Abstract
Introduction
The pentylenetetrazol (PTZ)-induced kindling model acts through the antagonism of central GABAA receptors and is one of the most widely used experimental animal models to study the characteristics of seizure development, behavioral manifestations and evaluation of antiseizure effects of existing and new drug candidates.
Methodology
In the current study, we investigated the impact of chronically administered levetiracetam (50 mg/kg) and sodium selenite (Sod.Se: 0.25 and 0.5 mg/kg) alone and in combination during the kindling process (21 days) in rats. Moreover, the behavioral changes (through the integration of a wide array of behavioral tests) and markers of oxidative stress in isolated brain homogenates were assessed in PTZ- kindled rats.
Results
The outcomes from the fully kindled rats revealed the increased seizure score and severity over time with marked behavioral deficits. However, the animals treated with the selected dose of LEV alone showed partial protection from epileptogenesis and amelioration (P < 0.05) of anxiety-like behavior (open filed, light/dark, elevated plus maze tests), cognitive impairment (y-maze, novel object recognition and water maze tests) and depression (sucrose preference test). Moreover, combining the LEV with sodium selenite resulted in a significant neuroprotective effect in comparison to monotherapy by reducing the disease progression and ameliorating behavioral outcomes. The combination of Sod.Se in a dose-dependent manner with LEV produced additive effects as maximum animals remained seizure-free compared to kindled rats (P < 0.05). The attenuation of PTZ induced oxidative stress was evident from the reduced malondialdehyde and elevated superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx) level with P < 0.05, as compared to control epileptic rats. These observed results of combination therapy might be due to the antioxidant and neuroprotective properties of Sod.Se, thus augmenting the seizure-modifying potentials of levetiracetam.
Conclusion
Overall, the current findings support the prominence of combining the Sod.Se with LEV, over monotherapy to deal with prevailing challenges of drug resistance and neuropsychiatric sufferings common in epileptic patients.
Keywords: Epilepsy, PTZ-kindling, Levetiracetam, Sodium selenite, Morris water maze
1. Introduction
Epilepsy is one of the most common complications of the nervous system characterized by unprovoked abnormal brain activity resulting in recurrent seizures. Depending on underlying brain dysfunctions, this neurological disorder comprises numerous etiologies including abrupt and excessive neuronal discharges that result in epileptogenesis (Stafstrom and Carmant, 2015). It affects all age groups but the occurrence is relatively higher in young and old populations (Beghi, 2020). According to WHO, around 50 million of the world’s populace is suffering from epilepsy of which 80% are residing in low-middle socioeconomic countries (Espinosa-Jovel et al., 2018). Epileptic patients also suffer from the burden of comorbidities like Alzheimer's, anxiety, depression, and dementia (Keezer et al., 2016) and literature reports that most epileptic patients suffer from at least one comorbid condition (Seidenberg et al., 2009). The prevalence of anxiety, dementia and depressive disorders is claimed to be eight times higher in epileptic patients as compared to the general population (Keezer et al., 2016).
The treatment of epilepsy has been a huge challenge for neurologists due to the high refractoriness of current therapy (Javaid et al., 2021, Javaid et al., 2021). The mainstay of therapy targets not only to protect the patient from seizure episodes but to improve the patient’s overall well-being by maintaining the psychosocial activities and evading the toxicities imposed by long-term drug use (Goldenberg, 2010). Unlike traditional antiepileptic drugs (AEDs) like phenytoin, valproate, and carbamazepine, the newer AEDs (levetiracetam, tiagabine, topiramate, lacosamide, and lamotrigine) have better safety profiles even with prolonged administration (Hanaya and Arita, 2016). Levetiracetam (LEV) is one of the newer AEDs whose mechanism of action is not completely understood. LEV does not mediate the GABAergic transmission and voltage-gated ion channels (Deshpande and DeLorenzo, 2014). Instead, it has an affinity for synaptic vesicular glycoprotein 2A (SV2A) thus modulating the discharge of neurotransmitters (Tan et al., 2017). This drug has been recently approved as adjunctive therapy to treat the primary generalized tonic-clonic seizures and myoclonic seizures with juvenile myoclonic epilepsy. LEV also appeared to be safer and more effective than phenobarbital in treating newborns with epilepsy (Grinspan et al., 2018). With its new mode of action, linear pharmacokinetics, little extrahepatic metabolism, and minimal interaction with other medicines, LEV offers an advantage over existing AEDs (Abou-Khalil, 2008).
Sodium selenite (Sod.Se) acts as a source of selenium, an inorganic element present in biological systems and serves as a precursor for antioxidant proteins (Kędzierska et al., 2018). The literature reports that selenium prevented the pentylenetetrazol (PTZ)-induced seizures, presumably through a prostaglandin E1 receptor-related mechanism (Rehni and Singh, 2013). In another study by Tawfik et al., the sildenafil in combination with selenium modulated the angiogenesis and combated the oxidative stress in PTZ-kindled mice (Tawfik et al., 2018). To assess the neuroprotective potential, Samad et al. reported the improved behavioral and biochemical outcomes by selenium supplementation in arsenic intoxicated rats (Samad et al., 2021).
The rodent kindling model is one of the most employed models to study the seizure-ameliorating potential of new therapies. Kindling with pentylenetetrazol (PTZ) involves the administration of sub-convulsive doses of PTZ that induces repetitive seizures in animals eventually leading to epileptogenesis (Bertram, 2007). Through working as an antagonist at the GABAA receptor complex, PTZ inhibits the GABAergic transmission resulting in hyperexcitability of neurons (Squires et al., 1984). Additionally, PTZ also induces behavior alterations in rodents indicating that this model also can mimic neuropsychiatric behavior (Kaur et al., 2016).
It is momentously important to find and authenticate the multi-targeting novel combinations effective in dealing with monotherapy-resistant epilepsy so that the early prevention of development and progression of epilepsy becomes possible. The evidence implicating the behavioral and biochemical manifestations in the PTZ-kindling model is also inadequate. In the light of these aforementioned facets, a chronic animal model of PTZ-induced kindling was employed to investigate the impact of LEV and Sod.Se as alone and in combination for possible seizure-modifying potential with subsequent assessment for behavioral and biochemical dynamics in the kindled rats.
2. Materials and methods
2.1. Drugs and chemicals
Levetiracetam and sodium selenite was obtained from the Life Pharmaceuticals Multan and PTZ from Sigma Aldrich. Levetiracetam (50 mg/kg) (Mazhar et al., 2017), sodium selenite (0.25, 0.5) (Kedzierska et al., 2018) and diazepam (2 mg/kg) (Muke et al., 2018) were dissolved in distilled water and injected via the intraperitoneal route (i.p.). Pentylenetetrazol (30 mg/kg) was dissolved in 0.9 percent saline and injected via the i.p. route (Giorgi et al., 1996). The dose of each drug used in the study was selected from the previously published literature.
2.2. Animals
Male adult Sprague Dawley rats weighing 150–250 g were purchased from the National Institute of Health Islamabad and accommodated in polycarbonate cages in the animal house of Faculty of Pharmacy, B.Z University Multan, Pakistan. All animals were maintained in a controlled and hygienic environment regulated at 25 °C and 12-h dark/light cycle with the availability of standard rodent food and water ad libitum. A total of 66 rats were used in this study and 4 rats were kept per cage. All animal studies were conducted after getting approval from the Department of Pharmacology Ethical Committee (02-PHL-S21, Dated 08-February 2021) BZU, Multan.
2.3. Animal grouping and treatments
A total of 66 rats were randomly divided into 8 experimental groups (n = 8 for all groups except PTZ control which comprise n = 10) named as control (received a single daily dose of 0.9% normal saline), PTZ control group (treated with PTZ 30 mg/kg on alternate days for 11 injections), LEV50 (treated with levetiracetam 50 mg/kg daily + PTZ), Diazepam as a positive control group (treated with diazepam 2 mg/kg daily + PTZ), Sod.Se 0.25 (treated with a once-daily dose of sodium selenite 0.25 mg/kg + PTZ), Sod.Se 0.5 (treated with a once-daily dose of sodium selenite 0.5 mg/kg + PTZ), LEV + Sod.Se 0.25 (treated with a freshly prepared combination of LEV 50 and sodium selenite 0.25 mg/kg daily), LEV + Sod.Se 0.5 + PTZ (treated with a combination of LEV 50 and sodium selenite 0.5 mg/kg daily).
2.4. PTZ-kindling protocol
Rats in the control group were administered PTZ in a sub-convulsive dose of 30 mg/kg via i.p. route on every alternate day for three weeks (21 days). Animals were observed for 30 min after each PTZ injection to evaluate seizure intensity. Seizure intensity was scored according to the stages as follows by using the modified Racine scale (de Souza et al., 2019).
Stage 1. No response
Stage 2. Hyperactivity
Stage 3. Repeated vertical movements or myoclonus jerking
Stage 4. Rearing, forelimb clonus
Stage 5. Tonic-clonic generalized seizures, wild running and jumping
Stage 6. Death
Animals were considered fully kindled when presented with three consecutive stage 4–5 seizures on exposure to PTZ (Nieoczym et al., 2021).
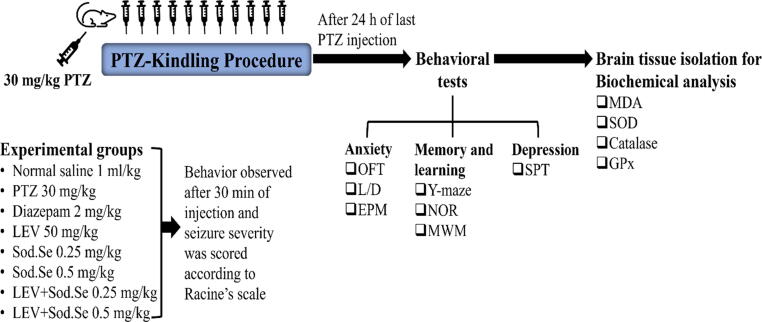
During the kindling phase, to the animals of all experimental groups, PTZ was administered on every alternate day for a total of 11 injections with once-daily dosing with their above-mentioned designated treatments. The animals of treatment groups were administered with PTZ after an hour of pre-treating with diazepam, LEV and Sod.Se. After 24 h of the last PTZ injection, animals were subjected to a series of behavioral experiments for assessment of anxiety, cognition and depression-like behavior as summarized in Fig. 1. To prevent any alteration in animal behavior, treatment was given to each group at least 12 h prior to behavioral testing. For each experimental group, the order of behavior test was designed from least to the most stressful. All experiments were carried out from 8:00 am to 6:00 pm and the cages were placed in a behavior room at least 1 h prior to the start of the experiment for acclimatization. After the completion of behavioral analysis, randomly chosen rats (n = 3) were decapitated and the whole brain was gently isolated to perform neurochemical analysis.
Fig. 1.
Experimental layout for PTZ-induced kindling and behavioral assessment for anxiety, memory and depression by using open field (OFT), light and dark (L/D), elevated plus maze (EPM), Y-maze, novel object recognition (NOR), Morris water maze (MWM) and sucrose preference (SPT) tests. Immediately after completion of behavioral experimentation, the dissected brains were biochemically evaluated for malondialdehyde (MDA), superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx).
2.5. Behavioral assessment
2.5.1. Open field test (OFT)
The OFT evaluates the effect of a drug on anxiety-like behavior, locomotor activity, and general explorative habits in rodents. For this purpose, an open field maze of dimension 80 × 80 × 45 cm was employed. On day 22nd of the study immediately after the kindling process, the animals were placed individually in the center of the maze and monitored for 5 min. Each rat was tested only once and between two rats, the maze was cleaned with 70% isopropyl alcohol to remove any scent clues from the previous animal (Malik et al.,2020, Parlar et al., 2020) The experimental videos were recorded and analyzed using a 1-month trial version of any-maze software and the parameters i.e. the number of entries and time spent in the central zone, as well as total distance traveled with the number of line crossings, were analyzed to evaluate the animal’s anxiety and locomotive activity, respectively.
2.5.2. Light and dark (L/D) box test
Rodents are tested for any unconditioned anxiety-like behavior as they have a natural drive to explore the novel environment with an innate aversion to brightly illuminated open areas (Haider et al., 2021). The test was performed in two-dimensional L/D apparatus (40 × 40 × 20 cm) with two connected compartments, one was brightly illuminated (aversive area/light zone), while the other was completely darkened (safe area/dark zone), with a small opening (10 × 10 cm) between them for animal crossing. On the 23rd day, each rat was placed in the light arena and allowed to roam freely for about 5 min to monitor the time spent in light and dark zones.
2.5.3. Elevated plus maze (EPM)
EPM test is used to evaluate anxiety-like behavior in rodents without prior conditioning of animals (Imran et al., 2020). The apparatus of EPM consists of a plus-shaped (+) elevated maze with two oppositely positioned open arms (50 × 10 cm), two oppositely positioned closed arms (50 × 10 × 30 cm) connected by a central platform (10 cm). The maze was elevated 60 cm above the floor. On the 24th day, each rat was placed in the central arena facing towards the open arm for 5 min. the whole experiment was recorded for each animal and the following parameters: Number of entries in open arm and closed arms and time spent in open arms and closed arms were analyzed using a 1-month trial version of any-maze software.
2.5.4. Y-maze (Spontaneous Alteration)
Y-maze spontaneous alternation behavior evaluates rodent spatial working memory. The experiment was carried out in a maze consisting of three arms (55 × 10 × 15 cm) positioned adjacently at an angle of 120°. On the 25th day, each rat was placed in the center of the maze and allowed to explore three arms freely for 5 min. Rodents preferably like to investigate previously un-visited and novel arms of the maze due to their innate curiosity to explore the new environment by remembering the arms previously visited (Alqahtani et al., 2020). Entries in each arm were observed and recorded for 5 min and percentage spontaneous alteration was calculated according to the formula
% Spontaneous alteration (SAP) = [(no. of alterations)/Total arm entries-2)] × 100
2.5.5. Novel object recognition (NOR) test
The NOR test is used to evaluate various aspects of memory and learning in rodents especially recognition memory. Due to their innate tendency, rodents will take up more time in exploring novel objects as compared to previously known/familiar objects. This test is used for assessing rodents' memory by examining their ability to differentiate between novel and familiar objects (Imran et al., 2020). The test was performed in an open box (80 × 80 × 40 cm). Two consecutive sessions were given on the 26th day of study. Firstly, in the acquisition phase rats were permitted to explore and familiarize themselves with two identical objects for 5 min. In the testing phase, rats were allowed to explore the same environment again for 5 min except one of the previously explored objects was replaced by a novel object. Discrimination index was calculated according to the formula: (time exploring novel object – time exploring familiar object)/ (time exploring novel object + time exploring familiar object).
2.5.6. Morris water maze (MWM) test
The MWM test is used to evaluate the long-term spatial memory in rodents. MWM test comprises three phases, training (27th-28th days), acquisition (29th-31th days), and probe trials (32nd day). The test was performed in a water-filled tank (150 × 50 cm) with any non-toxic opacifying agent such as milk divided into four quadrants (NE, SE, SW, NW) and a square platform (10 × 10 cm) was placed in one of the quadrants 2 cm below the water surface (Shakeel et al., 2020). To provide spatial reference points, proximal cues of different geometrical signs and colors were mounted on the internal side of the water tank while distal cues were mounted on wooden standees around the poles. During the training phase, for the first 2 days platform was kept visible and was placed in the southwest quadrant of a water tank. If animals could not find the platform in 2 min, then rats were slightly pushed towards the platform and allowed to stay for 10 sec for memory formation. During experimental trials in the subsequent three days, the platform was submerged 1 in. below water, and the ability of the animal to discover a hidden platform was assessed to measure the animal’s escape latency. On day 6, probe day, rats were tested in MWM without platform and were observed the number of entries and swimming duration in the targeted platform quadrant.
2.5.7. Sucrose preference test (SPT)
The sucrose preference test is used to evaluate depression-like behavior in animals and is a reward-based experiment (Liu et al., 2018). After the phase of 12 h of fasting, each rat was given free access to two bottles, one with 100 ml of simple tap water while the other containing 100 ml of 1% sucrose solution (Zhu et al., 2017). The quantities of tap water and 1% sucrose consumed were noted for 24 h. The reduced sucrose consumption indicates a disorder of reward behavior thus SPT is a reliable measure of assessing depression-like behavior in rodents. Sucrose preference percentage was calculated as follows: [(Sucrose solution intake)/ (sucrose solution intake + water intake) × 100 %.
2.6. Biochemical analysis
2.6.1. Brain tissue homogenate preparation
On the 34th day, immediately after completion of SPT, randomly selected animals (n = 3) from all experimental groups were dissected for brain isolation. These dissected brains were weighed and n homogenized separately in 1:10 w/v of 0.1 M phosphate buffer of pH 7.4 and centrifuged at 4000 rpm for 5 min at reduced temperature. After centrifugation, the pellet was discarded and brain tissue supernatant was isolated and used for the evaluation of biochemical parameters to associate the antioxidant potential of test treatments with protection from kindling progression and associated behavioral impairment (Samad et al., 2021).
2.6.2. Malondialdehyde assay (MDA)
For MDA content determination, 3 ml of brain homogenate along with TCA (trichloroacetic acid) and TBA (thiobarbituric acid) in 1:1 proportion were boiled for 15 min, cooled and centrifuged at 3500 rpm for 10 min (Parlar and Arslan, 2019). The absorbance of this mixture was noted at 532 nm by using a UV spectrophotometer and MDA content was expressed as µg MDA/g tissue.
2.6.3. Superoxide dismutase assay (SOD)
For the preparation of the reaction mixture, 0.5 ml of brain homogenate was mixed with 1 ml of 50 mM solution of sodium carbonate, 0.2 ml of 0.1 mM EDTA and 0.4 ml of 24 µm of NBT (nitro blue tetrazolium). To start a reaction that causes the conversion of NBT into insoluble formazan, 0.4 ml of 1 mM solution of HAC (hydroxylamine hydrochloride) was added and absorbance was noted at 570 nm (Naskar et al., 2009).
2.6.4. Catalase evaluation
0.1 ml brain tissue homogenate was mixed with 1 ml of 0.01 M phosphate buffer (pH 7.4) and 0.4 ml of 2 M hydrogen peroxide. The mixture was then incubated for 90 min at 37 °C followed by the addition of 2 ml of 5% potassium dichromate. After further incubation for 15 min, the absorbance was observed at 570 nm (Pari and Latha, 2004).
2.6.5. Glutathione peroxidase assay (GPx)
0.1 ml hydrogen peroxide (1 mM), 0.3 ml of 0.4 M phosphate buffer (pH 7.0), 0.2 ml reduced glutathione (2 mM) and 0.1 ml of 10 mM sodium azide was added to the 0.2 ml of brain supernatant. This mixture was incubated for 15 min at 37 °C followed by the addition of 0.5 ml of 10 % TCA. After centrifugation at 1500 rpm for 5 min, 0.1 ml of this mixture was combined with 0.2 ml of 0.3 mM Phosphate buffer and 0.7 ml of Ellman’s reagent (DTNB) and absorbance was noted at 420 nm (Flohé and Günzler, 1984).
2.7. Statistical analysis
Graph pad prism version 8.0 was used for statistical analysis of data. For the evaluation of behavioral and neurochemical data, one-way ANOVA followed by post-hoc Dunnett test was used except for the average seizure score and escape latencies noted in the water maze where two-way ANOVA followed by Tukey’s test was employed. All data were expressed as mean ± S.E.M. Results were considered statistically significant if P < 0.05.
3. Results
3.1. Impact of LEV and Sod.Se alone and in combination on PTZ-kindling progression in rats
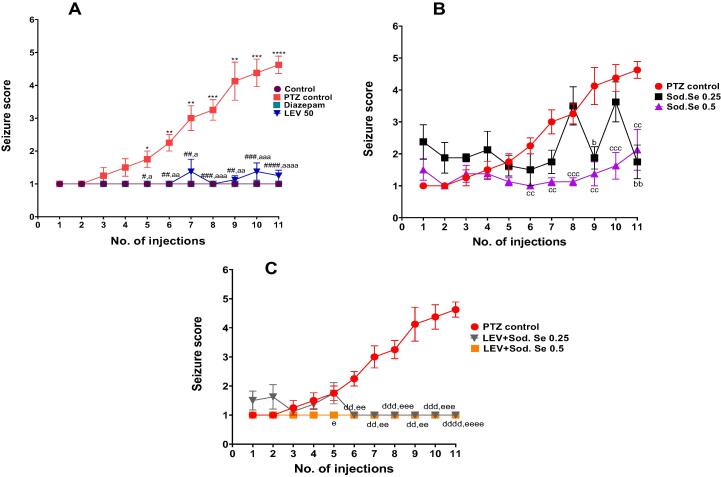
The continuous administration of sub convulsive doses of PTZ resulted in increased frequency and intensity of convulsions, assessed by using the Racine scale. The repetitive administration of PTZ 30 mg/kg i.p. every other day for a total of 11 injections resulted in the gradual escalation of convulsive activity culminating in stage 5 seizures as compared to normal control as shown in Fig. 2. The two-way ANOVA applied on the obtained data depicted a notable increase in average seizure score from 1st to 11th PTZ injection [F (28, 280) = 80.58, P < 0.0001] (Fig. 2A) and treatment with LEV suppressed the progression of kindling.
Fig. 2.
Impact of LEV 50 mg/kg and Sod.Se 0.25 and 0.5 mg/kg alone and in combination on progression of PTZ-induced kindling process. The animals were injected with PTZ 30 mg/kg every other day for 21 days and observed for 30 min after each PTZ injection for seizure scoring according to Racine scale and severity was expressed (A) comparison of control, diazepam and LEV groups with PTZ control, (B) comparison of Sod.Se 0.25 and 0.5 vs PTZ control, (C) comparison of LEV + Sod.Se 0.25 and 0.5 with PTZ control. Statistical analysis was performed using the two-way ANOVA followed by Dunnett’s test and all values are expressed as mean ± S.E.M (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 for control vs PTZ control rats, #P < 0.05, ##P < 0.01, ####P < 0.001 and ####P < 0.0001 for diazepam vs PTZ control, aP < 0.05, aaP < 0.01, aaaP < 0.001 and aaaaP < 0.0001 for LEV vs PTZ control, bP < 0.05 and bbP < 0.01 for Sod.Se 0.25 vs PTZ control, ccP < 0.01 and cccP < 0.001 for Sod.Se 0.5 vs PTZ control, ddP < 0.01, dddP < 0.001 and ddddP < 0.0001 for LEV + Sod.Se 0.25 vs PTZ control, eP < 0.05, eeP < 0.01, eeeP < 0.001 and eeeeP < 0.0001 for LEV + Sod.Se 0.5 vs PTZ control.
Furthermore, the two-way ANOVA compared the antiepileptic effects of Sod.Se 0.25 and 0.5 showed the significant variation among treatments [F (21,210) = 17.62, P < 0.0001] (Fig. 2B). Sod.Se 0.5 led to a reduced seizure score as compared to PTZ control while a lower dose did not exert significant antiepileptic effects. The seizure outcomes observed with the combination of LEV + Sod.Se was markedly different from the PTZ control group [F (21, 210) = 66.11, P < 0.0001] (Fig. 2C) as this polypharmacy caused more significant attenuation of enhanced seizure severity and most of the animals remained seizure-free and these effects were comparable with the anti-convulsive effects of diazepam.
3.2. OFt
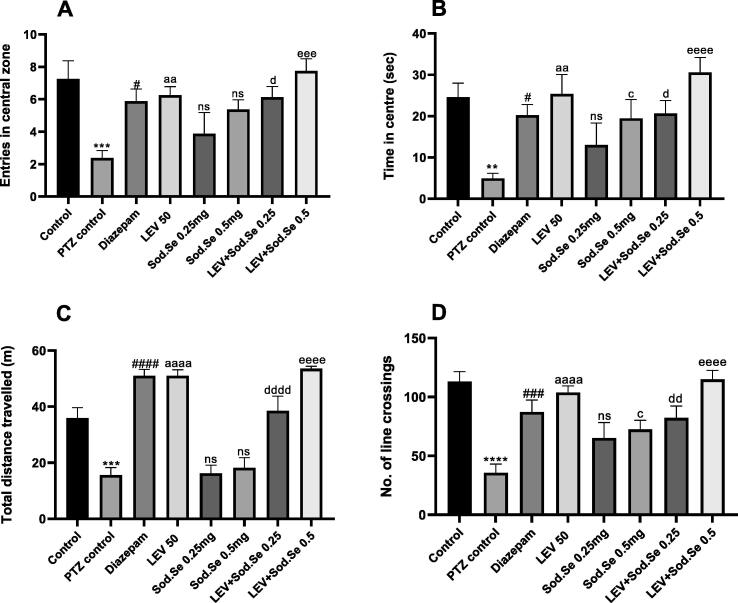
The rats were allowed to explore an open maze to evaluate their locomotor activity and anxiety-like behavior. The one-way ANOVA revealed a statistically significant difference between all groups for central zone entries [F (7, 56) = 4.523, P = 0.0005] and time spent there [F (7, 56) = 4.434, P = 0.0006] as shown in Fig. 3A and 3B. The PTZ-kindled rats showed more anxiety-like behavior. Compared to the PTZ control group, the rats exposed to the monotherapy with LEV and Sod.Se 0.5 mg/kg showed an increased preference for the central zone with P < 0.0095 and P = 0.0456, respectively. But the outcomes remained non-significant (P = 0.5037) in rats treated with Sod.Se 0.25. However, the combination of LEV with Sod.Se 0.25 and 0.5 mg/kg produced pronounced anxiolytic outcomes, dose-dependently with P = 0.0256 and P < 0.0001, respectively.
Fig. 3.
Outcomes of chronic pre-treatment with LEV and Sod.Se alone or in combination on anxiety-like behavior and locomotor activity in PTZ-kindled rats. The animals were pre-treated with LEV 50, Sod.Se 0.25 and 0.5 mg/kg and LEV + Sod.Se 0.25 and 0.5 and then assessed for anxious behavior in open field maze for 5 min by monitoring (A) entries in central zone, (B) time in centre, (C) total distance travelled and (D) number of line crossings. Statistical analysis was conducted by one-way ANOVA followed by Dunnett’s test and whole data was represented as mean ± S.E.M. (n = 8). **P < 0.01, ***P < 0.001 and ****P < 0.0001 comparison between control and PTZ control, #P < 0.05, ###P < 0.001, ####P < 0.0001 comparison between diazepam and PTZ control, aaP < 0.01 and aaaaP < 0.0001 comparison between LEV and PTZ control, cP < 0.05 comparison between Sod.Se 0.5 and PTZ control, dP < 0.05, ddP < 0.01 and ddddP < 0.0001 comparison between LEV + Sod.Se 0.25 and PTZ control, eeeP < 0.001 and eeeeP < 0.0001 comparison between LEV + Sod.Se 0.5 and PTZ control.
Additionally, the OFT was also used to assess the locomotor activity in rodents by estimating their distance traveled and the number of line crossings. The kindled rats (P = 0.0002) traveled less and remained motionless for most of the time, in comparison to normal control. The prohibition of this motionless behavior was noted in animals treated with monotherapy (PTZ vs LEV, P < 0.0001; PTZ vs Sod.Se 0.25, P = 0.9998 and PTZ vs Sod.Se 0.5, P = 0.9884) and polytherapy (PTZ vs LEV + Sod.Se 0.25, P < 0.0001; LEV + Sod.Se 0.5, P < 0.0001) as depicted in Fig. 3C and 3D.
3.3. L/D test
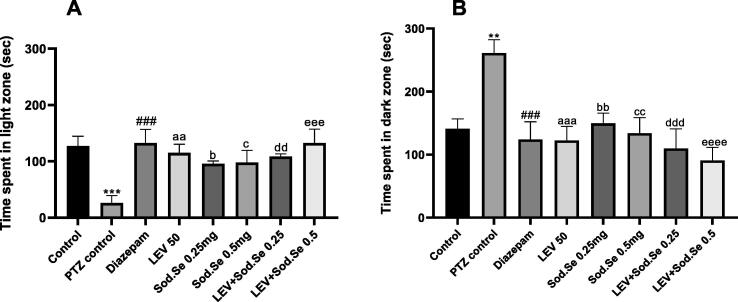
The statistical evaluation of data showed significance difference among groups for time spent in the light and dark zones with [F (7,56) = 4.008, P = 0.0013] and [F (7,56) = 5.022, P = 0.0002], respectively. The PTZ-kindled rats were found anxious and reluctant to explore the illuminated zone as their duration of stay in dark was comparatively longer (P = 0.0009) in comparison to normal control. The animals treated with test drugs were relatively more courageous as they spent more time in the light zone. In detail, the rats administered with LEV caused increased anxiolytic-like effect with P = 0. 004. The treatment with Sod.Se at both doses i.e., 0.25 and 0.5 mg/kg also caused increased fearlessness as depicted by the increased animal’s preference for the light zone with P = 0.0363 and P = 0.0292, respectively. However, this anxiety was further lessened by simultaneously treating the rats with LEV + Sod.Se (PTZ vs LEV + Sod.Se 0.25, P = 0.0085; PTZ vs LEV + Sod.Se 0.5, P = 0.0004) and outcomes were similar to diazepam (P = 0.0004) (Fig. 4A and 4B).
Fig. 4.
Assessment of anxiolytic impact of LEV and Sod.Se 0.25 and 0.5 mg/kg alone and in combination in PTZ-kindled rats. The animals were allowed to explore brightly illuminated arena and dark zone spontaneously by placing them in L/D box for 5 min and noted for (A) time spent in light zone and (B) time spent in dark zone. Statistical evaluation was performed by using one-way ANOVA followed by Dunnett’s test and data set was expressed in mean ± S.E.M (n = 8). ***P < 0.001 control vs PTZ control, ###P < 0.001 diazepam vs PTZ control, aaP < 0.01 and aaaP < 0.001 LEV vs PTZ control, bP < 0.05 and bbP < 0.01 Sod.Se 0.25 vs PTZ control, cP < 0.05 and ccP < 0.01 Sod.Se 0.5 vs PTZ control. ddP < 0.01 and dddP < 0.001 LEV + Sod.Se 0.25, eeeP < 0.001 and eeeeP < 0.0001 LEV + Sod.Se 0.5 vs PTZ control.
3.4. EPm
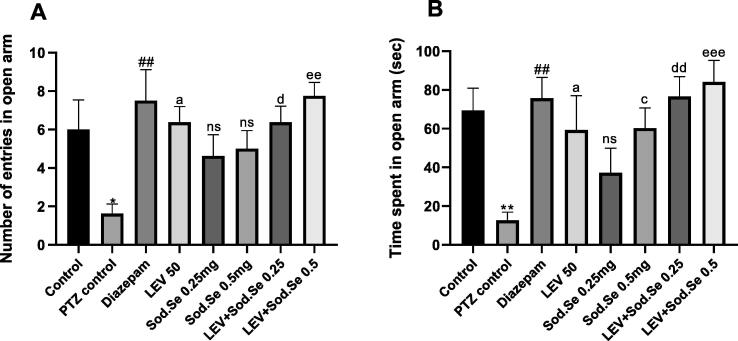
In EPM, one-way ANOVA demonstrated a significant difference between all groups for number of entries [F (7,56) = 3.302, P = 0.0052] and time spent [F (7,56) = 4.211, P = 0.0009] in open arms as shown in Fig. 5A and 5B. The post-hoc test revealed that monotherapy with LEV + Sod.Se 0.5 exerted a notable anxiogenic effect with P = 0.0340 and P = 0.0292, respectively. Though, the more pronounced anxiolytic effect was observed with combination therapy LEV + Sod.Se 0.25 and LEV + Sod.Se 0.5 as animals stayed longer in open arms with P = 0.0016 and P = 0.0004, as compared to PTZ control. Anyhow, the outcomes remained non-significant with Sod.Se 0.25 (P = 0.5106). Furthermore, treatment with LEV + Sod.Se 0.25 and Sod.Se 0.5 resulted in increased number of entries in open arms with P = 0.0164 and P = 0.0011, respectively as compared to PTZ-kindled rats.
Fig. 5.
Evaluation of anxiolytic effect of LEV and Sod.Se 0.25 and 0.5 mg/kg alone and in combination. Animals were placed in plus-shaped maze for 5 min to monitor (A) number of open arm entries and (B) time spent in open arms. The one-way ANOVA followed by Dunnett’s test was used and all data were expressed as mean ± S.E.M (n = 8). *P < 0.05, **P < 0.01 comparison between control and PTZ control, ##P < 0.01 comparison between diazepam and PTZ control, aP < 0.05 comparison between LEV and PTZ control, cP < 0.05 comparison between Sod.Se 0.5 and PTZ control, dP < 0.05 and dd < 0.01 comparison between LEV + Sod.Se 0.25 and PTZ control, eeP < 0.01 and eeeP < 0.001 comparison between LEV + Sod.Se 0.5 and PTZ control.
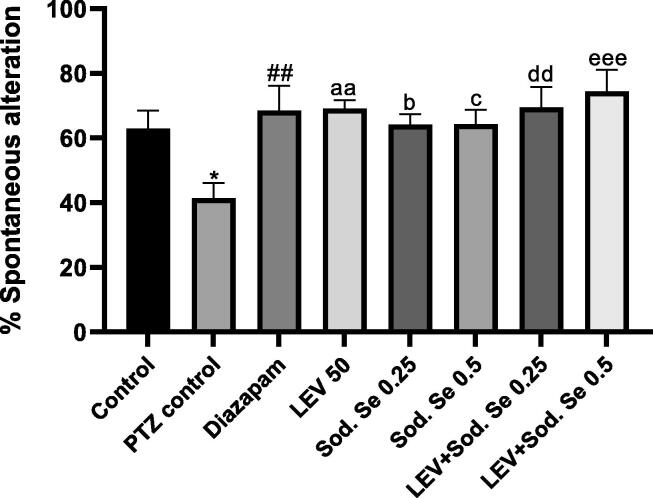
3.5. Y-maze test
The Y-maze test was used to assess the effect of test drugs as mono- and polypharmacy on animal’s short-term recognition capacity. A noticeable inter-group difference for spontaneous alternations was demonstrated by one-way ANOVA [F (7, 56) = 3.471, P = 0.0037]. The PTZ-kindled rats did not remember the immediately visited arm and tend to explore the pre-visited arms more often resulting in decreased spontaneous alternation (P = 0.0340) as compared to normal control. In comparison to PTZ control group, the animals administered with LEV, Sod.Se 0.25 and Sod.Se 0.5 mg/kg demonstrated increased spontaneous alternations with P = 0.0035, P = 0.0225 and P = 0.0219, respectively. However, the LEV + Sod.Se dual therapy caused an exceptional enhancement in spontaneous alternation behavior (PTZ vs LEV + Sod.Se 0.25, P = 0.0030 and PTZ vs LEV + Sod.Se 0.5, P = 0.0004) indicating the remarkable remembrance of previously visited arms of the Y-maze (Fig. 6).
Fig. 6.
Effect of LEV and Sod.Se 0.25 and 0.5 mg/kg alone and in combination was tested on cognition of P0TZ-kindled rats in Y-maze test. The freely moving animals for 5 min and % spontaneous alteration was calculated. The one-way ANOVA using Dunnett’s test was used and data were expressed in mean ± S.E.M. *P < 0.05 Control vs PTZ control, ##P < 0.01 diazepam vs PTZ control, aaP < 0.01 LEV vs PTZ control, bP < 0.05 Sod.Se 0.25 vs PTZ control, cP < 0.05 Sod.Se 0.5 vs PTZ control, ddP < 0.01 LEV + Sod.Se 0.25 vs PTZ control, eeeP < 0.001 LEV + Sod.Se 0.5 vs PTZ control.
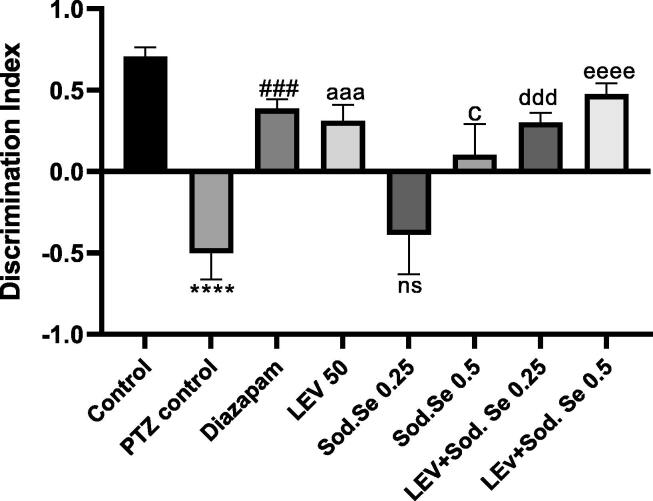
3.6. NOr
The animals were further tested for their memory by assessing their ability to discriminate the newly introduced object from previously familiarized one. The ANOVA represented marked statistical difference between all groups [F (7,56) = 9.774, P < 0.0001]. The animals treated with LEV and Sod.Se 0.5 mg/kg had better remembrance of familiarized object and they explored the novel object more than PTZ control with P = 0.0005 and P = 0.0135, respectively. However, the animals exposed to the combination of LEV + Sod.Se showed noticeably improved discrimination index with P = 0. 0006 and P < 0.0001 for PTZ vs LEV + Sod.Se 0.25 and LEV + Sod.Se 0.5, respectively (Fig. 7).
Fig. 7.
Assessment of impact of LEV and Sod.Se 0.25 and 0.5 mg/kg alone and in combination in novel object recognition in PTZ-kindled rats. The ability of rats to discriminate between familiar and novel objects was evaluated for 5 min and outcomes of discrimination index were compared by one-way ANOVA followed by Dunnett’s test and data were expressed as mean ± S.E.M. ****P < 0.0001 control vs PTZ control, ###P < 0.001 diazepam vs PTZ control, aaaP < 0.001 LEV vs PTZ control. cP < 0.05 Sod.Se 0.5 vs PTZ control. dddP < 0.001 LEV + Sod.Se 0.25 mg/kg vs PTZ control, eeeeP < 0.0001 LEV + Sod.Se vs PTZ control.
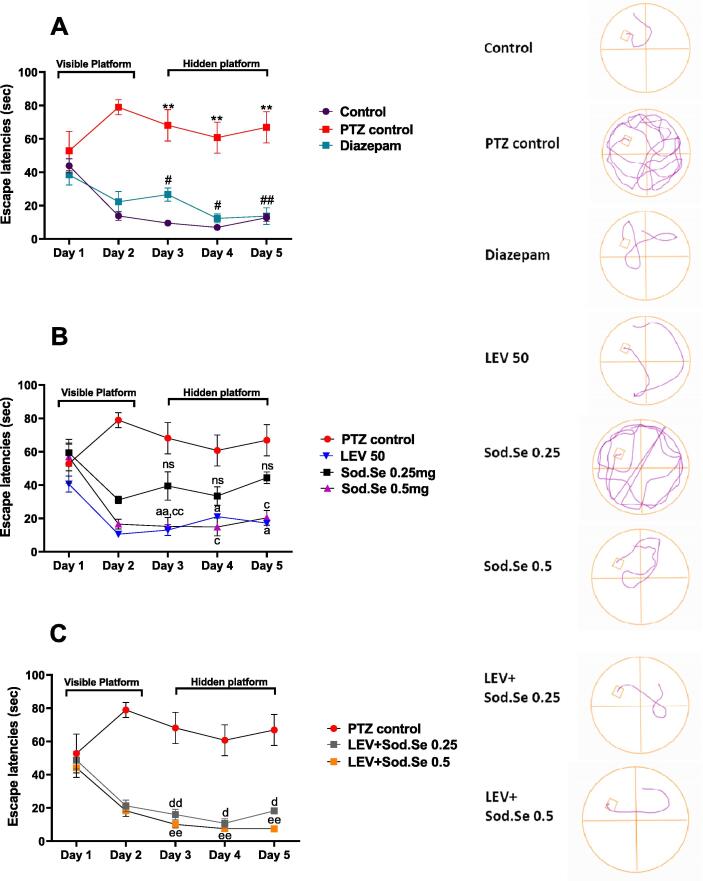
3.7. MWm
The water maze test examined the working and reference memory during six consecutive days. The two-way ANOVA depicted notable inter-group difference for escape latencies during initial five days [F (56,224) = 43.00, P < 0.0001]. After the initial two days of acquisition, the PTZ-kindled rats sustained the longer escape latencies (P = 0.0034 vs control group) during five consecutive days revealing the poor remembrance of platform location and prolonged swimming periods to locate the hidden platform as shown in Fig. 8A. The animals administered with LEV and Sod.Se 0.5 struggled comparatively less in locating the submerged rescue zone as shown from their decreased escape latencies with P = 0.0131 and P = 0.0166 as compared to kindled rats Fig. 8B. The animals chronically treated with LEV + Sod.Se showed dose-dependent cognition-enhancing capacity of combination as these animals locate the platform more quickly with P = 0.0047, as compared to PTZ-kindled rats Fig. 8C.
Fig. 8.
Impact of LEV and Sod.Se 0.25 and 0.5 mg/kg on capability PTZ-kindled animals to locate hidden platform in 2 min for 5 consecutive days in water maze test. Statistical analysis was conducted by two-way ANOVA followed by Tukey’s test and all data were expressed as mean ± S.E.M (n = 8). **P < 0.01 comparison between control and PTZ control, #P < 0.05 and ##P < 0.01 comparison between diazepam and PTZ control, aP < 0.05 and aaP < 0.01 comparison between LEV and PTZ control, cP < 0.05 and ccP < 0.01 comparison between Sod.Se 0.5 and PTZ control, dP < 0.05 and ddP < 0.01 comparison between LEV + Sod.Se 0.25 and PTZ control, eeP < 0.01 comparison between LEV + Sod.Se 0.5 and PTZ control.
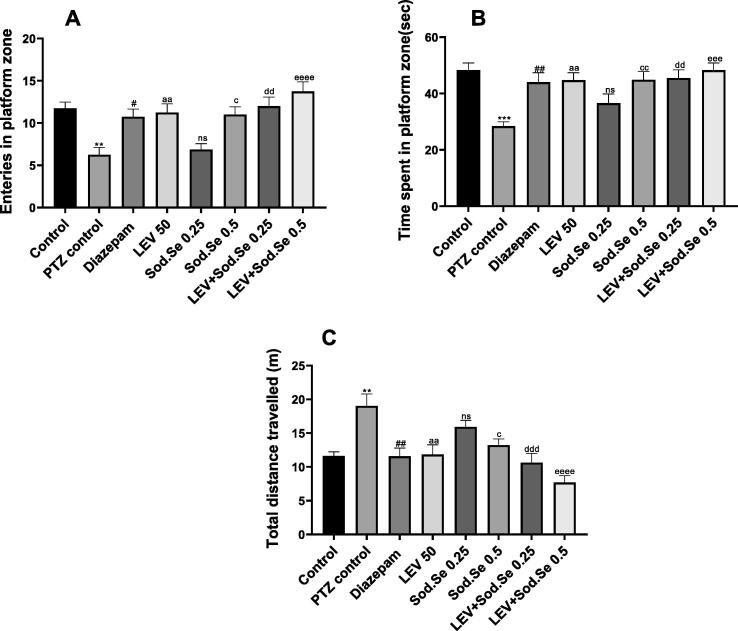
In the probe session, the animals were evaluated for their cognitive abilities by monitoring their remembrance of the platform zone. The one-way ANOVA revealed significant difference among all groups for number of entries [F (7, 56) = 7.692, P < 0.0001], time spent in target quadrant [F (7, 56) = 6.070, P < 0.0001] and total distance travelled [F (7, 56) = 8.173, P < 0.0001]. In detail, PTZ-kindled rats showed poor remembrance of rescue quadrant as their frequency of visits and duration of swim in target zone was significantly lesser than healthy animals as shown in Fig. 9A and 9B, respectively. However, these observed parameters were noticeably improved in treatment groups as LEV and Sod.Se (dose-dependently) depicted the memory-enhancement. The LEV and Sod.Se 0.5 mg/kg treated rats entered more often in platform zone with P = 0.0079 and P = 0.0139, respectively. Moreover, their duration of quadrant visit was also prolonged as compared to kindled rats with P < 0.01. But, in a dose-dependent manner, a combination of LEV + Sod.Se proved more efficient in halting memory impairment with a greater number of entries and prolonged stay in platform zone with P < 0.0001 and P = 0.0001, respectively.
Fig. 9.
Assessment of impact of LEV and Sod.Se 0.25 and 0.5 mg/kg alone and in combination on (A) entries in platform zone, (B) time spent in platform zone and (C) total distance travelled during probe session in water maze test. Statistical evaluation was conducted by using one-way ANOVA followed by Tukey’s test and each value in data was represented as mean ± S.E.M (n = 8). **P < 0.01 and ***P < 0.001 control vs PTZ control, #P < 0.05 and ##P < 0.01 diazepam vs PTZ control, aaP < 0.01 LEV vs PTZ control, cP < 0.05 Sod.Se 0.5 vs PTZ control, ddP < 0.01 and dddP < 0.001 LEV + Sod.Se 0.25 vs PTZ, eeeP < 0.001 and eeeeP < 0.0001 LEV + Sod.Se 0.5 vs PTZ control.
The kindled rats continued to spin in a whole water tank which was clear evidence of impaired memory and thigmotaxic behavior. Their total distance traveled on probe day was significantly longer (P = 0.0012) as compared to healthy control (Fig. 9C). The treatment with LEV and Sod.Se significantly protected from this behavior deficit with P = 0.0018 and P = 0.0218, respectively. Moreover, the combination of LEV + Sod.Se had prominent cognitive improvement over single treatment (PTZ vs LEV + Sod.Se 0.25, P = 0.0002 and PTZ vs LEV + Sod.Se 0.5, P < 0.0001).
3.8. SPt
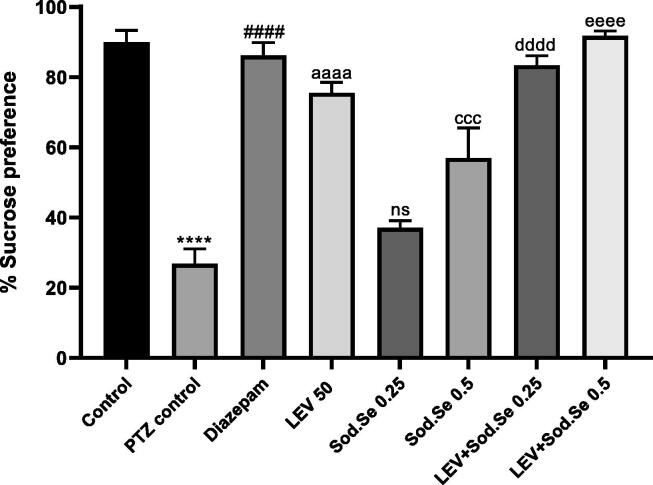
The statistical analysis demonstrated a marked difference in sucrose consumption in the PTZ control group as compared to all treatment groups [F (7,16) = 36.70, P < 0.0001]. The PTZ-kindled rats showed marked anhedonia which is the hallmark of depression-like behavior with P < 0.0001 as compared to normal control. The % sucrose consumption was higher for in animals treated with LEV and Sod.Se 0.5 mg/kg with P < 0.0001 and P = 0.0006, respectively. However, the animals chronically treated with LEV + Sod.Se 0.25 and 0.5 mg/kg remained depression-free as denoted by their increased sucrose preference (P < 0.0001) as compared to kindled rats (Fig. 10).
Fig. 10.
Effect of LEV and Sod.Se 0.25 and 0.5 mg/kg alone and in combination on depression-like behavior in PTZ-kindled rats by estimating the % preference for 1% sucrose solution within 24 h. The outcomes were evaluated by one-way ANOVA followed by Dunnett’s test and data were expressed as mean ± S.E.M (n = 8). ****P < 0.0001 control vs PTZ control, ####P < 0.0001 diazepam vs PTZ control, aaaaP < 0.0001 LEV vs PTZ control, cccP < 0.001 Sod.Se 0.5 vs PTZ control, ddddP < 0.0001 LEV + Sod.Se 0.25 vs PTZ control, eeeeP < 0.0001 LEV + Sod.Se 0.5 vs PTZ control.
3.9. Biochemical analysis
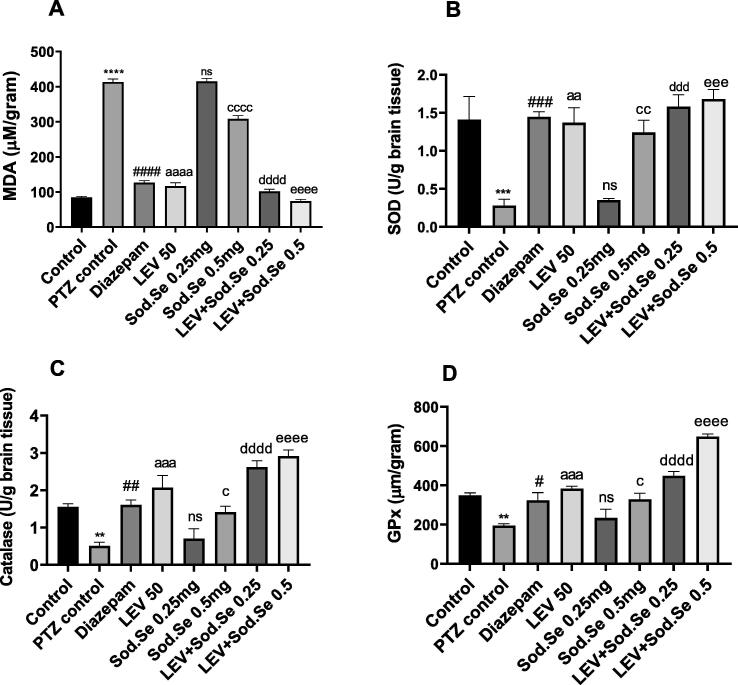
MDA is a well-known biomarker for assessing disease-induced oxidative stress (Gaweł et al., 2004). The statistical analysis revealed marked difference among all groups with [F (7,16) = 415.4, P < 0.0001]. The increased oxidative stress in the brains of PTZ-kindled rats leading to lipid peroxidation and neuronal damage was notable (P < 0.0001) as compared to normal control. The chronic administration with LEV and Sod.Se 0.5 significantly reduced MDA levels with P < 0.0001 and P < 0.0001. But, combing the LEV with Sod.Se caused the improvised reversion of oxidative build-up at both doses of Sod.Se with P < 0.0001 (Fig. 11A).
Fig. 11.
Neurochemical analysis of isolated brain depicting the impact of LEV and Sod.Se 0.25 and 0.5 as mono and polytherapy on levels of (A) malondialdehyde, (B) superoxide dismutase, (C) catalase, (D) glutathione peroxidase. The statistical analysis was performed by using one-way ANOVA followed by Dunnett’s test and data were represented as mean ± S.E.M. (n = 3). **P < 0.01, ***P < 0.001 and ****P < 0.0001 comparison between control and PTZ control, #P < 0.05 , ##P < 0.01, ###P < 0.001, and ####P < 0.0001 comparison between diazepam and PTZ control, aaP < 0.01, aaaP < 0.001, aaaaP < 0.0001 comparison between LEV and PTZ control, cP < 0.05, ccP < 0.01, ccccP < 0.0001 comparison between Sod.Se 0.5 and PTZ control, dddP < 0.001 and ddddP < 0.0001 comparison between LEV + Sod.Se 0.25 and PTZ control, eeeP < 0.01 and eeeeP < 0.0001 comparison between LEV + Sod.Se 0.5 and PTZ control.
SOD is a cellular enzyme known for its anti-oxidative, anti-inflammatory and neuroprotective functions (Aguiar et al., 2012). A highly significant difference in all groups with [F (7, 16) = 11.39, P < 0.0001] was detected by statistical evaluation. In un-treated kindled rats, SOD levels were depleted markedly as compared to normal control (P = 0.0008). Monotherapy with LEV and Sod.Se 0.5 mg/kg resulted in enhanced free radical scavenging and reduced oxidative stress in isolated brains as compared to the PTZ control group with P < 0.0012 and P < 0.0038, respectively but Sod.Se at 0.25 mg/kg didn’t produce any significant effects. The oxidative stress combating characteristics was imparted to Sod.Se (0.25 and 0.5 mg/kg) after combining it with LEV as this combination caused significant elevation of SOD (P = 0.0002) as compared to PTZ control group, thus validating the prominence of polytherapy as shown in Fig. 11B.
Similarly, the notable differences for all treatments were detected for catalase levels [F (7, 16) = 19.10, P < 0.0001]. This enzyme works as one of the endogenous antioxidant defense mechanisms (Munguía-Martínez et al., 2019) and its levels were found to be downregulated in PTZ-kindled rats as compared to the normal group with P = 0.0081. The chronic administration of LEV and Sod.Se 0.5 resulted in increased catalase levels as compared to the PTZ control group with P = 0.0055 and P = 0.0229. In comparison to PTZ-kindled rats, significant upregulation of catalase activity was observed in animals exposed to a cocktail of LEV + Sod.Se with P < 0.0001 as depicted in Fig. 11C.
GPx is an endogenous antioxidant enzyme involved in the termination reactions of reactive oxygen species. The ANOVA revealed significant inter-group difference with [ F (7, 16) = 28.26, P < 0.0001]. The PTZ caused the increased vulnerability of neurons to oxidative stress resulting from the accumulation of free radicals (P = 0.0043) as compared to normal control. In comparison to PTZ control treatment with LEV (P = 0.0007) and Sod.Se 0.5 (P = 0.0132) showed more antioxidant potential and neuroprotection. Dual therapy with LEV + Sod.Se 0.25 (P < 0.0001) and LEV + Sod.Se 0.5 (P < 0.0001) replenished GPx levels and ameliorated seizure-induced oxidative stress. However, treatment with Sod.Se 0.25 didn’t attenuate PTZ-induced oxidative stress with P = 0.8268 (Fig. 11D).
4. Discussion
The 30–40% of epileptic patients show resistance to monotherapy irrespective of therapeutic advancements resulting in increased acceptance of the polytherapy approach to manage the refractory type of epilepsy (Lee and Dworetzky, 2010, Łuszczki et al., 2003, Kwan and Brodie, 2000). In the present study, we compared the antiepileptic and neuroprotective potential of levetiracetam and sodium selenite alone and in combination in PTZ- kindled rats. PTZ-induced kindling involves repetitive administration of sub-convulsive doses of PTZ which eventually results in the generation of seizure activity by interrupting the GABAergic, Glutamatergic and antioxidant systems in the brain. PTZ-kindling is used as a chronic model of epilepsy model involving a sustained increase in seizure susceptibility causing molecular and cellular alterations contributing towards oxidative stress and neurodegenerative changes.
The first stage of the study comprised the exploration of the anticonvulsant potential of test drugs alone and in combination in the rats injected with PTZ (30 mg/kg, i.p.) on every alternate day for a total of 11 injections. The chronic administration with sub-convulsive doses of PTZ revealed increased seizure severity as compared to the healthy rats. However, treatment with LEV and Sod.Se hindered the kindling process dose-dependently as evident from comparatively reduced seizure score and mortality. The animals treated with LEV and Sod.Se alone depicted the seizures of stage 2–4 while the seizure development was more dominantly halted by combination as LEV + Sod.Se treated rats remained seizure-free during the kindling phase. The PTZ control rats showed myoclonic jerking and muscle twitching from the 7th injection which progressed to generalized tonic-clonic seizures at the 9th injection which ultimately led to the kindling of rats. However, the administration of LEV and Sod. Se was found capable to cause prominent latency in developing the myoclonic jerks and generalized seizures and polytherapy were found superior to monotherapy. These results are in agreement with the previous study carried out by Grover et al. in which LEV inhibited the development of PTZ-kindling in rodents (Gower et al., 1992). Similarly, Loscher et al. also reported the anti-epileptogenic effects of LEV in which drug inhibited the expression of seizures dose-dependently in the amygdala–kindled rats (Löscher et al., 1998). In the present study, this antiepileptic potential of LEV was intensified by combining it with Sod.Se as beneficial outcomes of selenium in preclinical studies have also been reported (Nazıroğlu, 2009, Rehni and Singh, 2013, Willmore and Rubin, 1981). Additionally, the selenium-deficient rats demonstrated more pronounced seizures and neuronal death in kainic acid-treated rodents (Savaskan et al., 2003). These findings have been supported by clinical pieces of evidence where intractable seizures in infants were managed with selenium supplementation (Ramaekers et al., 1994, Weber et al., 1991).
Patients with SE may experience a range of psychomotor symptoms with a high prevalence of anxiety (41). Approximately 45% of the patients who have epilepsy encounter anxiety which badly affects the quality of life (42). Therefore, we assessed comorbid anxiety-like behavior in animals immediately after inducing the brain insult through the kindling process. The PTZ control rats were found less exploring and more anxious in OFT, L/D and EPM as compared to the control group. This anxiety-like behavior was relatively ameliorated in animals exposed to LEV and Sod.Se, dose-dependently. But, the concomitant administration of LEV + Sod.Se resulted in a marked anxiolytic effect as animals had no distress towards open and lightened arena as compared to the PTZ control group. Our findings are in line with the previous preclinical studies where the action of LEV (Lamberty et al., 2003, Lamberty et al., 2001) and Sod.Se (Kędzierska et al., 2017) as anxiolytics has been reported by testing in various rodent’s behavioral models.
Furthermore, the animals were tested for memory and learning in the y-maze, NOR and MWM tests. The PTZ control rats showed impaired memory which was evident from their reduced spontaneous alternation, discrimination index and more thigmotaxic behavior in respective experiments. However, LEV and Sod.Se administration resulted in relative improvement of observed parameters but the animals receiving combined LEV + Sod.Se illustrated marked neuroprotection and cognition. Our findings are consistent with a preclinical study that reported the memory-improving potential of LEV in ketamine-exposed rats (Koh et al., 2018). Similarly, these animals were found least depressive as compared to PTZ control rats in SPT as well. Kędzierska et al. observed that sodium selenite worked synergistically with the antidepressants and diazepam in FST and EPM tests (Kędzierska et al., 2018). The kindling process is reported to have an association with the upregulation of brain BDNF mRNA levels (Elmér et al., 1998). In a study on LEV, Husum et al. noted that pre-treating the rats with LEV did not only delay the progression of kindling but also abolish the kindling-induced rise in BDNF mRNA. At this dose, LEV also increased the animal’s mobility in FST thus validating the antidepressant-like profile of this drug (Husum et al., 2004).
The neuroprotective effects of selenium are known to work by regulating the activity of selenoenzymes which exert protection from oxidative stress-mediated cell damage and resulting neurological disorders including epilepsy (Rehni and Singh, 2013, Sánchez-Elexpuru et al., 2017). Selenium regulates the phosphorylation of some important proteins involved in oxidative stress as well as reduces phosphorylation of tau proteins thus halting the neurofibrillary tangles formation and neurodegeneration resulting in improved memory and behavioral outcomes (Chen et al., 2014, Shultz et al., 2015). In various rodent models, the dephosphorylation of tau proteins by one of the selenium salts has been reported to suppress the epileptic seizures induced by various epileptogenic substances (Corcoran et al., 2010). Additionally, the nutritional deficiency of selenium is associated with various neuropsychiatric disorders including depression disorder, epilepsy and Alzheimer's disease. Thus, selenium may be hypothesized to exert an anti-convulsant activity in consonance with the endogenous selenoproteins. These above-mentioned characteristics of Sod.Se might be synergistically potentiating the neuroprotective effects of LEV, thus yielding the marked improvised neurobehavioral presentation of animals.
Imbalanced excitation and inhibition leading to hyperexcitability are some of the prominent pathological processes participating in neuronal loss in the epileptic brain (Scharfman, 2007). The exaggerated neuroexcitation causes elevated oxidative stress that further participates in the development of epilepsy (Aguiar et al., 2012). In our study, the reduced levels of superoxide dismutase, glutathione peroxidase and catalase and elevated malondialdehyde in the brains of PTZ-kindled rats were detected by biochemical testing which is consistent with the findings of claiming that PTZ-induced neuronal excitation causes increased oxidative stress (Eraković et al., 2003, Zhu et al., 2017b). However, the rats chronically administered with LEV mitigated this oxidative stress dose-dependently and outcomes are consistent with previous study reported by de Souza and colleauges who authenticated the antioxidant potential of LEV through in-vitro testing on brain homogenates (de Souza et al., 2019). Moreover, adding this newer AED with Sod.Se was worked efficiently in combating oxidative stress and these findings are supported by the previously reported preclinical findings where rats supplemented with Sod.Se for a week showed reduced cognitive deficit and oxidative stress (Ishrat et al., 2009). The reduced neuronal damage due to selenium supplementation might be attributed to the reduced lipid peroxidation leading to reduced generation of reactive nitrogen/oxygen species (Dominiak et al., 2016).
5. Conclusion
The findings of the present study suggested that LEV, a modulator of SV2A protein in the brain, is an auspicious option to halt the progression of epileptogenesis and epilepsy-related neuropsychological disorders. Indeed, the chronic administration of LEV (50 mg/kg) ameliorated anxiety, memory impairment and depression-like comorbid disarrays precipitated by PTZ-kindling in rats. Meanwhile, adding the Sod.Se with LEV showed dose-dependent synergistic anti-kindling effects with improved behavioral outcomes. Hence, in view of the increasing resistance towards antiepileptic drugs and the burden imposed by epilepsy-associated neuropsychiatric comorbidities, our findings indicate that combining the LEV with Sod.Se might be an effective therapeutic option to treat epilepsy. The mechanism of Sod.Se behind this neuroprotective potential might be due to its marvelous anti-oxidant characteristics. Though, future studies should be carried out to explore the detailed intrinsic pathways behind the anti-kindling role of Sod.Se that might be a fruitful adjunctive therapeutic strategy to deal with epilepsy and associated neurological challenges.
6. Ethics statement
All animal studies were performed after availing authorization from departmental ethical committee of BZU, Multan (02-PHL-S21, Dated 08-February 2021) and were accomplished by following instructions of the “Institute of Laboratory Animal Resources” (ILAR), Commission on Life Sciences, National Research Council (National Research Council, 1996).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors extended their appreciation to Distinguished Scientist Fellowship program at King Saud University, Riyadh, Saudi Arabia for funding this work through Research Supporting Project Number (RSP-2021/131).
Funding
This work was funded by Distinguished Scientist Fellowship program at King Saud University, Riyadh, Saudi Arabia through research supporting project Number (RSP-2021/131).
Availability of data and materials
The data of the current study are available from the corresponding author on reasonable request.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Faleh Alqahtani, Email: afaleh@ksu.edu.sa.
Imran Imran, Email: imran.ch@bzu.edu.pk.
References
- Abou-Khalil B. Levetiracetam in the treatment of epilepsy. Neuropsychiatr. Dis. Treat. 2008;4:507–523. doi: 10.2147/NDT.S2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar C.C.T., Almeida A.B., Araújo P.V.P., Abreu R.N.D.C.d., Chaves E.M.C., Vale O.C.d., Macêdo D.S., Woods D.J., Fonteles M.M.d.F., Vasconcelos S.M.M. Oxidative stress and epilepsy: Literature Review. Oxid. Med. Cell. Longev. 2012;2012:1–12. doi: 10.1155/2012/795259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqahtani F., Assiri M.A., Mohany M., Imran I., Javaid S., Rasool M.F., Shakeel W., Sivandzade F., Alanazi A.Z., Al-Rejaie S.S., Alshammari M.A., Alasmari F., Alanazi M.M., Alamri F.F., Fedele M. Coadministration of ketamine and perampanel improves behavioral function and reduces inflammation in acute traumatic brain injury mouse model. Biomed Res. Int. 2020;2020:1–12. doi: 10.1155/2020/3193725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020;54(Suppl. 2):185–191. doi: 10.1159/000503831. [DOI] [PubMed] [Google Scholar]
- Bertram E. The relevance of kindling for human epilepsy. Epilepsia. 2007;48(s2):65–74. doi: 10.1111/j.1528-1167.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Chen P., Wang L., Wang Y., Li S., Shen L., Liu Q., Ni J., Paudel H.K. Phosphoproteomic profiling of selenate-treated Alzheimer’s disease model cells. PLoS One. 2014;9(12):e113307. doi: 10.1371/journal.pone.0113307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran N.M., Martin D., Hutter-Paier B., Windisch M., Nguyen T., Nheu L., Sundstrom L.E., Costello A.J., Hovens C.M. Sodium selenate specifically activates PP2A phosphatase, dephosphorylates tau and reverses memory deficits in an Alzheimer’s disease model. J. Clin. Neurosci. 2010;17(8):1025–1033. doi: 10.1016/j.jocn.2010.04.020. [DOI] [PubMed] [Google Scholar]
- de Souza, A.G., Filho, A.J.M.C., Oliveira, J.V.S., de Souza, D.A.A., Lopes, I.S., de Carvalho, M.A.J., de Lima, K.A., Sousa, F.C.F., Vasconcelos, S.M.M., Macedo, D., Fonteles, M.M. de F., 2019. Prevention of pentylenetetrazole-induced kindling and behavioral comorbidities in mice by levetiracetam combined with the GLP-1 agonist liraglutide: Involvement of brain antioxidant and BDNF upregulating properties. Biomed. Pharmacother. 109, 429–439. https://doi.org/10.1016/j.biopha.2018.10.066. [DOI] [PubMed]
- Deshpande L.S., DeLorenzo R.J. Mechanisms of Levetiracetam in the control of status epilepticus and epilepsy. Front. Neurol. 2014;5:10. doi: 10.3389/FNEUR.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominiak A., Wilkaniec A., Wroczy|ski P., Adamczyk A. Selenium in the therapy of neurological diseases. where is it going? Curr. Neuropharmacol. 2016;14(3):282–299. doi: 10.2174/1570159X14666151223100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmér E., Kokaia Z., Kokaia M., Carnahan J., Nawa H., Lindvall O. Dynamic changes of brain-derived neurotrophic factor protein levels in the rat forebrain after single and recurring kindling-induced seizures. Neuroscience. 1998;83(2):351–362. doi: 10.1016/S0306-4522(97)00387-4. [DOI] [PubMed] [Google Scholar]
- Eraković V., Župan G., Varljen J., Simonić A. Pentylenetetrazol-induced seizures and kindling: changes in free fatty acids, superoxide dismutase, and glutathione peroxidase activity. Neurochem. Int. 2003;42:173–178. doi: 10.1016/S0197-0186(02)00070-0. [DOI] [PubMed] [Google Scholar]
- Espinosa-Jovel C., Toledano R., Aledo-Serrano Á., García-Morales I., Gil-Nagel A. Epidemiological profile of epilepsy in low income populations. Seizure. 2018;56:67–72. doi: 10.1016/J.SEIZURE.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Flohé L., Günzler W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–120. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Gaweł S., Wardas M., Niedworok E., Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004;57:453–455. [PubMed] [Google Scholar]
- Giorgi O., Carboni G., Frau V., Orlandi M., Valentini V., Feldman A., Corda M.G. Anticonvulsant effect of felbamate in the pentylenetetrazole kindling model of epilepsy in the rat. Naunyn. Schmiedebergs. Arch. Pharmacol. 1996;354:173–178. doi: 10.1007/BF00178717. [DOI] [PubMed] [Google Scholar]
- Goldenberg M.M. Overview of drugs used for epilepsy and seizures: etiology, diagnosis, and treatment. Pharm. Ther. 2010;35:392–415. [PMC free article] [PubMed] [Google Scholar]
- Gower A.J., Noyer M., Verloes R., Gobert J., Wülfert E. ucb L059, a novel anti-convulsant drug: pharmacological profile in animals. Eur. J. Pharmacol. 1992;222:193–203. doi: 10.1016/0014-2999(92)90855-X. [DOI] [PubMed] [Google Scholar]
- Grinspan Z.M., Shellhaas R.A., Coryell J., Sullivan J.E., Wirrell E.C., Mytinger J.R., Gaillard W.D., Kossoff E.H., Valencia I., Knupp K.G., Wusthoff C., Keator C., Ryan N., Loddenkemper T., Chu C.J., Novotny E.J., Millichap J., Berg A.T. Comparative effectiveness of levetiracetam vs phenobarbital for infantile epilepsy. JAMA Pediatr. 2018;172(4):352. doi: 10.1001/jamapediatrics.2017.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider M.S, Ashraf W., Javaid S., Rasool M.F, Rahman H.M.A, Saleem H., Anjum S.M.M, Siddique F., Morales-Bayuelo A., Kaya S., Alqahtani F., Alasmari F., Imran I. Chemical characterization and evaluation of the neuroprotective potential of Indigofera sessiliflora through in-silico studies and behavioral tests in scopolamine-induced memory compromised rats. Saudi J. Biol. Sci. 2021;28(8):4384–4398. doi: 10.1016/j.sjbs.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaya R., Arita K. The new antiepileptic drugs: their neuropharmacology and clinical indications. Neurol. Med. Chir. (Tokyo). 2016;56(5):205–220. doi: 10.2176/nmc.ra.2015-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husum H., Bolwig T.G., Sánchez C., Mathé A.A., Hansen S.L. Levetiracetam prevents changes in levels of brain-derived neurotrophic factor and neuropeptide Y mRNA and of Y1- and Y5-like receptors in the hippocampus of rats undergoing amygdala kindling: implications for antiepileptogenic and mood-stabilizing properties. Epilepsy Behav. 2004;5(2):204–215. doi: 10.1016/j.yebeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Imran I., Javaid S., Waheed A., Rasool M.F., Majeed A., Samad N., Saeed H., Alqahtani F., Ahmed M.M., Alaqil F.A. Grewia asiatica berry juice diminishes anxiety, depression, and scopolamine-induced learning and memory impairment in behavioral experimental animal models. Front. Nutr. 2020;7:587367. doi: 10.3389/fnut.2020.587367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T., Parveen K., Khan M.M., Khuwaja G., Khan M.B., Yousuf S., Ahmad A., Shrivastav P., Islam F. Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2009;1281:117–127. doi: 10.1016/J.BRAINRES.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Javaid S., Farooq T., Rehman Z., Afzal A., Ashraf W., Rasool M.F., Alqahtani F., Alsanea S., Alasmari F., Alanazi M.M., Alharbi M., Imran I. Dynamics of choline-containing phospholipids in traumatic brain injury and associated comorbidities. Int. J. Mol. Sci. 2021;22:11313. doi: 10.3390/IJMS222111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaid U., Javaid S., Ashraf W., Rasool M.F., Noman O.M., Alqahtani A.S., Majeed A., Shakeel W., Albekairi T.H., Alqahtani F., Imran I. Chemical profiling and dose-dependent assessment of fear reducing and memory-enhancing effects of Solanum virginianum in rats. Dose. Response. 2021;19:1–18. doi: 10.1177/1559325821998486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D., Pahwa P., Goel R.K. Protective effect of nerolidol against pentylenetetrazol-induced kindling, oxidative stress and associated behavioral comorbidities in mice. Neurochem. Res. 2016;41(11):2859–2867. doi: 10.1007/s11064-016-2001-2. [DOI] [PubMed] [Google Scholar]
- Kędzierska E., Dabkowska L., Obierzyński P., Polakowska M., Poleszak E., Wlaź P., Szewczyk K., Kotlińska J. Synergistic action of sodium selenite with some antidepressants and diazepam in mice. Pharmaceutics. 2018;10:270. doi: 10.3390/PHARMACEUTICS10040270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kędzierska E., Dudka J., Poleszak E., Kotlińska J.H. Antidepressant and anxiolytic-like activity of sodium selenite after acute treatment in mice. Pharmacol. Rep. 2017;69(2):276–280. doi: 10.1016/j.pharep.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Keezer M.R., Sisodiya S.M., Sander J.W. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(1):106–115. doi: 10.1016/S1474-4422(15)00225-2. [DOI] [PubMed] [Google Scholar]
- Koh M.T., Shao Y., Rosenzweig-Lipson S., Gallagher M. Treatment with levetiracetam improves cognition in a ketamine rat model of schizophrenia. Schizophr. Res. 2018;193:119–125. doi: 10.1016/J.SCHRES.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P., Brodie M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Lamberty Y., Falter U., Gower A.J., Klitgaard H. Anxiolytic profile of the antiepileptic drug levetiracetam in the Vogel conflict test in the rat. Eur. J. Pharmacol. 2003;469(1-3):97–102. doi: 10.1016/S0014-2999(03)01724-2. [DOI] [PubMed] [Google Scholar]
- Lamberty Y., Margineanu D.G., Klitgaard H. Effect of the new antiepileptic drug levetiracetam in an animal model of mania. Epilepsy Behav. 2001;2(5):454–459. doi: 10.1006/ebeh.2001.0254. [DOI] [PubMed] [Google Scholar]
- Löscher W., Hönack D., Rundfeldt H. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy - PubMed. J Pharmacol Exp Ther. 1998;284:474–479. PMID: 9454787. [PubMed] [Google Scholar]
- Lee J.W., Dworetzky B. Rational polytherapy with antiepileptic drugs. Pharmaceuticals. 2010;3(8):2362–2379. doi: 10.3390/PH3082362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.Y., Yin C.Y., Zhu L.J., Zhu X.H., Xu C., Luo C.X., Chen H., Zhu D.Y., Zhou Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018;13(7):1686–1698. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- Łuszczki J.J., Świader M., Parada-Turska J., Czuczwar S.J. Tiagabine synergistically interacts with gabapentin in the electroconvulsive threshold test in mice. Neuropsychopharmacology. 2003;28(10):1817–1830. doi: 10.1038/sj.npp.1300243. [DOI] [PubMed] [Google Scholar]
- Malik, H., Javaid, S., Rasool, M.F., Samad, N., Ahamad, S.R., Alqahtani, F., & Imran, I., 2020. Amelioration of scopolamine-induced amnesic, anxiolytic and antidepressant effects of Ficus benghalensis in behavioral experimental models. Medicina 56, 144. https://doi: 10.3390/medicina56030144. [DOI] [PMC free article] [PubMed]
- Mazhar, F., Malhi, S.M., Simjee, S.U., 2017. Comparative studies on the effects of clinically used anticonvulsants on the oxidative stress biomarkers in pentylenetetrazole-induced kindling model of epileptogenesis in mice. J. Basic Clin. Physiol. Pharmacol. 28, 31–42. https://doi.org/10.1515/JBCPP-2016-0034. [DOI] [PubMed]
- Muke S., Kaikini A., Peshattiwar V., Bagle S., Dighe V., Sathaye S. Neuroprotective effect of coumarin nasal formulation: kindling model assessment of epilepsy. Front. Pharmacol. 2018;9:992. doi: 10.3389/FPHAR.2018.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munguía-Martínez M.F., Nava-Ruíz C., Ruíz-Díaz A., Díaz-Ruíz A., Yescas-Gómez P., Méndez-Armenta M. Immunohistochemical study of antioxidant enzymes regulated by Nrf2 in the models of epileptic seizures (Ka and PTz) Oxid. Med. Cell. Longev. 2019;2019:1–8. doi: 10.1155/2019/1327986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naskar S., Islam A., Mazumder U.K., Saha P., Haldar P.K., Gupta M. In vitro and in vivo antioxidant potential of hydromethanolic extract of phoenix dactylifera fruits. J. Sci. Res. 2009;2:144–157. doi: 10.3329/JSR.V2I1.2643. [DOI] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. The National Academies Press (US); Washington, DC: 1996. [Google Scholar]
- Nazıroğlu M. Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem. Res. 2009;34(12):2181–2191. doi: 10.1007/s11064-009-0015-8. [DOI] [PubMed] [Google Scholar]
- Nieoczym D., Socała K., Zelek-Molik A., Pieróg M., Przejczowska-Pomierny K., Szafarz M., Wyska E., Nalepa I., Wlaź P. Anticonvulsant effect of pterostilbene and its influence on the anxiety- and depression-like behavior in the pentetrazol-kindled mice: behavioral, biochemical, and molecular studies. Psychopharmacology (Berl). 2021;238(11):3167–3181. doi: 10.1007/s00213-021-05933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pari L., Latha M.J. Protective role of Scoparia dulcis plant extract on brain antioxidant status and lipidperoxidation in STZ diabetic male Wistar rats. BMC Compl. Altern. Med. 2004;4:16. doi: 10.1186/1472-6882-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlar A., Arslan S.O. Resveratrol normalizes the deterioration of smooth muscle contractility after intestinal ischemia and reperfusion in rats associated with an antioxidative effect and modulating tumor necrosis factor alpha activity. Ann. Vasc. Surg. 2019;61:416–426. doi: 10.1016/J.AVSG.2019.06.027. [DOI] [PubMed] [Google Scholar]
- Parlar A., Arslan S.O., Çam S.A. Glabridin alleviates inflammation and nociception in rodents by activating bkca channels and reducing no levels. Biol. Pharm. Bull. 2020;43:884–897. doi: 10.1248/BPB.B20-00038. [DOI] [PubMed] [Google Scholar]
- Ramaekers V., Calomme M., Vanden Berghe D., Makropoulos W. Selenium deficiency triggering intractable seizures. Neuropediatrics. 1994;25(04):217–223. doi: 10.1055/S-2008-1073025. [DOI] [PubMed] [Google Scholar]
- Rehni A.K., Singh T.G. Selenium induced anticonvulsant effect: a potential role of prostaglandin E(1) receptor activation linked mechanism. J. Trace Elem. Med. Biol. 2013;27(1):31–39. doi: 10.1016/J.JTEMB.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Samad N., Rao T., Rehman M.H.u., Bhatti S.A., Imran I. Inhibitory effects of selenium on arsenic-induced anxiety-/depression-like behavior and memory impairment. Biol. Trace Elem. Res. 2021;200(2):689–698. doi: 10.1007/s12011-021-02679-1. [DOI] [PubMed] [Google Scholar]
- Sánchez-Elexpuru G., Serratosa J.M., Sánchez M.P. Sodium selenate treatment improves symptoms and seizure susceptibility in a malin-deficient mouse model of Lafora disease. Epilepsia. 2017;58(3):467–475. doi: 10.1111/epi.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan N.E., Bräuer A.U., Kühbacher M., Eyüpoglu I.Y., Kyriakopoulos A., Ninnemann O., Behne D., Nitsch R. Selenium deficiency increases susceptibility to glutamate-induced excitotoxicity. FASEB J. 2003;17(1):112–114. doi: 10.1096/fj.02-0067fje. [DOI] [PubMed] [Google Scholar]
- Scharfman H.E. The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep. 2007;7(4):348–354. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M., Pulsipher D.T., Hermann B. Association of epilepsy and comorbid conditions. Future Neurol. 2009;4(5):663–668. doi: 10.2217/fnl.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeel W., Javaid S., Anjum S.M.M., Rasool M.F., Samad N., Alasmari F., Alasmari A.F., Alaqil F.A., Alqarni S.A., Alotaibi F.M., Alqahtani F., Imran I. Time course evaluation of lacosamide alone and in polypharmacy on behavioral manifestations and oxidative stress in lithium-pilocarpine-induced model. J. Physiol. Pharmacol. 2020;71:547–564. doi: 10.26402/jpp.2020.4.10. [DOI] [PubMed] [Google Scholar]
- Squires R.F., Saederup E., Crawley J.N., Skolnick P., Paul S.M. Convulsant potencies of tetrazoles are highly correlated with actions on GABA/benzodiazepine/picrotoxin receptor complexes in brain. Life Sci. 1984;35(14):1439–1444. doi: 10.1016/0024-3205(84)90159-0. [DOI] [PubMed] [Google Scholar]
- Shultz S.R., Wright D.K., Zheng P., Stuchbery R., Liu S.-J., Sashindranath M., Medcalf R.L., Johnston L.A., Hovens C.M., Jones N.C., O’Brien T.J. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain. 2015;138(5):1297–1313. doi: 10.1093/brain/awv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom C.E., Carmant L. Seizures and epilepsy: an overview for neuroscientists. cold spring harb. Perspect. Med. 2015;5(6):a022426. doi: 10.1101/CSHPERSPECT.A022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Paquette V., Levine M., Ensom M.H.H. Levetiracetam clinical pharmacokinetic monitoring in pediatric patients with epilepsy. Clin. Pharmacokinet. 2017;56(11):1267–1285. doi: 10.1007/S40262-017-0537-1. [DOI] [PubMed] [Google Scholar]
- Tawfik K.M., Moustafa Y.M., El-Azab M.F. Neuroprotective mechanisms of sildenafil and selenium in PTZ-kindling model: Implications in epilepsy. Eur. J. Pharmacol. 2018;833:131–144. doi: 10.1016/J.EJPHAR.2018.05.035. [DOI] [PubMed] [Google Scholar]
- Weber G.F., Maertens P., Meng X., Pippenger C.E. Glutathione peroxidase deficiency and childhood seizures. Lancet. 1991;337(8755):1443–1444. doi: 10.1016/0140-6736(91)93130-2. [DOI] [PubMed] [Google Scholar]
- Willmore L.J., Rubin J.J. Antiperoxidant pretreatment and iron-induced epileptiform discharges in the rat: EEG and histopathologic studies. Neurology. 1981;31:63–69. doi: 10.1212/WNL.31.1.63. [DOI] [PubMed] [Google Scholar]
- Zhu X., Dong J., Han B., Huang R., Zhang A., Xia Z., Chang H., Chao J., Yao H. Neuronal nitric oxide synthase contributes to PTZ kindling-induced cognitive impairment and depressive-like behavior. Front. Behav. Neurosci. 2017;11:203. doi: 10.3389/FNBEH.2017.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Dong J., Han B., Huang R., Zhang A., Xia Z., Chang H., Chao J., Yao H. Neuronal nitric oxide synthase contributes to PTZ kindling epilepsy-induced hippocampal endoplasmic reticulum stress and oxidative damage. Front. Cell. Neurosci. 2017;11:377. doi: 10.3389/FNCEL.2017.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of the current study are available from the corresponding author on reasonable request.