-Tablets formulation containing SLS disintegrated within 165.7 ± 5.7 s compared to 237.4 ± 10.2 s for the tablet formula SA-Tablets.
3.3. In vitro dissolution
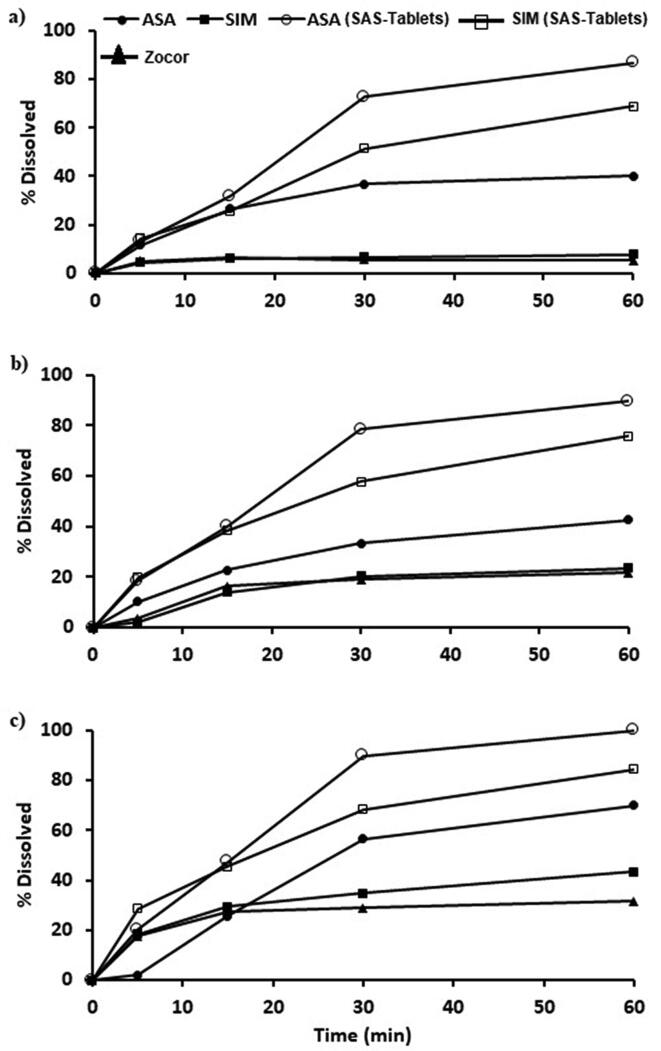
The in vitro dissolution of the prepared tablets containing SIM-ASA combinations compared with the commercial product were studied at three different pH values (1.2, 4.6, and 7.4), and the obtained data are illustrated in Fig. 1a–c.
Fig. 1.
The in vitro dissolution profiles of treated SIM and ASA from SAS-Tablets compared with its corresponding untreated drug and commercial product at three different pH a) pH 1.2; b) pH 4.6; c) pH 6.8.
In the acidic medium (pH 1.2), SIM exhibited very slow release from commercial tablet products and tablets containing aspirin combination in absence of SLS (SA-Tablets)). Only 5.22 % and 7.62 % of the loaded drug were dissolved during the first 60 min from these formulations, respectively as shown in Fig. 1. Also, aspirin exhibited slow in vitro dissolution from this tablet formula where only 39.92 % of the loaded aspirin were dissolved after 60 min. Incorporation of SLS in the SIM-ASA tablet combination (SAS-Tablets) resulted in enhancement of the dissolution of the two API (ASA and SIM) in the highly acidic medium (pH 1.2) where SIM and ASA showed 68.9% and 86.38% dissolution within the first 60 min respectively.
The in vitro dissolution rates of the two APIs in a medium of pH 4.6 followed the same pattern observed in the highly acidic medium (pH 1.2), but with a slight increment in the dissolution rates in all tested formulas as presented in Fig. 1b. The results revealed that 23.48% and 42.47% of SIM and ASA, respectively, were dissolved from the SA-Tablets formula. In the case of SAS-Tablets, a tablet formula that contains SLS, enhanced dissolution rates were observed for both APIs, in which 75.8% and 89.5% of the loaded SIM and ASA were dissolved, respectively.
The in vitro dissolution of SIM and ASA from tablet formula without SLS in pH 6.8 indicated a noticeable increase in the dissolution rates of the two APIs, in which 43.48% and 70.02% of the loaded SIM and ASA were dissolved within 60 min, Fig. 1c.
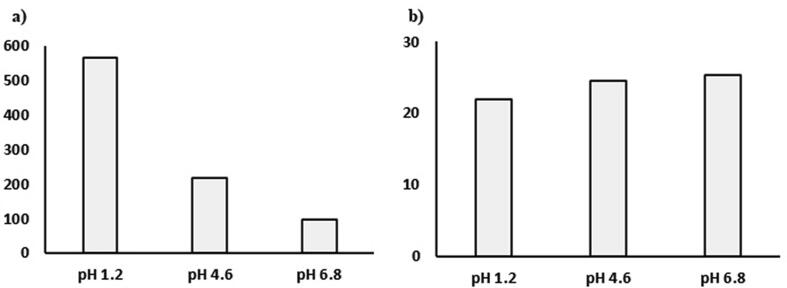
The dissolution profile of SIM was compared to its innovator product (Zocor) to determine the difference factor (f1) and the similarity factors (f2) in three different pH as shown in Fig. 2. The results showed that the f1 value was very high at pH 1.2 (565.9) and decreased to 96.6 at pH 6.8. In the case of similarity factor f2, the values reach 100 when the dissolution profiles are similar and decreased when the dissimilarity increase. The f2 value for SIM was less than 30 at different pH indicating the dissimilarity between the dissolution profile between the treated and the marketed product.
Fig. 2.
The difference factor f1 (a) and the similarity factor f2 (b) of treated SIM compared to commercial product Zocor®.
3.4. In-vivo anti-ulcer activity
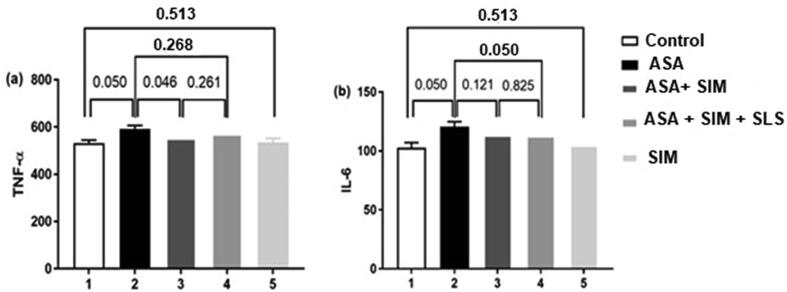
3.4.1. Expression of TNF-α and IL-6 in plasma
The serum levels of TNF-α and IL-6 in different groups of rats are shown in Fig. 3. The levels of TNF-α were significantly high in the ASA administrated group compared with rats in the control group and the ASA-SIM treated group. While there was no significant change between ASA administrated group and the ASA-SIM-SLS treated group. The IL-6 levels were significantly higher in ASA administrated group in comparison with a control group and ASA-SIM-SLS group. However, there was no significant change between the ASA administrated group and the AS-SIM.
Fig. 3.
TNF-α and IL-6 serum levels in different experimental groups, values are expressed as means ± SE (n = 7). P-value indicates significance at p ≤ 0.05.
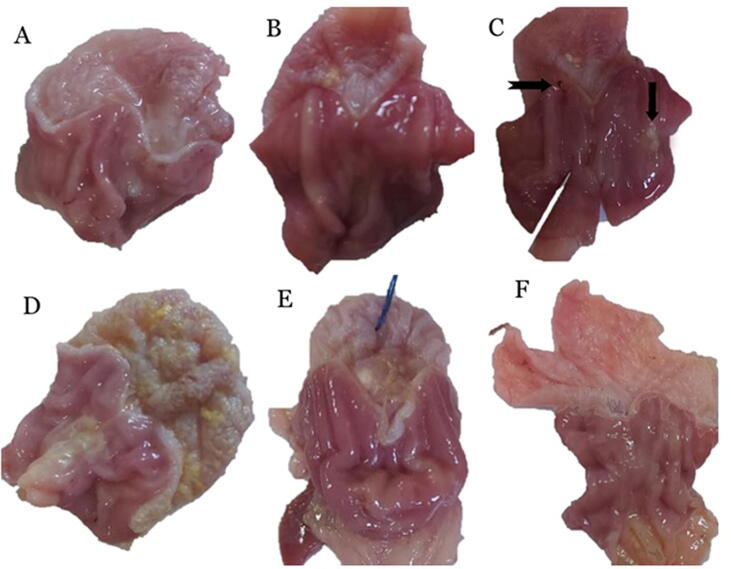
3.4.2. Gross examination
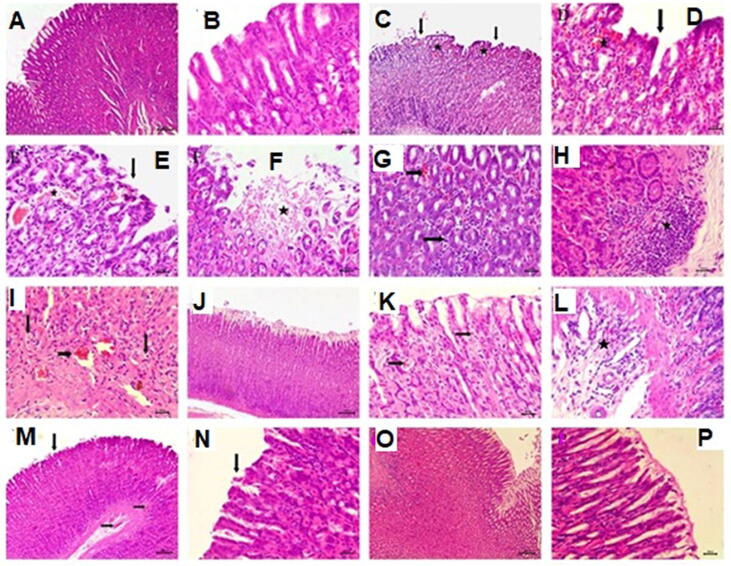
Gross examination of stomach sections of control rat which consists of the forestomach and glandular stomach presented in (Fig. 4). The mucosal surface of the forestomach showed normal whitish-brown coloration, while the glandular part was divided into the fundus with reddish mucosa and the pylorus as a relatively whitish mucosa (Fig. 4a). Hyperemia, small hemorrhagic lesion, necrotic foci, and ulcers were observed in the glandular part of the stomach of the ASA administrated group (Fig. 4b& c). In the SA-Tablets group, the wall of the forestomach was thicker than usual, while, the glandular area showed slight hyperemia (Fig. 4d). In addition, stomachs of the SAS-Tablets group showed slight hyperemic glandular areas (Fig. 4e). Moreover, SIM treated group showed no injury of the gastric mucosa (Fig. 4f).
Fig. 4.
Gross examination of gastric mucosa a) control rats showing normal gastric mucosa. b) ASA administrated group showing hyperemia. c) ASA administrated group showing small hemorrhagic lesion (notched arrow), necrotic foci (arrow), and ulcers (white arrow). d) ASA + SIM treated group showing slight hyperemia in glandular part with thickening of the forestomach wall e)ASA + SIM + SDS treated group showing mild hyperemia in glandular part with the absence of ulcers. f) Control simvastatin group showing no injury of the gastric mucosa.
The ulcer score and index of all gross lesions observed were summarized in Table 3.
Table 3.
Demonstrates the ulcer scores and ulcer index in the stomach of the different experimental groups.
| Control | ASA | SA-Tablets | SAS-Tablets | SIM | |
|---|---|---|---|---|---|
| Rat1 | 0 | 3 | 3 | 0 | 0 |
| Rat2 | 0 | 2 | 0 | 1 | 0 |
| Rat3 | 0 | 2 | 0 | 1 | 0 |
| Rat4 | 0 | 3 | 2 | 1 | 0 |
| Rat5 | 0 | 2 | 1 | 0 | 0 |
| Rat6 | 0 | 2 | 2 | 2 | 0 |
| Ulcer index | 0 | 2.3 | 1.3 | 0.8 | 0 |
3.4.3. Histopathological examination
Microscopic examination of H&E stained tissue sections from the stomach of the control rats’ revealed histological features of the normal gastric mucosa (Fig. 5a). The gastric mucosa lined with gastric epithelium exhibited normal architecture with gastric pits (Fig. 5b). Histopathological examination of stomach sections from the ASA administrated group at different parts of the glandular gastric mucosa, mostly in the fundic and pyloric regions of the stomach revealed extensive ulcerative and hemorrhagic gastric changes in all 6 rats (Fig. 5c, d, and e). The whole mucosal surface showed the presence of focal areas of deep ulcers including almost the entire thickness of the mucosa; moreover necrosis and desquamation of the lining epithelium of gastric glands (Fig. 5f). These changes accompanied by vascular changes include hemorrhagic inflammation characterized by interstitial hemorrhage infiltrated with mononuclear inflammatory cells between gastric glands (Fig. 5g). Intestinal inflammation also presented as the presence of the focal area of mononuclear inflammatory cells between gastric glands and muscular mucosae (Fig. 5h). The gastric glands lost their normal histological architecture presented by vacuolar degeneration of gastric epithelium of lower parts of gastric glands associated with mucosal hyperemia (Fig. 5i).
Fig. 5.
Histopathological examination of gastric mucosa A) control rats showing normal gastric mucosa bar = 100B) control rats showing normal gastric mucosa lined with normal gastric epithelium bar = 50C) ASA administrated group showing focal ulcerative necrosis in the gastric mucosa (arrow), interstitial mucosal hemorrhage (star) bar = 100. D &E) higher magnification showing necrosis and desquamation of the lining gastric epithelial (arrows), interstitial hemorrhage (star) bar = 50. F) ASA administrated group showing focal areas of deep ulcers with necrosis and desquamation of the lining epithelium of gastric glands (star) bar = 50. G) ASA administrated group showing mucosal hemorrhage (notched arrow) infiltrated with inflammatory cells (arrow) bar = 50. H) ASA administrated group showing the focal area of mononuclear inflammatory cells between gastric glands and muscular mucosae (star). G) ASA administrated group showing vacuolar degeneration of gastric epithelium of gastric glands (arrow), mucosal hyperemia (notched arrow) bar = 50. J) ASA-SIM treated group showing intact gastric mucosa bar = 100. K) ASA-SIM treated group showing mild hyperemia (arrow) bar = 50 L) ASA + SIM treated group showing mononuclear cellular infiltration in the submucosa (star) bar = 50. M) ASA-SIM-SLS treated group showing slight desquamation of gastric epithelium (arrow), mucosal and submucosal hyperemia (notched arrow) bar = 100. N) Higher magnification showing slight desquamation of gastric epithelium (arrow) bar = 50. O) Control SIM group showing normal gastric mucosa bar = 100. P) Higher magnification showing normal gastric mucosa bar = 50. H&E.
Microscopic examination of tissue sections of the stomach in the SA-Tablets treated group revealed that 5 examined rats out of 6 showed intact gastric epithelium with mild gastric alterations (Fig. 5j). These alterations were manifested by slight hyperemia in mucosa with mononuclear cellular infiltration in the submucosa (Fig. 5k, l). However, the SAS-Tablets treated group exhibited very mild gastric changes. These changes are characterized by slight desquamation of gastric epithelium accompanied by slight mucosal and submucosal hyperemia (Fig. 5m, n). Regarding the microscopic examination of SIM, the group showed normal gastric mucosa with normal gastric epithelium (Fig. 5o, p). The incidence of histopathological findings of the stomach in the experimental groups is summarized in (Table 4).
Table 4.
Incidence of histopathological lesions in the stomach of the experimental groups.
| Lesions | Control | ASA administrated GP | SA-Tablets treated Gp | SAS-Tablets Gp | SIM Gp | |
|---|---|---|---|---|---|---|
|
Necrosis and desquamation of epithelium | - (100%) |
++++ (100%) |
++ (50%) |
+ (25%) |
- (100%) |
| Hyperemia | - (100%) |
+++ (75%) |
+++ (75%) |
++ (75%) |
- (100%) |
|
| Hemorrhage | - (100%) |
++ (100%) |
+ (25%) |
- (100%) |
- (100%) |
|
|
B. Mucosal glandular changes: |
Degeneration of the lining epithelium |
- (100%) |
+++ (50%) |
++ (50%) |
++ (25%) |
- (100%) |
| Loss of histological architecture of the glands. |
- (100%) |
+++ (50%) |
+ (25%) |
+ (25%) |
- (100%) |
|
|
C.Submucosal changes: |
Inflammatory cellular infiltration | - (100%) |
+++ (75%) |
++ (75%) |
+ (25%) |
- (100%) |
– No lesions; + slight lesions; ++ moderate lesions; +++ severe lesions; ++++ very severe lesions. Percentages represent the no. of affected rats in each group.
3.4.4. Morphometric analysis
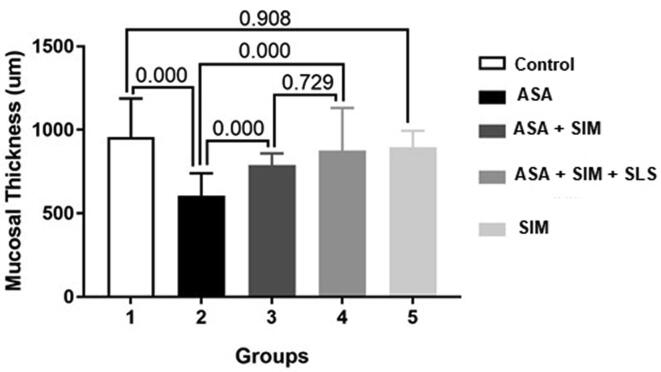
Data of the morphometrical analysis of the stomach in all experimental groups confirmed the previously observed results by H&E stained tissue sections and was shown (Fig. 6). The examined rats of the ASA administrated group showed a reduction in the thickness of gastric mucosa compared to the control group and other treated groups at several microscopic fields. In ASA administrated group, rats showed the mean mucosal length was (607.10 ± 133.80) compared to that of the control group (957.05 ± 231.20) indicating a significant decrease. The thickness of mucosa significantly increased in both SA-Tablets and SAS-Tablets treated groups when compared with ASA administrated group, and no significant differences were found between the two groups. There was no significant change in the thickness of mucosa of the stomach between the control and SIM group indicating also the protective effect of SIM alone.
Fig. 6.
The mucosal thickness in different experimental groups, values are expressed as means ± SE (n = 7). P-value indicates significance at p ≤ 0.05.
3.4.5. Immunohistochemical examination
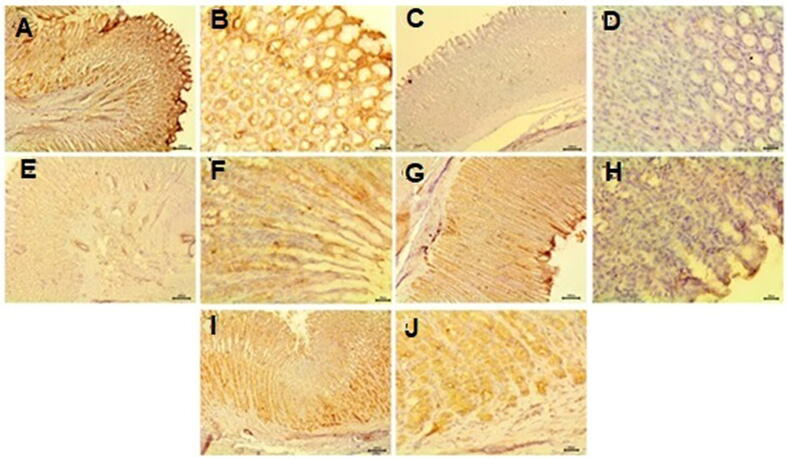
Immunohistochemical examination of immunoreactivity of COX-1 in gastric mucosa of control rats showed strong positive expressions of COX-1 with intense brown color (Fig. 7a &b). Negative expression of COX-1 reaction was observed in ASA administrated group (Fig. 7c &d). However, SIM-ASA treated group revealed mild positive expression of COX-1 reaction (Fig. 7e & f). Moderate positive expression of COX-1 reaction detected in SAS-Tablets treated group (Fig. 7g &h). In addition, SIM alone treated rats exhibited moderate strong positive expressions of COX-1 (Fig. 7i &j).
Fig. 7.
Immunohistochemical examination of immunoreactivity of COX-1 gastric mucosa A& B) control rats showing strong positive expressions of COX-1. C&D) ASA administrated group showing negative expression of COX-1 reaction. E&F) SIM-ASA treated group showing mild positive expression of COX-1 reaction. G&H) SIM-ASASLS treated group showing moderate positive expression of COX-1 reaction. J) Simvastatin treated rats showing moderate positive expressions of COX-1. (A, C, E, G&I bar = 100) (B, D, F, H&J are higher magnification bar = 20).
4. Discussion
4.1. Tablets evaluation
The tablets were evaluated for hardness, weight variation, friability, and content uniformity. The evaluated tablets met the Pharmacopeia requirement with an AV of less than 15. The tablet also showed good hardness and friability.
4.2. In vitro disintegration
The time required for tablets to disintegrate into small particles was evaluated by determining the disintegration time. The disintegration time was ranked as follows; SAS-Tablets < SA-tablets < commercial SIM tablets. The presence of SLS in SAS-Tablets resulted in decreasing the disintegration time of SAS-Tablets compared to tablets not containing SLS (SA-Tablets) as well as the commercial tablet. This finding might be due to increased wettability of tablets containing SLS and enhanced the contact between tablet surface and disintegration media, which in turn allowed rapid tablet disintegration (Duna et al., 2018).
4.3. In vitro dissolution
The dissolution test was performed in three different pH; 1.2, 4.6, and 6.8. It was observed that the presence of SLS significantly enhanced the dissolution rate of both API; SIM, and ASA in the different pH. In slightly alkaline media (pH 6.8) which might be due to increased drug solubility in this pH medium (Voelker and Hammer, 2012, O'Neil, 2013).
On the other hand, the drugs exhibited pronounced enhanced dissolution rates from SAS-Tablets. SIM exhibited initial dissolution of 28.5 after min, and 84.5% at the end of the dissolution period, while ASA dissolution rate was 100% after 60 min, with an initial dissolution rate of 20.29 after 5 min. The enhanced dissolution rates of both SIM and ASA might be due to that SLS is a surfactant commonly used in pharmaceutical formulations to improve drug dissolution (De Waardet al., 2008, Shokriet al., 2008). Israr et al. (2014) found that cefuroxime dissolution result from tablet formulation containing 1% SLS in higher pH dissolution media (4.6 and 6.8) was significantly higher in comparison with SA-Tablets. Levy and Gumtowthe (1963) showed that the enhancing effect of sodium lauryl sulfate on drug dissolution rate is might not be only due to tablet microenvironmental pH modification or micellar solubilization, but rather to the improved solvent penetration into tablets and their component granules, which causes greater availability of drug surface.
By calculating the similarity factor f2, the results showed a dissimilarity between the SAS-Tablets and commercial product of SIM. This dissimilarity in the dissolution profile is due to the enhancement that occurred in the dissolution of SIM from SAS-Tablets which retained the presence of SLS.
4.4. In-vivo anti-ulcer activity
4.4.1. Expression of TNF-α and IL-6 in plasma
Aspirin-induced gastric damage accompanied with multistage pathogenic events plays a role in the development of inflammation and ulcers such as the development of oxygen species (ROS), vascular permeability, and inflammatory cells (Jainu and Devi, 2006, Kato and Takeuchi, 2002) These pathogenic events indicate that the mucosal tissue injury is accompanied by the induction of proinflammatory cytokines interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) (Slomiany and Slomiany, 2001). The rise in the production of TNF-α due to an increase in neutrophil-derived superoxide generation stimulates the production of IL-1, leading to neutrophil accumulation (Kokura et al., 2000). In the present study, ASA-SIM treated group decreased the TNF-α level. However, ASA-SIM-SLS treated group decreased IL-6 level. TNF-α and IL-6 levels showed no significant change between both the control and SIM groups, suggesting that the gastroprotective effect of SIM may be dependent on its inhibitory effect on neutrophil infiltration and the neutrophil-associated TNF- α and IL-6 response (Carvalho et al., 2016). These results were agreeing with other studies on alendronate and 5-fluorouracil-induced gastric mucosal injury in rats (Carvalhoet al., 2016, Medeiroset al., 2018). Several studies have shown that SIM inhibits cell migration at the site of inflammation, and this protective effect is associated with decreased production of pro-inflammatory cytokines (Silva et al., 2014). In addition, a combination of SLS in ASP-SIM tablets resulted in raising the environmental pH around the dissolved drugs, resulting in enhancing their dissolution accompanied with protection against gastric irritating due to increasing pH of the stomach-contacting medium.
4.4.2. Gross examination
The gross examination of stomach sections of the ASA administrated group showed hyperemia, small hemorrhagic lesion, necrotic foci, and ulcers. Similar results were obtained by (Mahmoud and Abd El-Ghaffar, 2019), who reported that the wall of the stomach was thinner with aspirin administration than control, while the glandular area showed hyperemia and hemorrhagic lesions covered with coagulated blood which were shallow and linear. The observed gross lesions in the present work were confirmed by histopathological examination. Extensive ulcerative and hemorrhagic gastric changes were observed at different parts of the glandular gastric mucosa in ASA administrated group. These lesions were characterized by focal areas of deep ulcers including necrosis and desquamation of the lining epithelium of gastric glands accompanied by hemorrhagic inflammation and the gastric glands lost their normal histological architecture. Moreover, the morphometric analysis of the mucosal thickness in the ASA administrated group showed a significant reduction in the thickness of gastric mucosa compared to the control group. These results were consistent with previous studies of the potential of aspirin on gastric mucosa in experimental animals (Sebai et al., 2014). Aspirin considers one of the “barrier-breaking” agents that cause intramucosal histamine release by mast cells, with subsequent vascular congestion, and edema (Kauffman, 1989).
4.4.3. Histopathological examination
Microscopic examination of the stomach in the SA-Tablets treated group showed intact gastric epithelium with mild gastric alterations. Though, the SLS containing Tablets treated group exhibited very mild gastric changes. In addition, the thickness of mucosa increased significantly in both groups when compared with ASA administrated group. Pretreatment with SIM and SLS protected gastric mucosa against aspirin-induced injury as mentioned previously. Several studies confirmed the protective effect of SIM on gastric mucosa (Heeba et al., 2009). Simvastatin (SIM) is a widely prescribed statin with anti-inflammatory and antioxidant properties and also, reduced the free acidity inside the gastric lumen (Scalia et al., 2001, Franzoniet al., 2003). The gastroprotective effect of tablets containing SLS appeared due to raising gastric pH by the presence of the surfactant, in addition to stimulating the secretion of mucus only causing turbidity of gastric contents which become viscous (Komarov et al., 1950).
4.4.4. Morphometric analysis
Data of the morphometrical analysis of the stomach in all experimental groups confirmed the previously observed results. The gastric mucosa thickness was significantly reduced with ASA gp due to the irritation caused by aspirin. Other gp showed an insignificant difference between each other and control gp.
4.4.5. Immunohistochemical examination
An examination of immunoreactivity of COX-1 in gastric mucosa in the present study showed a decrease of COX-1 expression in the ASA administrated group. SIM-ASA treated group caused a mild increase in COX-1 reaction. The amelioration becomes clearer in the SAS-Tablets treated group as the COX-1 reaction was moderate expression. COX-1 is normally expressed in the stomach and is responsible for the production of prostaglandins (PGs) involved in mucosal defense (Jackson et al., 2000). Aspirin causes gastric injury by inhibiting the synthesis of endogenous PGs (Takeuchi and Amagase, 2018) COX-1 is responsible for the mucosal blood flow, which normally provides an adequate supply of nutrients and oxygen for epithelial cells to secrete mucous and bicarbonate so, COX-1 inhibition causes decreases in the secretion of bicarbonate and mucous, and increases the gastric acid (Fornai et al., 2005). The method by which aspirin inhibits COX enzyme activity is through acetylating serine deposit in the active site of the COX enzyme. The covalently adapting is causing a conformational change in the COX1 enzyme, making it unable to oxidize arachidonic acid (Mabrok and Mohamed, 2019). The ameliorative effect of Simvastatin against gastric injuries due to aspirin may be due to the antioxidant effect of simvastatin which causes the increase of PGs causing maintenance of gastric mucosa and increase of mucin concentrations (Heeba et al., 2009).
5. Conclusion
The present study showed the role of SLS in enhancing the dissolution rates of SIM and ASA in directly compressed tablets containing the two APIs. In addition, SLS reduced the rats’ gastric irritation effects of the two drugs even though their dissolution rates in acidic pH medium have been enhanced. The study also revealed that concomitant administration of SIM and ASA cold be achieved with maximized drug dissolution and minimized gastric irritation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through Research Group number RG-1441-391
Footnotes
Peer review under responsibility of King Saud University.
References
- Alizadeh M.N., Shayanfar A., Jouyban A. Solubilization of drugs using sodium lauryl sulfate: Experimental data and modeling. J. Mol. Liq. 2018;268:410–414. [Google Scholar]
- Alshora, D., Ibrahim, M.A., Alanazi, F.K., 2016. Nanotechnology from particle size reduction to enhancing aqueous solubility. In: AM Grumezescu, editor. Surface chemistry of nanobiomaterials. Application of nanobiomaterials. Volume 3. New York: Elsevier, p. 163–191.
- Arnett, D.K., Blumenthal, R.S., Albert, M.A., Buroker, A.B., Goldberger, Z.D., Hahn, E.J., Himmelfarb, C.D., Khera, A., Lloyd-Jones, D., McEvoy, J.W., Michos, E.D., Miedema, M.D., Muñoz, D., Smith, S.C., Virani, S.S., Williams, K.A., Yeboah, J., Ziaeian, B., 2019. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 742, e177–e232
- Badawy S.I.F., Hussain M.A. Microenvironmental pH Modulation in Solid Dosage Forms. J. Pharm. Sci. 2007;96:948–959. doi: 10.1002/jps.20932. [DOI] [PubMed] [Google Scholar]
- Bancroft, J. D., Stevens, A.I., 1982. Theory and Practice of histological techniques. 2nd. Churchill Livingston, 338-439.
- Carvalho N.S., Silva M.M., Silva R.O., Nicolau L.A., Araújo T.S., Costa D.S., Medeiros J.V.R. Protective effects of simvastatin against alendronate-induced gastric mucosal injury in rats. Dig. Dis. Sci. 2016;61:400–409. doi: 10.1007/s10620-015-3890-7. [DOI] [PubMed] [Google Scholar]
- Clara M.V., Mendoza D.C., Castaño H.N. Effects of D-002 on aspirin-induced ulcers and neutrophil infiltration on the gastric mucosa. Revista Cubana de Farmacia. 2012;46(2012):249–258. [Google Scholar]
- Chaudhari, F.M., Puttewar, T.Y., Patil, R.Y. 2015. Solubility Enhancement of Aspirin by Solid Dispersion Method. Human. 5, 208–218.
- De Waard H., Hinrichs W., Visser M., Bologna C., Frijlink H. Unexpected differences in dissolution behavior of tablets prepared from solid dispersions with a surfactant physically mixed or incorporated. Int. J. Pharm. 2008;349:66–73. doi: 10.1016/j.ijpharm.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Desai D., Wong B., Huang Y., Ye Q., Tang D., Guo H., Huang M., Timmins P. Surfactant-Mediated Dissolution of Metformin Hydrochloride Tablets: Wetting Effects Versus Ion Pairs Diffusivity. J. Pharm. Sci. 2014;103:920–926. doi: 10.1002/jps.23852. [DOI] [PubMed] [Google Scholar]
- Duna J., Osei-Yeboahb F., Boulasb P., Linb Y., Sun C.C. A systematic evaluation of dual functionality of sodium lauryl sulfate as a tablet lubricant and wetting enhancer. Int. J. Pharm. 2018;552:139–147. doi: 10.1016/j.ijpharm.2018.09.056. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency, Background review for sodium laurilsulfate used as an excipient. 2015.
- Fornai M., Natale G., Colucci R., Tuccori M., Carazzina G., Antonioli L., Del Tacca M. Mechanisms of protection by pantoprazole against NSAID-induced gastric mucosal damage. Naunyn Schmiedebergs Arch. Pharmacol. 2005;372:79–87. doi: 10.1007/s00210-005-1075-1. [DOI] [PubMed] [Google Scholar]
- Franzoni F., Quinones-Galvan A., Regoli F., Ferrannini E., Galetta F. A comparative study of the in vitro antioxidant activity of statins. Int. J. Cardiol. 2003;90:317–321. doi: 10.1016/s0167-5273(02)00577-6. [DOI] [PubMed] [Google Scholar]
- Gouardhane A.P., Kadam N.V., Dutta S. Review on enhancement of solubilization process. Am. J. Drug Discov. Dev. 2014;4:134–152. [Google Scholar]
- Heeba G.H., Hassan M.K., Amin R.S. Gastroprotective effect of simvastatin against indomethacin-induced gastric ulcer in rats: role of nitric oxide and prostaglandins. Eur. J. Pharmacol. 2009;607:188–193. doi: 10.1016/j.ejphar.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Hennekens, C.H., Sacks, F.M., Tonkin, A., Jukema, J.W., Byington, R.P., Pitt, B., Berry, D.A., Berry, S.M., Ford, N.F., Walker, A.J., Natarajan, K., Sheng-Lin, C., Fiedorek, F.T., Belder, R., 2004. Additive benefits of pravastatin and aspirin to decrease risks of cardiovascular disease: randomized and observational comparisons of secondary prevention trials and their meta-analyses. Arch Intern Med. 164, 40–44. [DOI] [PubMed]
- Israr F., Mahmood Z.A., Hassan F., Hasan S.M.F., Jabeen S. Formulation design and evaluation of Cefuroxime axetil 125 mg immediate release tablets using different concentration of sodium lauryl sulphate as solubility enhancer. Braz. J. Pharm. Sci. 2014;50:943–953. [Google Scholar]
- Jackson L.M., Wu K.C., Mahida Y.R., Jenkins D., Hawkey C.J. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut. 2000;47:762–770. doi: 10.1136/gut.47.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainu M., Devi C.S.S. Gastroprotective action of Cissus quadrangularis extract against NSAID induced gastric ulcer: role of proinflammatory cytokines and oxidative damage. Chem. Biol. Interact. 2006;161:262–270. doi: 10.1016/j.cbi.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Jiang, T., Han, N., Zhao, B., Xie, Y., Wang, S. 2012. Enhanced dissolution rate and oral bioavailability of simvastatin nanocrystal prepared by sonoprecipitation. Drug Dev. Ind. Pharm. 38, 1230–1239. [DOI] [PubMed]
- Kato S., Takeuchi K. Alteration of gastric ulcerogenic and healing responses in rats with adjuvant-induced arthritis. Jpn J. Pharmacol. 2002;89:1–6. doi: 10.1254/jjp.89.1. [DOI] [PubMed] [Google Scholar]
- Kauffman G. Aspirin-induced gastric mucosal injury: lessons learned from animal models. Gastroenterology. 1989;96:606–614. doi: 10.1016/s0016-5085(89)80056-3. [DOI] [PubMed] [Google Scholar]
- Khan K.A. The concept of dissolution efficiency. J. Pharm. Pharmacol. 1975;27:48–49. doi: 10.1111/j.2042-7158.1975.tb09378.x. [DOI] [PubMed] [Google Scholar]
- Kokura S., Wolf R.E., Yoshikawa T., Granger D.N., Aw T.Y. T-lymphocyte-derived tumor necrosis factor exacerbates anoxia-reoxygenation-induced neutrophil-endothelial cell adhesion. Circ. Res. 2000;86:205–213. doi: 10.1161/01.res.86.2.205. [DOI] [PubMed] [Google Scholar]
- Komarov S.A., Shay H., Siplet H., Gruenstein M. A study of the effects and mechanism of action of sodium dodecyl sulphate on gastric secretion in rats. Br. J. Pharmacol. Chemother. 1950;5:1–8. doi: 10.1111/j.1476-5381.1950.tb00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.-J., Lee S.-W., Choi H.-G., Kim C.-K. Bioavailability of cyclosporin A dispersed in sodium lauryl sulfate–dextrin based solid microspheres. Int. J. Pharm. 2001;218:125–131. doi: 10.1016/s0378-5173(01)00621-4. [DOI] [PubMed] [Google Scholar]
- Levy L., Gumtow R.H. Effect of certain tablet formulation factors on dissolution rate of the active ingredient III. Tablet Lubricants. J Pharm Sci. 1963;52:1139–1144. doi: 10.1002/jps.2600521209. [DOI] [PubMed] [Google Scholar]
- Mabrok H.B., Mohamed M.S. Induction of COX-1, suppression of COX-2 and pro-inflammatory cytokines gene expression by moringa leaves and its aqueous extract in aspirin-induced gastric ulcer rats. Mol. Biol. Rep. 2019;46:4213–4224. doi: 10.1007/s11033-019-04874-9. [DOI] [PubMed] [Google Scholar]
- MacAllister C.G., Andrews F.M., Deegan E., Ruoff W., Olovson S.G. A scoring system for gastric ulcers in the horse. Equine Vet. J. 1997;29:430–433. doi: 10.1111/j.2042-3306.1997.tb03154.x. [DOI] [PubMed] [Google Scholar]
- Mahmoud Y.I., Abd El-Ghffar E.A. Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed. Pharmacother. 2019;109:314–321. doi: 10.1016/j.biopha.2018.10.118. [DOI] [PubMed] [Google Scholar]
- Martin A. Physical pharmacy. Lea & Febiger; Philadelphia: 1993. Diffusion and Dissolution; pp. 324–361. [Google Scholar]
- Medeiros A.D.C., Azevedo Í.M., Lima M.L., Araújo I., Moreira M.D. Effects of simvastatin on 5-fluorouracil-induced gastrointestinal mucositis in rats. Rev Col Bras Cir. 2018;45:1–8. doi: 10.1590/0100-6991e-20181968. [DOI] [PubMed] [Google Scholar]
- Margulis-Goshen, K., Magdassi, S. 2009. Formation of simvastatin nanoparticles from microemulsion. Nanomed: Nanotechnol. Biol. Med. 5, 274–281. [DOI] [PubMed]
- Moore J., Flanner H. Mathematical comparison of dissolution profiles. Pharm Technol. 1996;20:64–74. [Google Scholar]
- . Nokhodchi, A., Norouzi-Sani, S., Siahi-Shadbad, M.-R., Lotfipoor, F., Saeedi, M., 2002. The effect of various surfactants on the release rate of propranolol hydrochloride from hydroxypropylmethylcellulose (HPMC)-Eudragit matrices. Eur. J. Pharm. Biopharm. 349–356. [Google Scholar] [CrossRef] [DOI] [PubMed]
- O'Neil M.J. Royal Society of Chemistry; Cambridge, UK: 2013. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals; p. 1587. [Google Scholar]
- Scalia R., Gooszen M.E., Jones S.P., Hoffmeyer M., Rimmer D.M., 3rd, Trocha S.D., Huang P.L., Smith M.B., Lefer A.M., Lefer D.J. Simvastatin exerts both anti-inflammatory and cardioprotective effects in apoliprotein E-deficient mice. Circulation. 2001;103:2598–2603. doi: 10.1161/01.cir.103.21.2598. [DOI] [PubMed] [Google Scholar]
- Sebai H., Jabri M.A., Souli A., Hosni K., Selmi S., Tounsi H., Sakly M. Protective effect of Artemisia campestris extract against aspirin-induced gastric lesions and oxidative stress in rat. Rsc Adv. 2014;4(91):49831–49841. [Google Scholar]
- Shay H., Komarov S.A., Siplet H., Gruenstein M. An evaluation of some antacid and antipeptic agents in the prevention of gastric ulceration in the rat. Am. J. Dig. Dis. 1947;14:99–104. doi: 10.1007/BF03001073. [DOI] [PubMed] [Google Scholar]
- Shanthala, H.K, Jayaprakash, H.V, Radhakrishna, M., Gowda, BH, Paul, K., Shankar, S.J, Ahmed, M.G., Sanjana, A. 2021. Enhancement of solubility and dissolution rate of acetylsalicylic acid via co-crystallization technique: a novel ASA-valine cocrystal. Int J App Pharm 13, 199–205.
- Shokri J., Ahmadi P., Rashidi P., Shahsavari M., Rajabi-Siahboomi A. A. Nokhodchi, Swellable elementary osmotic pump (SEOP): an effective device for delivery of Poorly- water-soluble drugs. Eur. J. Pharm. Biopharm. 2008;68:289–297. doi: 10.1016/j.ejpb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Silva, R.O., Lucett,i L.T., Wong, D.V., Aragão, K.S., Junior, E.M., Soares, P.M., Barbosa, A.L., Ribeiro, R.A., Souza, M.H., Medeiros, J.V., 2014. Alendronate induces gastric damage by reducing nitric oxide synthase expression and NO/ cGMP/KATP signaling pathway. Nitric Oxide. 40, 22–30 [DOI] [PubMed]
- Slomiany B.L., Slomiany A. Role of ERK and p38 mitogen activated protein kinase cascades in gastric mucosal inflammatory responses to H. pylori lipopolysaccharide. IUBMBLife. 2001;51:315–320. doi: 10.1080/152165401317190833. [DOI] [PubMed] [Google Scholar]
- Suvarna, K.S., Layton, C., Bancroft, J.D., 2018. (Eds.). Bancroft's theory and practice of histological techniques E-Book. Elsevier Health Sciences.
- Swarnakar S., Ganguly K., Kundu P., Banerjee A., Maity P., Sharma A.V. Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. J. Biol. Chem. 2005;280:9409–9415. doi: 10.1074/jbc.M413398200. [DOI] [PubMed] [Google Scholar]
- Schachter, M. 2005. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam. Clin. Pharmacol. 19, 117–125. [DOI] [PubMed]
- Takeuchi K., Amagase K. Roles of cyclooxygenase, prostaglandin E2 and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract. Curr. Pharm. Des. 2018;24:2002–2011. doi: 10.2174/1381612824666180629111227. [DOI] [PubMed] [Google Scholar]
- Taupitz T., Klein S. Can biorelevant media be simplified by using SLS and tween 80 to replace bile compounds? Open Drug Deliv. J. TODDJ. 2013;4:30–37. [Google Scholar]
- United States Pharmacopeia 2011: USP 34; the National Formulary: Nf 29. Rockville, MD: United States Pharmacopeial Convention, 2010.
- Voelker M., Hammer M. Dissolution and pharmacokinetics of a novel micronized aspirin Formulation. Inflammopharmacol. 2012;20:225–231. doi: 10.1007/s10787-011-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienbergen H., Senges J., Gitt A.K. Should We Prescribe Statin and Aspirin for Every Diabetic Patient? Diabetes Care. 2008;31:S222–S225. doi: 10.2337/dc08-s253. [DOI] [PubMed] [Google Scholar]
- https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Access 22/8/2021.
- Zayed, G., 2014. Dissolution rate enhancement of ketoprofen by surface solid dispersion with colloidal silicon dioxide. UJPB. 2, 33-38