Summary
Background:
Compared to the exhaustive study of transgenerational programming of obesity and diabetes through exposures in the prenatal period, postnatal programming mechanisms are understudied, including the the potential role of breast milk composition linking maternal metabolic status (BMI and diabetes) and offspring growth, metabolic health and future disease risk.
Methods:
This narrative review will principally focus on four emergent bioactive compounds [microRNA’s (miRNA), lipokines/signaling lipids, small molecules/metabolites and fructose] that, until recently were not known to exist in breast milk. The objective of this narrative review is to integrate evidence across multiple fields of study that demonstrate the importance of these compositional elements of breast milk during lactation and the subsequent effect of breast milk components on the health of the infant.
Results:
Current knowledge on the presence of miRNA’s, lipokines/signaling lipids, small molecules/metabolites and fructose in breast milk and their associations with infant outcomes is compelling, but far from resolved. Two themes emerge: 1) maternal metabolic phenotypes are associated with these bioactives and 2) though existing in milk at low concentrations, they are also associated with offspring growth and body composition.
Conclusion:
Breast milk research is gaining momentum though we must remain focused in understanding how non-nutritive bioactive components are affected by the maternal phenotype, how they subsequently impact infant outcomes. Though early, there is evidence to suggest fructose is associated with fat mass in the 1st months of life where as 12,13 diHOME (brown fat activator) and betaine are negatively associated with early adiposity and growth.
Keywords: lactation, milk, bioactives, growth, obesity, metabolic programming
1 ∣. INTRODUCTION
Though breast milk is a complex biological fluid with known benefits for maternal and infant health as compared to formula, the compositional components responsible for these effects are little known. A recent (November 2021) PubMed search revealed ~20% more published articles on bovine milk composition as compared to human milk composition. This only highlights the gap that currently exists in the field and the accumulating evidence linking specific milk components with infant growth and the potential to protect from obesity and diabetes later in life and overall long-term health. Despite attention to breastfeeding and health, considerable gaps currently exist between what is shown epidemiologically about the benefits of human milk and the scientific evidence demonstrating the specific features of milk underlying its purported benefits 1. It is important that the scientific community continues to conduct rigorous analyses and review of the current state of the field so that a better understanding of the cross-talk between maternal factors, human milk composition and infant outcomes can be determined. Building upon prior reviews, this narrative review will synthesize the current state of ‘novel’ human milk component research, specifically focusing on nutrients and bioactive components that until recently were not known to exist in human milk 2,3.
1.1 ∣. Outline of review
Topics covered in this narrative review are as follows: 1) the theoretical framework for lactational programming of infant organ development, growth and later health outcomes; 2) possible mechanisms for absorption of milk ‘bioactives’ in the infant gut; 3) review of keystone animal cross-fostering studies that demonstrate plausibility for compounds in milk that are altered with maternal metabolic dysfunction and are bioactive for the offspring; 4) review of recent ‘novel’ milk bioactives including microRNA’s, lipokines/signaling lipids, small molecules/metabolites and fructose and 5) recommendations for study approaches and new research areas needed to advance the field. This section will not discuss hormones, adipokines or HMO’s given they have been the most studied and reported on to date.
2 ∣. LACTATIONAL PROGRAMMING OF OFFSPRING METABOLIC HEALTH
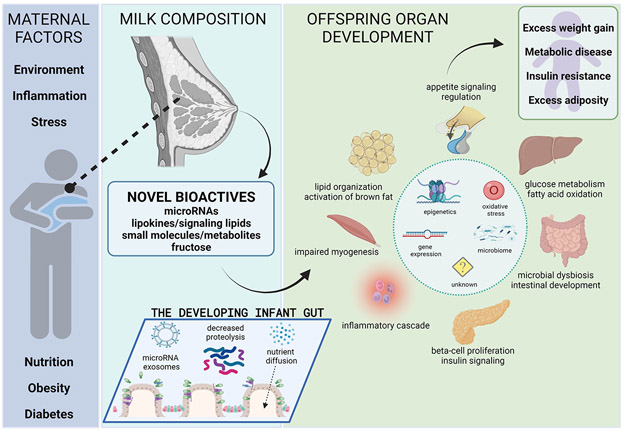
The concept of programming during critical windows of development was first hypothesized by Barker describing the “developmental origins of health and disease” 4. The field of developmental programming has rapidly expanded with both animal models and human studies identifying complex influences across the perinatal and postnatal period with a focus on the first 1000 days of life 2,5. Within this period of rapid growth and development of critical and vulnerable organ systems, a major factor in long term programming for metabolic disease risk is postnatal nutrition 6,7. The lactation window defines a key period where offspring survival is dependent on maternal milk with milk providing dynamic bioactive factors and nutrients that contribute to programming and future health (Figure 1) 2. Bartol termed the early exposure of biologically active factors through milk consumption and the importance on offspring health, the “lactocrine hypothesis” 8.
Figure 1.
Overview of lactational programming. Created with BioRender.com
The lactational period is a critical period open to insults, with differentiation of fragile organ-systems continuing during the early post-natal period, therefore setting the stage for heightened risk of future disease 2. Offspring organ development spans the prenatal through postnatal period including key developmental and maturation events occurring in the first days to months after birth ranging from completion of adipocyte development in the first months to ongoing islet and hepatic development until beyond ~2 years of life, after weaning has occurred 2,9. Regulation of energy homeostasis, metabolism and appetite is governed by the ongoing development of the hypothalamus, liver, muscle, pancreas, gut and adipose tissue that continues during the postnatal stage, but with timing that varies between atricial (incapable of movement after hatching, needing extensive parental care) and precocial (capable of movement upon hatching) species 10. While organ development and differentiation are well-organized, controlled and regulated processes, disruptions or alterations in signaling events precipitated by milk factors can impact downstream development 11. Proposed programming factors in breast milk include, but are not limited to, macronutrients, lipid species, amino acids, hormones (insulin, leptin, ghrelin, adiponectin, cortisol), adipokines, growth factors, metabolites, prebiotics, probiotics, cytokines, chemokines, anti-microbials, pro- inflammatory and anti-inflammatory signaling molecules, vitamins, toxins, cells, milk fat globule membranes, microRNAs, nucleotides and oligosaccharides 12,13. Emergent data is beginning to explore and identify mechanisms by which these milk components may potentially serve as mediators in programming offspring tissues and organ systems. The proposed mechanisms include epigenetic modifications 10,14, induction of oxidative stress 15, alterations in gene expression and translation through microRNAs 16,17 and infant intestinal microbial colonization 18.
3 ∣. MECHANISTIC FRAMEWORK FOR THE SYSTEMIC BIOACTIVITY OF MILK COMPONENTS IN THE INFANT
For non-nutrient milk components to play a causal role in infant health, they must remain “bioactive” after ingestion. First, although most macromolecules are degraded by digestive enzymes and are absorbed in the form of nutrients, some do resist both the low pH of the gastric fluid and proteolytic enzyme hydrolysis, allowing them to reach the small intestinal lumen. This is particularly the case for infants, in that the infant gut resists proteolysis due to early milk being high in protease inhibitors, immaturity of pancreatic enzymes, and the infant stomach having a higher pH than in later life 19. Second, the paracellular pathway allows diffusion of small molecules through tight junction pores and other structures in between the intestinal epithelial cells 20. Paracellular diffusion is a particularly important non-digestive absorptive pathway characteristic of the neonatal gut for macronutrients is 21. Third, transcellular transport of large particles, including microbes, has been ascribed to intestinal M cells as well as dendritic cells that can extend dendrites between epithelial cells to ample soluble antigens in the lumen allowing transport into the circulation 20. Finally, certain milk components, like microRNAs, are protected in exosomal vesicles which are also resistant to degradation 22 . Collectively, these biological/physiological pathways/mechanisms demonstrate unique aspects of newborn and milk physiology that allow a variety of large and small molecules in human milk to retain function and bioactivity in the infant gut and to be transported into the infant circulation.
4 ∣. CROSS-FOSTERING STUDIES
The study of the role of breast milk in infant development is complicated given the dynamic and multifaceted composition of milk, which is influenced by maternal health, maternal nutrition, stress, environmental exposures, stage of lactation, time of day, and individualization based on offspring needs 23. Animal models allow the specific study of exposures during discrete time periods during lactation, which is challenging to demonstrate in human studies. The granularity of these animal models provides conclusive evidence that milk composition confers metabolic signals that can either prevent or drive diabetes/obesity in the offspring.
Through maternal diet alteration (fat, carbohydrate, proteins, specific fatty acids), artificial rearing, cross fostering, litter size reduction and direct neonatal diet supplementation, researchers are able to narrow their focus to outcomes that result from exposures solely during the period of lactation in animal models 24-27. Mouse models have demonstrated milk composition is altered in the setting of maternal nutrition during lactation and maternal disease 23,28-30. We performed a recent review looking at these outcomes in depth 2. Since that time, additional mouse and rat models have shown that dietary exposures during lactation alter milk composition. Maternal high fat diet isolated to the lactation period, for example, resulted in milk alterations with increased milk fat, energy, cholesterol, omega-6/omega-3 polyunsaturated fatty acid ratio, and altered long chain fatty acid composition. In lactational high fat diet models offspring had resulting abnormal lipid accumulation in white adipose tissue and liver, adipocyte hypertrophy, insulin resistance, and divergent gut microbial diversity levels, all signs of offspring metabolic compromise 18,24-26,31,32. Monks et al set out to dissect the effects of maternal obesity versus maternal high fat diet during the lactation period. They found that pups reared by lean dams on a high fat diet during lactation had the fastest rate of growth with early increased visceral fat accumulation and inflammation, implicating maternal diet having a stronger impact on metabolic outcomes than maternal obesity in this mouse model 32. On the other hand, a rat model of caloric restriction during lactation identified higher levels of adiponectin and myo-inositol in milk 27,33. The changes in milk composition due to caloric restriction lead to changes in offspring long term health outcomes including lower body weight and better glucose control. These experiments carefully demonstrate the strong impact of maternal diet during lactation on rodent offspring.
Cross-fostering models looking at the effect maternal obesity plays during lactation, show a clear and consistent effect of increased obesity in offspring from control mothers fostered onto obese dams 34-36. These studies all reported an increased absolute offspring body weight with some even showing an increased relative fat mass (i.e. %fat) when obese dams suckled pups of lean dams. The main culprits in the increased somatic growth of the offspring suckled by obese dams is thought to be increased breast milk insulin, with leptin showing mixed findings 37,38. A possible target for these changes may be neuronal and lead to long-term alterations in food intake. Pups exposed to maternal diabetes during lactation by cross-fostering onto a gestational diabetes mellitus mother at birth had abnormal programming of both structure and function of hypothalamic neurons that resulted in a delayed growth pattern during the neonatal period 39. Collectively these cross-fostering studies (and others) demonstrate exposure to ‘obesogenic-diabetic’ milk during lactation to be linked with negative offspring future health. Confirmation is bi-directional in that maternal health via milk composition is a stronger driver (perhaps the strongest) in conferring the pup phenotype than in utero exposure. Gorski et al. reported offspring from obese dams fostered onto lean dams had improved insulin sensitivity (though no changes in body weight were observed) 35.
Translating these animal model findings to human research is challenging, however, given the limited ability to control for confounders such as maternal environmental exposures and offspring exposures during pregnancy in humans. A direct study of infant diets is also difficult as there is an inability to randomize to alternative nutrition interventions for infants, given the known benefits of providing human milk.
4 ∣. REVIEW OF NON-NUTRITIVE COMPONENTS
Extensive evaluation of the complex components in human milk and their associations with infant metabolic outcomes remains limited in current research. Previous review articles have importantly highlighted the roles of nutritive (fat, protein, carbohydrate) and non-nutritive (insulin, leptin, adiponectin, ghrelin, interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), toxins) components of human milk in lactational programming through animal models and human studies. Of the more thoroughly studied hormones, leptin (an appetite regulator), has shown early inverse associations with infant growth trajectory. However, the influences on human offspring outcomes remain inconsistent for many other non-nutritive factors which are limited by the small sample size, inability to control confounding factors, and limited number of studies. Beyond these factors, there are newly discovered human milk components that appear to have a more consistent relationships to infant growth. This review will synthesize the evidence for emerging bioactive compounds in human milk including miRNAs, lipokines/signaling lipids, small molecules/metabolites and fructose. Table 1 summarizes predominantly human studies of these novel factors focusing on studies during the window of lactation and programming outcomes.
Table 1.
Summary of predominantly human studies of novel factors focusing on studies during the window of lactation and programming outcomes
| Bioactive Component |
Author (Year) |
Species (n) |
Milk Collection Standardized |
Lactation Time Point(s) |
Maternal Influence | Infant Outcomes | Weakness/Limitations |
|---|---|---|---|---|---|---|---|
| miRNA | Xi (2016) | Human (n=86) | No | 2-5d, 3 mo | miRNA-30B, let-7a, miRNA-378 in colostrum & mature milk are negatively correlated with maternal BMI (pre-pregnancy & late pregnancy), weight Let-7a in mature milk is negative correlated with late pregnancy maternal weight No miRNA differences with gestational diabetes mellitus or gestational age. |
miRNA-30B & miRNA-378 were higher in colostrum for girls | No maternal diet data No infant anthropometrics |
| Zamanillo (2019) | Human (n=59) | Yes | 1mo, 2mo, 3mo | NW mothers had negative correlations between milk miRNAs expression Leptin/adiponectin levels observed in NW but not in OW/OB mothers |
In NW mothers, infant BMI at 2 years negatively correlated with miRNA at 2m (miR-103, miR-17, miR-181a, miR-222, miR-let7c, miR-146b). No infant growth correlations with OW/OB mothers |
No maternal diet data Infant growth at 2 years & maternal BMI were obtained by maternal recall |
|
| Shah (2021) | Human (n=60) | Yes | 1mo & 3mo | Decreased miRNA (miR-148a, miR-30b) abundance at 1mo in OW/OB mothers compared to NW mothers | miR-184a was negatively associated with infant weight, FM and FFM at 1m miR-32 was negatively associated with 1m infant weight miR-30b abundance was positively associated with 1m weight, %fat & fat mass. No significant associations were observed with 1m milk miRNA & 6m infant anthropometrics, or between 3m milk miRNA and infant anthropometrics at 3m or 6m |
No maternal diet data | |
| Lipokines&Signaling lipids | Brezinova (2018) | Human (n=53) & Mouse | No | 72 hr | PAHSAs levels were positively associated with weight gain in pregnancy. Obese mothers had lower total PAHSA and 5-PAHSA levels |
No outcomes examined | No maternal diet data. No infant outcomes examined |
| Bruun (2018) | Human (n=100) | No | 4mo | Only adjusted for maternal BMI | The low weight z-score group had higher concentrations of oleoylethanolamide, steroylethanolamide, palitoylethanolamide vs infants in the high weight z-score. Lower skinfold thickness & weight gain per day were associated with higher milk steroylethanolamide |
No early lactation samples. No maternal diet data. Storage techniques altered concentration of N-acylethanolamides. |
|
| Wolfs (2021) | Human (n=58) | Yes | 1mo, 3mo, 6mo | 12,13-diHOME positively associated with height, however no association with maternal pre-pregnancy BMI or gestational weight gain 12,13-diHOME concentration increased in milk after exercise at 1mo postpartum |
12,13-diHOME was positively associated with birth weight for length and BMI z-score, and negatively associated with in BMI & weight for length z-score over the first 6 months of life 12,13-diHOME was negatively associated with subcutaneous and overall adiposity at 1mo No association between infant sex and milk 12,13-diHOME levels High milk succinate was associated with lower infant BMI at 6mo |
No maternal diet data. | |
| Small molecules & Metabolites | Isganaitis (2019) | Human (n=35) | Yes | 1mo, 6mo | Maternal BMI was associated with 10 differing metabolites at 1mo 20 differing metabolites at 6mo 1mo, maternal obesity was linked to differences in human milk orotate & 1,5-anhydroglucitol 6mo maternal obesity was associated with increased acylcarnitine, monosaccharide, & 1,5-anhydroglucitol |
Adenine positively correlated with infant weight at 1mo, while X-19656 (an unidentified metabolite) was negative correlated 1mo, 5-methylthioadenosine was positively correlated with infant %fat and maternal BMI. No correlations between milk metabolites with infant weight or adiposity and maternal BMI at 6mo |
No maternal diet data. |
| Prentice (2019) | Human (n=619) | Yes | 4-8wks | SCFAs were not associated with maternal BMI Mothers exclusively breastfeeding had higher milk butyrate |
Butyrate was negatively associated with infant weight between 3mo-12mo Milk formic acid levels were negative associated with BMI over the first 2 years of life Acetate was negatively associated with skinfold thickness at 3mo |
No maternal diet data. | |
| Saben (2020) | Human (n=159) | Yes | 2wk, 2mo, 6mo | Maternal BMI & %fat positively correlated with amount of human milk monosaccharides & sugar alcohols | 6mo milk mannose, lyxitol & shikimic acid showed a positive association with infant fat mass & %fat | No maternal diet data. | |
| Ribo (2021) | Human (n=143) & Mouse | Yes | 1mo | Did not report maternal influence on milk betaine | Human milk betaine concentration was inversely associated with infant weight-for-length z-score at 1mo & 12mo | No maternal diet data | |
| In mice supplemented with betaine, a fivefold increase in betaine milk content was observed | In mice, early-exposure to betaine in lactation was associated with decreased adiposity & improved glucose homeostasis in adult mice. Betaine intake transiently increases offspring Akkermansia species during early life, which is linked to lower gut inflammation in mice |
||||||
| Fructose | Goran (2017) | Human (n=25) | Yes | 1mo, 6mo | Maternal BMI was used as a covariate | Milk fructose was associated with high infant body weight, lean mass, FM, weight for length z-score, & bone mineral content at 6mo | No maternal diet data. |
| Berger (2018) | Human (n=41) | Yes | 6 wks | HFCS-sweetened beverage increased milk fructose for up to 5 hrs without an impact on milk glucose or lactose. | None were collected | No infant outcomes No maternal diet data |
|
| Berger (2020) | Human (n=88) | NA | NA | No differences in maternal fructose or sugar-sweetened beverage + juice intake based on maternal pre-pregnancy BMI | Infant 24mo cognitive development scores were inversely correlated with fructose consumption by their mothers at 1mo postpartum No association at 6mo postpartum |
Homogenous sample (limited to Hispanic mother-infant dyads) No milk compositional analysis was performed |
4.1 ∣. miRNAs
A rather new class of bioactive components discovered in human milk and now being investigated are miRNAs. miRNAs are small, non-coding, single-stranded RNAs that modulate gene regulation by inhibiting mRNA translation and protein synthesis. Genes targeted by human milk miRNAs can impact many pathways, particularly carbohydrate and energy metabolism, immune function and brain development 40. Human milk is one of the richest sources of RNA and miRNAs amongst all body fluids 17. Most human milk miRNAs are transported and encapsulated by the lipid bilayer of extracellular vesicles, predominantly in exosomes. Exosomal miRNAs in breast milk are thereby protected from the enzymatic degradation that would occur in the digestive tract 41, allowing uptake by intestinal epithelial cells 22,42.
Multiple factors such as maternal nutrition, exercise, and maternal disease states (e.g. obesity and diabetes) can impact the abundance and constituency of human milk miRNAs. Studies investigating the role of maternal metabolic status on human milk miRNA composition are sparse. A study performed by Xi et al shows that some miRNAs involved in adipogenesis (miRNAs-let-7a, 378 and 30b) are negatively correlated with maternal pre-pregnancy BMI 43. Further, a study performed by Zamanillo et al showed that maternal obesity altered the breast milk supply of some miRNAs, their association with milk bioactive factors such as leptin and adiponectin, and their impact on infant BMI at two years of age 44. Our group has recently shown the abundance of selected miRNAs involved in important metabolic pathways such as insulin signaling and adipogenesis (miRNAs-148a-3p and 30b-5p) are decreased in human milk exosomes obtained from overweight or obese women 45. In the same study, these miRNAs were also associated with infant weight and adiposity at 1 month of age suggesting potential role of miRNAs in regulating infant growth and body composition. Mechanistic studies into the effects of human milk miRNAs on growth, development and early infant programming are lacking. Future mechanistic studies are needed to examine the uptake and targets of ingested human milk miRNAs, not only in the infant gut but also in the metabolically active tissues such as liver, pancreas identifying their functional role.
4.2 ∣. LIPOKINES AND SIGNALING LIPIDS
Based on accumulating data over the last 15 years, certain lipids are now recognized as having hormone-like effects in the regulation of insulin sensitivity, pancreatic beta cell function, inflammation and other cellular processes, leading Hotamisligli and colleagues to coin the term “lipokine” 46. Recent studies indicate that human milk is an abundant source of lipokines and are associated with infant growth and metabolism. For example, fatty acid esters of hydroxy fatty acids (FAHFAs) are endogenous lipids with known insulin-sensitizing potential and anti-inflammatory properties 47,48. Recently, Brezinova et al studied the role of a specific FAHFA, palmitic acid hydroxystearic acid in the milk of normal weight and obese women 49. Their findings were quite interesting in that: a) for the first time the presence of FAHFs in breast milk were detected (previously, it was thought FAHFAs only existed in serum and white adipose tissue) and b) palmitic acid hydroxystearic acid was significantly lower in milk from obese mothers. These findings perhaps, represent a pathway where milk from obese mothers whom were shown to have lower concentrations of anti-inflammatory and pro-insulin sensitizing FAHFAs provide a double-hit to their offspring, with the first hit occurring in utero and the second during lactation.
The regulation of food intake and appetite are in part regulated by palmitoylethanolamide (PEA), N-acylethanolamine lipids oleoylethanolamide (OEA) and stearoylethanolamide (SEA) 50. Human milk concentrations of these appetite regulators were studied in mother/infant dyads from the Odense Child Cohort (an unselected, prospective birth cohort in Odense, Denmark) 51. Breast milk was collected at 4-months in 100 infants divided equally into one of two groups, low weight-for-age Z-score (L-WAZ score) or high weight-for-age Z-score (H-WAZ score). Key findings from this study were: a) OEA, SEA and PEA concentrations were lower in the H-WAZ group vs. the L-WAZ group (p < 0.05); and b) higher concentrations of SEA were associated with lower triceps skinfold thickness and lower weight gain per day (birth to date of testing). These data demonstrate that key appetite regulators are not only present in breast milk but may play a part in driving growth and fat deposition.
More recently, certain lipokines have been shown to play a central role in the regulation of thermogenesis and brown adipose tissue function. For example, the oxylipin 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME) was shown to be released into plasma in response to cold exposure, and to stimulate brown adipose tissue fuel uptake and thermogenesis 52. Our group recently identified 12,13-diHOME in human milk and reported an inverse correlation between abundance of this oxylipin and infant adiposity 53. Interestingly, maternal moderate exercise resulted in a significant increase in milk 12,13-diHOME, raising the intriguing possibility that maternal interventions might enhance milk content of obesegenic-protecting factors 54. Further reinforcing the important, emerging connection between breastmilk and adipose tissue metabolism in infancy, the lab of Tamas Roszer recently described an elegant mechanism by which alkylglycerol-type ether lipids in human milk play a role in downregulating the shift from brown-fat like metabolically active “beige” adipocytes to lipid-storing “white” adipocytes 55. Alkylglycerol lipids are metabolized by adipose tissue macrophages, triggering beige adipocyte development in the infant via a signaling cascade involving IL-6 and STAT3. Notably, such alkylglycerol lipids are unique to human milk, and are largely absent from infant formulas and adult diets. Together, these data suggest that human milk influences childhood obesity risk via lipid-dependent effects on adipose tissue metabolism.
4.3 ∣. SMALL MOLECULES AND METABOLITES
With the advent of high throughput “metabolomics” platforms, which allow the comprehensive quantification of up to 1000 small molecules and metabolic intermediates in a small biological sample, there has been increasing interest in how the human milk metabolome may be influenced by maternal phenotype, and how these small molecules in turn may regulate infant growth and metabolism. Metabolomic analyses have proved to be a valuable approach for biomarker discovery in developmental programming and metabolism research 56. Considered “downstream” from the genome, transcriptome, and proteome, the metabolome also can be influenced by the nutritional environment and the gut microbiome. As such, metabolomics integrates multiple aspects of metabolic health, providing a sensitive research tool. Analyses of human milk have to date identified an association of maternal BMI with the milk metabolome. The first report on the human milk metabolome, published in 2013 by Smilowitz and colleagues, detected 65 low-molecular-weight metabolites including carbohydrates, oligosaccharides, amino acids, fatty acids, vitamins, and nucleotides. A key finding was wide inter-individual variation in the amount and composition of human milk oligosaccharides 57. This is especially intriguing given the observation that human milk oligosaccharides play a role in immune regulation, bacterial defenses, and infant growth 58.
More recently, our group used an untargeted metabolomics platform to analyze associations among the human milk metabolome, maternal obesity, and infant body composition 59. At 1 month postpartum, only 10 of 275 detectable metabolites differed between overweight/obese and lean women at a significance threshold of P < 0.05; at 6 months postpartum 20 of 275 metabolites were altered with P<0.05. Although the number of altered metabolites was relatively small, it was notable that many of the metabolites that were altered by maternal obesity belonged to the same chemical class, with enrichment of purine nucleotide derivatives, and human milk oligosaccharides. Indeed, milk content of the nucleotide adenine was positively correlated both with maternal BMI, and with infant adiposity and fat accrual (DXA) between 1 and 6 months postpartum. This is intriguing considering clinical trial data and meta-analyses indicating that nucleotide supplementation of cow’s milk based infant formulas results in accelerated postnatal weight gain 60. Thus, human milk nucleotides may represent an “obesigenic” factor in human milk.
In 2021, Saben et al. published the largest study on the metabolomic composition of human milk reported to date 61. The metabolome of human milk was analyzed at 2 weeks, 2 months, and 6 months postpartum in women with normal (BMI < 25.0) vs. obese (BMI ≥ 30.0) pre-pregnancy BMI. This repeated-sampling approach allowed the authors to identify a time-dependent effect of maternal obesity on milk composition; they report a significant interaction of maternal BMI and time on milk content of metabolites related to amino acid metabolism. Their findings suggest that maternal BMI alters the evolution of human milk during the postnatal period from “transitional” to “mature” milk. Notably, 111 metabolites, or nearly a third of the 355 metabolites that were identified using their GC-MS approach, were significantly associated with maternal BMI (P < 0.05), with milk from obese mothers having higher abundance of monosaccharides and sugar alcohols. In turn, a proportion of the differentially abundant metabolites (e.g., mannose, lyxitol, shikimic acid) were associated with infant adiposity. These results support the possibility that components of human milk may be mediators of mother–child transmission of obesity risk, although these relationships would need to be validated using experimental studies in in vitro or in vivo systems.
From a mechanistic perspective, the human milk metabolome has the potential to influence infant growth via several distinct mechanisms, including direct interactions between individual milk biochemicals and infant intestinal or immune cells, nutritional effects (e.g. providing higher or lower amounts of essential amino acids or vitamins), effects of absorbed metabolites on distant organs, effects on the developing infant microbiome, and other pathways. The possibility of metabolome – microbiome interactions is supported by a recent study by Ribo et al., which found reduced levels of betaine (a one-carbon metabolite previously linked to cardiovascular and diabetes risk 62,63) in milk from obese mothers 64. Betaine supplementation of mouse dams resulted in increased betaine content in milk, and long-term improvements in metabolic health of offspring including decreased adiposity and improved glucose tolerance, via a microbiome-dependent mechanism. The role of milk components in regulating offspring energy metabolism is suggested by Prentice et al. who showed that milk short chain fatty acid butyrate content was protective against excess infant weight gain and adiposity development in the first year of life 65.
In sum, the milk metabolome is altered in the setting of maternal obesity and many metabolites have been linked to infant health outcomes. The extent to which modifiable risk factors such as gestational weight gain, glucose homeostasis, or exercise might affect the milk metabolome, and whether metabolomic fingerprints of maternal obesity might be reversible, will be important areas for future studies.
4.4 ∣. FRUCTOSE
There is evidence that maternal dietary intake during lactation may modify and/or introduce nutrients into human milk that are then transmitted to the nursing infant. However, recent data in this realm are limited, as few studies have collected detailed dietary assessments of mothers during the postnatal period. Dynamic changes in the food environment may inadvertently expose nursing infants to novel constituents of Western-style diets that may not be ideal at this life stage. For example, a randomized cross-over trial revealed that mothers who consumed a sugar-sweetened beverage containing fructose had significantly higher concentrations of milk fructose, as compared to those consuming a control beverage 66. Indeed, mothers who consumed the fructose-containing beverage had increased concentrations of human milk fructose above baseline which persisted up to five hours after intake 66. Infant exposure to milk fructose through human milk, even at low levels, has been associated with greater fat mass and lean mass in nursing infants at 6 months of age 67 as well as poorer neurodevelopmental outcomes at 2 years 68. These findings may inform dietary recommendations for mothers during the period of exclusive human milk feeding, as high fructose corn syrup is not a naturally-occurring sugar in human milk.
5 ∣. CURRENT STATE AND RECOMMENDATIONS TO ADVANCE THE FIELD
Based on the current state of the literature we have identified 5 domains in human milk research that limit our collective knowledge and need to be breached to propel the field forward. Theoretically, milk is of immense importance as a carrier of metabolic cues between parental and offspring generations, over and above as its importance as a nutrient source. While human data is still limited, results from animal models are more conclusive 2, suggesting numerous impacts during the highly plastic period of early development, including the shaping of the early infant gut, immune system and key metabolic systems responsible for glucose and weight regulation. In the hopes of propelling the field forward, we propose some solutions to identified weaknesses in the field.
-
Limited statistical power. To date, most studies in the literature are sorely underpowered, do not take into account important covariates and many times are samples of convenience. These issues decrease inference and the ability to make definitive conclusions.
Action needed: Adequately and appropriately powered studies to definitively answer study hypothesis. Consortia of individual studies with similar sampling methods should be established and funded to combine harmonizable data to address the above hypotheses in large numbers of mothers and infants/children.
-
Cross-sectional study designs limiting determination of temporality. Currently most studies capture one time point or in some cases two over several months (though rarely) but few are carried out past 3 months. We acknowledge these sorts of studies are hard to implement and execute but are sorely needed to advance the field and understand compositonal changes in milk over time.
Action needed: Conduct longitudinal studies that include colostrum, transitional milk and mature milk to 6-12 months (but 6 months if at all possible), with repetitive and synchronized collections of both maternal serum (to gauge maternal inflammation, nutrient status, etc), human milk, and infant outcomes.
-
Poor coordination between basic animal models and human study designs. Experimental studies in animals provide needed mechanistic precision and causal support for lactational programming, where randomization of women to most dietary or lactational interventions would be unethical. However, human studies are always needed given species variation in milk composition, infant nutrient requirements, metabolic physiology, early growth and development and life history characteristics. Further, human studies are needed to appropriately judge the relative and absolute contributions on the infant of the clustered and socially determined maternal conditions mentioned above (e.g., obesity, diet quality and breast feeding duration). Animal models should also take into consideration mixed feedings and timing of introduction of solid foods, both of which are important influences for many babies.
Action needed: Basic scientists and human clinical scientists must work more closely together on shared aims, with iterative and cross-species replication/experimentation, in order to build a base for causal evidence of lactational programming and make machanistic discoveries that are properly rooted in human epidemiology and have the potential for translation.
-
Lack of consideration of for maternal metabolic phenotypes (gestational weight gain, obesity status and diabetes status) during pregnancy and lactation. Few studies take into account maternal obesity and/or diabetes status and their impact on the composition of breastmilk. Expansion and cooperation between pregnancy studies and infant prospective cohorts would also enhance the information that can be gathered from these costly and time-intensive study designs. This information would also allow investigators to parse out lactational versus gestational programming effects and how the lactation exposures may modify gestational stressors.
Action needed: Per routine, all milk studies would collect clinicaly relevant pre-pregnancy and pregnancy data, such as BMI, weight gain, glucose control and other metabolic markers.
-
Poor and or lack of collection of maternal diet assessment during lactation. Though the scientific community as a whole recognizes the importance in the use of nutritional surveys, the practical assessment in the dietary intake on an individual level is challenging in the best of circumstances, partly due to the complexity and frequently changing nature of dietary habits 69. The ultimate goal of dietery assessment is to better understand the impact of dietary intake of specific nutrients on the composition of milk and the outcome in the infant.
Action needed: Human milk researchers should partner with registered dietitians or other nutrition researchers to determine how to sample maternal dietary habits in prospective mother-infant studies.
6 ∣. Concluding Statements
It is important that as the field moves forward, we remain in unwavering pursuit of a detailed knowledge of breast milk, the complex suite of factors/signals impacting its composition and the molecular roles that identified and yet to be identified human milk factors play on child obesity and other aspects of long-term offspring health.
FUNDING INFORMATION
DAF, EWD and PKB are supported by the National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH) under Award Numbers R01HD080444 and K99HD098288; while BG is supported by the National Institute for Diabetes, Digestive and Kidney Diseases of the NIH (NIDDK) under Award Number R56DK121787 and EI supported by NIDDK 5U01DK061230-15 (PI: Drews/Zeitler) and (P30DK036836 –PI: George King). LE, KS, SV and GP do not have any funding to report. The opinions expressed are those of the authors and do not necessarily represent those of the NIH or any other organization
Footnotes
CONFLICT OF INTEREST STATEMENTS
The authors declare that they have no competing interests.
REFERENCES
- 1.Casazza K, Brown A, Astrup A, et al. Weighing the Evidence of Common Beliefs in Obesity Research. Crit Rev Food Sci Nutr. Dec 6 2015;55(14):2014–53. doi: 10.1080/10408398.2014.922044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellsworth L, Harman E, Padmanabhan V, Gregg B. Lactational programming of glucose homeostasis: a window of opportunity. Reproduction (Cambridge, England). Aug 2018;156(2):R23–r42. doi: 10.1530/rep-17-0780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fields DA, Schneider CR, Pavela G. A narrative review of the associations between six bioactive components in breast milk and infant adiposity. Obesity (Silver Spring). Jun 2016;24(6):1213–21. doi: 10.1002/oby.21519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ. The fetal and infant origins of adult disease. BMJ. Nov 17 1990;301(6761):1111. doi: 10.1136/bmj.301.6761.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson PO. How Sweet It Is: Sweeteners in Breast Milk. Breastfeed Med. Jan/Feb 2019;14(1):14–16. doi: 10.1089/bfm.2018.0228 [DOI] [PubMed] [Google Scholar]
- 6.Schwarzenberg SJ, Georgieff MK, Committee On N. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics. Feb 2018;141(2)doi: 10.1542/peds.2017-3716 [DOI] [PubMed] [Google Scholar]
- 7.Mameli C, Mazzantini S, Zuccotti GV. Nutrition in the First 1000 Days: The Origin of Childhood Obesity. Int J Environ Res Public Health. Aug 23 2016;13(9)doi: 10.3390/ijerph13090838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartol FF, Wiley AA, Bagnell CA. Epigenetic programming of porcine endometrial function and the lactocrine hypothesis. Reprod Domest Anim. Jul 2008;43 Suppl 2:273–9. doi: 10.1111/j.1439-0531.2008.01174.x [DOI] [PubMed] [Google Scholar]
- 9.Bagnell CA, Bartol FF. Review: Maternal programming of development in the pig and the lactocrine hypothesis. Animal. Dec 2019;13(12):2978–2985. doi: 10.1017/S1751731119001654 [DOI] [PubMed] [Google Scholar]
- 10.Marousez L, Lesage J, Eberle D. Epigenetics: Linking Early Postnatal Nutrition to Obesity Programming? Nutrients. Dec 5 2019;11(12)doi: 10.3390/nu11122966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puttabyatappa M, Cardoso RC, Padmanabhan V. Effect of maternal PCOS and PCOS-like phenotype on the offspring's health. Mol Cell Endocrinol. Nov 5 2016;435:29–39. doi: 10.1016/j.mce.2015.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gila-Diaz A, Arribas SM, Algara A, et al. A Review of Bioactive Factors in Human Breastmilk: A Focus on Prematurity. Nutrients. Jun 10 2019;11(6)doi: 10.3390/nu11061307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. Feb 2013;60(1):49–74. doi: 10.1016/j.pcl.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartwig FP, Loret de Mola C, Davies NM, Victora CG, Relton CL. Breastfeeding effects on DNA methylation in the offspring: A systematic literature review. PLoS One. 2017;12(3):e0173070. doi: 10.1371/journal.pone.0173070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conceicao EP, Franco JG, Oliveira E, et al. Oxidative stress programming in a rat model of postnatal early overnutrition--role of insulin resistance. J Nutr Biochem. Jan 2013;24(1):81–7. doi: 10.1016/j.jnutbio.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 16.Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. Mar 1 2010;1(1):7. doi: 10.1186/1758-907X-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsaweed M, Hartmann PE, Geddes DT, Kakulas F. MicroRNAs in Breastmilk and the Lactating Breast: Potential Immunoprotectors and Developmental Regulators for the Infant and the Mother. Int J Environ Res Public Health. 2015;12(11):13981–4020. doi: 10.3390/ijerph121113981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Zhang Y, Miller D, et al. Microbial Reconstitution Reverses Early Female Puberty Induced by Maternal High-fat Diet During Lactation. Endocrinology. Feb 1 2020;161(2)doi: 10.1210/endocr/bqz041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonnerdal B Bioactive proteins in human milk: mechanisms of action. J Pediatr. Feb 2010;156(2 Suppl):S26–30. doi: 10.1016/j.jpeds.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 20.Menard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. May 2010;3(3):247–59. doi: 10.1038/mi.2010.5 [DOI] [PubMed] [Google Scholar]
- 21.Wada Y, Lonnerdal B. Bioactive peptides derived from human milk proteins--mechanisms of action. J Nutr Biochem. May 2014;25(5):503–14. doi: 10.1016/j.jnutbio.2013.10.012 [DOI] [PubMed] [Google Scholar]
- 22.Liao Y, Du X, Li J, Lonnerdal B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol Nutr Food Res. Nov 2017;61(11)doi: 10.1002/mnfr.201700082 [DOI] [PubMed] [Google Scholar]
- 23.Vogt MC, Paeger L, Hess S, et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell. Jan 30 2014;156(3):495–509. doi: 10.1016/j.cell.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuduki T, Kitano Y, Honma T, Kijima R, Ikeda I. High dietary fat intake during lactation promotes development of diet-induced obesity in male offspring of mice. J Nutr Sci Vitaminol (Tokyo). 2013;59(5):384–92. doi: 10.3177/jnsv.59.384 [DOI] [PubMed] [Google Scholar]
- 25.Tsuduki T, Yamamoto K, Hatakeyama Y, Sakamoto Y. High dietary cholesterol intake during lactation promotes development of fatty liver in offspring of mice. Mol Nutr Food Res. May 2016;60(5):1110–7. doi: 10.1002/mnfr.201500736 [DOI] [PubMed] [Google Scholar]
- 26.Zhao M, Li Y, Yao H, et al. Sex-specific Alterations in Serology and the Expression of Liver FATP4 Protein in Offspring Exposed to High-Fat Diet during Pregnancy and/or Lactation. Lipids. Mar 2018;53(3):301–311. doi: 10.1002/lipd.12029 [DOI] [PubMed] [Google Scholar]
- 27.Palou M, Torrens JM, Castillo P, Sanchez J, Palou A, Pico C. Metabolomic approach in milk from calorie-restricted rats during lactation: a potential link to the programming of a healthy phenotype in offspring. Eur J Nutr. Apr 2020;59(3):1191–1204. doi: 10.1007/s00394-019-01979-6 [DOI] [PubMed] [Google Scholar]
- 28.Korotkova M, Gabrielsson B, Hanson LA, Strandvik B. Maternal dietary intake of essential fatty acids affects adipose tissue growth and leptin mRNA expression in suckling rat pups. Pediatr Res. Jul 2002;52(1):78–84. doi: 10.1203/00006450-200207000-00015 [DOI] [PubMed] [Google Scholar]
- 29.Hadley KB, Guimont-Desrochers F, Bailey-Hall E, Salem N Jr., Yurko-Mauro K, Field CJ, Supplementing dams with both arachidonic and docosahexaenoic acid has beneficial effects on growth and immune development. Prostaglandins Leukot Essent Fatty Acids. Nov 2017;126:55–63. doi: 10.1016/j.plefa.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 30.Kucia M, Langhammer M, Gors S, et al. High-protein diet during gestation and lactation affects mammary gland mRNA abundance, milk composition and pre-weaning litter growth in mice. Animal. Feb 2011;5(2):268–77. doi: 10.1017/S1751731110001734 [DOI] [PubMed] [Google Scholar]
- 31.de Los Rios EA, Ruiz-Herrera X, Tinoco-Pantoja V, et al. Impaired prolactin actions mediate altered offspring metabolism induced by maternal high-fat feeding during lactation. FASEB J. Jun 2018;32(6):3457–3470. doi: 10.1096/fj.201701154R [DOI] [PubMed] [Google Scholar]
- 32.Monks J, Orlicky DJ, Stefanski AL, et al. Maternal obesity during lactation may protect offspring from high fat diet-induced metabolic dysfunction. Nutr Diabetes. Apr 25 2018;8(1):18. doi: 10.1038/s41387-018-0027-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexandre-Gouabau MC, David-Sochard A, Royer AL, Parnet P, Paille V. Moderate High Caloric Maternal Diet Impacts Dam Breast Milk Metabotype and Offspring Lipidome in a Sex-Specific Manner. Int J Mol Sci. Jul 30 2020;21(15)doi: 10.3390/ijms21155428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oben JA, Mouralidarane A, Samuelsson AM, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. Jun 2010;52(6):913–20. doi: 10.1016/j.jhep.2009.12.042 [DOI] [PubMed] [Google Scholar]
- 35.Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. Am J Physiol Regul Integr Comp Physiol. Sep 2006;291(3):R768–78. doi:00138.2006 [pii] 10.1152/ajpregu.00138.2006 [DOI] [PubMed] [Google Scholar]
- 36.Reifsnyder PC, Churchill G, Leiter EH. Maternal environment and genotype interact to establish diabesity in mice. Genome Res. Oct 2000;10(10):1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vickers MH, Gluckman PD, Coveny AH, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. Oct 2005;146(10):4211–6. doi: 10.1210/en.2005-0581 [DOI] [PubMed] [Google Scholar]
- 38.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. Apr 2 2004;304(5667):108–10. doi: 10.1126/science.1095004 [DOI] [PubMed] [Google Scholar]
- 39.Fahrenkrog S, Harder T, Stolaczyk E, et al. Cross-fostering to diabetic rat dams affects early development of mediobasal hypothalamic nuclei regulating food intake, body weight, and metabolism. J Nutr. Mar 2004;134(3):648–54. [DOI] [PubMed] [Google Scholar]
- 40.Lonnerdal B Human Milk MicroRNAs/Exosomes: Composition and Biological Effects. Nestle Nutr Inst Workshop Ser. 2019;90:83–92. doi: 10.1159/000490297 [DOI] [PubMed] [Google Scholar]
- 41.Zempleni J, Aguilar-Lozano A, Sadri M, et al. Biological Activities of Extracellular Vesicles and Their Cargos from Bovine and Human Milk in Humans and Implications for Infants. J Nutr. Jan 2017;147(1):3–10. doi: 10.3945/jn.116.238949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melnik BC, Kakulas F, Geddes DT, et al. Milk miRNAs: simple nutrients or systemic functional regulators? Nutr Metab (Lond). 2016;13:42. doi: 10.1186/s12986-016-0101-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xi Y, Jiang X, Li R, Chen M, Song W, Li X. The levels of human milk microRNAs and their association with maternal weight characteristics. Eur J Clin Nutr. Apr 2016;70(4):445–9. doi: 10.1038/ejcn.2015.168 [DOI] [PubMed] [Google Scholar]
- 44.Zamanillo R, Sanchez J, Serra F, Palou A. Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI. Nutrients. Oct 28 2019;11(11)doi: 10.3390/nu11112589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah KB, Chernausek SD, Garman LD, et al. Human Milk Exosomal MicroRNA: Associations with Maternal Overweight/Obesity and Infant Body Composition at 1 Month of Life. Nutrients. Mar 27 2021;13(4)doi: 10.3390/nu13041091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. Sep 19 2008;134(6):933–44. doi: 10.1016/j.cell.2008.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yore MM, Syed I, Moraes-Vieira PM, et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. Oct 9 2014;159(2):318–32. doi: 10.1016/j.cell.2014.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liberati-Cizmek AM, Bilus M, Brkic AL, et al. Analysis of Fatty Acid Esters of Hydroxyl Fatty Acid in Selected Plant Food. Plant Foods Hum Nutr. Jun 2019;74(2):235–240. doi: 10.1007/s11130-019-00728-8 [DOI] [PubMed] [Google Scholar]
- 49.Brezinova M, Kuda O, Hansikova J, et al. Levels of palmitic acid ester of hydroxystearic acid (PAHSA) are reduced in the breast milk of obese mothers. Biochim Biophys Acta Mol Cell Biol Lipids. Feb 2018;1863(2):126–131. doi: 10.1016/j.bbalip.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 50.Witkamp RF. The role of fatty acids and their endocannabinoid-like derivatives in the molecular regulation of appetite. Mol Aspects Med. Dec 2018;64:45–67. doi: 10.1016/j.mam.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 51.Bruun S, Gouveia-Figueira S, Domellof M, et al. Satiety Factors Oleoylethanolamide, Stearoylethanolamide, and Palmitoylethanolamide in Mother's Milk Are Strongly Associated with Infant Weight at Four Months of Age-Data from the Odense Child Cohort. Nutrients. Nov 13 2018;10(11)doi: 10.3390/nu10111747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lynes MD, Leiria LO, Lundh M, et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med. May 2017;23(5):631–637. doi: 10.1038/nm.4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolfs D, Lynes MD, Tseng YH, et al. Brown Fat-Activating Lipokine 12,13-diHOME in Human Milk Is Associated With Infant Adiposity. J Clin Endocrinol Metab. Jan 23 2021;106(2):e943–e956. doi: 10.1210/clinem/dgaa799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roszer T Further Evidence that Breast Milk Lipids Control Adiposity. J Clin Endocrinol Metab. Mar 8 2021;106(3):e1458–e1459. doi: 10.1210/clinem/dgaa910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu H, Dilbaz S, Cossmann J, et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J Clin Invest. May 13 2019;130:2485–2499. doi: 10.1172/JCI125646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perng W, Villamor E, Shroff MR, et al. Dietary intake, plasma homocysteine, and repetitive element DNA methylation in the Multi-Ethnic Study of Atherosclerosis (MESA). Nutr Metab Cardiovasc Dis. Jun 2014;24(6):614–22. doi: 10.1016/j.numecd.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smilowitz JT, O'Sullivan A, Barile D, German JB, Lonnerdal B, Slupsky CM. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr. Nov 2013;143(11):1709–18. doi: 10.3945/jn.113.178772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinde K, Lewis ZT. MICROBIOTA. Mother's littlest helpers. Science. Jun 26 2015;348(6242):1427–8. doi: 10.1126/science.aac7436 [DOI] [PubMed] [Google Scholar]
- 59.Isganaitis E, Venditti S, Matthews TJ, Lerin C, Demerath EW, Fields DA. Maternal obesity and the human milk metabolome: associations with infant body composition and postnatal weight gain. Am J Clin Nutr. Jul 1 2019;110(1):111–120. doi: 10.1093/ajcn/nqy334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, Mu S, Xu X, Shi Z, Shen L. Effects of dietary nucleotide supplementation on growth in infants: a meta-analysis of randomized controlled trials. Eur J Nutr. Apr 2019;58(3):1213–1221. doi: 10.1007/s00394-018-1640-2 [DOI] [PubMed] [Google Scholar]
- 61.Saben JL, Sims CR, Piccolo BD, Andres A. Maternal adiposity alters the human milk metabolome: associations between nonglucose monosaccharides and infant adiposity. Am J Clin Nutr. Nov 11 2020;112(5):1228–1239. doi: 10.1093/ajcn/nqaa216 [DOI] [PubMed] [Google Scholar]
- 62.Walford GA, Ma Y, Clish C, et al. Metabolite Profiles of Diabetes Incidence and Intervention Response in the Diabetes Prevention Program. Diabetes. May 2016;65(5):1424–33. doi: 10.2337/db15-1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porcu E, Gilardi F, Darrous L, et al. Triangulating evidence from longitudinal and Mendelian randomization studies of metabolomic biomarkers for type 2 diabetes. Sci Rep. Mar 18 2021;11(1):6197. doi: 10.1038/s41598-021-85684-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ribo S, Sanchez-Infantes D, Martinez-Guino L, et al. Increasing breast milk betaine modulates Akkermansia abundance in mammalian neonates and improves long-term metabolic health. Sci Transl Med. Mar 31 2021;13(587)doi: 10.1126/scitranslmed.abb0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prentice PM, Schoemaker MH, Vervoort J, et al. Human Milk Short-Chain Fatty Acid Composition is Associated with Adiposity Outcomes in Infants. J Nutr. May 1 2019;149(5):716–722. doi: 10.1093/jn/nxy320 [DOI] [PubMed] [Google Scholar]
- 66.Berger PK, Fields DA, Demerath EW, Fujiwara H, Goran MI. High-Fructose Corn-Syrup-Sweetened Beverage Intake Increases 5-Hour Breast Milk Fructose Concentrations in Lactating Women. Nutrients. May 24 2018;10(6)doi: 10.3390/nu10060669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goran MI, Martin AA, Alderete TL, Fujiwara H, Fields DA. Fructose in Breast Milk Is Positively Associated with Infant Body Composition at 6 Months of Age. Nutrients. Feb 16 2017;9(2)doi: 10.3390/nu9020146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berger PK, Plows JF, Jones RB, et al. Associations of maternal fructose and sugar-sweetened beverage and juice intake during lactation with infant neurodevelopmental outcomes at 24 months. Am J Clin Nutr. Dec 10 2020;112(6):1516–1522. doi: 10.1093/ajcn/nqaa255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Webb D, Leahy MM, Milner JA, et al. Strategies to optimize the impact of nutritional surveys and epidemiological studies. Adv Nutr. Sep 1 2013;4(5):545–7. doi: 10.3945/an.113.004259 [DOI] [PMC free article] [PubMed] [Google Scholar]