Abstract
Background:
Drug addiction is a complex brain disorder that is characterized by craving, withdrawal, and relapse, which can be perpetuated by social stress. Stemming from an acute life event, chronic stress, or trauma in a social context, social stress has a major role in the initiation and trajectory of substance use. Preclinical literature shows that early life stress exposure and social isolation facilitate and enhance drug self-administration. Epidemiological evidence links childhood adversity to increased risk for drug use and demonstrates that cumulative stress experiences are predictive of substance use severity in a dose-dependent manner. Stress and drug use induce overlapping brain alterations leading to downregulation or deficits in brain reward circuitry, thereby resulting in greater sensitization to the rewarding properties of drugs. Though stress in the context of addiction has been studied at the neural level, a gap in our understanding of the neural underpinnings of social stress in humans remains.
Methods:
We conducted a systematic review of in vivo structural and functional neuroimaging studies to evaluate the neural processes associated with social stress in individuals with substance use disorder. Results were considered in relation to participants’ history of social stress and with regard to the effects of social stress induced during the neuroimaging paradigm.
Results:
An exhaustive search yielded 21 studies that matched inclusion criteria. Social stress induces broad structural and functional neural effects in individuals with substance use disorder throughout their lifespan and across drug classes. A few patterns emerged across studies: (1) many of the brain regions altered in individuals who were exposed to chronic social stress and during acute stress induction have been implicated in addiction networks (including the prefrontal cortex, insula, hippocampus, and amygdala); (2) individuals with childhood maltreatment and substance use history had decreased gray matter or activation in regions of executive functioning (including the medial prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex), the hippocampal complex, and the supplementary motor area; and (3) during stress-induction paradigms, activation in the anterior cingulate cortex, caudate, and amygdala was most commonly observed.
Conclusions/Implications:
A distinct overlap is shown between social stress-related circuitry and addiction circuitry, particularly in brain regions implicated in drug-seeking, craving, and relapse. Given the few studies that have thoroughly investigated social stress, the evidence accumulated to date needs to be replicated and extended, particularly using research designs and methods that disentangle the effects of substance use from social stress. Future clinical studies can leverage this information to evaluate the impact of exposure to trauma or adverse life events within substance use research. Expanding knowledge in this emerging field could help clarify neural mechanisms underlying addiction risk and progression to guide causal-experimental inquiry and novel prevention and treatment strategies.
Keywords: Early and lifetime adversity, Human drug addiction, Neuroimaging, Social stress, Substance use disorder
1. Introduction
Substance use has profound individual, societal, and economic impacts (Gomes et al. 2018a; Substance Abuse and Mental Health Services Administration 2017; Volkow 2017; Hedegaard et al. 2017), yet effective evidence-based treatments to circumvent this public health emergency are limited (Volkow 2017). Individuals with substance use disorders (SUD) frequently use drugs to relieve physical and/or emotional pain, a pattern that can lead to increased morbidity and mortality (Blum et al. 2013; Gomes et al. 2018b). A major source of emotional pain experienced by individuals with SUD is linked to chronic social stress (rejection/isolation; childhood maltreatment; loss of a caregiver) (Lawson et al. 2013; Stein et al. 2007), which may shape neural and physiological responses to drugs and exacerbate illness risk (Quinn et al. 2016; Conroy et al. 2009). Indeed, negative social emotions inclusive of social stress have been associated with activation of the opioid system which is intricately related to systems mediating physical pain (Stein et al. 2007; Eisenberger et al. 2003; Dewall et al. 2010).
Clinical and epidemiological evidence has long shown the involvement of stress induced by social factors as a critical contributor to the initiation and progression of addiction in humans (Sinha 2001; Sandi and Haller 2015; Jordan and Andersen 2017). Neurobiological evidence that links social stress and drug use stems primarily from animal models that have provided mechanistic insights about the impact of the neural stress system on reward circuits that mediate drug-seeking and taking behaviors through negative reinforcement (e.g. self-medication, withdrawal avoidance) (Koob 2008). While this knowledge provides a foundation for the neural underpinnings of how social stress can potentially trigger drug use and relapse, direct neurobiological insights about the human brain are of significant importance. The development of in vivo neuroimaging tools in recent decades has begun to examine social stress in addiction. This review evaluated such neuroimaging studies in an attempt to provide possible neural-mechanistic clarity into the impact of social stress in SUD. Knowledge gleaned may carry important translational utility to guide causal-experimental inquiry and possible intervention development.
1.1. Stress: Definition and Overview of Neurobiology
Stress may be defined as a real or interpreted threat to the physiological or psychological integrity of an individual that impacts behavior, subjective experience, and cognitive function (McEwen 2017a; Levine 2005; de Kloet et al. 2005). The term “stress” refers to a disruption in equilibrium resulting in a cascade of physiological and behavioral responses to reinstate allostasis (e.g., achieving stability through change) (McEwen 2017a; Koob and Schulkin 2019). This complex multidimensional concept involves perception (of stress stimuli), appraisal (processing of stress), response (to challenging or threatening events or stimuli), and eventually adaptation (Lazarus and Folkman 1984; Sinha 2008). While it has been recognized that stress could have positive impacts (“good” and “tolerable” stress promote resilience/growth experiences via allostasis), “toxic” stress (hereafter referred to as “stress”) is likely to result in pathophysiology due to cumulative burden with higher levels of allostatic load/overload (overuse and dysregulation that exacerbate disease processes) (McEwen 2017a).
The stress response is mainly mediated by stress hormones including corticotropin-releasing factor (CRF) and cortisol that is released by the hypothalamic–pituitary–adrenal (HPA) axis and adrenal cortex; and catecholamines, and norepinephrine (or noradrenaline) released by the adrenal medulla and sympathetic nerves (McEwen 2013; McEwen and Gianaros 2011). Peripheral stress hormones provide feedback to the brain, regulating the activity of the HPA axis. This negative feedback loop depends on the activation of two types of glucocorticoid receptors in the brain: high-affinity mineralocorticoid receptors, activated by lower concentrations of cortisol, preventing further release of CRF; and low-affinity glucocorticoid receptors, activated by higher concentrations of cortisol, and resulting in the opposite effect, an increase in the release of CRF (McEwen 2013; McEwen and Gianaros 2011). Stress-related imbalance involves additional systemic physiological responses via neuroendocrine, autonomic, cardiovascular/immune, and metabolic mediators. Inter-individual variation in vulnerability or resilience to stress are complex but partially relate to genetic background through stress-related gene expression (Agorastos et al. 2019) and epigenetic modifications (e.g., DNA methylation associated with early life stress (Meaney and Szyf 2005)).
The experience of stress and dysregulation of stress hormones affect structural and functional neuroplasticity (e.g., dendritic remodeling and synapse turnover) (McEwen 2013; McEwen and Gianaros 2011). However, the stress experience, whether acute or chronic, induces different effects that have been characterized in brain regions such as the hippocampus, amygdala, and prefrontal cortex (PFC) that mediate fear-related memories, working memory, and self-regulatory behaviors. Traumatic stressors induce region-specific neuroplasticity effects that may lead to profound behavioral impact. In the PFC, chronic stress causes medial PFC (mPFC) neurons to debranch and shrink dendrites, a process associated with cognitive rigidity, whereas orbitofrontal cortical neurons expand dendrites that may be related to increased vigilance (Radley et al. 2004; Liston et al. 2006; McEwen 2017b). Additional acute and chronic stress-related neural alterations (Vyas et al. 2002; Bennur et al. 2007; Lau et al. 2017) are implicated in increased anxiety, posttraumatic stress disorder (PTSD)-like behaviors, and social avoidance (Bennur et al. 2007; Lau et al. 2017), conditions that are closely tied to drug-seeking and drug-using behaviors.
1.2. Social Stress and Substance Use: Preclinical, Clinical, and Neurobiological Context
Stress is a well-known contributor to drug use and relapse vulnerability. On the behavioral level, drug use was proposed as a coping strategy to alleviate acute and chronic stress (Wills and Shiffman 1986; Conger 1956; Khantzian 1985). Initially used to modulate tension, with repeated administration, drug use may become a frequent response for mood enhancement. On the neurobiological level, stress and drug use involve overlapping brain changes (Ruisoto and Contador 2019) where drug addiction has been viewed as an allostatic disorder (Koob and Schulkin 2019). Stress and drug use lead to downregulation or deficits in the brain reward circuitry resulting in greater sensitization to the rewarding properties of drugs (Koob and Schulkin 2019; Koob and Le Moal 1997). The effects of stress on the reward system enhance the reward and craving induced by drugs or drug cues and increase the motivation to use drugs compulsively (Piazza and Le Moal 1998). Stress-induced hyperactivity of the HPA axis and upregulation of the amygdala is involved in negative emotionality during withdrawal and drives drug-seeking and taking behaviors through negative reinforcement (Belujon and Grace 2011). The hippocampus is also down-regulated by both stress and drug use leading to further impairments of memory and emotion regulation (Belujon and Grace 2011). Importantly, stress leads to surges of dopamine and norepinephrine in the PFC, resulting in impairments of the executive function network, thereby disrupting decision making and the ability to inhibit relapse, the hallmark of drug addiction (Mather and Lighthall 2012). Collectively, these models provide explanations for the crucial roles of stress in the transition from casual substance use to SUD.
Converging lines of evidence indicate that the stressors correlated with increased risk of substance use were primarily social in nature (versus other forms of stress, such as economic or natural disasters) (Thoits 2010). In animal models, individual differences in susceptibility to cocaine use within a population, measured by the availability of dopamine D2 receptors, may be mediated by social dominance rank (Morgan et al. 2002). Moreover, early life stress, induced through neonatal or maternal separation, facilitates drug self-administration, while social defeat stress or social isolation escalates drug use (Sinha 2001). Conversely, operant access to social reward in addicted rats prevented compulsive drug self-administration and incubation of craving and relapse (Venniro et al. 2018).
Studies in humans extend these findings. Epidemiological evidence links childhood adversity to increased risk for cannabis, cocaine, and prescription drug use among adolescents (Carliner et al. 2016; Khoury et al. 2010). In adults, alcohol and drug use can contribute to unstable housing or homelessness; likewise, increased duration of homelessness was shown to be associated with increased risk of substance use (Kipke et al. 1997). Furthermore, the cumulative number of stress events an individual experienced was predictive of alcohol and drug use severity in a dose-dependent manner (Sinha 2008; Turner and Lloyd 2003; Lloyd and Turner 2008). Collectively, these findings indicate that chronic exposure to social stress, trauma, and/or an adverse life event enhances an individual’s vulnerability to substance use (Sinha 2008).
Animal models of stress have been a useful tool to investigate mechanisms of substance use through which stress alters neuroplasticity and animal behavior in a social context (Mumtaz et al. 2018). However, in vivo neuroimaging models are limited in clinical studies, leading to a gap in the understanding of the brain effects of social stress in SUD. This chapter presents the current state of knowledge of the neural underpinnings of social stress in human drug addiction. This review considers the multi-faceted roles of social stress in SUD. It seeks to understand how the prevalence of chronic and/or acute social stress may impact the neural underpinnings of SUD vulnerability and its trajectory. Studies are contextualized within the scope of preclinical, neurobiological, and clinical findings. Future directions are discussed to inform priorities for enhancing the knowledge base of this emerging field, which could progress prevention targets and opportunities for novel treatments.
1.3. Search Method
We searched Pubmed and the Neurosynth database (www.neurosynth.org) (Yarkoni et al. 2011), for relevant studies published between January 1, 2000, and July 1, 2021. A combination of the following keywords was used: social stress, trauma, childhood trauma, childhood maltreatment, social isolation, rejection, drug addiction, human drug addiction, substance use, substance use disorder, cocaine, heroin, opioid, nicotine, alcohol, cannabis, marijuana, imaging, neuroimaging, structural integrity, functional magnetic resonance imaging (fMRI), functional connectivity, resting-state functional connectivity, paradigm. Studies were included if they met the following criteria: (1) conducted neuroimaging (functional connectivity, structural or functional magnetic resonance imaging); (2) studied a substance using population with a history of social stress and/or employed a stress-inducing paradigm in a substance using population; and (3) used standardized methods to assess for SUD and/or its severity.
Twenty-one studies were identified and summarized in Table 1. Studies are grouped by the social stress characteristics of the sample first, and the type of stress-induction paradigm used second.
Table 1.
Summary of neuroimaging studies of social stress in human drug addiction
| Reference | Social stress type (measure) | Substance use/disorder | Sample | Paradigm (social stress or other) | Imaging tool/procedure | Analysis type | Prime brain regions | Brain-behavior associations |
|---|---|---|---|---|---|---|---|---|
| Past social stress/trauma | ||||||||
| (Van Dam et al. 2014) | Childhood maltreatment (CTQ) | Alcohol, cocaine, and/or cannabis | 79 in-treatment SUD; 98 CON | N/A | 3T MRI VBM | Whole-brain; ROI | CM (controlling for SUD): ↓L hippocampus, ↓parahippocampus, ↓anterior fusiform gyrus |
↓CM-related gray matter volume, ↑severity of substance use after initial relapse |
| (Bachi et al. 2018) | Childhood maltreatment (CTQ) | Cocaine | 24 CUD + CM; 23 CUD-CM; 29 CON | N/A | 3T MRI VBM | Whole-brain | CUD + CM > CON| CUD-CM: ↓R lateral OFC CUD + CM > CON: ↓middle temporal gyrus | ↓R lateral OFC, ↑depression and ↓constraint |
| (Elton et al. 2015) | Childhood maltreatment (CTQ) | Cocaine | 20 male CUD + CM; 18 male CUD-CM | Guided imagery induction. Post-task: anxiety, craving | 3T fMRI BOLD | Whole-brain; ROI | Stress > neutral script (CUD + CM): ↓L dorsal-anterior precuneus, ↓L supplementary motor area CTQ score-by-craving rating interaction: ↓rostral ACC | ↓L precuneus and ↓L supplementary motor area, ↑physical and emotional abuse |
| (Ieong and Yuan 2018) | Childhood maltreatment (Adverse Childhood Experiences; (Felitti et al. 1998)) | Heroin and/or nicotine | 7 heroin (abstinent >3 months)-nicotine dependence +CM; 7 nicotine dependence +CM; 7 CON | Reading the mind in the eyes | Continuous wave; functional near-infrared spectroscopy; Resting-state FC | ROI | Heroin-nicotine dependence > nicotine dependence: ↑OFC, ↑mPFC, ↓dorsolateral PFC | N/A |
| (Casement et al. 2015) | Stressful life events in late adolescence (Life Event Questionnaire for Adolescents (Masten et al. 1994) and Interpersonal Problem Situations Inventory for Urban Adolescents; (Farrell et al. 1998)) | Alcohol (problematic use) | 152 males | Reward-guess task | 3T fMRI BOLD | ROI | Stress scores; severity of alcohol use, association with reward: Anticipation of reward and reward outcome = ↓mPFC | ↓mPFC, ↑cumulative life stress events and ↑severity of alcohol use |
| (Poppa et al. 2019) | PTSD (lifetime sexual trauma; Life Stressors Checklist-Revised; (Wolfe and Kimerling 1997)) | Methamphetamine and/or cocaine | 14 female SUD-PTSD; 29 female SUD | Interoceptive-exteroceptive attention | 3T fMRI FC of task-modulated networks | Whole-brain | SUD-PTSD > SUD: ↓OFC network located in the mid-posterior insula, angular gyrus, precuneus, and lateral prefrontal cortex areas | ↓OFC network strength, ↑sexual violence exposure across all participants |
| (Gawrysiak et al. 2017) | Trauma history (lifetime; Addiction Severity Index; (McLellan et al. 1992)) | Cocaine | 18 CUD-trauma; 16 CUD | N/A | 3T fMRI Resting-stateFC | Seed-based (amygdala) | Trauma: ↑amygdala and limbic-striatal regions connectivity | N/A |
| Social stress induction | ||||||||
| (Seo et al. 2011) | N/A | Alcohol (social drinking) | 20 males; 23 females |
Guided imagery induction | 3T fMRI BOLD | Whole-brain | Stress script (both): ↑L dorsal striatum Stress script (male > female): ↑mPFC, ↑rostral ACC, ↑posterior insula, ↑putamen, ↑amygdala ↑hippocampus, ↑parahippocampal gyrus |
↑dorsal striatum, ↑craving in males |
| (Li et al. 2005) | N/A | Cocaine | 17 male CUD; 10 female CUD |
Guided imagery induction. Post-task: Anxiety, craving | 1.5T fMRI BOLD | Whole-brain; ROI | Stress script (both): ↑mid temporal gyrus Stress script (female > male): ↑middle, medial, inferior frontal cortical areas, ↑anterior cingulate, ↑insula, ↑middle and inferior frontal cortices, ↑R posterior cingulate cortex | ↑L anterior cingulate and ↑R posterior cingulate cortices, ↓craving rating during stress imagery in female CUDs |
| (Li et al. 2006) | N/A | Cocaine | 17 male CUD; 10 female CUD |
Guided imagery induction | 1.5T fMRI BOLD | ROI | Stress script (both): ↑R inferior frontal cortex, ↑mPFC, ↑R anterior cingulate | ↑mPFC and ↓L posterior cingulate cortex, ↓socialization in female CUDs |
| (Sinha et al. 2005) | N/A | Cocaine | 20 CUD; 8 CON |
Guided imagery induction | 1.5T fMRI BOLD | Whole-brain | Stress script (CUD): ↓ medial orbitofrontal/anterior cingulate region, ↓R postcentral gyrus, ↓R fusiform gyrus, ↓L precentral gyrus, ↓medial FG, ↓L hippocampus, ↑caudate, ↑ dorsal striatum | ↑caudate and ↑dorsal striatum, ↑stress-induced cocaine craving ratings |
| (Kober et al. 2017) | N/A | Nicotine | 11 smokers in mindfulness training; 12 smokers in freedom from smoking treatment |
Guided imagery induction | 1.5T fMRI BOLD | Whole-brain | Stress script (both): ↑↓cluster in amygdala, anterior insula, mid insula, hippocampus, parahippocampal gyrus, thalamus, middle occipital gyrus, midbrain, cerebellum, and R posterior insula; ↑↓cluster across cuneus/precuneus, posterior cingulate cortex | ↑neural activity during stress, ↓post-treatment cigarettes per day reduction. Protracted effect in similar brain regions at 3 months follow-up |
| (Maurage et al. 2012) | N/A | Alcohol | 22 AUD; 22 CON |
Cyberball | 3T fMRI BOLD | Whole-brain | AUD > CON (social exclusion): ↑R insula, ↓R ventral PFC, ↓L medial FG AUD > CON (re-inclusion post social exclusion): ↑L dorsal ACC, ↑parahippocampal gyrus |
N/A |
| (Hanlon et al. 2018) | N/A | Cocaine | 18 CUD; 25 CON |
Cyberball | 3T fMRI BOLD | Whole-brain | CUD > CON (social exclusion > inclusion): ↑R medial FG/pregenual cingulate ↑L ventral FG, ↑R caudate |
N/A |
| (Bach et al. 2019a, b) | N/A | Opioid | 19 OMT; 20 CON |
Cyberball | 3T fMRI VBM | Whole-brain; ROI | OMT > CON (VBM): ↓anterior insula, ↓L inferior FG, ↓R frontal lobe, ↓R inferior FG | ↓L insula, ↓reduced feelings of inclusion during inclusion trial and ↑feelings of exclusion during inclusion trial. ↓L insula, ↑social interaction anxiety symptoms |
| (Bach et al. 2019a, b) | N/A | Opioid | 17 OMT; 21 CON |
Cyberball | 3 T fMRI BOLD | Whole-brain | OMT > CON (social exclusion and inclusion): ↓ superior and middle occipital and lingual gyri, ↓ cunei, ↓L fusiform gyrus | N/A |
| (Beard et al. 2021) | N/A | Alcohol and/or cannabis use | 181 adolescents | Cyberball | 3T fMRI BOLD | Whole-brain: ROI | Exclusion > inclusion: ↑dorsal ACC, ↑subgenual ACC, ↑anterior insula |
↓dorsal ACC and ↑anxiety, ↑substance use later in adolescence ↓dorsal ACC and ↑distress (game-related), ↑substance use later in adolescence ↑anterior insula, ↑substance use in females |
| (Charlet et al. 2014) | N/A | Alcohol | 33 AUD; 33 CON |
Facial emotion recognition (aversive faces) | 3T fMRI BOLD & VBM | ROI | AUD > CON (BOLD): ↓ FG, ↑L rostral ACC, ↑L anterior cingulate cortex/ medial FG, ↑R precuneus AUD > CON (VBM): ↓FG, ↓ rostral ACC, ↓frontal regions (ACC, insula, temporal, occipital gyri) | ↑L rostral ACC, ↑days of abstinence and ↓days of binge drinking. ↑FG activation, ↑lifetime drinking |
| (Bedi et al. 2009) | N/A | MDMA (administration) | 9 individuals with past MDMA use | Facial emotion recognition (aversive faces) | 3T fMRI BOLD | Whole-brain; ROI | MDMA 1.5 mg/kg > MDMA 0.75 mg/kg |Placebo (angry > neutral faces): ↓L amygdala MDMA 0.75 mg/kg > MDMA 1.5 mg/kg|Placebo (happy > neutral): ↑R ventral striatum | ↑R ventral striatum, no effect on self-reported sociability in MDMA (0.75 mg/kg) |
| (Flanagan et al. 2019) | N/A | Alcohol, cannabis and/or cocaine | 10 couples (n = 20) w/one partner w/ hazardous drinking or SUD | Relationship conflict | 3T fMRI BOLD | ROI | Female > male (conflict > neutral): ↑R amygdala-L inferior FG and mid-cingulate connectivity, ↑L OFC-R mid temporal gyrus connectivity Male > female (conflict > neutral): ↑R OFC-R, amygdala/hippocampus connectivity Group (conflict): ↑R OFC-R frontal pole connectivity | ↑R amygdala and L prefrontal cortex FC during the conflict or neutral cues, ↑intimate partner violence victimization and perpetration |
| (Dagher et al. 2009) | N/A | Nicotine | 15 nicotine smokers | Montreal imaging stress task. Post-task: Cue-reactivity | 3 T fMRI BOLD | Whole-brain; ROI | Stressful > non-stressful: ↓hippocampus, ↓amygdala, ↓bed nucleus of the stria terminalis, ↓hypothalamus, ↓nucleus accumbens Stressful > non-stressful (smoking cue-reactivity): ↑dorsomedial PFC, ↑dorsal caudate, ↑dorsomedial thalamus, ↑hippocampus |
↑hippocampus, ↑amygdala, and ↑nucleus accumbens, ↑cue-reactivity and ↑ craving response |
Notes: Childhood Trauma Questionnaire (CTQ; (Bernstein et al. 2003)) Substance use disorder (SUD); Control (CON); Magnetic resonance imaging (MRI); Voxel-based morphometry (VBM); Childhood maltreatment (CM); Left (L); Cocaine use disorder (CUD); Right (R); Orbitofrontal cortex (OFC); Functional magnetic resonance imaging (fMRI); Blood-oxygen-level-dependent (BOLD); Region-of-interest (ROI); Anterior cingulate cortex (ACC); Functional connectivity (FC); Prefrontal cortex (PFC); Medial PFC (mPFC); Post-traumatic stress disorder (PTSD); Frontal gyrus (FG); Alcohol use disorder (AUD); Opioid methadone treatment (OMT); 3,4-Methylenedioxymethamphetamine (MDMA)
2. Early and Lifetime Adversity
Early and lifetime adversity encompass a wide range of stressors with varying magnitudes of severity. Individuals may be affected by a single traumatic event, a series of stressful events, chronic stress, live in a socially dysfunctional environment, and more. The developmental timing of the stress exposure and the amount and duration of stress exposure can affect an individual’s vulnerability to SUD and their disorder trajectory (Teicher et al. 2016). This section describes studies in individuals with SUD who have experienced chronic stress or trauma within a social context in the early periods of their life (before the age of 18; see Fig. 1, top panel) or throughout their life (see Fig. 1, bottom panel).
Fig. 1.
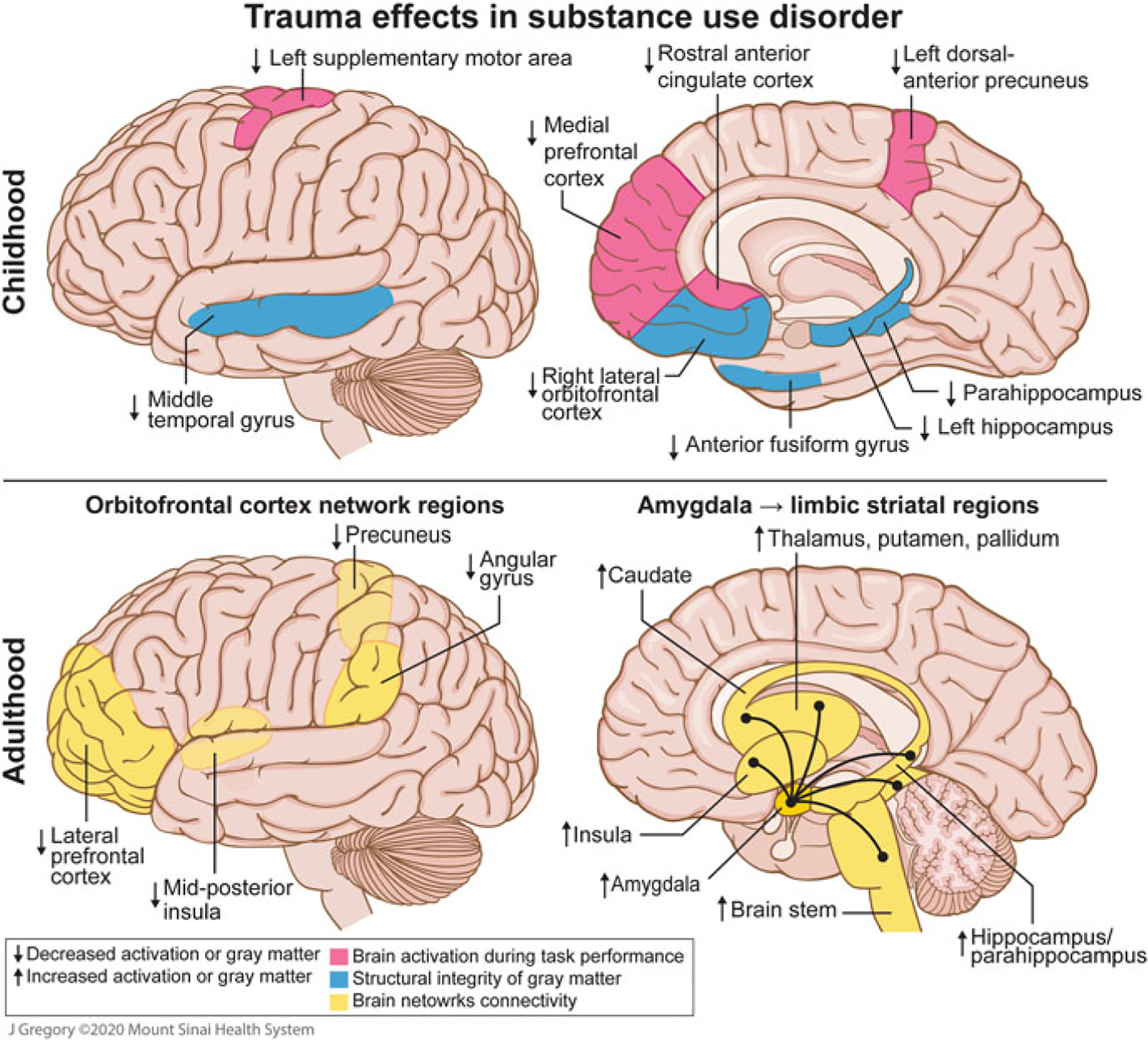
Putative neural underpinnings of childhood and lifetime trauma in substance use disorder. Images depicting the potential effects of exposure to childhood maltreatment on gray matter volume or concentration and functional activity, and the effects of exposure to trauma during adulthood on brain connectivity. Top left and right: Childhood maltreatment was associated with decreased gray matter volume in the left hippocampus, parahippocampus, and anterior fusiform gyrus in individuals with substance use (alcohol, cocaine, and/or cannabis) (Van Dam et al. 2014) as well as decreased gray matter concentration in the right lateral orbitofrontal cortex and middle temporal gyrus in individuals with cocaine use disorder (CUD) (Bachi et al. 2018). Social stress (induction) was associated with decreased activation in the left supplementary motor area, rostral anterior cingulate cortex, and left dorsal-anterior precuneus, in individuals with past childhood maltreatment and CUD (Elton et al. 2015). Life stress during adolescence and severity of alcohol use was associated with decreased activation in the medial prefrontal cortex during reward processing in males (Casement et al. 2015). Bottom left: In adulthood, females with posttraumatic stress disorder, primarily from sexual trauma, and substance use (methamphetamine and/or cocaine) had reduced orbitofrontal cortex task-modulated functional connectivity in the lateral prefrontal cortex, mid-posterior insula, angular gyrus, and precuneus, during the interoceptive-exteroceptive attention task (Poppa et al. 2019). Bottom right: In individuals with CUD and trauma history, enhanced amygdala resting-state functional connectivity with limbic-striatal regions was observed (Gawrysiak et al. 2017)
2.1. Social Stress in Early Life
Studies that examined early life stress typically focused on the effects of childhood maltreatment in individuals with SUD, which includes physically, emotionally, and sexually abusive events and incidences of physical and emotional neglect (Van Dam et al. 2014; Bachi et al. 2018; Elton et al. 2015; Ieong and Yuan 2018). Extending this line of inquiry beyond the specific definition of childhood trauma, one study assessed cumulative stressful life experiences during adolescence (ages 15–18) with stressors ranging from poor performance in school to arguments with family members (Casement et al. 2015).
Several studies report structural brain alterations in individuals with SUD and early life trauma, which in turn may contribute to behaviors underlying SUD (see Fig. 1, top panel). Decreased hippocampal, parahippocampal, and anterior fusiform gyrus gray matter volume in a sample with alcohol, cannabis, and/or cocaine use was associated with childhood maltreatment while controlling for SUD status (Van Dam et al. 2014). Altered structural integrity in regions of the hippocampal complex overlaps with findings from preclinical studies on early life stress (Lupien et al. 2009; McEwen 2010), thus providing an important translational link. Altered reward-based learning, anxiety, depression, and/or addiction may be mediated by deficits in the hippocampal complex (Van Dam et al. 2014; Hyman and Malenka 2001). Moreover, reduced gray matter concentration in the right lateral orbitofrontal cortex (OFC) observed in individuals with cocaine use disorder (CUD) with a history of high childhood trauma was correlated with increased depression and reduced constraint (Bachi et al. 2018). The OFC (and other prefrontal regions) are highly linked to drug-seeking motivation (however, decreased motivation for other goals), awareness and interoception, decision making, learning and memory, and salience attribution (Goldstein and Volkow 2011). Deficits in the right OFC/superior temporal gyrus were observed in individuals with childhood maltreatment history and without addiction in an extensive meta-analysis (Lim et al. 2014). Evidence of decreased gray matter in the OFC in addiction literature is abundant too, however, these brain morphology effects, previously attributed to substance use, could be shaped in a premorbid stage by early exposure to trauma or an additive effect of both characteristics (Bachi et al. 2018).
Functional outcomes provide further clarity on neural pathways connecting early life stress contributions to drug-seeking behaviors. Individuals with CUD and high trauma showed decreased activation in the left dorsal-anterior precuneus and left supplementary motor area during the stress portion of a stress-induction paradigm (as compared to the baseline neutral script) (Elton et al. 2015). Diminished responses in the dorsal-anterior precuneus and supplementary motor area in individuals with childhood maltreatment history suggest a functional compromise in the ability to engage parietal-motor networks and controlled versus automated action selection in response to stressful experiences (Elton et al. 2015; Nachev et al. 2007). Similarly, stress-induction led to a loss in volitional control of behavior, indicating it could be an intermediate mechanism in a nonlinear pathway, beginning in childhood adversity and leading to SUD (Hosking and Winstanley 2011). An interaction between maltreatment severity and drug craving indicated reduced activity in the rostral anterior cingulate cortex (Elton et al. 2015), a region important for emotional conflict resolution (Etkin et al. 2006). This deactivation suggests that childhood maltreatment may attenuate a key mechanism of conflict resolution vital for adaptive stress responses (Elton et al. 2015). Hence, the anterior cingulate cortex, a region identified as a central “hub” for addiction-related neural networks (Zhao et al. 2020), may also mediate the relationship between social stress and craving. These findings are broadened in a longitudinal fMRI study of male participants, which found that higher cumulative stressful life events during adolescence were associated with problematic alcohol use and decreased mPFC activation during a reward task (Casement et al. 2015). Previous neuroimaging research found that acute psychosocial stressors resulted in a significant decrease in reward-related responses in the mPFC (Ossewaarde et al. 2011), however, this study is the first to show a link between cumulative life stress and blunted mPFC reward response (Casement et al. 2015). As the mPFC plays an important role in reward processing and drug reinstatement (Perry et al. 2011), it is plausible that chronic stress during adolescence blunts neural mechanisms that help regulate alcohol-motivated behavior; thus, stress exposure diminishes mPFC response to naturally rewarding events and heightens the perceived benefits of alcohol use (Koob and Le Moal 1997; Casement et al. 2015).
Beyond enhancing drug-seeking behaviors, early exposure to trauma may increase the risk of relapse in individuals with SUD. Childhood maltreatment predicted a shorter time to drug relapse and gray matter volume reductions in the hippocampal complex were correlated to greater severity of substance use after initial relapse in individuals with SUD (Van Dam et al. 2014). Activation of the occipital cortex in response to drug cues was attributed to modulation of visual attention to the conditional motivational properties of drug cues in individuals with CUD (Hogarth et al. 2009). This heightened response leads to enhanced drug craving responses, and likely increases the potential to relapse (Elton et al. 2015). The processes of drug-seeking and drug use behaviors are proposed to be automatically engaged during stress (Pierce and Vanderschuren 2010), thereby supporting the observation that acute social stress and/or chronic exposure to childhood adversity is linked to risk of relapse (Elton et al. 2015).
Taken together, these in vivo neuroimaging findings suggest that early life stress has widespread effects on individuals suffering from addiction at the brain, behavioral, and clinical levels, that lead to long-lasting marks. Examination of structural integrity in gray matter and task-based brain activation revealed that individuals with childhood maltreatment and substance use disorder have decreased volume or activation in key regions of executive function and the hippocampal complex (see Fig. 1, top panel). Interestingly, these results are consistent with studies examining structural and functional alterations in individuals with childhood trauma and without substance use history (Teicher et al. 2012, 2016; Hanson et al. 2015; Andersen et al. 2008; Carrion et al. 2007; Teicher and Samson 2016). As such, it is difficult to disentangle the neural effects of early life stress from the neural effects of SUDs. The observed differences in brain structure and function have been associated with SUD severity, risk of relapse, depression, and other important clinical symptomatology, indicating that individuals with SUD and a history of early life stress may represent a clinically and biologically distinct phenotype (Bachi et al. 2018; Dannlowski et al. 2012). There is likely overlap between brain regions implicated in early life stress and substance use, however, the severity and pathology may be expected to be worse in individuals with childhood trauma and SUD given their clinical differences. Within this subset of studies that explored the neural underpinnings of childhood maltreatment in SUD, the mesocorticolimbic pathways which are central to SUD are impacted by a history of early life trauma. These neural alterations may contribute to an individual’s pattern of SUD, drug-related behaviors, and clinical symptomatology that might inform models of trauma-informed treatment.
2.2. Social Stress in Adulthood/Lifetime
While significant attention in the field has focused on the impact of stress during childhood, the neurobiological correlates of trauma exposure in adult SUD or cumulative lifetime chronic stress are understudied. We provide an overview of cross-sectional studies that contrast brain connectivity in adult individuals with SUD and healthy controls.
Sexual trauma in adulthood is a significant stressor that has long-lasting consequences. In females with polysubstance use (primarily methamphetamine and cocaine), those with a PTSD diagnosis showed diminished functional connectivity of the OFC network in the precuneus, mid-posterior insula, lateral prefrontal cortex, and angular gyrus during an interoception task (Poppa et al. 2019) (see Fig. 1, bottom left). These observations are consistent with prior studies of posttraumatic stress in individuals without substance use history, which found reduced orbitofrontal or ventromedial prefrontal cortex activity during trauma-related tasks (Daigre et al. 2015; Moser et al. 2015). Moreover, across the sample of females with SUD, OFC network strength was inversely associated with sexual violence exposure, which accounted for an additional variance beyond the effects of PTSD. This indicates that not only is diminished OFC network strength predictive of PTSD, the OFC is functionally sensitive to the cumulative effects of sexual trauma in females with SUD (Poppa et al. 2019). Individuals with CUD and a history of trauma showed enhanced amygdala resting-state functional connectivity with limbic-striatal regions (Gawrysiak et al. 2017) (see Fig. 1, bottom right). Hyperactivity in the amygdala, a region implicated in fear processing and learning in response to threat, has been linked to traumatic stress (Brown et al. 2014; Lanius et al. 2006); increased amygdala response to evocative cues has been observed in individuals with PTSD and anxiety disorders (Patel et al. 2012; Shin and Liberzon 2010). Additionally, amygdala resting-state functional connectivity has been sensitive enough to distinguish between CUD patients (Gu et al. 2010) and heroin-dependent patients (Ma et al. 2010) from healthy controls, therefore these results may reveal further within-group heterogeneity. The impact of prior adversity on resting-state functional connectivity emphasizes the relevance of these brain regions as potential biomarkers for clinical vulnerability (Gawrysiak et al. 2017). These findings of functional connectivity, both task-modulated and at rest, indicate that individuals who experienced trauma in adulthood and are battling SUD may have attenuated connectivity in brain regions involved in executive function and fear processing (Poppa et al. 2019; Gawrysiak et al. 2017).
2.3. Social Stress in Early Life and Adulthood: Cumulative Interacting Dysregulations
While the majority of social stress studies in SUD populations have focused on a particular lifetime period (e.g., childhood/adulthood), notably exposure to stress in early life is associated with heightened vulnerability to social stress in adulthood (Miller et al. 2011). Early life stress during critical periods of neurodevelopment could have broad effects on networks related to the stress response and thus may lead to an evolving phenotype with altered allostatic processes and reduced adaptability to stress later in life (Agorastos et al. 2019; Taylor 2010). A chronically impaired stress response involves enduring hyper- or hypo-activation of the stress system and altered glucocorticoid signaling, alterations in emotional and autonomic reactivity, circadian rhythm disruption, functional and structural changes in the brain, immune and metabolic dysregulation, and epigenetic modifications [see review: 25]. The extent of subsequent vulnerability to later-life stress involves factors such as the timing, duration, intensity/severity, and type of early life stress as well as other later-life challenges, such as type of additional stressors, coping strategies, support systems, lifestyle, and aging (Agorastos et al. 2019; Teicher et al. 2016; Teicher and Samson 2016). Although most findings point to a causal relationship between early life stress and psychobiological maladaptation in later life, the precise developmental trajectories and their temporal coincidence remain unclear (Agorastos et al. 2019).
3. Stress-Induction Paradigms
In addition to studying the long-term consequences of stress, the neural responsivity during an acute stress condition is also of importance in understanding neural systems particularly sensitive to stress. Such knowledge is accomplished by studies with simultaneous in vivo neuroimaging and stress-inducing tasks performed in a social context. Such tasks include guided imagery induction, social ball tossing, facial emotion recognition, psychosocial stress, and relationship conflict. Neural responses elicited during these tasks have been used to predict clinical outcomes in SUD as summarized in Table 1 and Fig. 2.
Fig. 2.
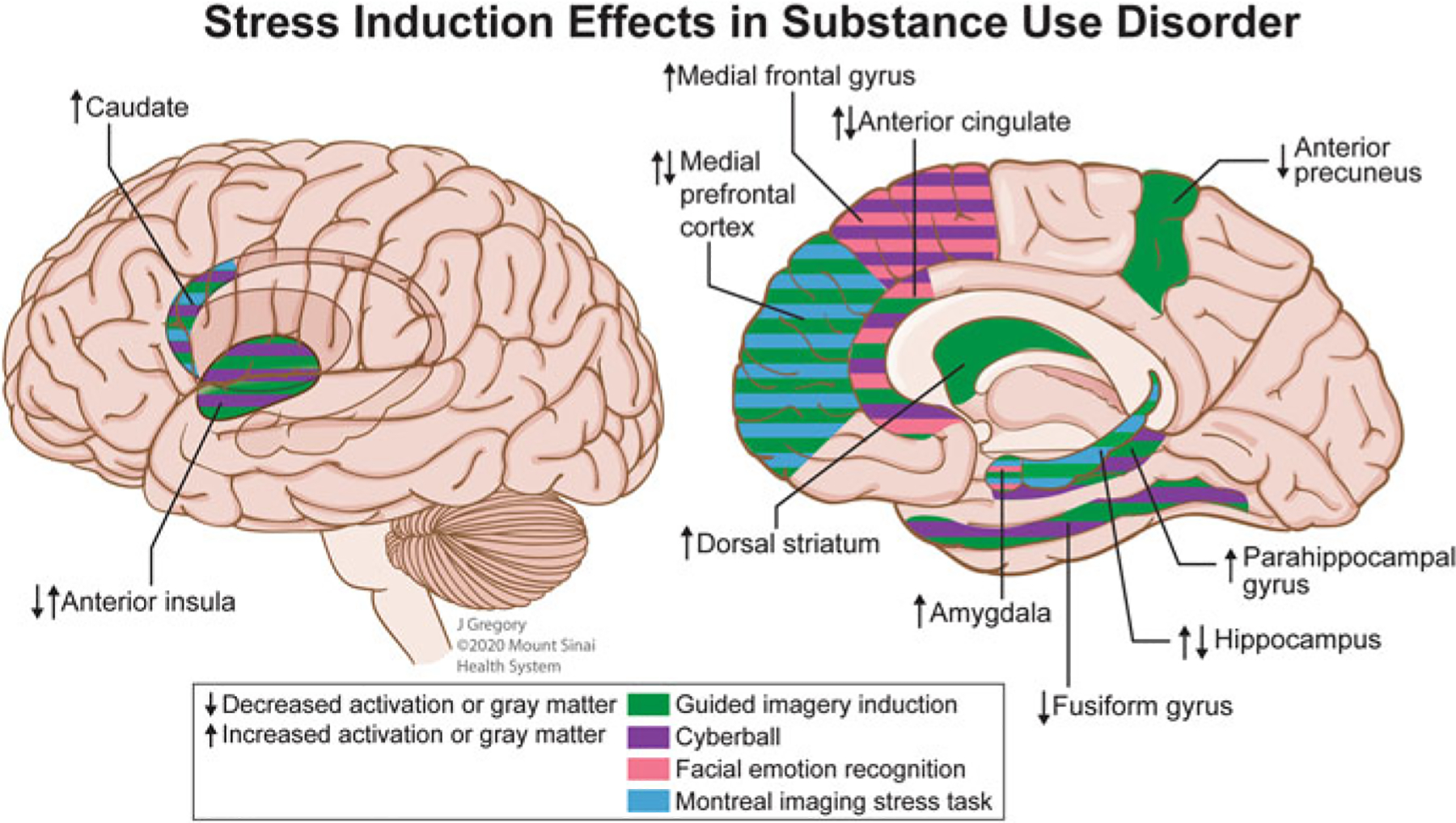
Putative neural underpinnings of stress induction in substance use disorder. Images depicting the potential effects of exposure to task-induced social stress on functional activation that was consistent between two or more studies. During the stress script portion of the Guided Imagery Induction task, increased activation was observed in critical brain areas implicated in addiction including the dorsal striatum in social drinkers (Seo et al. 2011) and individuals with cocaine use disorder (CUD) (Sinha et al. 2005); the medial prefrontal cortex (Li et al. 2006), anterior cingulate (Elton et al. 2015; Sinha et al. 2005; Li et al. 2006), and caudate (Sinha et al. 2005) in individuals with CUD; the hippocampus, parahippocampal gyrus, anterior insula, amygdala, and precuneus in nicotine smokers in mindfulness or cognitive behavioral treatment programs was associated with a lower smoking reduction (Kober et al. 2017). Decreased activation was observed during the stress script portion of the task in individuals with CUD in the anterior precuneus (Elton et al. 2015), hippocampus, and fusiform gyrus (Sinha et al. 2005). In the social exclusion portion of the Cyberball task, increased activation in the caudate and medial frontal gyrus was observed in individuals with CUD compared to social inclusion (Hanlon et al. 2018). Reduced dorsal anterior cingulate cortex response to social exclusion in adolescents with high anxiety predicted increased substance use later in adolescence (Beard et al. 2021). Re-inclusion after social exclusion resulted in increased activation of the parahippocampal gyrus and anterior cingulate in individuals with AUD (Maurage et al. 2012). Social exclusion and inclusion were both associated with decreased activation in the fusiform gyrus (Bach et al. 2019b) and decreased anterior insula volume was associated with reduced feelings of inclusion during the inclusion trial and increased feelings of exclusion during exclusion (Bach et al. 2019a) in opioid maintenance treatment patients. Angry faces, as compared to neutral faces, during a facial emotion recognition task, elicited decreased amygdala response in healthy individuals administered a high MDMA dose (Bedi et al. 2009). In a modified Hariri faces-task, aversive faces-face shapes resulted in increased anterior cingulate/medial frontal gyrus and precuneus activation in AUD patients (Charlet et al. 2014). The Montreal Stress Imaging Task, performed in nicotine smokers, demonstrated decreased activation in the hippocampus, and amygdala; following stress, an increased neural response to drug cues in the medial prefrontal cortex and caudate was observed (Dagher et al. 2009)
3.1. Guided Imagery Induction: Stress Script
The guided imagery induction uses a scene development interview to calibrate the response to a stressful scenario (Miller et al. 1987; Sinha et al. 1992). Stress scripts are based on a recent personal event and are followed by a neutral script that is developed based on the participant’s description of a personal neutral situation. Participants listen to an audio recording of these personalized stress, neutral, and in some situations, drug use, scenarios while in the MRI scanner. Studies contrast brain activation during the stress or drug use scripts to activation during the neutral stimuli.
Results from such studies report increased frontostriatal and frontolimbic activity during subjective social stress as important predictors of craving. Increased activation in the dorsal striatum (Seo et al. 2011) and caudate (Sinha et al. 2005) were associated with greater post-stress craving scores in individuals with CUD and social drinkers. As the dorsal striatum has been associated with learned habits and procedural learning (Berke and Hyman 2000), stress-induced craving related to dorsal striatum/caudate activity highlights the habitual nature of substance use and suggests that chronic stress may alter habit-based decision making (Dias-Ferreira et al. 2009). An inverse relationship was found between anterior cingulate and posterior cingulate cortices activity and craving in females with CUD (Li et al. 2005; Li et al. 2006), suggesting the distinct role of the cingulate cortices in modulating stress-induced craving (Goldstein and Volkow 2002) and that females may employ more verbal coping strategies than males when experiencing stress (McCrae and Costa 1986). Furthermore, decreased posterior cingulate cortex and increased mPFC activity during stress were associated with low socialization scores in females with CUD (Li et al. 2006). This finding may reflect that the posterior cingulate cortex was engaged during emotional distress and the mPFC is involved with the suppression of emotional distress, thereby suggesting that reduced socialization impacts the processing of negative emotional stimuli (Li et al. 2006; Phan et al. 2005). In addition to cocaine and alcohol use, nicotine addiction has been studied in relation to acute stress using the guided imagery induction. Activity in the amygdala, insula, and hippocampal regions in nicotine smokers undergoing mindfulness or cognitive behavioral treatment was negatively correlated with post-treatment reduction in smoking (Kober et al. 2017). As the amygdala and insula have been implicated in drug craving (Chase et al. 2011; Garavan 2010; Jasinska et al. 2014; Mihov and Hurlemann 2012), this suggests that smoking reduction treatments may reduce feelings of craving that could lead to positive clinical outcomes (Kober et al. 2017).
Due to its subjective nature, the effect of performing the guided imagery task can elicit an experience of enhanced memories and emotional activation related to past traumas. Structural and functional alternations across early and lifetime adversity involved similar brain regions as those engaged by this paradigm, indicating that these brain regions may have a predisposed vulnerability to the effects of social stress (see Table 1, and Figs. 1 and 2).
3.2. Social Ball Tossing: Cyberball
The social ball-tossing paradigm, called “Cyberball,” is an online task where participants are informed that they are playing with actual people nearby to enhance the authenticity of the social context. The paradigm induces states of social inclusion, exclusion, and re-inclusion (after social exclusion) (Williams and Jarvis 2006). During social exclusion, the task has been shown to engage heightened insula activity in individuals with alcohol use disorder (AUD), which suggests an association between AUD and higher emotional reactions to ostracism (Maurage et al. 2012). Functional activation and structural deficits of the insula have demonstrated the pertinence of this region in aspects underlying SUD, particularly in indexing a higher emotional reaction to social rejection (Eisenberger et al. 2003; Moor et al. 2012). A similar outcome was found in individuals with OUD (Bach et al. 2019a). Decreased gray matter volume in the anterior insula in opioid methadone treatment patients was associated with stronger feelings of exclusion during the exclusion trial and reduced feelings of inclusion during the inclusion trial. Moreover, insula gray matter volume was negatively correlated with social interaction anxiety symptoms, thereby highlighting the role of this brain region in emotion- and anxiety-processing (Bach et al. 2019a).
Select frontal regions, as well as limbic-related regions (e.g., parahippocampal gyrus), have also been implicated in the processing of feelings of social ostracism (Thoits 2010; Maurage et al. 2012; Bach et al. 2019a; Hanlon et al. 2018). The frontal gyri, including the inferior frontal gyrus in individuals with OUD (Bach et al. 2019a) and medial frontal gyrus in individuals with AUD and CUD (Maurage et al. 2012; Hanlon et al. 2018), were shown to be involved in the processing of negative affect during social ostracism. Intriguingly, opposite activations were observed in individuals with AUD versus CUD in this region: individuals with AUD had reduced response in the medial frontal gyrus (Maurage et al. 2012), whereas individuals with CUD had a greater response (Hanlon et al. 2018), indicating possible drug-specific neural effects. AUD was also associated with increased activation in the dorsal anterior cingulate and parahippocampal gyrus during re-inclusion after exclusion (Maurage et al. 2012). As the frontal gyrus, anterior cingulate, and parahippocampal gyrus have previously been identified as potential treatment targets (Konova et al. 2013), these findings extend the translational relevance of these brain regions.
Recent literature suggests that neural sensitivity in the anterior cingulate induced by the Cyberball paradigm was predictive of increased substance use in adolescents (Beard et al. 2021). In particular, blunted dorsal anterior cingulate cortex response to exclusion was associated with an increased risk for substance use in adolescents with high anxiety (Beard et al. 2021). This highlights that neural response to social stress, especially the anterior cingulate cortex, may be a vulnerability marker for the propensity of substance use.
3.3. Facial Emotion Recognition
Facial emotion recognition tasks are used to assess the recognition of basic facial expressions. In some tasks, brain activation during emotional expressions is contrasted with neutral expressions (Ekman and Friesen 1976; Bedi et al. 2009); in others, brain activation while matching faces (selecting the face which matches the target face) is counterbalanced with neutral shapes (Hariri et al. 2002; Charlet et al. 2014). Through these paradigms, the neural response to viewing socially threatening faces (fearful, angry, disgusted) was analyzed in individuals with SUD.
Acute substance use dysregulated the processing of social signals (Bedi et al. 2009), whereas chronic substance use in treatment-seekers affected neural response in regions implicated in childhood maltreatment (Charlet et al. 2014) (see Fig. 1, top panel, and Fig. 2) and predicted positive treatment outcomes. Healthy individuals administered a high dose of ±3,4 Methylenedioxymethamphetamine (MDMA) responded to angry faces with decreased activation in the left amygdala, while those treated with low dose MDMA responded to happy faces with enhanced ventral striatum activation (Bedi et al. 2009), a region predictive of reward signals (Knutson and Cooper 2005). Collectively, these results indicate that MDMA increased sociability, even in situations of social threat (Bedi et al. 2009) which is in line with enhanced empathy and prosocial behavior induced by MDMA. Abstinent individuals with AUD displayed strong left rostral anterior cingulate cortex, left mPFC, and right precuneus response to aversive facial stimuli versus neutral shapes (Charlet et al. 2014), regions implicated in childhood and adult trauma effects (Elton et al. 2015; Casement et al. 2015; Poppa et al. 2019) (see Figs. 1 and 2). The anterior cingulate cortex, a brain region implicated in emotion regulation (Phelps and LeDoux 2005; Kienast et al. 2008), was positively associated with days of abstinence and negatively associated with binge drinking, suggesting that it may represent a resilience factor that protects against relapse in patients recovering from AUD (Charlet et al. 2014).
3.4. Relationship Conflict: In the Context of Intimate Partner Violence
A relationship conflict task explores sex differences to social stress and conflict resolution in individuals in a romantic relationship by requiring the couple to work toward a resolution together (Flanagan et al. 2019). Similar to the guided imagery induction, the script for this task is personalized to the participant; a topic of relationship difficulty is identified by each partner, and a recording of the couple discussing the topic is played in the MRI scanner. Brain response during conflict resolution with a partner is contrasted with brain response during the neutral script (recording of participant discussing their morning routine) in couples with one partner engaging in hazardous drinking or meeting DSM-IV criteria for SUD (primarily cocaine or cannabis) (Flanagan et al. 2019). Results from the neuroimaging study show that for both sexes the amygdala is functionally engaged during the relationship conflict (Flanagan et al. 2019). However, females show greater functional connectivity between the right amygdala-left inferior frontal cortex, whereas males have stronger functional connectivity between the OFC-right amygdala/hippocampus (Flanagan et al. 2019). Intimate partner violence, highly prevalent and salient in SUD populations (Afifi et al. 2009; Chermack et al. 2008; Leonard and Homish 2008), is associated with increased functional connectivity between the right amygdala-left prefrontal cortex. These findings suggest that the amygdala circuitry plays a crucial role in both males and females with substance use during processing relationship conflict (Flanagan et al. 2019).
3.5. Psychosocial Stress: Montreal Imaging Stress Task
Psychosocial stress is induced by the Montreal Imaging Stress Task (Dedovic et al. 2005), based on the Trier Mental Challenge Task (Kirschbaum et al. 1993), by having participants solve difficult mental arithmetic problems on a computer with external pressure. This includes adjusting the time limit to ensure a >50% failure rate, having direct negative feedback from one of the investigators, and showcasing performance progress on the monitor. The combination of these external pressures creates an anxiety-producing social environment during an already challenging task. Neural activation during the Montreal Imaging Stress Task was contrasted with a non-stress control version of the task in nicotine smokers (Dagher et al. 2009). Widespread deactivations in limbic and paralimbic systems, notably in the hippocampus, amygdala, and nucleus accumbens were observed (Dagher et al. 2009). The deactivations predicted smoking cue-activity and craving in brain areas controlling attention and motivation, thereby again suggesting mesocorticolimbic circuits underlie stress in addiction and relapse (Dagher et al. 2009).
In summary, task-related fMRI activation during a range of social contexts has provided insights into neural circuits affecting acute stress response in relation to SUD. The different stress paradigms – guided imagery induction, social ball tossing, facial emotion recognition, relationship conflict, and psychosocial stress – revealed activations in distinct regions and overlapping regions predictive of addiction trajectory (Fig. 2). A few patterns emerged among these tasks: (1) there was an overlap between regions implicated in SUD or behaviors related to drug use, and the processing of social stress, (e.g., anterior insula, amygdala, hippocampus, and medial prefrontal cortex); (2) the anterior cingulate cortex, caudate, and amygdala activations were most commonly observed among stress paradigms; and (3) greater activation to drug cues and higher reporting of craving emerged post-stress. Paradigm-specific trends emerged as well. During the stress script paradigm, hypoactivation was observed in regions involved in emotion regulation, while hyperactivation was observed in regions involved in drug craving and substance use-related behaviors (Elton et al. 2015; Seo et al. 2011; Sinha et al. 2005; Li et al. 2006; Kober et al. 2017). Feelings of social exclusion elicited by Cyberball produced increased neural responses in frontal regions involved in processing negative affect, while decreased responses in frontal regions were associated with regulating feelings of social ostracism (Maurage et al. 2012; Bach et al. 2019a; Hanlon et al. 2018; Bach et al. 2019b). The aversive face paradigm induced hyperactivation in brain regions implicated in control, decision making, and higher-order executive functioning (Bedi et al. 2009; Charlet et al. 2014).
4. Future Directions
Main results from 21 neuroimaging studies were reviewed, corroborated, and ultimately deemed instrumental in identifying key brain regions that are dysregulated in individuals with early and lifetime social stress with SUD, and those involved in the processing of social stress stimuli in individuals with substance use. These findings should, however, be considered in light of methodological limitations in this emerging research area. The fact that the body of knowledge is based on only 21 studies to explore the intersection of substance use/disorder and social stress should question the generalizability of these findings.
One of the main challenges of investigating social stress in SUD is disentangling the effects of social stress from the effects of substance use. Most studies utilized a cross-sectional design comparing individuals with substance use history and trauma history to healthy controls. Only a few included a sample with substance use history and no trauma history or healthy individuals with trauma history. Moreover, none of the studies did all of the above. The absence of a healthy control group with trauma and an SUD group without trauma means that it is impossible to confirm the unique neural effects of social stress in individuals with SUD. Many of the structural and functional alterations identified in the early and lifetime studies corroborated findings from previous research on stress or trauma in samples without substance use history, indicating that these effects may be trauma-specific. Studies of social stress in adulthood largely did not account for histories of childhood maltreatment in their sample, diminishing the possibility to identify effects of cumulative stress dysregulation. The neuroimaging studies exploring the acute response to stress in SUD also failed to account for past trauma history, except for one study (Elton et al. 2015). Without acknowledging that prior stress may impact current stress response, a complete and accurate depiction of the neural underpinnings of stress in SUD cannot be determined. To disentangle the brain effects due to addiction versus social stress, future studies must include a control group with high trauma and no substance use history.
In the same vein, the effects of non-social stress must be disentangled from social stress. Capturing social stress in a natural setting is a difficult undertaking. It requires longitudinal studies and in vivo neuroimaging tasks that include naturalistic stimuli related to an individual’s social context. However, rather than developing novel in vivo neuroimaging paradigms, extending subjective neuroimaging paradigms such as the Guided Imagery Induction, and supplementing them with ecological momentary assessments (EMA) is a more pragmatic approach (Stone and Shiffman 1994). EMA enables data collection during the daily life of a person and has the potential to capture important information (feelings, thoughts, emotions, cravings, and more) related to social stress. Such methodological advances could guide further research.
The neurobiological sex differences in response to social stress in SUD populations is another area that requires further exploration. As sex-specific risk factors for mental illness disproportionately affect females, particularly sexual violence (Flanagan et al. 2019; Oram et al. 2017), further analysis into sex-specific effects is warranted. While currently limited, some studies are suggesting important sex differences. For example, in healthy participants, fMRI studies revealed different neurobiological alterations associated with psychological stress and during processing social stress in females as compared to males (Wang et al. 2007; Goldfarb et al. 2019). A few of the studies summarized in this chapter also suggest unique neural effects of social stress in females versus males with alcohol or cocaine use associated with craving and reduced social functioning (Seo et al. 2011; Li et al. 2005; Li et al. 2006). However, the sex differences in neurobiological signatures associated with social stress have not been fully characterized in SUD. The clear roles that sex plays in various aspects of stress sensitivity and mental illness emphasize the importance of expanding knowledge of the female brain that could also lead to enhanced sex-specific interventions.
In addition to the issues raised above, several questions remain. How do brain alterations in SUD with a history of chronic stress or induced (acute) stress differ depending on drug class? What are the unique neural pathways of social stress in SUD as compared with other types of stress (e.g., stress associated with withdrawal)? What targeted treatments may be suitable to address neural and behavioral impairments resulting from social stress in SUD? Systematic research on these questions through rigorous clinical studies in diverse addictions samples accounting for past trauma as well as measuring stress response could contribute to a greater understanding of the multi-faceted role of social stress in SUD.
5. Conclusion
Neuroimaging studies provide remarkable insight into the effects of social stress on brain structure, function, and connectivity in SUD. As summarized in this chapter, social stress history and induction alter brain regions implicated in drug-seeking, taking, and craving pathways. These results lead to a few hypotheses: (1) chronic social stress leads to neural alterations underlying a propensity for drug use; (2) brain effects previously attributed to drug use may be, at least in part, impacted also by stress exposure; (3) brain regions impacted by chronic substance use affects stress response thereby modulating social function; and (4) synergistic effects of social stress and SUD may result in further increased SUD severity and social dysfunction. The burgeoning interest in the “social factors of addiction” will hopefully accelerate our understanding of how an individuals’ exposure to adverse life events, trauma, or chronic stress affects their substance use trajectory. Solidifying the knowledge of neural pathways of social stress in SUD is crucial to inform causal research, prevention, and targeted addiction treatments.
Acknowledgements
Funding:
National Institute on Drug Abuse—Grant K23DA045928 (KB); The Addiction Institute of Mount Sinai, and Department of Psychiatry support.
References
- Afifi TO, MacMillan H, Cox BJ, Asmundson GJ, Stein MB, Sareen J (2009) Mental health correlates of intimate partner violence in marital relationships in a nationally representative sample of males and females. J Interpers Violence 24(8):1398–1417. 10.1177/0886260508322192 [DOI] [PubMed] [Google Scholar]
- Agorastos A, Pervanidou P, Chrousos GP, Baker DG (2019) Developmental trajectories of early life stress and trauma: a narrative review on neurobiological aspects beyond stress system dysregulation. Front Psych 10(118). 10.3389/fpsyt.2019.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH (2008) Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 20(3):292–301. 10.1176/jnp.2008.20.3.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Frischknecht U, Klinkowski S, Bungert M, Karl D, Vollmert C, Vollstadt-Klein S, Lis S, Kiefer F, Hermann D (2019a) Higher social rejection sensitivity in opioid-dependent patients is related to smaller insula gray matter volume: a voxel-based morphometric study. Soc Cogn Affect Neurosci 14(11):1187–1195. 10.1093/scan/nsz094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Frischknecht U, Bungert M, Karl D, Vollmert C, Vollstadt-Klein S, Lis S, Kiefer F, Hermann D (2019b) Effects of social exclusion and physical pain in chronic opioid maintenance treatment: fMRI correlates. Eur Neuropsychopharmacol 29(2):291–305. 10.1016/j.euroneuro.2018.11.1109 [DOI] [PubMed] [Google Scholar]
- Bachi K, Parvaz MA, Moeller SJ, Gan G, Zilverstand A, Goldstein RZ, Alia-Klein N (2018) Reduced orbitofrontal gray matter concentration as a marker of premorbid childhood trauma in cocaine use disorder. Front Hum Neurosci 12:51. 10.3389/fnhum.2018.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard SJ, Hastings PD, Ferrer E, Robins RW, Guyer AE (2021) Neural response to social exclusion moderates the link between adolescent anxiety symptoms and substance use. Biol Psychiatry Cogn Neurosci Neuroimaging. 10.1016/j.bpsc.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H (2009) Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology 207(1):73–83. 10.1007/s00213-009-1635-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA (2011) Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci 1216:114–121. 10.1111/j.1749-6632.2010.05896.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S (2007) Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience 144(1):8–16. 10.1016/j.neuroscience.2006.08.075 [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25(3):515–532. 10.1016/s0896-6273(00)81056-9 [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W (2003) Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse Negl 27(2):169–190. 10.1016/s0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- Blum J, Gerber H, Gerhard U, Schmid O, Petitjean S, Riecher-Rössler A, Wiesbeck GA, Borgwardt SJ, Walter M (2013) Acute effects of heroin on emotions in heroin-dependent patients. Am J Addict 22(6):598–604. 10.1111/j.1521-0391.2013.12025.x [DOI] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, Mid-Atlantic MW, McCarthy G, Morey RA (2014) Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology 39(2):351–359. 10.1038/npp.2013.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carliner H, Keyes KM, McLaughlin KA, Meyers JL, Dunn EC, Martins SS (2016) Childhood trauma and illicit drug use in adolescence: population-based national comorbidity survey replication - adolescent supplement study. J Am Acad Child Adolesc Psychiatry 55 (8):701–708. 10.1016/j.jaac.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Reiss AL (2007) Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics 119(3):509–516. 10.1542/peds.2006-2028 [DOI] [PubMed] [Google Scholar]
- Casement MD, Shaw DS, Sitnick SL, Musselman SC, Forbes EE (2015) Life stress in adolescence predicts early adult reward-related brain function and alcohol dependence. Soc Cogn Affect Neurosci 10(3):416–423. 10.1093/scan/nsu061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet K, Schlagenhauf F, Richter A, Naundorf K, Dornhof L, Weinfurtner CE, Konig F, Walaszek B, Schubert F, Muller CA, Gutwinski S, Seissinger A, Schmitz L, Walter H, Beck A, Gallinat J, Kiefer F, Heinz A (2014) Neural activation during processing of aversive faces predicts treatment outcome in alcoholism. Addict Biol 19(3):439–451. 10.1111/adb.12045 [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L (2011) The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry 70(8):785–793. 10.1016/j.biopsych.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermack ST, Murray RL, Walton MA, Booth BA, Wryobeck J, Blow FC (2008) Partner aggression among men and women in substance use disorder treatment: correlates of psychological and physical aggression and injury. Drug Alcohol Depend 98(1–2):35–44. 10.1016/j.drugalcdep.2008.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger JJ (1956) Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol 17:296–305 [PubMed] [Google Scholar]
- Conroy E, Degenhardt L, Mattick RP, Nelson EC (2009) Child maltreatment as a risk factor for opioid dependence: comparison of family characteristics and type and severity of child maltreatment with a matched control group. Child Abuse Negl 33(6):343–352. 10.1016/j.chiabu.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D (2009) An acute psychosocial stress enhances the neural response to smoking cues. Brain Res 1293:40–48. 10.1016/j.brainres.2009.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigre C, Rodriguez-Cintas L, Tarifa N, Rodriguez-Martos L, Grau-Lopez L, Berenguer M, Casas M, Roncero C (2015) History of sexual, emotional or physical abuse and psychiatric comorbidity in substance-dependent patients. Psychiatry Res 229(3):743–749. 10.1016/j.psychres.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H (2012) Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71(4):286–293. 10.1016/j.biopsych.2011.10.021 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6(6):463–475. 10.1038/nrn1683 [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, Pruessner JC (2005) The Montreal imaging stress task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatr Neurosci 30(5):319–325 [PMC free article] [PubMed] [Google Scholar]
- Dewall CN, Macdonald G, Webster GD, Masten CL, Baumeister RF, Powell C, Combs D, Schurtz DR, Stillman TF, Tice DM, Eisenberger NI (2010) Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci 21(7):931–937. 10.1177/0956797610374741 [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N (2009) Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325(5940):621–625. 10.1126/science.1171203 [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD (2003) Does rejection hurt? An FMRI study of social exclusion. Science 302(5643):290–292. 10.1126/science.1089134 [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV (1976) Pictures of facial affect. Consulting Psychologists Press, Palo Alto [Google Scholar]
- Elton A, Smitherman S, Young J, Kilts CD (2015) Effects of childhood maltreatment on the neural correlates of stress- and drug cue-induced cocaine craving. Addict Biol 20(4):820–831. 10.1111/adb.12162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J (2006) Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51 (6):871–882. 10.1016/j.neuron.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Farrell AD, Ampy LA, Meyer AL (1998) Identification and assessment of problematic interpersonal situations for urban adolescents. J Clin Child Psychol 27(3):293–305. 10.1207/s15374424jccp2703_6 [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am J Prev Med 14(4):245–258. 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Flanagan JC, Yonce S, Calhoun CD, Back SE, Brady KT, Joseph JE (2019) Preliminary development of a neuroimaging paradigm to examine neural correlates of relationship conflict. Psychiatry Res Neuroimaging 283:125–134. 10.1016/j.pscychresns.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H (2010) Insula and drug cravings. Brain Struct Funct 214(5–6):593–601. 10.1007/s00429-010-0259-8 [DOI] [PubMed] [Google Scholar]
- Gawrysiak MJ, Jagannathan K, Regier P, Suh JJ, Kampman K, Vickery T, Childress AR (2017) Unseen scars: cocaine patients with prior trauma evidence heightened resting state functional connectivity (RSFC) between the amygdala and limbic-striatal regions. Drug Alcohol Depend 180:363–370. 10.1016/j.drugalcdep.2017.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb EV, Seo D, Sinha R (2019) Sex differences in neural stress responses and correlation with subjective stress and stress regulation. Neurobiol Stress 11:100177. 10.1016/j.ynstr.2019.100177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159 (10):1642–1652. 10.1176/appi.ajp.159.10.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12(11):652–669. 10.1038/nrn3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN (2018a) The burden of opioid-related mortality in the United States. JAMA Netw Open 1(2):e180217. 10.1001/jamanetworkopen.2018.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN (2018b) The burden of opioid-related mortality in the United States. JAMA Netw Open 1(2):e180217–e180217. 10.1001/jamanetworkopen.2018.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y (2010) Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage 53(2):593–601. 10.1016/j.neuroimage.2010.06.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Shannon EE, Porrino LJ (2018) Brain activity associated with social exclusion overlaps with drug-related frontal-striatal circuitry in cocaine users: a pilot study. Neurobiol Stress 10:100137–100137. 10.1016/j.ynstr.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, Davidson RJ (2015) Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry 77(4):314–323. 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR (2002) The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage 17(1):317–323. 10.1006/nimg.2002.1179 [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Warner M, Minino AM (2017) Drug overdose deaths in the United States, 1999–2016, NCHS data brief no. 294. National Center for Health Statistics, Hyattsville [Google Scholar]
- Hogarth L, Dickinson A, Duka T (2009) Detection versus sustained attention to drug cues have dissociable roles in mediating drug seeking behavior. Exp Clin Psychopharmacol 17(1):21–30. 10.1037/a0014957 [DOI] [PubMed] [Google Scholar]
- Hosking J, Winstanley CA (2011) Impulsivity as a mediating mechanism between early-life adversity and addiction: theoretical comment on Lovic et al. (2011). Behav Neurosci 125 (4):681–686. 10.1037/a0024612 [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC (2001) Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2(10):695–703. 10.1038/35094560 [DOI] [PubMed] [Google Scholar]
- Ieong HF, Yuan Z (2018) Emotion recognition and its relation to prefrontal function and network in heroin plus nicotine dependence: a pilot study. Neurophotonics 5(2):025011. 10.1117/1.NPh.5.2.025011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y (2014) Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev 38:1–16. 10.1016/j.neubiorev.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Andersen SL (2017) Sensitive periods of substance abuse: early risk for the transition to dependence. Dev Cogn Neurosci 25:29–44. 10.1016/j.dcn.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ (1985) The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 142(11):1259–1264. 10.1176/ajp.142.11.1259 [DOI] [PubMed] [Google Scholar]
- Khoury L, Tang YL, Bradley B, Cubells JF, Ressler KJ (2010) Substance use, childhood traumatic experience, and posttraumatic stress disorder in an urban civilian population. Depress Anxiety 27(12):1077–1086. 10.1002/da.20751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Buchholz HG, Smolka MN, Grunder G, Cumming P, Kumakura Y, Bartenstein P, Dolan RJ, Heinz A (2008) Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci 11(12):1381–1382. 10.1038/nn.2222 [DOI] [PubMed] [Google Scholar]
- Kipke MD, Montgomery SB, Simon TR, Iverson EF (1997) “Substance abuse” disorders among runaway and homeless youth. Subst Use Misuse 32(7–8):969–986. 10.3109/10826089709055866 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH (1993) The ‘trier social stress test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28 (1–2):76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC (2005) Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol 18(4):411–417. 10.1097/01.wco.0000173463.24758.f6 [DOI] [PubMed] [Google Scholar]
- Kober H, Brewer JA, Height KL, Sinha R (2017) Neural stress reactivity relates to smoking outcomes and differentiates between mindfulness and cognitive-behavioral treatments. NeuroImage 151:4–13. 10.1016/j.neuroimage.2016.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Goldstein RZ (2013) Common and distinct neural targets of treatment: changing brain function in substance addiction. Neurosci Biobehav Rev 37(10 Pt 2):2806–2817. 10.1016/j.neubiorev.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2008) A role for brain stress systems in addiction. Neuron 59(1):11–34. 10.1016/j.neuron.2008.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278 (5335):52–58. 10.1126/science.278.5335.52 [DOI] [PubMed] [Google Scholar]
- Koob GF, Schulkin J (2019) Addiction and stress: an allostatic view. Neurosci Biobehav Rev 106:245–262. 10.1016/j.neubiorev.2018.09.008 [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm R, Lanius U, Pain C (2006) A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J Psychiatr Res 40(8):709–729. 10.1016/j.jpsychires.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Lau T, Bigio B, Zelli D, McEwen BS, Nasca C (2017) Stress-induced structural plasticity of medial amygdala stellate neurons and rapid prevention by a candidate antidepressant. Mol Psychiatry 22(2):227–234. 10.1038/mp.2016.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KM, Back SE, Hartwell KJ, Maria MM-S, Brady KT (2013) A comparison of trauma profiles among individuals with prescription opioid, nicotine or cocaine dependence. Am J Addict/Am Acad Psychiatr Alcohol Addict 22(2):127–131. 10.1111/j.1521-0391.2013.00319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus R, Folkman S (1984) Stress, appraisal, and coping. Springer, New York [Google Scholar]
- Leonard KE, Homish GG (2008) Predictors of heavy drinking and drinking problems over the first 4 years of marriage. Psychol Addict Behav 22(1):25–35. 10.1037/0893-164X.22.1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S (2005) Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology 30(10):939–946. 10.1016/j.psyneuen.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Li CS, Kosten TR, Sinha R (2005) Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry 57 (5):487–494. 10.1016/j.biopsych.2004.11.048 [DOI] [PubMed] [Google Scholar]
- Li CS, Kosten TR, Sinha R (2006) Antisocial personality and stress-induced brain activation in cocaine-dependent patients. Neuroreport 17(3):243–247. 10.1097/01.wnr.0000199471.06487.a2 [DOI] [PubMed] [Google Scholar]
- Lim L, Radua J, Rubia K (2014) Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am J Psychiatry 171(8):854–863. 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS (2006) Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci Off J Soc Neurosci 26 (30):7870–7874. 10.1523/jneurosci.1184-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DA, Turner RJ (2008) Cumulative lifetime adversities and alcohol dependence in adolescence and young adulthood. Drug Alcohol Depend 93(3):217–226. 10.1016/j.drugalcdep.2007.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10(6):434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR (2010) Addiction related alteration in resting-state brain connectivity. NeuroImage 49(1):738–744. 10.1016/j.neuroimage.2009.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Neemann J, Andenas S (1994) Life events and adjustment in adolescents: the significance of event Independence, desirability, and chronicity. J Res Adolesc 4(1):71–97. 10.1207/s15327795jra0401_5 [DOI] [Google Scholar]
- Mather M, Lighthall NR (2012) Risk and reward are processed differently in decisions made under stress. Curr Dir Psychol Sci 21(1):36–41. 10.1177/0963721411429452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Philippot P, Heeren A, Vermeulen N, Mahau P, Delperdange C, Corneille O, Luminet O, de Timary P (2012) Disrupted regulation of social exclusion in alcohol-dependence: an fMRI study. Neuropsychopharmacology 37(9):2067–2075. 10.1038/npp.2012.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, Costa PT (1986) Personality, coping, and coping effectiveness in an adult sample. J Pers 54(2):385–404. 10.1111/j.1467-6494.1986.tb00401.x [DOI] [Google Scholar]
- McEwen BS (2010) Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci 1204(Suppl):E38–E59. 10.1111/j.1749-6632.2010.05568.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2013) The brain on stress: toward an integrative approach to brain, body, and behavior. Perspect Psychol Sci 8(6):673–675. 10.1177/1745691613506907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2017a) Stress: homeostasis, rheostasis, reactive scope, allostasis and allostatic load. In: Reference module in neuroscience and biobehavioral psychology. Elsevier. 10.1016/B978-0-12-809324-5.02867-4 [DOI] [Google Scholar]
- McEwen BS (2017b) Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks) 1. 10.1177/2470547017692328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ (2011) Stress- and allostasis-induced brain plasticity. Annu Rev Med 62 (1):431–445. 10.1146/annurev-med-052209-100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M (1992) The fifth edition of the addiction severity index. J Subst Abus Treat 9(3):199–213. 10.1016/0740-5472(92)90062-s [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M (2005) Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci 7(2):103–123. 10.31887/DCNS.2005.7.2/mmeaney [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihov Y, Hurlemann R (2012) Altered amygdala function in nicotine addiction: insights from human neuroimaging studies. Neuropsychologia 50(8):1719–1729. 10.1016/j.neuropsychologia.2012.04.028 [DOI] [PubMed] [Google Scholar]
- Miller GA, Levin DN, Kozak MJ, Cook EW, McLean A, Lang PJ (1987) Individual differences in imagery and the psychophysiology of emotion. Cognit Emot 1(4):367–390. 10.1080/02699938708408058 [DOI] [Google Scholar]
- Miller GE, Chen E, Parker KJ (2011) Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull 137(6):959–997. 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor BG, Guroglu B, Op de Macks ZA, Rombouts SA, Van der Molen MW, Crone EA (2012) Social exclusion and punishment of excluders: neural correlates and developmental trajectories. NeuroImage 59(1):708–717. 10.1016/j.neuroimage.2011.07.028 [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA (2002) Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci 5(2):169–174. 10.1038/nn798 [DOI] [PubMed] [Google Scholar]
- Moser DA, Aue T, Suardi F, Kutlikova H, Cordero MI, Rossignol AS, Favez N, Rusconi Serpa S, Schechter DS (2015) Violence-related PTSD and neural activation when seeing emotionally charged male-female interactions. Soc Cogn Affect Neurosci 10(5):645–653. 10.1093/scan/nsu099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz F, Khan MI, Zubair M, Dehpour AR (2018) Neurobiology and consequences of social isolation stress in animal model-a comprehensive review. Biomed Pharmacother 105:1205–1222. 10.1016/j.biopha.2018.05.086 [DOI] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O’Neill K, Husain M, Kennard C (2007) The role of the pre-supplementary motor area in the control of action. NeuroImage 36(Suppl 2):T155–T163. 10.1016/j.neuroimage.2007.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram S, Khalifeh H, Howard LM (2017) Violence against women and mental health. Lancet Psychiatry 4(2):159–170. 10.1016/s2215-0366(16)30261-9 [DOI] [PubMed] [Google Scholar]
- Ossewaarde L, Qin S, Van Marle HJ, van Wingen GA, Fernandez G, Hermans EJ (2011) Stress-induced reduction in reward-related prefrontal cortex function. NeuroImage 55(1):345–352. 10.1016/j.neuroimage.2010.11.068 [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA (2012) Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36(9):2130–2142. 10.1016/j.neubiorev.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT (2011) Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev 65(2):124–149. 10.1016/j.brainresrev.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME (2005) Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry 57(3):210–219. 10.1016/j.biopsych.2004.10.030 [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE (2005) Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48(2):175–187. 10.1016/j.neuron.2005.09.025 [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M (1998) The role of stress in drug self-administration. Trends Pharmacol Sci 19(2):67–74. 10.1016/s0165-6147(97)01115-2 [DOI] [PubMed] [Google Scholar]
- Pierce RC, Vanderschuren LJ (2010) Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev 35(2):212–219. 10.1016/j.neubiorev.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppa T, Droutman V, Amaro H, Black D, Arnaudova I, Monterosso J (2019) Sexual trauma history is associated with reduced orbitofrontal network strength in substance-dependent women. Neuroimage Clin 24:101973. 10.1016/j.nicl.2019.101973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn K, Boone L, Scheidell JD, Mateu-Gelabert P, McGorray SP, Beharie N, Cottler LB, Khan MR (2016) The relationships of childhood trauma and adulthood prescription pain reliever misuse and injection drug use. Drug Alcohol Depend 169:190–198. 10.1016/j.drugalcdep.2016.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH (2004) Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125(1):1–6. 10.1016/j.neuroscience.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Ruisoto P, Contador I (2019) The role of stress in drug addiction. An integrative review. Physiol Behav 202:62–68. 10.1016/j.physbeh.2019.01.022 [DOI] [PubMed] [Google Scholar]
- Sandi C, Haller J (2015) Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci 16(5):290–304. 10.1038/nrn3918 [DOI] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R (2011) Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp 32(11):1998–2013. 10.1002/hbm.21165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I (2010) The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35(1):169–191. 10.1038/npp.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology 158 (4):343–359. 10.1007/s002130100917 [DOI] [PubMed] [Google Scholar]
- Sinha R (2008) Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141:105–130. 10.1196/annals.1441.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lovallo WR, Parsons OA (1992) Cardiovascular differentiation of emotions. Psychosom Med 54(4):422–435. 10.1097/00006842-199207000-00005 [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE (2005) Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology 183(2):171–180. 10.1007/s00213-005-0147-8 [DOI] [PubMed] [Google Scholar]
- Stein DJ, van Honk J, Ipser J, Solms M, Panksepp J (2007) Opioids: from physical pain to the pain of social isolation. CNS Spectr 12(9):669–670, 672–664. 10.1017/s1092852900021490 [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S (1994) Ecological momentary assessment (Ema) in behavioral medicine. Ann Behav Med 16(3):199–202. 10.1093/abm/16.3.199 [DOI] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2017) Key substance use and mental health indicators in the United States: results from the 2016 national survey on drug use and health (HHS publication no. SMA 17–5044, NSDUH series H-52). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville [Google Scholar]
- Taylor SE (2010) Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci 107(19):8507–8512. 10.1073/pnas.1003890107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA (2016) Annual research review: enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry 57(3):241–266. 10.1111/jcpp.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A (2012) Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci U S A 109(9):E563–E572. 10.1073/pnas.1115396109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K (2016) The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 17(10):652–666. 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- Thoits PA (2010) Stress and health: major findings and policy implications. J Health Soc Behav 51 (Suppl):S41–S53. 10.1177/0022146510383499 [DOI] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA (2003) Cumulative adversity and drug dependence in young adults: racial/ethnic contrasts. Addiction 98(3):305–315. 10.1046/j.1360-0443.2003.00312.x [DOI] [PubMed] [Google Scholar]
- Van Dam NT, Rando K, Potenza MN, Tuit K, Sinha R (2014) Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiat 71(8):917–925. 10.1001/jamapsychiatry.2014.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, Morales M, Epstein DH, Shaham Y (2018) Volitional social interaction prevents drug addiction in rat models. Nat Neurosci 21 (11):1520–1529. 10.1038/s41593-018-0246-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND (2017) Federal efforts to combat the opioid crisis: a status update on CARA and other initiatives. House Committee on Energy and Commerce, National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), Rockville. https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2017/federal-efforts-to-combat-opioid-crisis-status-update-cara-other-initiatives [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci Off J Soc Neurosci 22(15):6810–6818. 10.1523/jneurosci.22-15-06810.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA (2007) Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci 2(3):227–239. 10.1093/scan/nsm018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD, Jarvis B (2006) Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behav Res Methods 38(1):174–180. 10.3758/bf03192765 [DOI] [PubMed] [Google Scholar]
- Wills TA, Shiffman S (1986) Coping and substance use: a conceptual framework. Academic Press, San Diego [Google Scholar]
- Wolfe J, Kimerling R (1997) Gender issues in the assessment of posttraumatic stress disorder. In: Assessing psychological trauma and PTSD. The Guilford Press, New York, pp 192–238 [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011) Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8(8):665–670. 10.1038/nmeth.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sallie SN, Cui H, Zeng N, Du J, Yuan T, Li D, De Ridder D, Zhang C (2020) Anterior cingulate cortex in addiction: new insights for neuromodulation. Neuromodulation. 10.1111/ner.13291 [DOI] [PubMed] [Google Scholar]


